Disc Granulation Process of Carbonation Lime Mud as a Method of Post-Production Waste Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Testing Properties and Chemical Composition of Carbonation Lime Mud
2.2. Testing Properties and Chemical Composition of Carbonation Lime Mud
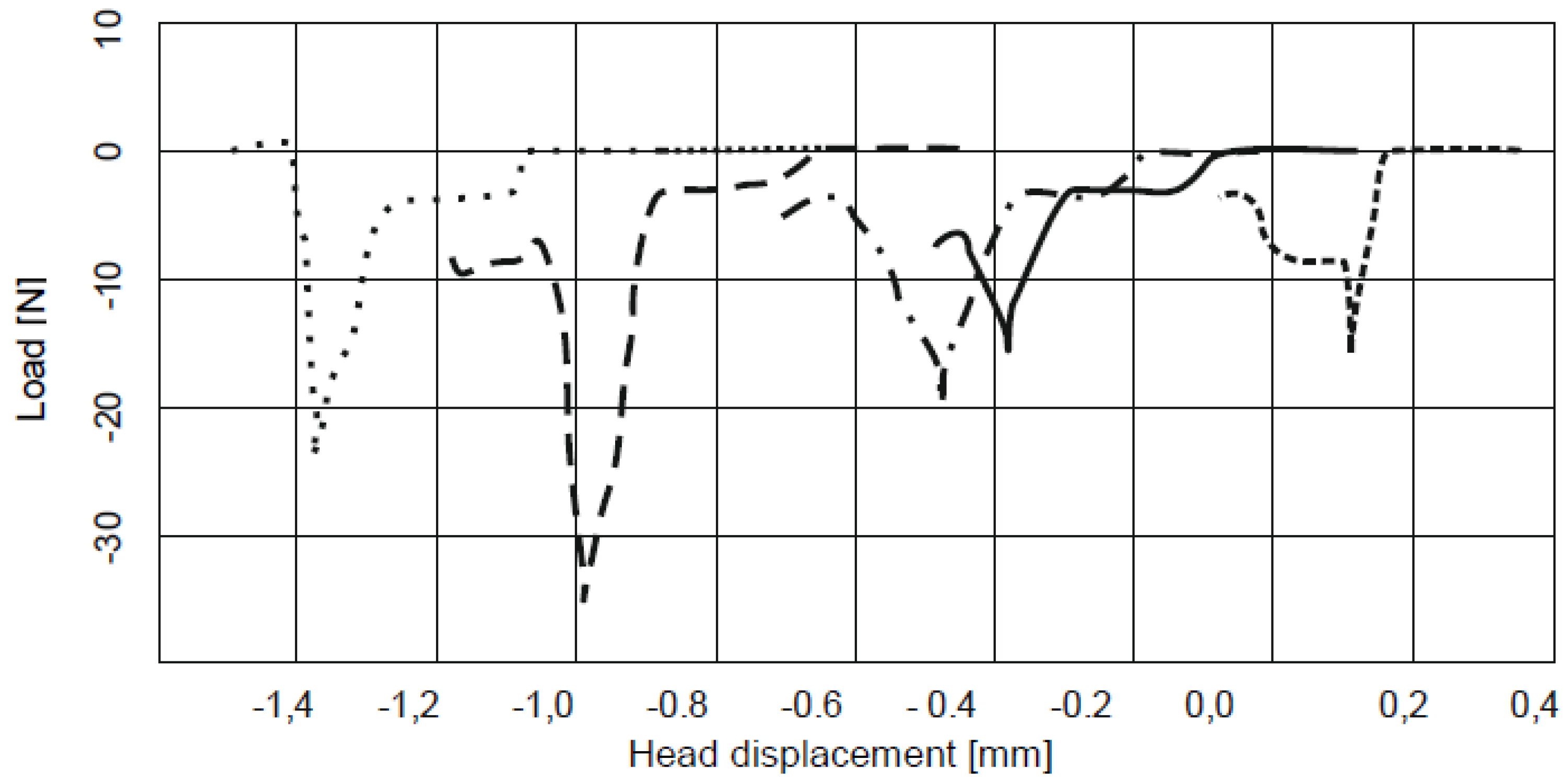
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-hamid, A.; Azza, R.; Mansour, S.F. Competency of some soil amendments used for improvement of extreme salinity of sahl ‚ el-tina soil (north-sinai). J. Soil Sci. Agric. Eng. 2011, 2, 649–667. [Google Scholar] [CrossRef]
- Lutin, F.; Bailly, M.; Bar, D. Process improvements with innovative technologies in the starch and sugar industries. Desalination 2002, 148, 121–124. [Google Scholar] [CrossRef]
- Timmer, V.R.; Teng, Y.; Pedlar, J. Soil and leaf analysis of fertilized sugar maple stands after ice storm damage. For. Chron. 2003, 79, 99–105. [Google Scholar] [CrossRef]
- Quina, M.J.; Bordado, J.; Quinta-Ferreira, R. Treatment and use of air pollution control residues from MSW incineration: An overview. Waste Manag. 2008, 28, 2097–2121. [Google Scholar] [CrossRef] [PubMed]
- Hafid, H.S.; ’Aini, A.R.N.; Mokhtar, M.N.; Talib, A.T.; Baharuddin, A.S.; Shah, U.K.M. Over production of fermentable sugar for bioethanol production from carbohydrate-rich Malaysian food waste via sequential acid-enzymatic hydrolysis pretreatment. Waste Manag. 2017, 67, 95–105. [Google Scholar] [CrossRef]
- Karray, R.; Karray, F.; Loukil, S.; Mhiri, N.; Sayadi, S. Anaerobic co-digestion of Tunisian green macroalgae Ulva rigida with sugar industry wastewater for biogas and methane production enhancement. Waste Manag. 2016, 61, 171–178. [Google Scholar] [CrossRef]
- Ozkan, L.; Erguder, T.H.; Demirer, G. Investigation of the effect of culture type on biological hydrogen production from sugar industry wastes. Waste Manag. 2010, 30, 792–798. [Google Scholar] [CrossRef]
