Potential of Waste Biomass from the Sugar Industry as a Source of Furfural and Its Derivatives for Use as Fuel Additives in Poland
Abstract
1. Introduction
2. Furfural
- PN-EN ISO 14040:2009 Environmental management. Life Cycle Assessment. Principles and structure. This standard covers the basic principles and structure of an LCA without describing the method in detail.
- PN-EN ISO 14044:2009 Environmental management. Life Cycle Assessment. Requirements and guidelines.
3. Furfuryl Alcohol (FA) and Tetrahydrofurfuryl Alcohol (THFA)
- from developing countries—694,478,000 kg, which was 5.36% of the total mass. China was the only developing country that supplied furfuryl and tetrahydrofurfuryl alcohol.
- from other developed countries—4,827,918,000 kg, which constituted 37.28% of the total mass. The main importer in this group of countries was South Africa, supplying 97.75% by weight. The remaining imports came from countries such as Switzerland, Canada, Norway, and the United States of America.
- from the European Union—7,428,604,000 kg, which constituted 57.36% of the total weight. Among the EU countries, Belgium was the main importer, supplying 91.51% of imports from EU countries. Other imports came from countries such as Austria, the Netherlands, France, Germany, Italy, and the United Kingdom.
4. Results
5. Materials and Methods
5.1. Lignocellulosic Plant Material
5.2. Acidic Hydrolysis of Biomass
5.3. Catalytic Reduction of Furfural
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- EU. Communication of the European Commission on Integrated Product Policy; EU: Brussels, Belgium, 2003; Volume 302. [Google Scholar]
- Tao, L.; Aden, A. The economics of current and future biofuels. In Vitro Cell. Dev. Biol. Plant 2009, 45, 199–217. [Google Scholar] [CrossRef]
- Hira, A. Sugar rush: Prospects for a global ethanol market. Energy Policy 2011, 39, 6925–6935. [Google Scholar] [CrossRef]
- Bos, H.L.; Meesters, K.P.H.; Conijn, S.G.; Corre, W.J.; Patel, M.K. Accounting for the constrained availability of land: A comparison of bio-based ethanol, polyethylene, and PLA with regard to non-renewable energy use and land use. Biofuels Bioprod. Biorefin. 2012, 6, 146–158. [Google Scholar] [CrossRef]
- De Jong, W.; Marcotullio, G. Overview of biorefineries on co-production of furfural, existing concepts and novel developments. Int. J. Chem. Reactor Eng. 2010, 8, A69. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Makkee, M.; Van Diepen, A.E. Chemical Process Technology, 2nd ed.; John Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
- Silva, J.F.L.; Selicani, M.A.; Junqueira, T.L.; Klein, B.C.; Vaz Junior, S.; Bonomi, A. Integrated furfural and first generation bioethanol production: Process simulation and technoeconomic analysis. Braz. J. Chem. Eng. 2017, 34, 623–634. [Google Scholar] [CrossRef]
- Shittu, A. Catalytic Conversion of Hemicellulosic Sugars into Furfural in Ionic Liquid Media. Master’s Thesis, The University of Toledo, Toledo, OH, USA, 2010. [Google Scholar]
- Win, D.T. Furfural—Gold from Garbage. AU J. Technol. 2005, 8, 185–190. [Google Scholar]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sus. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of liquid alkanes by aqueous-phase processing of biomass derived carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef]
- Xu, W.; Xia, Q.; Zhang, Y.; Guo, Y.; Wang, Y.; Lu, G. Effective Production of Octane from Biomass Derivatives under Mild Conditions. ChemSusChem 2011, 4, 1758–1761. [Google Scholar] [CrossRef]
- Malinowski, A.; Wardzińska, D. Katalityczna konwersja furfuralu do biokomponentów paliwowych. Chemik 2012, 66, 982–990. [Google Scholar]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Dong, S.Y.; Shi, Q.L.; Ma, Y.S.; Mou, Q.P.; Zhang, J.L.; Luan, B. Gasoline Octane Number Enhancer and Preparation Method Thereof. CN102746919A, 24 October 2012. [Google Scholar]
- Nikul’shin, P.A.; Ershov, M.A.; Grigor’yeva, E.V.; Tarazanov, S.V.; Kuzentsova, S.N.; Repina, O.V. Furfural derivatives as fuel components. Chem. Tech. Fuels Oil 2020, 55, 720–725. [Google Scholar] [CrossRef]
- Furfural Market by Raw Material (Sugarcane Bagasse, Corncob, Rice Husk and Others), Application (Derivatives (Furfural Alcohol and Other Derivatives), Solvent) and Region (Asia-Pacific, Americas, Europe, Middle East and Africa)—Global Forecast to 2024; Report ID: 4847017; Research and Market: Dublin, Ireland, 2019.
