Molecular-Scale Considerations of Enhanced Oil Recovery in Shale
Abstract
1. Introduction
2. Methods
2.1. Modeling Approach
2.2. Simulation Details
3. Results
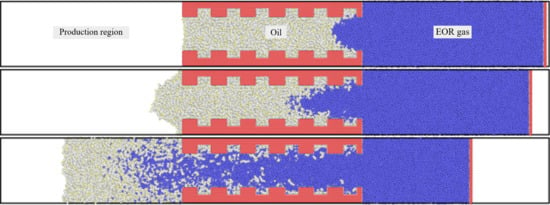
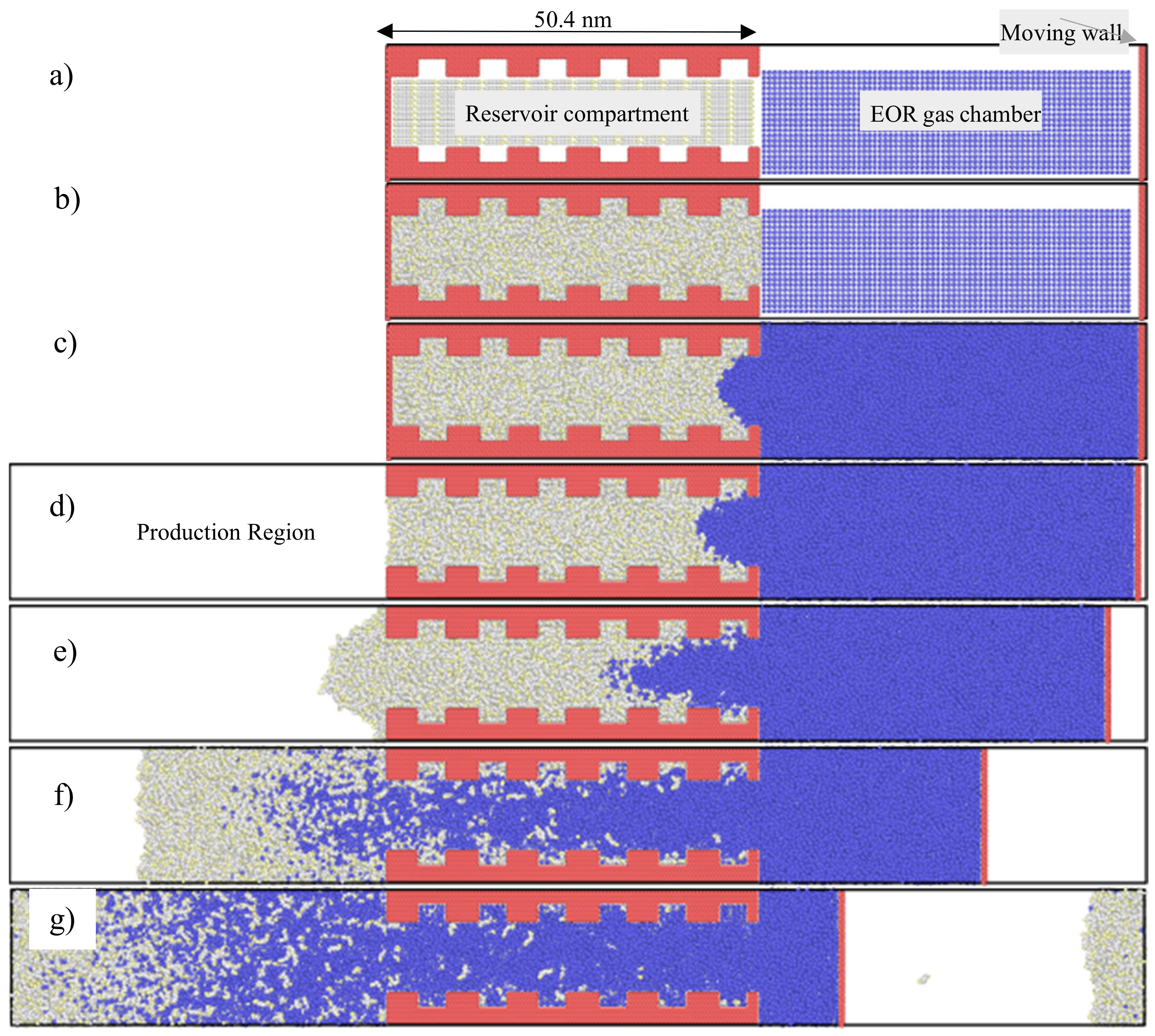
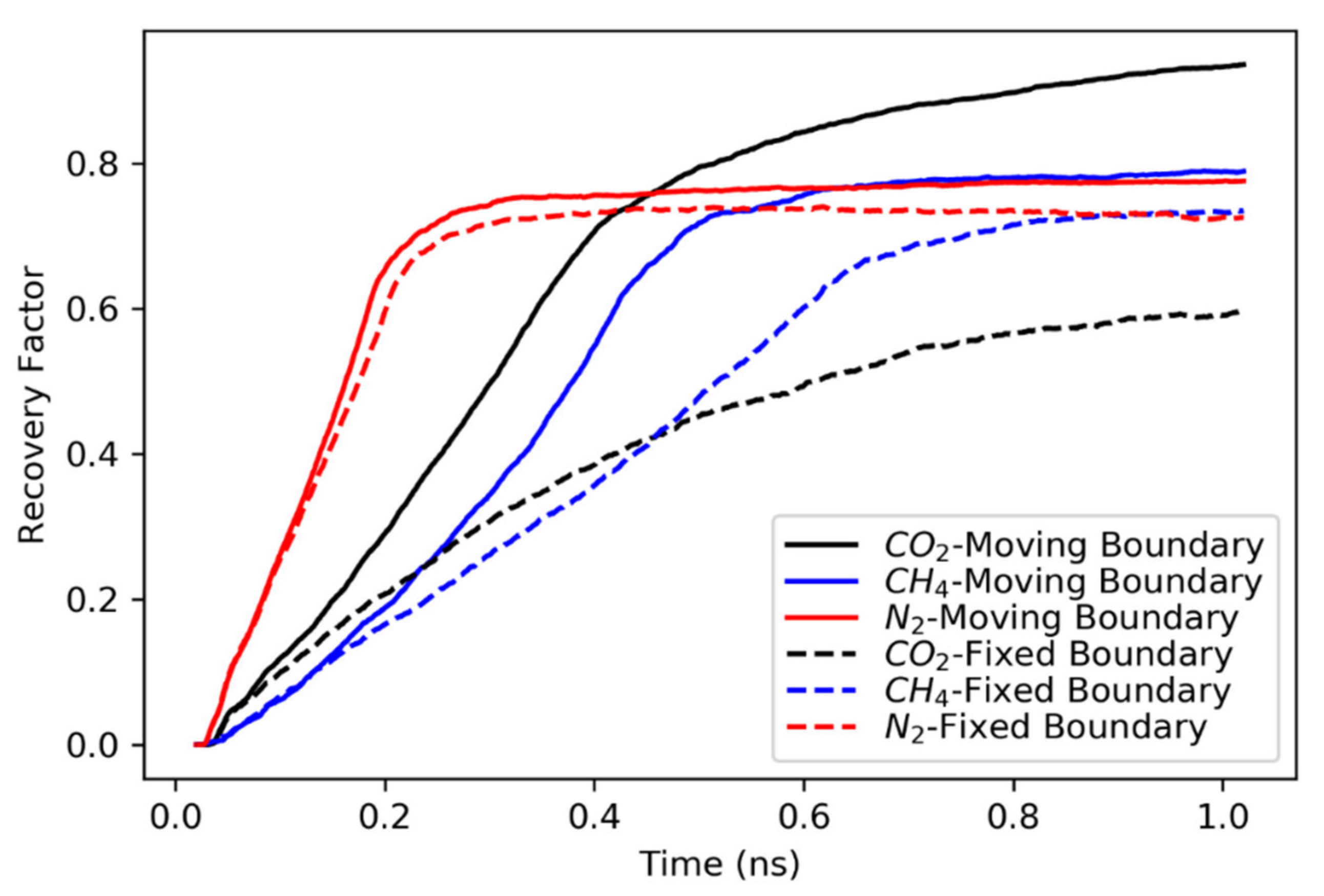
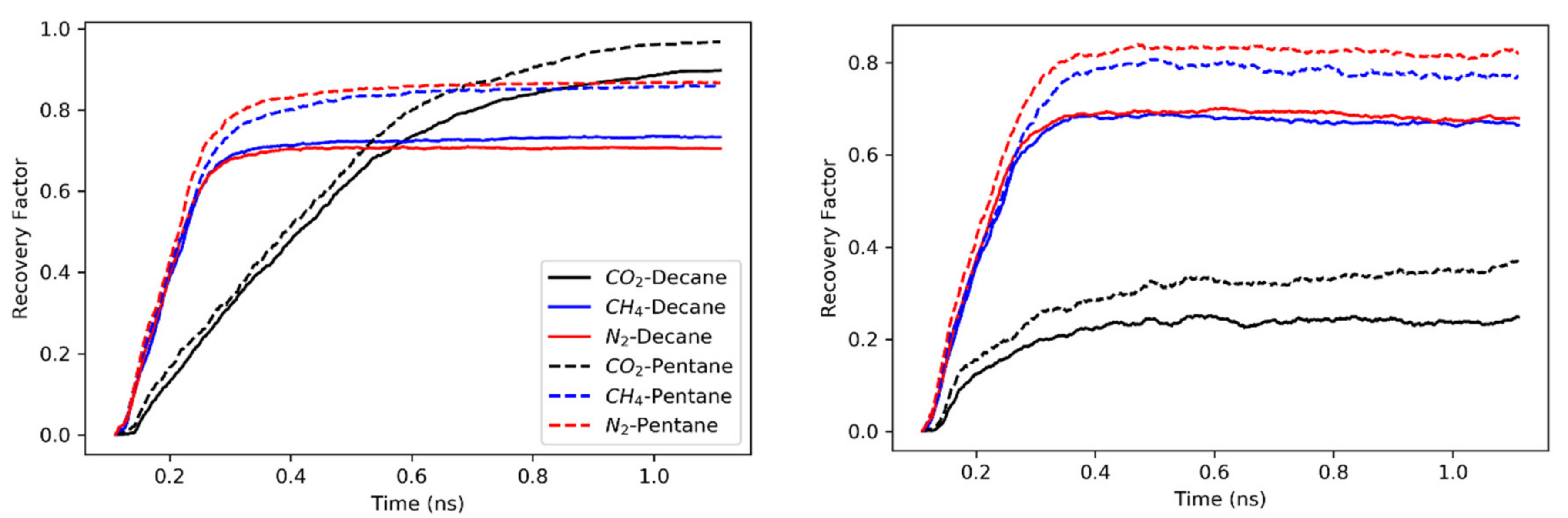
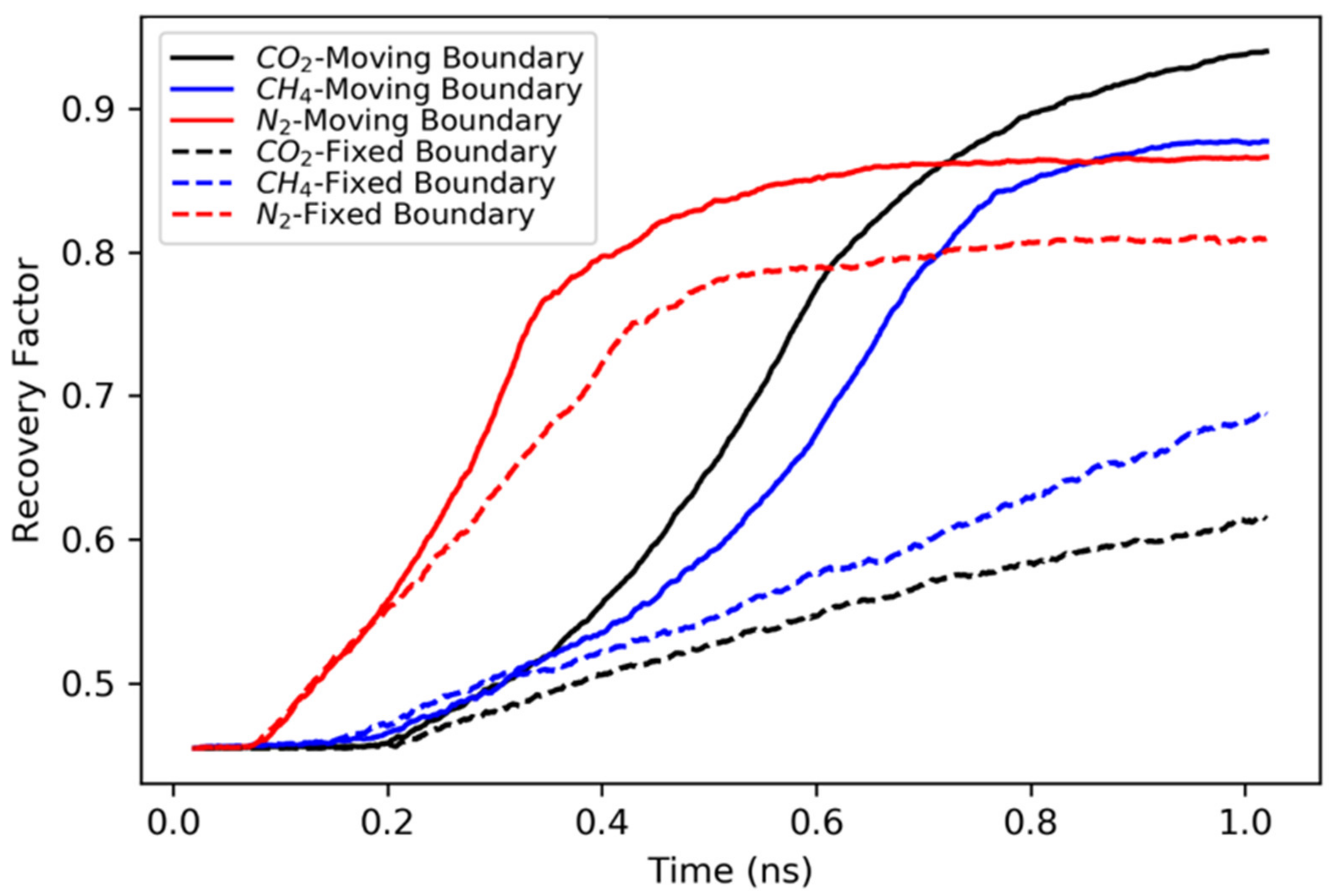