- Kirby, M.; Theodorou, M.K.; Brizuela, C.M.; Huntington, J.A.; Powles, J.; Wilkinson, R.G. The anaerobic digestion of pig carcase with or without sugar beet pulp, as a novel on-farm disposal method. Waste Manag. 2018, 75, 251–260. [Google Scholar] [CrossRef]
- Kantiranis, N. Re-cycling of sugar-ash: A raw feed material for rotary kilns. Waste Manag. 2004, 24, 999–1004. [Google Scholar] [CrossRef]
- Satisha, G.; Devarajan, L. Effect of amendments on windrow composting of sugar industry pressmud. Waste Manag. 2007, 27, 1083–1091. [Google Scholar] [CrossRef]
- Hillion, M.-L.; Moscoviz, R.; Trably, E.; Leblanc, Y.; Bernet, N.; Torrijos, M.; Escudié, R. Co-ensiling as a new technique for long-term storage of agro-industrial waste with low sugar content prior to anaerobic digestion. Waste Manag. 2018, 71, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Paleckienė, R.; Sviklas, A.M.; Šlinkšienė, R. The Role of Sugar Factory Lime on Compound Fertilizer Properties. Pol. J. Environ. Stud. 2007, 16, 423–426. [Google Scholar]
- Sims, A.L.; Windels, C.E.; Bradley, C.A. Content and Potential Availability of Selected Nutrients in Field Applied Sugar Beet Factory Lime. Commun. Soil Sci. Plant Anal. 2010, 41, 438–453. [Google Scholar] [CrossRef]
- Sviklas, A.; Paletskene, R. Physicochemical Principles of Synthesis of Liquid Fertilizers Based on Potassium Hydrophosphate. Russ. J. Appl. Chem. 2004, 77, 521–526. [Google Scholar] [CrossRef]
- Sviklas, A.; Shlinkshene, R. Liquid Fertilizers Based on Dolomite, Nitric Acid, and Ammonia. Russ. J. Appl. Chem. 2003, 76, 1885–1890. [Google Scholar] [CrossRef]
- Chłopek, M.; Dzik, T.; Hryniewicz, M. Determining the grip angle in a granulator with a flat matrix. Maint. Reliab. 2014, 16, 337–340. [Google Scholar]