- Allied Market Research, Furfural Market Report. Available online: https://www.alliedmarketresearch.com (accessed on 11 October 2017).
- Clauser, N.M.; Gutiérez, S.; Area, M.C.; Felissia, F.E.; Vallejos, M.E. Techno-economic assessment of caroxylic acids, furfural, and pellet production ion a pine sawdust biorefinery. Biofuels Bioprod. Bioref. 2018, 12, 997–1012. [Google Scholar] [CrossRef]
- Polish Central Statistical Office. Foreign Trade Database from 2004–2016; Polish Central Statistical Office: Warsaw, Poland, 2017.
- Ministry of Agriculture and Rural Development. Rolnictwo i Gospodarka Żywnościowa w Polsce, Collective Work Edited by the Institute of Agricultural and Food Economics; Institute of Agricultural and Food Economics: Warsaw, Poland, 2019.
- Daniel, Z.; Juliszewski, T.; Kowalczyk, Z.; Malinowski, M.; Sobol, Z.; Wrona, P. Metoda szczegółowej klasyfikacji odpadów z sektora rolniczego i rolno-spożywczego. Infrastrukt. Ekol. Terenów Wiej. 2012, 2/IV, 141–152. [Google Scholar]
- Modelska, M.; Binczarski, M.; Dziugan, P.; Karski, S.; Witońska, I. Furfural obtained from waste biomass as a substrate in selective furfuryl alcohol catalytic production. In Proceedings of the 4th International Conference Sustainable Postharvest and Food Technologies—INOPTEP and 27th National Conference Processing and Energy in Agriculuture—PTEP, Divčibare, Serbia, 19–24 April 2015; pp. 165–169. [Google Scholar]
- Modelska, M.; Binczarski, M.; Dziugan, P.; Karski, S.; Witońska, I. From industrial waste biomass to valuable chemical compounds. In Proceedings of the 29th EFFoST Conference Food Science Research and Innovation: Delivering Sustainable Solutions to the Global Economy and Society, Athens, Greece, 10–12 November 2015; Volume I, pp. 237–242. [Google Scholar]
- Modelska, M.; Berlowska, J.; Kręgiel, D.; Cieciura, W.; Antolak, H.; Tomaszewska, J.; Binczarski, M.; Szubiakiewicz, E.; Witońska, I. Concept for recycling waste biomass from the sugar industry for chemical and biotechnological purposes. Molecules 2017, 22, 1544. [Google Scholar] [CrossRef]
- Produkcja Cukru w Polsce, Kampania 2019/2020. Available online: http://www.agroindustry.pl/index.php/2020/04/07/produkcja-cukru-w-polsce-kampania-20192020/ (accessed on 18 October 2020).