3.1. Concurrent Displacement
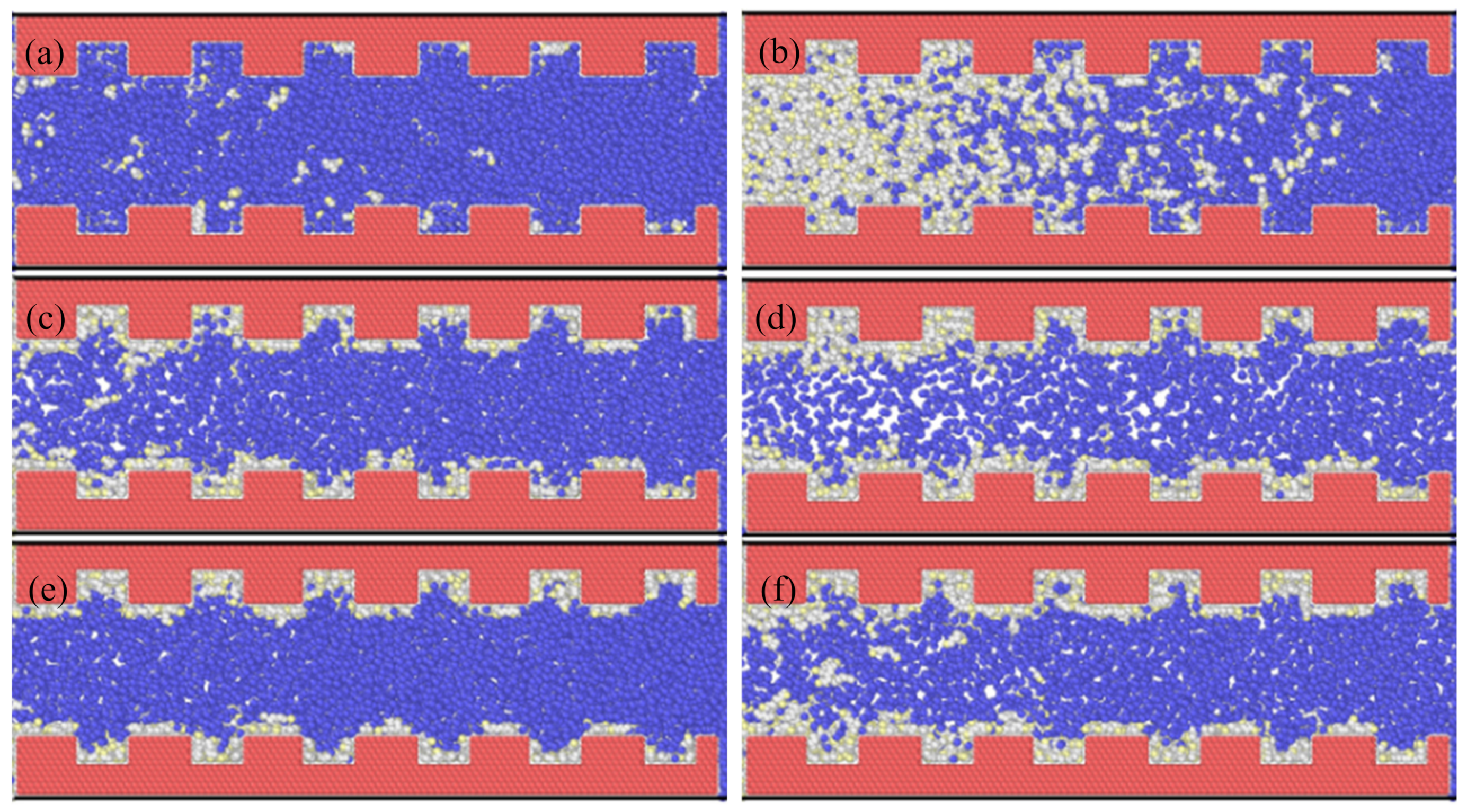
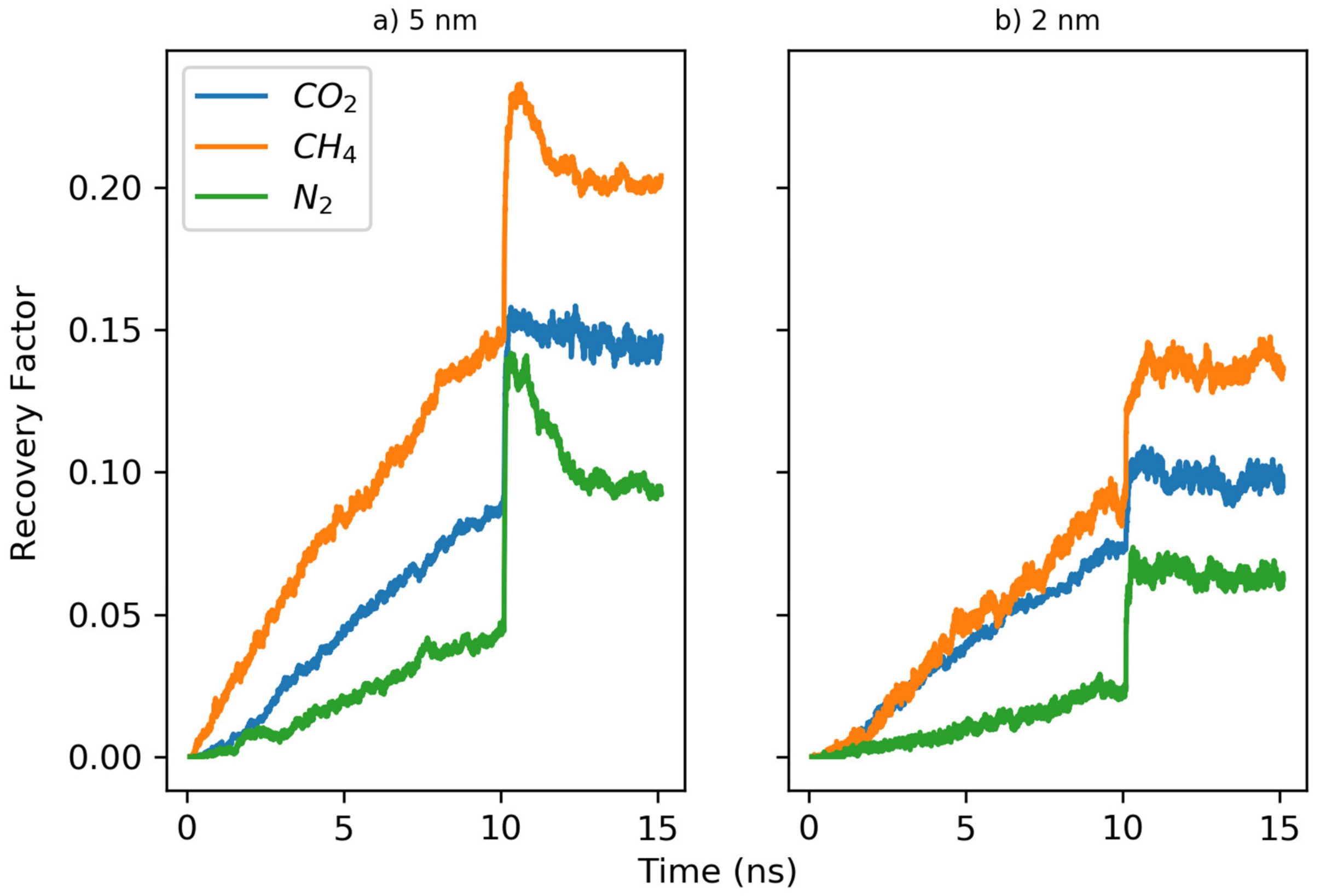
3.2. Countercurrent Displacement
4. Conclusions
- Confinement enhances the adsorption of hydrocarbons to the pore surface, which hinders both concurrent and counter-current displacements.
- All gases are more efficient in displacing hydrocarbons in concurrent displacement relative to counter-current displacement. While the recovery factors observed in counter-current displacements are usually less than 20%, the concurrent displacement could reach up to 90%.
- Nitrogen usually exhibited faster breakthrough regardless of the hydrocarbons’ type, pore size and the boundary conditions for the concurrent displacement. Interestingly, the limited diffusion and miscibility of nitrogen in hydrocarbons led to faster recovery in the case of concurrent displacement, while the opposite was observed for counter-current displacement. On the other hand, methane yielded better recovery for counter-current displacement.