- Obidziński, S. Utilization of post-production waste of potato pulp and buckwheat hulls in the form of pellets. Pol. J. Environ. Stud. 2014, 23, 1391–1395. [Google Scholar]
- Obidziński, S. Pelletization process of postproduction plant waste. Int. Agrophysics 2012, 26, 279–284. [Google Scholar] [CrossRef]
- Chansataporn, W.; Nopharatana, M. Effects of binder content and drum filling degree on cassava pearl granulation using drum granulator. Asian J. Food Agro. Ind. 2009, 2, 739–748. [Google Scholar]
- Sobiecka, E.; Obraniak, A.; Antizar-Ladislao, B. Influence of mixture ratio and pH to solidification/stabilization process of hospital solid waste incineration ash in Portland cement. Chemosphere 2014, 111, 18–23. [Google Scholar] [CrossRef]
- Ding, J.; Ma, S.; Shen, S.; Xie, Z.; Zheng, S.; Zhang, Y. Research and industrialization progress of recovering alumina from fly ash: A concise review. Waste Manag. 2017, 60, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Obraniak, A.; Gluba, T.; Ławińska, K.; Derbiszewski, B. Minimisation of environmental effects related with storing fly ash from combustion of hard coal. Environ. Prot. Eng. 2018, 44, 177–189. [Google Scholar] [CrossRef]
- Teixeira, S.R.; Pena, A.; Miguel, A. Briquetting of charcoal from sugar-cane bagasse fly ash (scbfa) as an alternative fuel. Waste Manag. 2010, 30, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Ileleji, K.E.; Li, Y.; Ambrose, R.K.; Doane, P.H. Experimental investigations towards understanding important parameters in wet drum granulation of corn stover biomass. Powder Technol. 2016, 300, 126–135. [Google Scholar] [CrossRef]
- Maxim, R.; Fu, J.S.; Pickles, M.J.; Salman, A.; Hounslow, M.; Hounslow, M.J. Modelling effects of processing parameters on granule porosity in high-shear granulation. Granul. Matter 2004, 6, 131–135. [Google Scholar] [CrossRef]