- Gawryszczak, M. Europejski i Światowy Rynek Cukru. Konferencja Pokampanijna Stowarzyszenia; Techników Cukrowników: Warsaw, Poland, 15 February 2019. [Google Scholar]
- Draycott, A.P. Sugar Beet; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 1–8. [Google Scholar]
- Rule, D.C.; Koch, D.W.; Jones, R.R.; Kercher, C.J. Brassica and sugar beet forages for lambs—Growth performance of lambs and composition of forage and dock-fat fatty acids. J. Product. Agric. 1991, 4, 29–33. [Google Scholar] [CrossRef]
- Aufrère, J.; Michalet-Doreau, B. Comparison of methods for predicting digestibility of feeds. Anim. Feed Sci. Technol. 1988, 20, 203–218. [Google Scholar] [CrossRef]
- Aramrueang, N.; Zicari, S.M.; Zhang, R. Response surface optimization of enzymatic hydrolysis of sugar beet leaves into fermentable sugars for bioethanol production. Adv. Biosci. Biotechnol. 2017, 8, 51–67. [Google Scholar] [CrossRef]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef]
- Olmos, J.C.; Hansen, M.E.Z. Enzymatic depolymerization of sugar beet pulp: Production and characterization of pectin and pectic-oligosaccharides as a potential source for functional Carbohydrates. Chem. Eng. J. 2012, 192, 29–36. [Google Scholar] [CrossRef]
- Micard, V.; Renard, C.M.G.C.; Thibault, J.-F. Influence of pretreatments on enzymatic degradation of a cellulose-rich residue from sugar-beet pulp. LWT Food Sci. Technol. 1997, 30, 284–291. [Google Scholar] [CrossRef]
- Ward, D.P.; Cárdenas-Fernández, M.; Hewitson, P.; Ignatova, S.; Lye, G.J. Centrifugal partition chromatography in a biorefinery context: Separation of monosaccharides from hydrolysed sugar beet pulp. J. Chromatogr. A 2015, 1411, 84–91. [Google Scholar] [CrossRef]
- Hamley-Bennett, C.; Lye, G.J.; Leak, D. Selective fractionation of sugar beet pulp for release of fermentation and chemical feedstocks; optimisation of thermo-chemical pre-treatment. Bioresour. Technol. 2016, 209, 259–264. [Google Scholar] [CrossRef]
- Jensen, J.; Morinelly, J.; Aglan, A.; Mix, A.; Shonnard, D.R. Kinetic characterization of biomass dilute sulfuric acid hydrolysis: Mixtures of hardwoods, softwood, and switchgrass. A IChE J. 2008, 54, 1637–1645. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yabushita, M.; Komanoya, T.; Hara, K.; Fujita, I.; Fukuoka, A. High-yielding one-pot synthesis of glucose from cellulose using simple activated carbons and trace hydrochloric acid. ACS Catal. 2013, 3, 581–587. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech. 2013, 3, 415–431. [Google Scholar] [CrossRef]
- Yang, W.; Li, P.; Bo, D.; Chang, H. The optimization of formic acid hydrolysis of xylose in furfural production. Carbohydr. Res. 2012, 357, 53–61. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, K.H. Acidic Pretreatment. Pretreatment of Biomass. Proc. Technol. 2015, 3, 27–50. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.; Marianou, A.; Michailof, C.; Kalogianni, A.; Lappas, A. Lignocellulosic Biomass Fractionation as a Pretreatment Step for Production of Fuels and Green Chemicals. Waste Biomass Valoriz. 2015, 6, 781–790. [Google Scholar] [CrossRef]
- Nhien, L.C.; Van Duc Long, N.; Kim, S.; Lee, M. Techno-economic assessment of hybrid extraction and distillation processes for furfural production from lignocellulosic biomass. Biotechnol. Biofuels 2017, 10, 812017. [Google Scholar] [CrossRef]
- Tallarico, S.; Costanzo, P.; Bonacci, S.; Macario, A.; Di Gioia, M.L.; Nardi, M.; Procopio, A.; Oliverio, M. Combined Ultrasound/Microwave Chemocatalytic Method for Selective Conversion of Cellulose into Lactic Acid. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chambon, F.; Rataboul, F.; Pinel, C.; Cabiac, A.; Guillon, E.; Essayem, N. Cellulose hydrothermal conversion promoted by heterogeneous Brønsted and Lewis acids: Remarkable efficiency of solid Lewis acids to produce lactic acid. Appl. Catal. B Environ. 2011, 105, 171–181. [Google Scholar] [CrossRef]