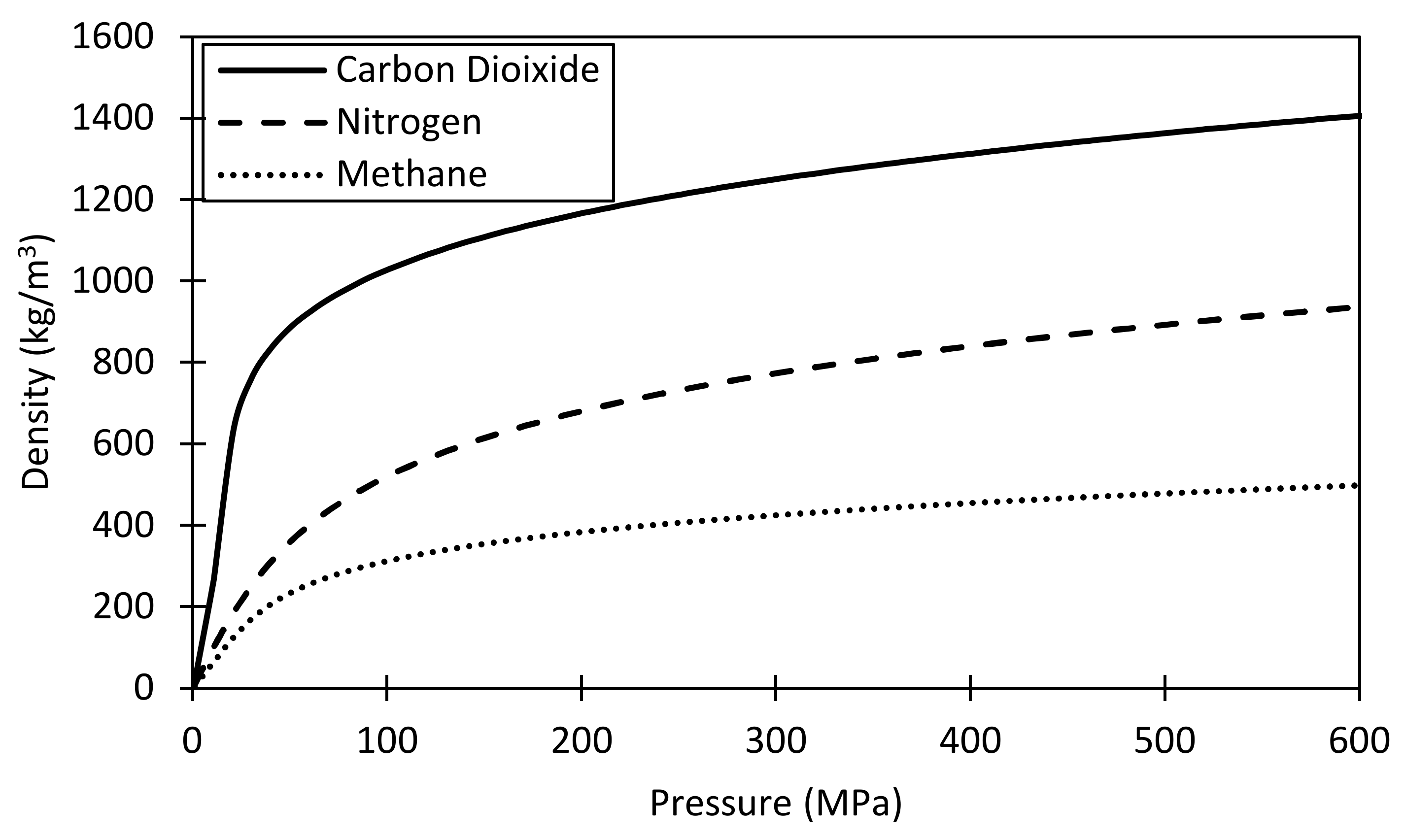
- Carbon dioxide proved more efficient in extracting the hydrocarbons from rough pores (from the grooves) if enough pressure was maintained. Having favorable adsorption characteristics and capability to improve the hydrocarbon mobility, carbon dioxide provides the best candidate. However, constant pressure support is needed to overcome the unstable displacement front.
Author Contributions
Funding
Conflicts of Interest
References
- Mehana, M.; El-monier, I. Shale characteristics impact on Nuclear Magnetic Resonance (NMR) fluid typing methods and correlations. Petroleum 2016, 2, 138–147. [Google Scholar] [CrossRef]
- Hughes, J.D. Energy: A reality check on the shale revolution. Nature 2013, 494, 307. [Google Scholar] [CrossRef]
- Middleton, R.; Carey, B.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef]
- King, G.E. Thirty years of gas shale fracturing: What have we learned? In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 19–22 September 2010; Society of Petroleum Engineers: Richardson, TX, USA, 2010.
- King, G.E. Hydraulic fracturing 101: What every representative, environmentalist, regulator, reporter, investor, university researcher, neighbor and engineer should know about estimating frac risk and improving frac performance in unconventional gas and oil wells. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 6–8 February 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Clark, A.J. Determination of recovery factor in the Bakken formation, Mountrail County, ND. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009; Society of Petroleum Engineers: Richardson, TX, USA, 2009. [Google Scholar]
- LeFever, J.A.; Helms, L.D. Bakken Formation Reserve Estimates; North Dakota Geological Survey: Bismarck, ND, USA, 2006. [Google Scholar]
- Green, D.W.; Willhite, G.P. Enhanced oil recovery. In Doherty Memorial Fund of AIME; Henry, L., Ed.; Society of Petroleum Engineers: Richardson, TX, USA, 1998; Volume 6. [Google Scholar]
- Lake, L.W. Enhanced Oil Recovery. 1989. Available online: https://store.spe.org/Enhanced-Oil-Recovery--P436.aspx (accessed on 22 September 2020).