- Obraniak, A.; Gluba, T. A model of granule porosity changes during drum granulation. Physicochem. Probl. Miner. Process. 2011, 46, 219–228. [Google Scholar]
- Ramachandran, R.; Chaudhury, A. Model-based design and control of a continuous drum granulation process. Chem. Eng. Res. Des. 2012, 90, 1063–1073. [Google Scholar] [CrossRef]
- Abrahamsson, P.; Björn, I.N.; Rasmuson, A. Parameter study of a kinetic-frictional continuum model of a disk impeller high-shear granulator. Powder Technol. 2013, 238, 20–26. [Google Scholar] [CrossRef]
- Ławińska, K.; Obraniak, A.; Modrzewski, R. Granulation Process of Waste Tanning Shavings. Fibres Text. East. Eur. 2019, 27, 107–110. [Google Scholar] [CrossRef]
- Obraniak, A.; Gluba, T. A Model of agglomerate formation during bed wetting in the process of disc granulation. Chem. Process. Eng. 2012, 33, 153–165. [Google Scholar] [CrossRef]
- Gluba, T.; Obraniak, A. Nucleation and granule formation during disc granulation process. Physicochem. Probl. Miner. Process. 2012, 48, 113–120. [Google Scholar]
- Scott, A.; Hounslow, M.J.; Instone, T. Direct evidence of heterogeneity during high-shear granulation. Powder Technol. 2000, 113, 205–213. [Google Scholar] [CrossRef]
- Sidor, J. Vibratory granulators Granulatory wibracyjne. Przemysł Chemiczny 2015, 1, 137–140. [Google Scholar] [CrossRef]
- Feliks, J. Granulation of dolomite and limestone in the vibratory granulator. Przemysl Chemiczny 2015, 94, 771–773. [Google Scholar]
- Schab, S.; Biskupski, A.; Rusek, P. Process for production of a urea superphosphate fertilizer under continuous feeding of raw materials. Przemysl Chemiczny 2016, 95, 1000–1002. [Google Scholar]
- Obraniak, A.; Lawinska, K. Spectrophotometric analysis of disintegration mechanisms (abrasion and crushing) of agglomerates during the disc granulation of dolomite. Granul. Matter 2017, 20, 7. [Google Scholar] [CrossRef]