- Dziugan, P.; Binczarski, M.; Modelska, M.; Witonska, I.; Sadowski, A. Recovery of municipal green bio-waste by the way of chemical transformation into valuable chemical products: Intermediates of bio polymers, green solvents and bio components of fuels. Logistyka Odzysku 2015, 3, 87–89. [Google Scholar]
- Modelska, M.; Binczarski, M.; Kaminski, Z.J.; Karski, S.; Kolesińska, B.; Mierczynski, P.; Severino, C.J.; Stanishevsky, A.; Witońska, I.A. Bimetallic Pd-Au/SiO2 Catalysts for Reduction of Furfural in Water. Catalysts 2020, 10, 444. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Lesiak, M.; Binczarski, M.; Karski, S.; Maniukiewicz, W.; Rogowski, J.; Szubiakiewicz, E.; Berlowska, J.; Dziugan, P.; Witońska, I. Hydrogenation of furfural over Pd–Cu/Al2O3 catalysts. The role of interaction between palladium and copper on determining catalytic properties. J. Mol. Catal. A Chem. 2014, 395, 337–348. [Google Scholar] [CrossRef]
- Anthonia, E.E.; Philip, H.S. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 2015, 3, 42–47. [Google Scholar] [CrossRef]
- Rogowski, J.; Andrzejczuk, M.; Berłowska, J.; Binczarski, M.; Kręgiel, D.; Kubiak, A.; Modelska, M.; Szubiakiewicz, E.; Stanishevsky, A.; Tomaszewska, J.; et al. WxC-β-SiC Nanocomposite Catalysts Used in Aqueous Phase Hydrogenation of Furfural. Molecules 2017, 22, 2033. [Google Scholar] [CrossRef]
- Vom Stein, T.; Klankermayer, J.; Leitner, W. Tailor-Made Fuels and Chemicals from Biomass in Catalysis for the Conversion of Biomass and its Derivatives. In Max Planck Research Library for the History and Development of Knowledge; Behrens, M., Datye, A.K., Eds.; Neopubli GmbH: Berlin, Germany, 2013. [Google Scholar]
- Merat, N.; Godowa, C.; Gaset, A. High selective production of tetrahydrofurfuryl alcohol: Catalytic hydrogenation of furfural and furfuryl alcohol. J. Chem. Technol. Biotechnol. 2007, 48, 145–159. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Huang, Z.; Yuan, G. Tuning catalytic selectivity of liquid-phase hydrogenation of furfural via synergistic effects of supported bimetallic catalysts. Appl. Catal. A 2015, 500, 23–29. [Google Scholar] [CrossRef]
- Szubiakiewicz, E.; Modelska, M.; Brzezińska, M.; Binczarski, M.J.; Severino, C.J.; Stanishevsky, A.; Witońska, I. Influence of modification of supported palladium systems by polymers: PVP, AMPS and AcrAMPS on their catalytic properties in the reaction of transformation of biomass into fuel bio-components. Fuel 2020, 271, 117584–117596. [Google Scholar] [CrossRef]
- Rogowski, J.; Binczarski, M.; Dziugan, P.; Karski, S.; Modelska, M.; Witonska, I.; Kubiak, A. Sposób Wytwarzania Katalizatora Nanokompozytowego Katalizującego Reakcję Redukcji Furfuralu do Alkoholu Tetrahydrofurfurylowego w Fazie Wodnej. Patent Office of the Republic of Poland PL 412726, 15 June 2015. [Google Scholar]
- Binczarski, M.; Kaminski, Z.; Karski, S.; Kolesinska, B.; Modelska, M.; Witonska, I. Sposób Wytwarzania Mieszaniny Ciekłych Dodatków Paliwowych z Furfuralu Oraz Sposób Wytwarzania Katalizatora Palladowo-Złotowego Stosowanego w Tym Procesie. Patent Office of the Republic of Poland PL 431048, 5 September 2019. [Google Scholar]
- Tetrahydrofurfuryl Alcohol (THFA) from Furfuryl Alcohol. Available online: https://dalinyebo.com/furfural-and-its-many-by-products/tetrahydrofurfuryl-alcohol-thfa/?fbclid=IwAR191qUuf8HhSHlOuTIIDkQ8O_1__ZK82XaSWI424Ea6gsX00eZIk10aZkw (accessed on 18 October 2020).