- Lake, L.W.; Johns, R.; Rossen, W.R.; Pope, G.A. Fundamentals of Enhanced Oil Recovery. 2014. Available online: https://store.spe.org/Fundamentals-ofEnhanced-Oil-Recovery-P921.aspx (accessed on 22 September 2020).
- Chalmers, G.R.; Ross, D.J.; Bustin, R.M. Geological controls on matrix permeability of Devonian Gas Shales in the Horn River and Liard basins, northeastern British Columbia, Canada. Int. J. Coal Geol. 2012, 103, 120–131. [Google Scholar] [CrossRef]
- Yu, W.; Wu, K.; Zuo, L.; Tan, X.; Weijermars, R. Physical models for inter-well interference in shale reservoirs: Relative impacts of fracture hits and matrix permeability. In Proceedings of the Unconventional Resources Technology Conference, San Antonio, TX, USA, 1–3 August 2016. [Google Scholar]
- Alfarge, D.; Wei, M.; Bai, B. IOR methods in unconventional reservoirs of North America: Comprehensive review. In Proceedings of the SPE Western Regional Meeting, Bakersfield, CA, USA, 23–27 April 2017; Society of Petroleum Engineers: Richardson, TX, USA, 2017. [Google Scholar]
- Du, F.; Nojabaei, B. A review of gas injection in shale reservoirs: Enhanced oil/gas recovery approaches and greenhouse gas control. Energies 2019, 12, 2355. [Google Scholar] [CrossRef]
- Zhang, Y.; Di, Y.; Shi, Y.; Hu, J. Cyclic CH4 injection for enhanced oil recovery in the Eagle Ford shale reservoirs. Energies 2018, 11, 3094. [Google Scholar] [CrossRef]
- Meng, X.; Meng, Z.; Ma, J.; Wang, T. Performance Evaluation of CO2 Huff-n-Puff Gas Injection in Shale Gas Condensate Reservoirs. Energies 2018, 12, 42. [Google Scholar] [CrossRef]
- Assef, Y.; Almao, P.P. Evaluation of Cyclic Gas Injection in Enhanced Recovery from Unconventional Light Oil Reservoirs: Effect of Gas Type and Fracture Spacing. Energies 2019, 12, 1370. [Google Scholar] [CrossRef]
- Yu, W.; Lashgari, H.; Sepehrnoori, K. Simulation study of CO2 huff-n-puff process in Bakken tight oil reservoirs. In Proceedings of the SPE Western North American and Rocky Mountain Joint Meeting, Denver, CO, USA, 17–18 April 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Yu, W.; Lashgari, H.R.; Wu, K.; Sepehrnoori, K. CO2 injection for enhanced oil recovery in Bakken tight oil reservoirs. Fuel 2015, 159, 354–363. [Google Scholar] [CrossRef]
- Sheng, J.J.; Chen, K. Evaluation of the EOR potential of gas and water injection in shale oil reservoirs. J. Unconv. Oil Gas. Resour. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Sheng, J.J. Enhanced oil recovery in shale reservoirs by gas injection. J. Nat. Gas. Sci. Eng. 2015, 22, 252–259. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Yu, X.; Wang, C.; Yao, B.; Wang, S.; Winterfeld, P.H.; Wang, X.; Yang, Z.; Wang, Y. Advances in improved/enhanced oil recovery technologies for tight and shale reservoirs. Fuel 2017, 210, 425–445. [Google Scholar] [CrossRef]
- Alharthy, N.; Teklu, T.W.; Kazemi, H.; Graves, R.M.; Hawthorne, S.B.; Braunberger, J.; Kurtoglu, B. Enhanced oil recovery in liquid-rich shale reservoirs: Laboratory to field. SPE Reserv. Eval. Eng. 2018, 21, 137–159. [Google Scholar] [CrossRef]
- Hoffman, B.T. Comparison of various gases for enhanced recovery from shale oil reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Sheng, J.J. Increase liquid oil production by huff-n-puff of produced gas in shale gas condensate reservoirs. J. Unconv. Oil Gas. Resour. 2015, 11, 19–26. [Google Scholar] [CrossRef]
- Fragoso, A.; Wang, Y.; Jing, G.; Aguilera, R. Improving recovery of liquids from shales through gas recycling and dry gas injection. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Quito, Ecuador, 18–20 November 2015; Society of Petroleum Engineers: Richardson, TX, USA, 2015. [Google Scholar]
- Jin, L.; Hawthorne, S.; Sorensen, J.; Pekot, L.; Kurz, B.; Smith, S.; Heebink, L.; Herdegen, V.; Bosshart, N.; Torres, J. Advancing CO2 enhanced oil recovery and storage in unconventional oil play—Experimental studies on Bakken shales. Appl. Energy 2017, 208, 171–183. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Tovar, F.D.; Eide, O.; Graue, A.; Schechter, D.S. Experimental investigation of enhanced recovery in unconventional liquid reservoirs using CO2: A look ahead to the future of unconventional EOR. In Proceedings of the SPE Unconventional Resources Conference, The Woodlands, TX, USA, 1–3 April 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Gamadi, T.; Sheng, J.; Soliman, M.; Menouar, H.; Watson, M.; Emadibaladehi, H. An experimental study of cyclic CO2 injection to improve shale oil recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, New Orleans, LA, USA, 30 September–2 October 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Yu, Y.; Sheng, J.J. Experimental evaluation of shale oil recovery from Eagle Ford core samples by nitrogen gas flooding. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar]