- Gluba, T.; Olejnik, T.P.; Obraniak, A. Technology for producing washing agent in continuous process. Przemysl Chemiczny 2015, 94, 1370–1374. [Google Scholar]
- Dzikuć, M.; Kuryło, P.; Dudziak, R.; Szufa, S.; Dzikuć, M.; Godzisz, K. Selected Aspects of Combustion Optimization of Coal in Power Plants. Energies 2020, 13, 2208. [Google Scholar] [CrossRef]
- Szufa, S.; Wielgosiński, G.; Piersa, P.; Czerwińska, J.; Dzikuć, M.; Adrian, Ł.; Lewandowska, W.; Marczak, M. Torrefaction of Straw from Oats and Maize for Use as A Fuel and Additive to Organic Fertilizers—TGA Analysis, Kinetics as Products for Agricultural Purposes. Energies 2020, 13, 2064. [Google Scholar] [CrossRef]
- Jewiarz, M.; Wróbel, M.; Mudryk, K.; Szufa, S. Impact of the Drying Temperature and Grinding Technique on Biomass Grindability. Energies 2020, 13, 3392. [Google Scholar] [CrossRef]
- Szufa, S.; Dzikuć, M.; Adrian, Ł.; Piersa, P.; Romanowska-Duda, Z.; Lewandowska, W.; Marczak, M.; Błaszczuk, A.; Piwowar, A. Torrefaction of oat straw to use as solid biofuel, an additive to organic fertilizers for agriculture purposes and activated carbon—TGA analysis, kinetics. E3S Web Conf. 2020, 154, 02004. [Google Scholar] [CrossRef]
- Szufa, S.; Adrian, Ł.; Piersa, P.; Romanowska-Duda, Z.; Ratajczyk-Szufa, J. Torrefaction process of millet and cane using batch reactor. In Renewable Energy Sources: Engineering, Technology, Innovation, Springer Proceedings in Energy; Wróbel, M., Jewiarz, M., Szlęk, A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 371–379. [Google Scholar]
- Szufa, S.; Adrian, Ł.; Piersa, P.; Romanowska-Duda, Z.; Grzesik, M.; Cebula, A.; Kowalczyk, S. Experimental studies on energy crops torrefaction process using batch reactor to estimate torrefaction temperature and residence time. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer: Cham, Switzerland, 2018; pp. 365–373. ISBN 978-3-319-72370-9. [Google Scholar] [CrossRef]