- Hann, J.P.; Stevenson, P.A. Fuel Compositions. U.S. Patent US20070094919A1, 1 February 2007. [Google Scholar]
- Lacome, T.; Montagne, X.; Delfort, B.; Paille, F. Diesel Fuel Compositions Containing Oxygenated Compounds Derived from Tetrahydrofurfuryl. U.S. Patent US20020053161A1, 9 May 2002. [Google Scholar]
- Constable, G.A.; Carlson, N.L.; Heelan, G.A.; Oehr, K.H. Method of Upgrading Heavy Crude Oil. WO2008124912A1, 23 October 2008. [Google Scholar]
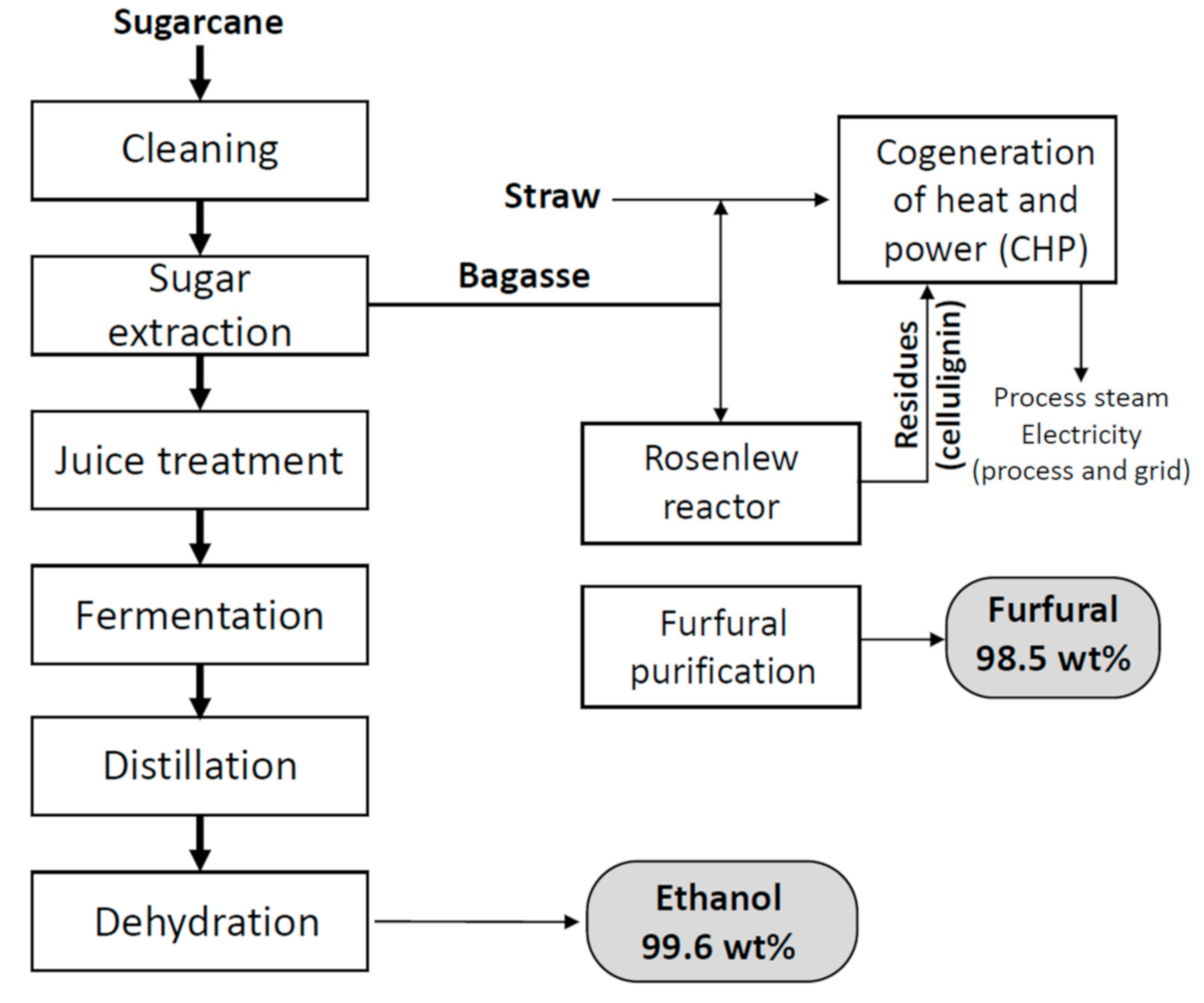

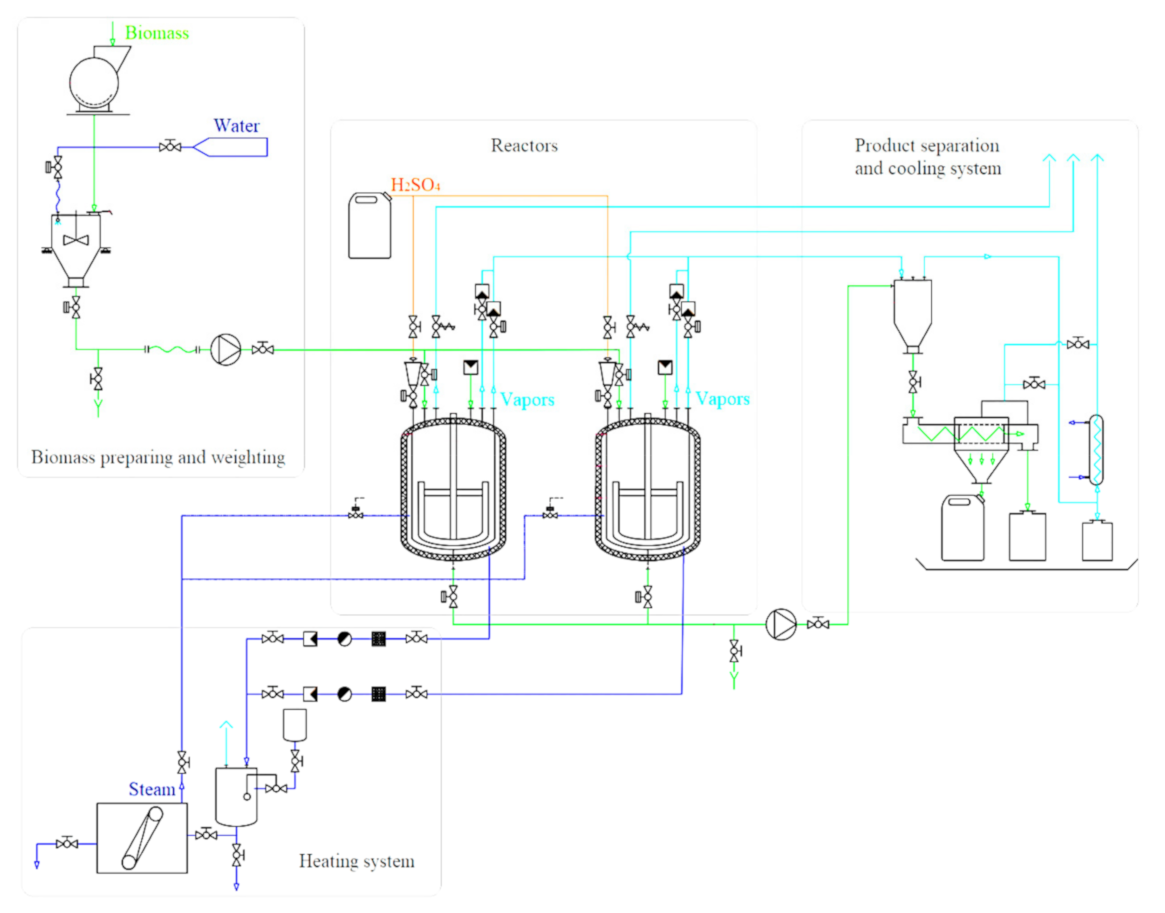

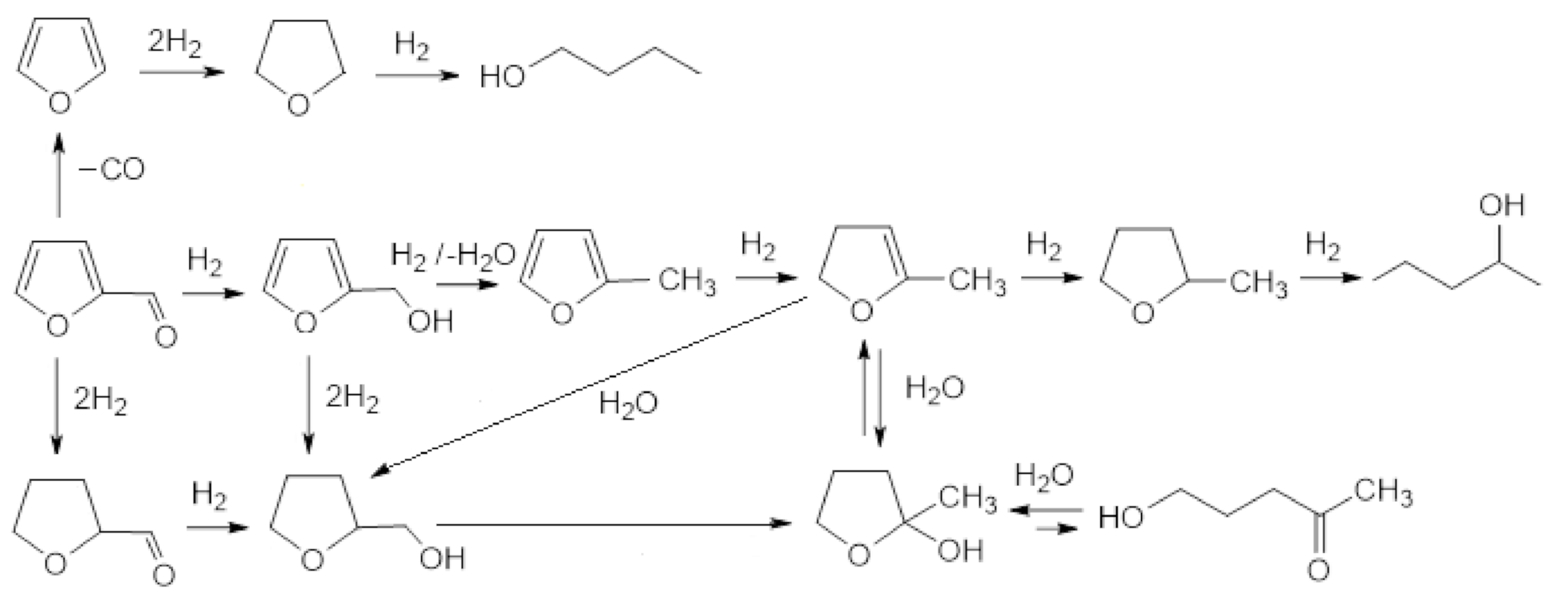
| Production Process | % Weight of Raw Material Not Used in End Products |
|---|---|
| Making Yoghurt | 2–6 |
| Making Canned Fruit and Vegetables | 5–30 |
| Making Canned Fish | 30–65 |
| Making Fruit and Vegetable Juices | 30–50 |
| Poultry Slaughter and Processing of Poultry Meat | 31–38 |
| Pig Slaughter and Pork Processing | 35 |
| Cattle Slaughter and Beef Processing | 40–52 |
| Manufacture of Vegetable Oils | 40–70 |
| Manufacture of Corn Starch | 41–43 |