- Nguyen, P.; Carey, J.W.; Viswanathan, H.S.; Porter, M. Effectiveness of supercritical-CO2 and N2 huff-and-puff methods of enhanced oil recovery in shale fracture networks using microfluidic experiments. Appl. Energy 2018, 230, 160–174. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Jin, L.; Kurz, B.A.; Miller, D.J.; Grabanski, C.B.; Sorensen, J.A.; Pekot, L.J.; Bosshart, N.W.; Smith, S.A.; Burton-Kelly, M.E. Integrating petrographic and petrophysical analyses with CO2 permeation and oil extraction and recovery in the Bakken Tight oil formation. In Proceedings of the SPE Unconventional Resources Conference, Calgary, AB, Canada, 15–16 February 2017; Society of Petroleum Engineers: Richardson, TX, USA, 2017. [Google Scholar]
- Todd, H.B.; Evans, J.G. Improved oil recovery IOR pilot projects in the Bakken formation. In Proceedings of the SPE Low Perm Symposium, Denver, CO, USA, 5–6 May 2016; Society of Petroleum Engineers: Richardson, TX, USA, 2016. [Google Scholar]
- Liu, G.; Sorensen, J.; Braunberger, J.; Klenner, R.; Ge, J.; Gorecki, C.; Steadman, E.; Harju, J. CO2-based enhanced oil recovery from unconventional reservoirs: A case study of the Bakken formation. In Proceedings of the SPE Unconventional Resources Conference, The Woodlands, TX, USA, 1–3 April 2014; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Pankaj, P.; Mukisa, H.; Solovyeva, I.; Xue, H. Enhanced oil recovery in eagle ford: Opportunities using huff-n-puff technique in unconventional reservoirs. In Proceedings of the SPE Liquids-Rich Basins Conference-North America, Midland, TX, USA, 5–6 September 2018; Society of Petroleum Engineers: Richardson, TX, USA, 2018. [Google Scholar]
- Kerr, E.; Venepalli, K.K.; Patel, K.; Ambrose, R.; Erdle, J. Use of Reservoir Simulation to Forecast Field EOR Response-An Eagle Ford Gas Injection Huff-N-Puff Application. In Proceedings of the SPE Hydraulic Fracturing Technology Conference and Exhibition, The Woodlands, TX, USA, 4–6 February 2020; Society of Petroleum Engineers: Richardson, TX, USA, 2020. [Google Scholar]
- Rassenfoss, S. Shale EOR Works, But Will It Make a Difference? J. Pet. Technol. 2017, 69, 34–40. [Google Scholar] [CrossRef]
- Lan, Y.; Yang, Z.; Wang, P.; Yan, Y.; Zhang, L.; Ran, J. A review of microscopic seepage mechanism for shale gas extracted by supercritical CO2 flooding. Fuel 2019, 238, 412–424. [Google Scholar] [CrossRef]
- Mehana, M.; Fahes, M.; Huang, L. The Density of Oil/Gas Mixtures: Insights from Molecular Simulations. SPE J. 2018, 23, 1798–1808. [Google Scholar] [CrossRef]
- Mehana, M.; Fahes, M.; Huang, L. Asphaltene Aggregation in Oil and Gas Mixtures: Insights from Molecular Simulation. Energy Fuels 2019, 33, 4721–4730. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Liu, H. Molecular dynamics simulations about adsorption and displacement of methane in carbon nanochannels. J. Phys. Chem. C 2015, 119, 13652–13657. [Google Scholar] [CrossRef]
- Wang, T.; Tian, S.; Li, G.; Sheng, M.; Ren, W.; Liu, Q.; Zhang, S. Molecular simulation of CO2/CH4 competitive adsorption on shale kerogen for CO2 sequestration and enhanced gas recovery. J. Phys. Chem. C 2018, 122, 17009–17018. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, H.; Qi, N.; Li, Y. Molecular insights into the enhanced shale gas recovery by carbon dioxide in kerogen slit nanopores. J. Phys. Chem. C 2017, 121, 10233–10241. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Zhang, J.; Xiao, S.; Zhang, Z.; Shen, Y.; Sun, B.; He, J. Displacement mechanism of oil in shale inorganic nanopores by supercritical carbon dioxide from molecular dynamics simulations. Energy Fuels 2016, 31, 738–746. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, Z.; Luo, K.H. Insights into recovery of multi-component shale gas by CO2 injection: A molecular perspective. Fuel 2020, 267, 117247. [Google Scholar] [CrossRef]
- Zhang, M.; Zhan, S.; Jin, Z. Recovery mechanisms of hydrocarbon mixtures in organic and inorganic nanopores during pressure drawdown and CO2 injection from molecular perspectives. Chem. Eng. J. 2020, 382, 122808. [Google Scholar] [CrossRef]
- Neil, C.W.; Mehana, M.; Hjelm, R.P.; Hawley, M.E.; Watkins, E.B.; Mao, Y.; Viswanathan, H.; Kang, Q.; Xu, H. Reduced methane recovery at high pressure due to methane trapping in shale nanopores. Commun. Earth Environ. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Santos, J.E.; Mehana, M.; Wu, H.; Prodanovic, M.; Kang, Q.; Lubbers, N.; Viswanathan, H.; Pyrcz, M.J. Modeling nanoconfinement effects using active learning. J. Phys. Chem. C 2020, 124, 22200–22211. [Google Scholar] [CrossRef]
- Lubbers, N.; Agarwal, A.; Chen, Y.; Son, S.; Mehana, M.; Kang, Q.; Karra, S.; Junghans, C.; Germann, T.C.; Viswanathan, H.S. Modeling and scale-bridging using machine learning: Nanoconfinement effects in porous media. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- NIST. National Institute of Standards and Technology (NIST) Chemistry WebBook. 2009. Available online: https://webbook.nist.gov/chemistry/fluid/ (accessed on 5 October 2020).