- Szufa, S.; Romanowska-Duda, B.Z.; Grzesik, M. Torrefaction proces of the Phragmites Communis growing in soil contaminated with cadmium. In Proceedings of the 20th European Biomass Conference and Exibition, Milan, Italy, 18–22 June 2014; pp. 628–634, ISBN 978-88-89407-54-7. [Google Scholar] [CrossRef]
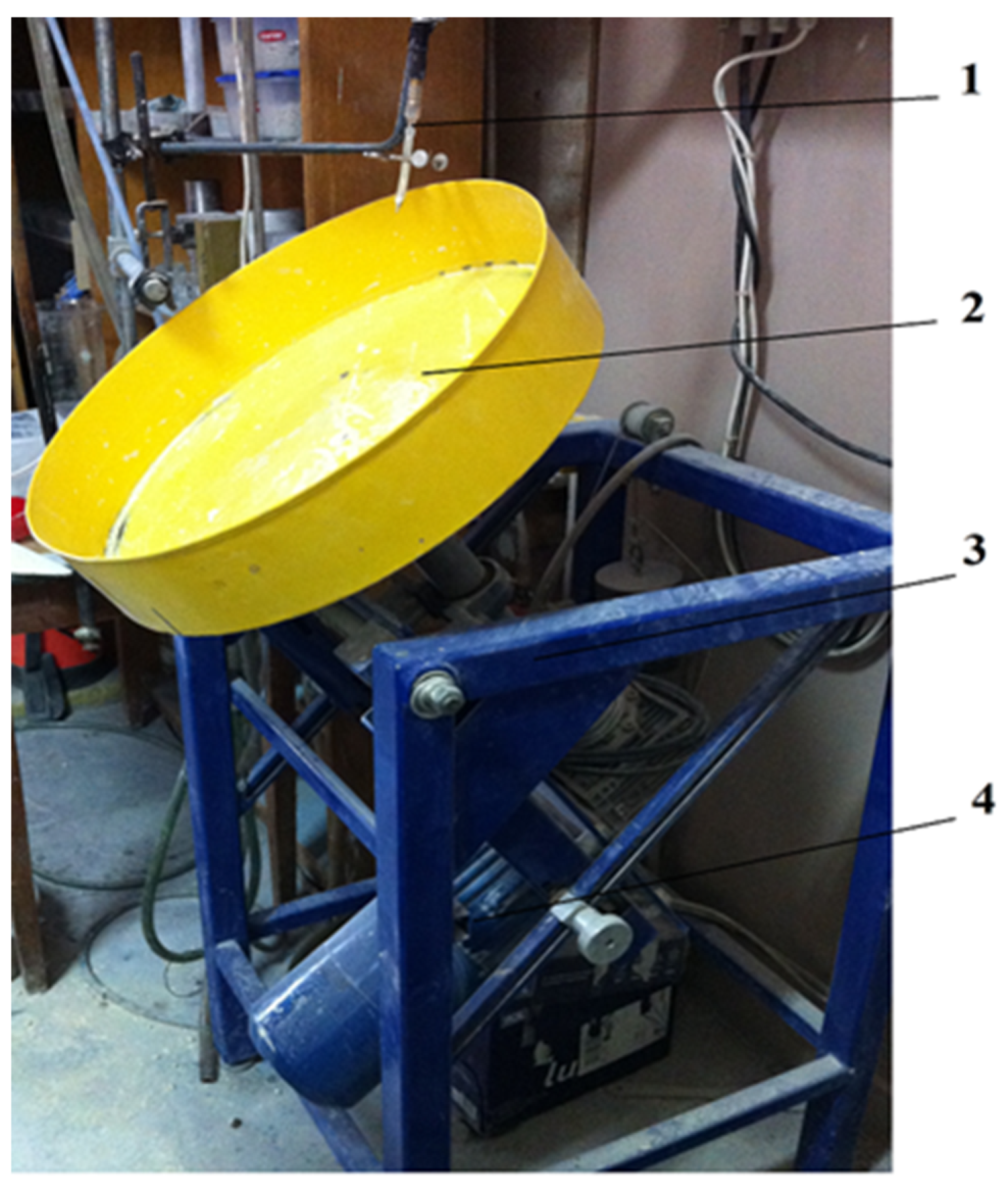
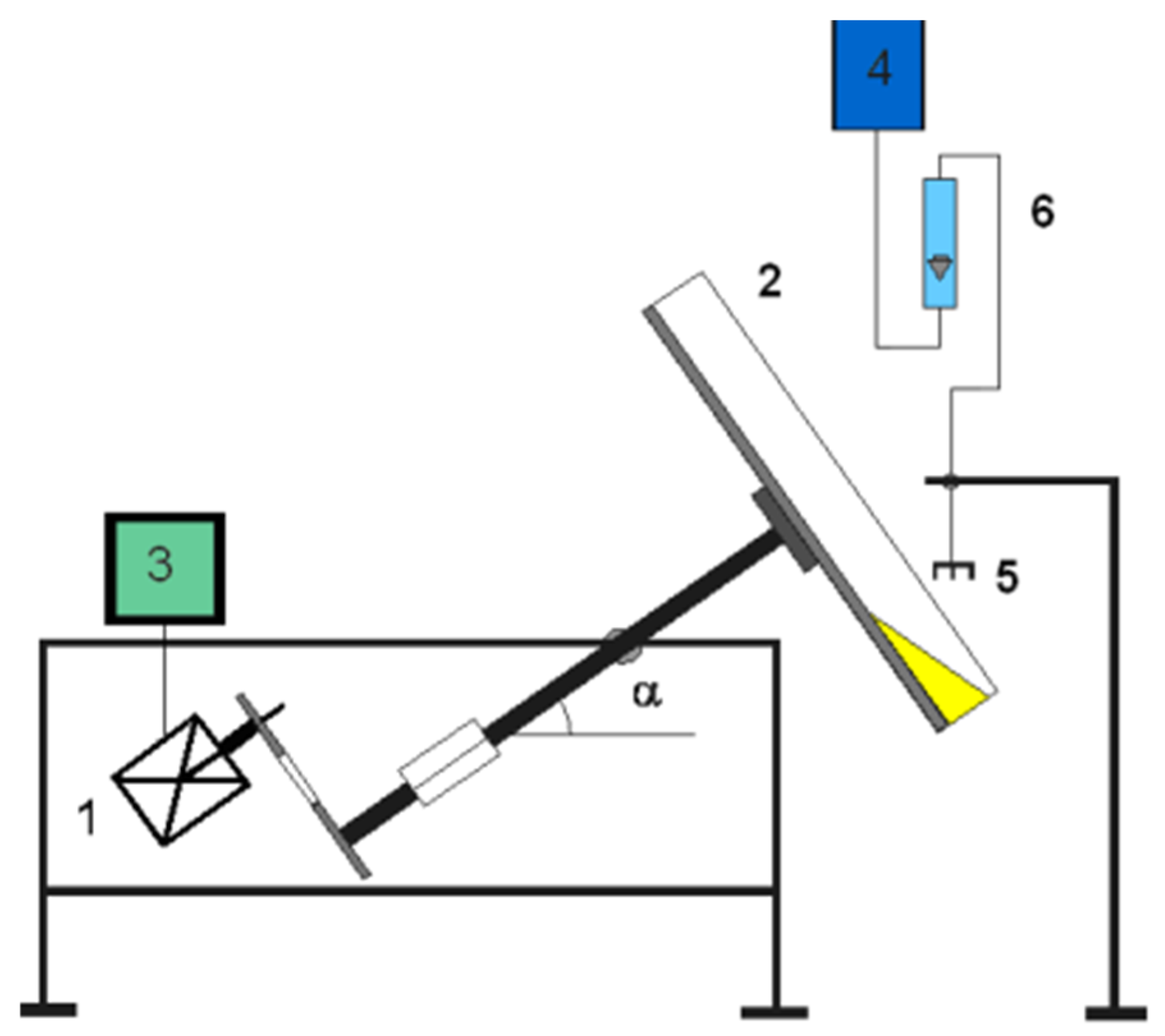

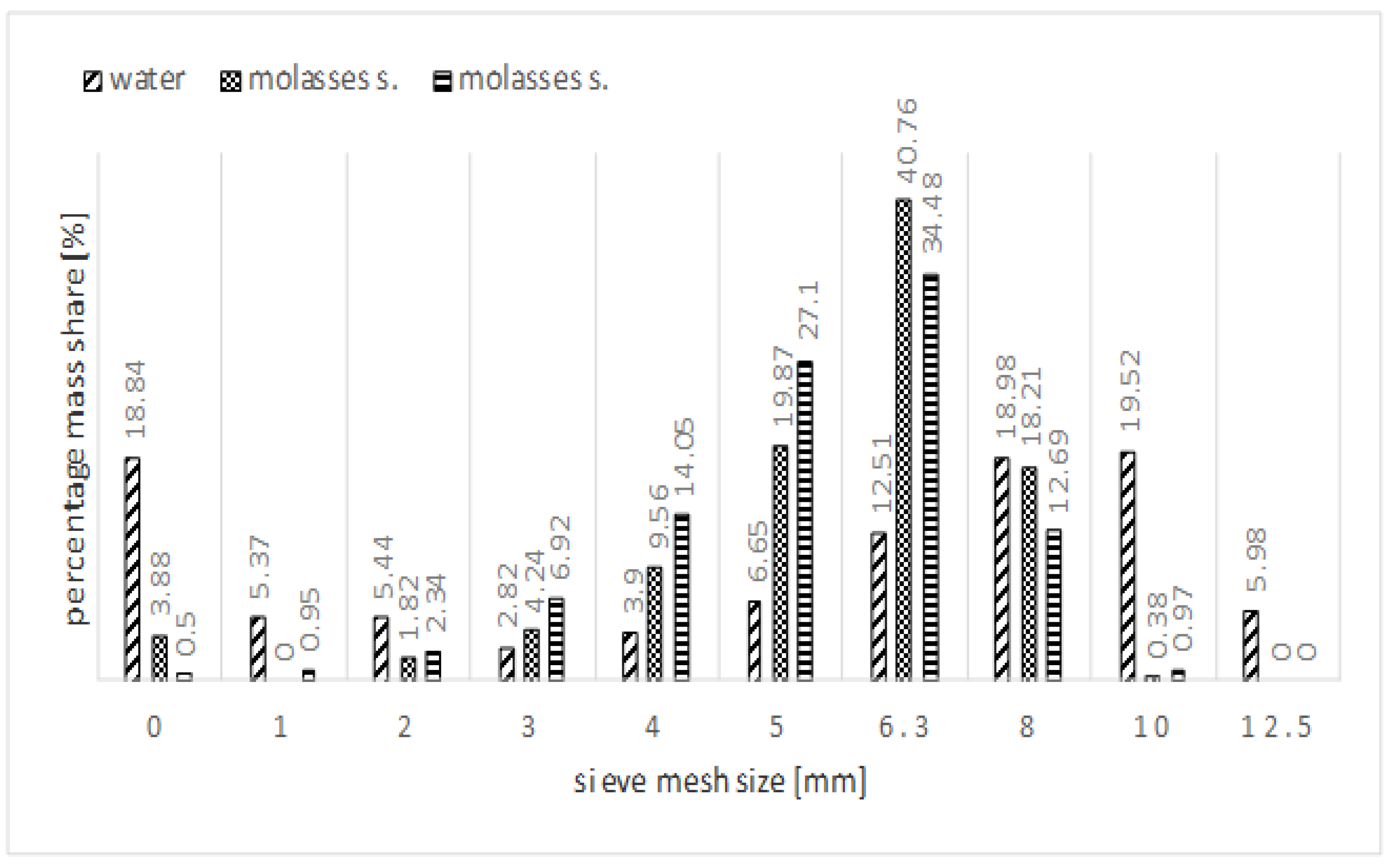
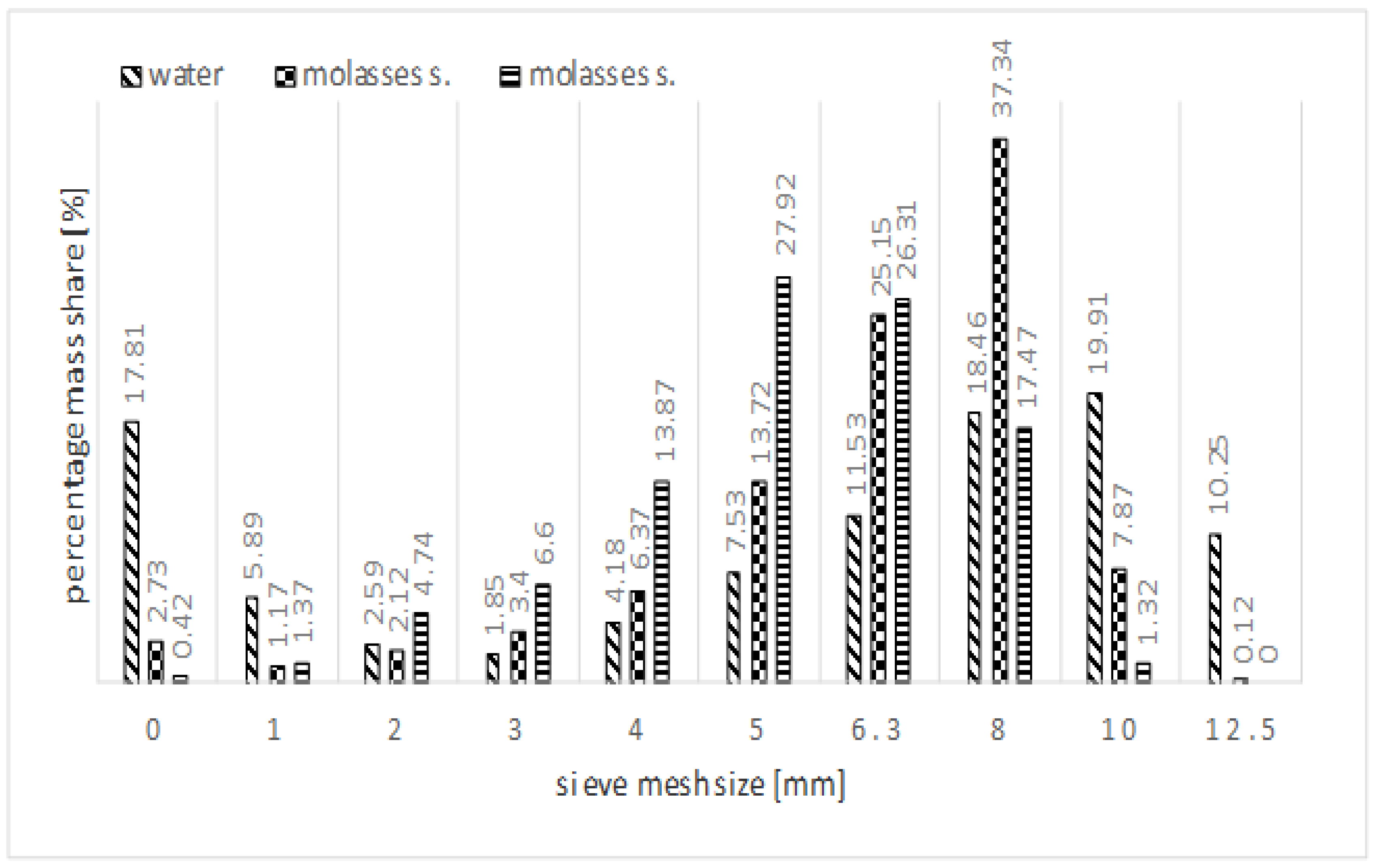


| Property | Value |
|---|---|
| Range of changes in grain size (mm) | 0–0.56 |
| Average grain size (mm) | 0.12 |
| Tangent of the angle of natural repose | 1.34 |
| Bulk density (kg/m3) | 1131 |
| Humidity | 38% |
| Element | Content | Unit |
|---|---|---|
| Zinc | 15.3 | mg/kg dry matter |
| Nickel | 0.89 | mg/kg dry matter |
| Lead | 0.19 | mg/kg dry matter |
| Cadmium | 0.06 | mg/kg dry matter |
| Copper | 4.6 | mg/kg dry matter |
| Magnesium | 18.6 | mg/kg dry matter |
| Mercury | 0.003 | mg/kg dry matter |
| Chromium | 4.11 | mg/kg dry matter |
| Boron | 0.03 | mg/kg dry matter |
| Potassium | 0.06 | % dry matter |
| Phosphorus | 0.15 | % dry matter |
| Calcium | 45.10 | % dry matter |
| Trial Number | Raw Material | Wetting Time tn (min) | Granulation Time tg (min) | Material Used for Powdering | Type of Wetting Liquid |
|---|---|---|---|---|---|
| 1 | mud | 6.0 | 30.0 | gypsum | water |
| 2 | mud | 4.0 | 30.0 | gypsum | water |
| 3 | mud | 8.0 | 17.0 | - | water |
| 4 | mud, gypsum | 6.0 | 15.0 | gypsum | water |
| 5 | mud, gypsum | 5.0 | 11.0 | gypsum | water |
| 6 | mud, gypsum | 4.0 | 8.5 | - | water |
| 7 | mud | 5.0 | 8.0 | gypsum | water |
| 8 | mud | 5.0 | 10.0 | gypsum | water |
| 9 | mud | 5.0 | 8.0 | dolomite | water |