| Making Fish Fillets, Salted Fish | 50–75 |
| Wine Production | 20–30 |
| Manufacture of Wheat Starch | 50 |
| Manufacture of Starch from Potatoes | 80 |
| Manufacture of Cheese | 85–90 |
| Making Sugar from Sugar Beet | 86 |
| Biomass Hydrolysis | ||||
|---|---|---|---|---|
| Number of Reactors (cs) | 1 | 2 | 3 | 4 |
| Reactors capacity (m3) | 40 | 80 | 120 | 160 |
| Productivity of furfural/year (t) | 226 | 454 | 680 | 907 |
| Profitability analysis (PLN) | ||||
| Estimated investment outlays | 5,000,000 | 6,500,000 | 8,000,000 | 9,500,000 |
| Sales revenue/year | 1,587,000 | 3,175,000 | 4,763,000 | 6,350,000 |
| Operating profit after tax (10 years) | 420,000 | 4,954,000 | 9,480,000 | 14,022,000 |
| Net present value (NPV) of discounted cash flows (DCF) | (−1,889,000) | 6000 | 1,902,000 | 3,797,000 |
| Raw Material | Dry Matter (%) | Raw Material Weight (kg) (Dry Matter of Raw Material (kg)) | Water Weight (kg) | H2SO4 Conc. (%) | Time (h) | Temp. (°C) | Furfural Conc. (g/kg) | Yield of Furfural (%) |
|---|---|---|---|---|---|---|---|---|