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Jewett, A.I.; Zhuang, Z.; Shea, J.E. Moltemplate a coarse-grained model assembly tool. Biophys. J. 2013, 104, 169. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Fang, T.; Wang, M.; Gao, Y.; Zhang, Y.; Yan, Y.; Zhang, J. Enhanced oil recovery with CO2/N2 slug in low permeability reservoir: Molecular dynamics simulation. Chem. Eng. Sci. 2019, 197, 204–211. [Google Scholar] [CrossRef]
- Pereira, L.M.; Chapoy, A.; Burgass, R.; Tohidi, B. Measurement and modelling of high pressure density and interfacial tension of (gas+ n-alkane) binary mixtures. J. Chem. 2016, 97, 55–69. [Google Scholar] [CrossRef]
- Tong, J.; Gao, W.; Robinson, R.L.; Gasem, K.A. Solubilities of nitrogen in heavy normal paraffins from 323 to 423 K at pressures to 18.0 MPa. J. Chem. Eng. Data 1999, 44, 784–787. [Google Scholar] [CrossRef]
- Georgiadis, A.; Llovell, F.; Bismarck, A.; Blas, F.J.; Galindo, A.; Maitland, G.C.; Trusler, J.M.; Jackson, G. Interfacial tension measurements and modelling of (carbon dioxide+ n-alkane) and (carbon dioxide+ water) binary mixtures at elevated pressures and temperatures. J. Supercrit. Fluids 2010, 55, 743–754. [Google Scholar] [CrossRef]
- Zamudio, M.; Schwarz, C.; Knoetze, J. Phase equilibria of branched isomers of C10-alcohols and C10-alkanes in supercritical carbon dioxide. J. Supercrit. Fluids 2011, 59, 14–26. [Google Scholar] [CrossRef]
- Nourozieh, H.; Bayestehparvin, B.; Kariznovi, M.; Abedi, J. Equilibrium properties of (carbon dioxide+ n-decane+ n-octadecane) systems: Experiments and thermodynamic modeling. J. Chem. Eng. Data 2013, 58, 1236–1243. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.; Li, H.; Wang, Q.; Ye, H.; Qin, H. Molecular simulation of CO2 sequestration and enhanced gas recovery in gas rich shale: An insight based on realistic kerogen model. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13–16 November 2017; Society of Petroleum Engineers: Richardson, TX, USA, 2017. [Google Scholar]
- Fang, T.; Zhang, Y.; Liu, J.; Ding, B.; Yan, Y.; Zhang, J. Molecular insight into the miscible mechanism of CO2/C10 in bulk phase and nanoslits. Int. J. Heat Mass Transf. 2019, 141, 643–650. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Sheng, J.J. Effect of the injection pressure on enhancing oil recovery in shale cores during the CO2 huff-n-puff process when it is above and below the minimum miscibility pressure. Energy Fuels 2017, 31, 3856–3867. [Google Scholar] [CrossRef]
- Li, L.; Su, Y.; Hao, Y.; Zhan, S.; Lv, Y.; Zhao, Q.; Wang, H. A comparative study of CO2 and N2 huff-n-puff EOR performance in shale oil production. J. Pet. Sci. Eng. 2019, 181, 106174. [Google Scholar] [CrossRef]
- Tovar, F.D.; Barrufet, M.A.; Schechter, D.S. Enhanced Oil Recovery in the Wolfcamp Shale by Carbon Dioxide or Nitrogen Injection: An Experimental Investigation. SPE J. 2020. [Google Scholar] [CrossRef]
- Choudhary, N.; Nair, A.K.N.; Ruslan, M.F.A.C.; Sun, S. Bulk and interfacial properties of decane in the presence of carbon dioxide, methane, and their mixture. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Potoff, J.J.; Siepmann, J.I. Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. AIChE J. 2001, 47, 1676–1682. [Google Scholar] [CrossRef]
| LJ | Ɛ (Kcal/Mole) | σ (Å) |
|---|---|---|
| C | 0.068443 | 3.407 |
| CO2 | 0.717017 | 3.72 |
| CH4 | 0.294 | 3.73 |
| N2 | 0.18918 | 3.75 |
| CH3 | 0.175 | 3.905 |
| CH2 | 0.118 | 3.905 |
| Bond | Kb (Kcal/mole) | b0 (Å) |
| CH3-CH2 CH2-CH2 | 260 | 1.526 |
| Angle | Ka (Kcal/mole) | θ (°) |
| CH3-CH2-CH2 CH2-CH2-CH2 | 63 | 112.4 |
| # | Pore Width (nm) | Hydrocarbon Mixture | Boundary Condition | EOR Gas |
|---|---|---|---|---|
| 1 | 7 | Decane | Moving | CO2 |
| 2 | CH4 | |||
| 3 | N2 | |||
| 4 | Fixed | CO2 | ||
| 5 | CH4 | |||
| 6 | N2 | |||
| 7 | Decane and Pentane | Moving | CO2 | |
| 8 | CH4 | |||
| 9 | N2 | |||
| 10 | Fixed | CO2 | ||
| 11 | CH4 | |||
| 12 | N2 | |||
| 13 | 5 | Decane | Fixed | CO2 |
| 14 | CH4 | |||
| 15 | N2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehana, M.; Kang, Q.; Viswanathan, H. Molecular-Scale Considerations of Enhanced Oil Recovery in Shale. Energies 2020, 13, 6619. https://doi.org/10.3390/en13246619
Mehana M, Kang Q, Viswanathan H. Molecular-Scale Considerations of Enhanced Oil Recovery in Shale. Energies. 2020; 13(24):6619. https://doi.org/10.3390/en13246619
Chicago/Turabian StyleMehana, Mohamed, Qinjun Kang, and Hari Viswanathan. 2020. "Molecular-Scale Considerations of Enhanced Oil Recovery in Shale" Energies 13, no. 24: 6619. https://doi.org/10.3390/en13246619
APA StyleMehana, M., Kang, Q., & Viswanathan, H. (2020). Molecular-Scale Considerations of Enhanced Oil Recovery in Shale. Energies, 13(24), 6619. https://doi.org/10.3390/en13246619