| 10 | mud | 5.5 | 8.0 | dolomite | water |
| 11 | mud | 6.0 | 15.0 | chalk | water |
| 12 | mud | 6.0 | 14.0 | chalk | water |
| 13 | mud | 5.5 | 12.0 | limestone flour | water |
| 14 | mud | 6.0 | 12.0 | limestone flour | water |
| 15 | mud | 6.0 | 12.0 | dry mud | water |
| 16 | mud | 7.0 | 12.0 | dry mud | water |
| 17 | mud | 7.5 | 10.0 | gypsum | 33% solution of molasses |
| 18 | mud | 7.5 | 10.0 | dolomite | 33% solution of molasses |
| 19 | mud | 5.0 | 12.0 | chalk | 33% solution of molasses |
| 20 | mud | 5.0 | 10.0 | limestone flour | 33% solution of molasses |
| 21 | mud | 7.5 | 15.0 | - | 33% solution of molasses |
| 22 | mud | 3.0 | 12.0 | gypsum | 66% solution of molasses |
| 23 | mud | 4.0 | 12.0 | dolomite | 66% solution of molasses |
| 24 | mud | 4.0 | 12.0 | chalk | 66% solution of molasses |
| 25 | mud | 4.0 | 10.0 | limestone flour | 66% solution of molasses |
| 26 | mud | 3.5 | 6.0 | mud | 66% solution of molasses |
| 27 | mud | 4 | 6.0 | mud | 66% solution of molasses |
| 28 | mud | 4.5 | 6.0 | mud | 66% solution of molasses |
| Trial Number | Material Used for Powdering | Liquid Used for Wetting | Fraction Size (mm) | Average Force Destroying Granules (N) |
|---|---|---|---|---|
| 18 | gypsum | 33% solution of molasses | 4.0 | 4 |
| 5.0 | 6 | |||
| 6.3 | 4 | |||
| 8.0 | 6 | |||
| 10.0 | 7 | |||
| 19 | dolomite | 33% solution of molasses | 4.0 | 4 |
| 5.0 | 8 | |||
| 6.3 | 5 | |||
| 8.0 | 9 | |||
| 10.0 | 8 | |||
| 20 | chalk | 33% solution of molasses | 4.0 | 4 |
| 5.0 | 3 | |||
| 6.3 | 5 | |||
| 8.0 | 8 | |||
| 10.0 | 12 | |||
| 21 | limestone flour | 33% solution of molasses | 4.0 | 3 |
| 5.0 | 4 | |||
| 6.3 | 4 | |||
| 8.0 | 5 | |||
| 10.0 | 8 | |||
| 22 | - | 33% solution of molasses | 4.0 | 4 |
| 5.0 | 4 | |||
| 6.3 | 4 | |||
| 8.0 | 4 | |||
| 10.0 | 8 | |||
| 23 | gypsum | 66% solution of molasses | 4.0 | 9 |