| Sugar Beet Pulp | 19.71 19.71 23.34 23.34 19.71 | 10 (1.971) 10 (1.971) 10 (2.334) 10 (2.334) 20 (3.942) | 50 50 50 50 40 | 0.5 2.0 5.0 0.5 0.5 | 2 2 2 2 2 | 140 140 140 120 140 | 1.596 1.754 2.136 1.762 2.286 | 4.86 5.34 5.49 4.53 3.48 |
| Sugar Beet Leaves | 11.75 11.75 11.75 11.75 11.75 | 10 (1.175) 10 (1.175) 10 (1.175) 10 (1.175) 20 (2.350) | 50 50 50 50 40 | 0.5 0.5 0.5 2.0 0.5 | 2 2 2 2 2 | 160 140 120 140 140 | 0.497 0.359 0.298 0.467 0.496 | 2.53 1.83 1.52 2.38 1.27 |
| Furfural | 5% Cu/Al2O3 | 5% Pd/Al2O3 | ||||
|---|---|---|---|---|---|---|
| X (%) | SFA (%) | STHFA (%) | X (%) | SFA (%) | STHFA (%) | |
| Commercial | 81 | 100 | 0 | 100 | 28 | 72 |
| From sugar beet pulp hydrolysate | 90 | 100 | 0 | 100 | 31 | 69 |
| From sugar beet leaves hydrolysate | 78 | 100 | 0 | 100 | 26 | 74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modelska, M.; Binczarski, M.J.; Dziugan, P.; Nowak, S.; Romanowska-Duda, Z.; Sadowski, A.; Witońska, I.A. Potential of Waste Biomass from the Sugar Industry as a Source of Furfural and Its Derivatives for Use as Fuel Additives in Poland. Energies 2020, 13, 6684. https://doi.org/10.3390/en13246684
Modelska M, Binczarski MJ, Dziugan P, Nowak S, Romanowska-Duda Z, Sadowski A, Witońska IA. Potential of Waste Biomass from the Sugar Industry as a Source of Furfural and Its Derivatives for Use as Fuel Additives in Poland. Energies. 2020; 13(24):6684. https://doi.org/10.3390/en13246684
Chicago/Turabian StyleModelska, Magdalena, Michal J. Binczarski, Piotr Dziugan, Szymon Nowak, Zdzisława Romanowska-Duda, Adam Sadowski, and Izabela A. Witońska. 2020. "Potential of Waste Biomass from the Sugar Industry as a Source of Furfural and Its Derivatives for Use as Fuel Additives in Poland" Energies 13, no. 24: 6684. https://doi.org/10.3390/en13246684
APA StyleModelska, M., Binczarski, M. J., Dziugan, P., Nowak, S., Romanowska-Duda, Z., Sadowski, A., & Witońska, I. A. (2020). Potential of Waste Biomass from the Sugar Industry as a Source of Furfural and Its Derivatives for Use as Fuel Additives in Poland. Energies, 13(24), 6684. https://doi.org/10.3390/en13246684