| 5.0 | 11 | |||
| 6.3 | 25 | |||
| 8.0 | 26 | |||
| 10.0 | 25 | |||
| 24 | dolomite | 66% solution of molasses | 4.0 | 13 |
| 5.0 | 16 | |||
| 6.3 | 21 | |||
| 8.0 | 24 | |||
| 10.0 | 46 | |||
| 25 | chalk | 66% solution of molasses | 4.0 | 15 |
| 5.0 | 13 | |||
| 6.3 | 29 | |||
| 8.0 | 42 | |||
| 10.0 | 12 | |||
| 26 | limestone flour | 66% solution of molasses | 4.0 | 11 |
| 5.0 | 18 | |||
| 6.3 | 20 | |||
| 8.0 | 28 | |||
| 10.0 | 27 | |||
| 27 | carbonation lime mud | 66% solution of molasses | 4.0 | 11 |
| 5.0 | 12 | |||
| 6.3 | 17 | |||
| 8.0 | 22 | |||
| 10.0 | 34 | |||
| 28 | carbonation lime mud | 66% solution of molasses | 4.0 | 11 |
| 5.0 | 11 | |||
| 6.3 | 13 | |||
| 8.0 | 14 | |||
| 10.0 | 37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ławińska, K.; Szufa, S.; Obraniak, A.; Olejnik, T.; Siuda, R.; Kwiatek, J.; Ogrodowczyk, D. Disc Granulation Process of Carbonation Lime Mud as a Method of Post-Production Waste Management. Energies 2020, 13, 3419. https://doi.org/10.3390/en13133419
Ławińska K, Szufa S, Obraniak A, Olejnik T, Siuda R, Kwiatek J, Ogrodowczyk D. Disc Granulation Process of Carbonation Lime Mud as a Method of Post-Production Waste Management. Energies. 2020; 13(13):3419. https://doi.org/10.3390/en13133419
Chicago/Turabian StyleŁawińska, Katarzyna, Szymon Szufa, Andrzej Obraniak, Tomasz Olejnik, Robert Siuda, Jerzy Kwiatek, and Dominika Ogrodowczyk. 2020. "Disc Granulation Process of Carbonation Lime Mud as a Method of Post-Production Waste Management" Energies 13, no. 13: 3419. https://doi.org/10.3390/en13133419
APA StyleŁawińska, K., Szufa, S., Obraniak, A., Olejnik, T., Siuda, R., Kwiatek, J., & Ogrodowczyk, D. (2020). Disc Granulation Process of Carbonation Lime Mud as a Method of Post-Production Waste Management. Energies, 13(13), 3419. https://doi.org/10.3390/en13133419






