Characterization of Metallic Interconnects Extracted from Solid Oxide Fuel Cell Stacks Operated up to 20,000 h in Real Life Conditions: The Air Side
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Operating Parameters
2.2. Samples Preparation and Characterization
3. Results
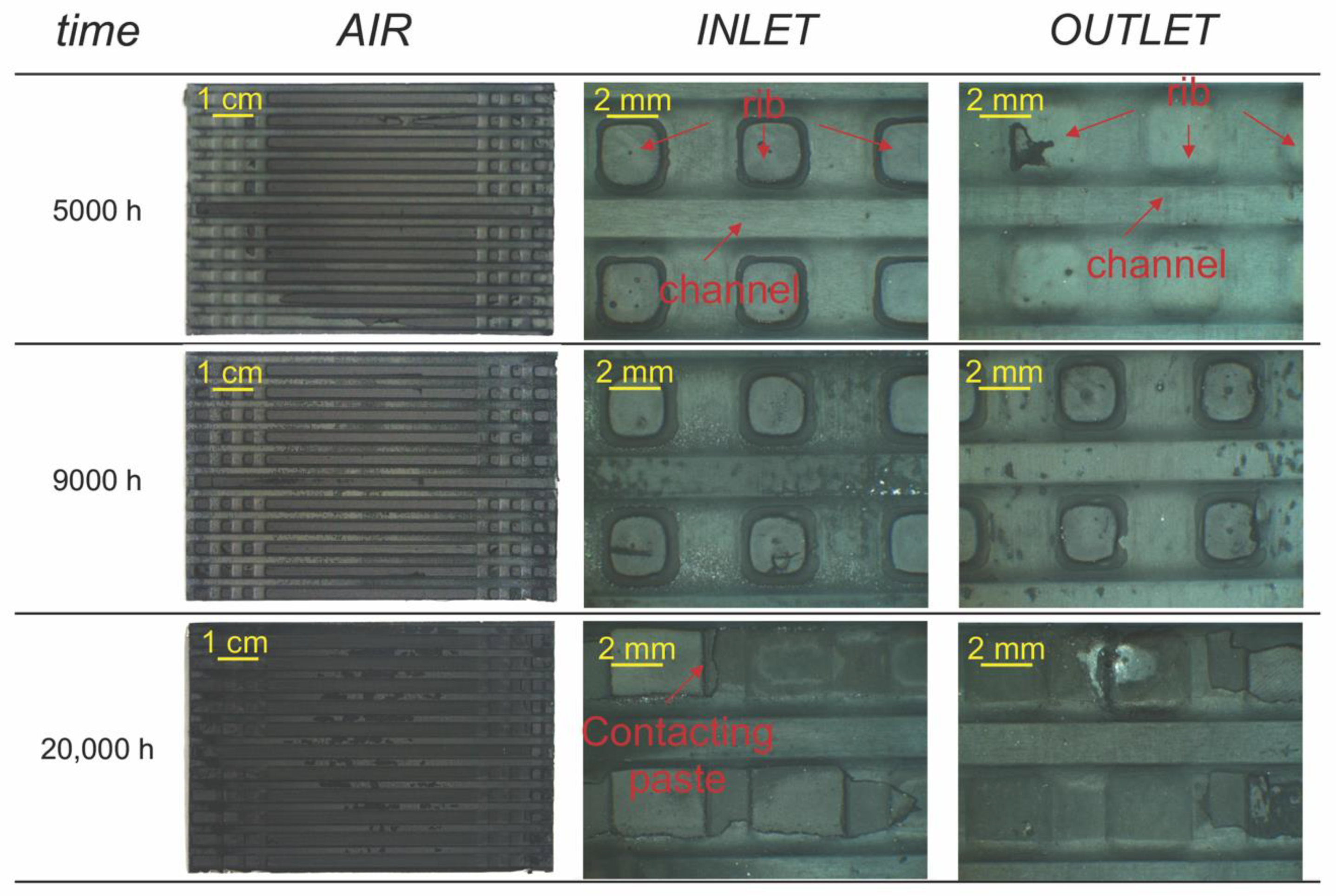
3.1. Surface Observations of the MIC
3.2. Cross Sections
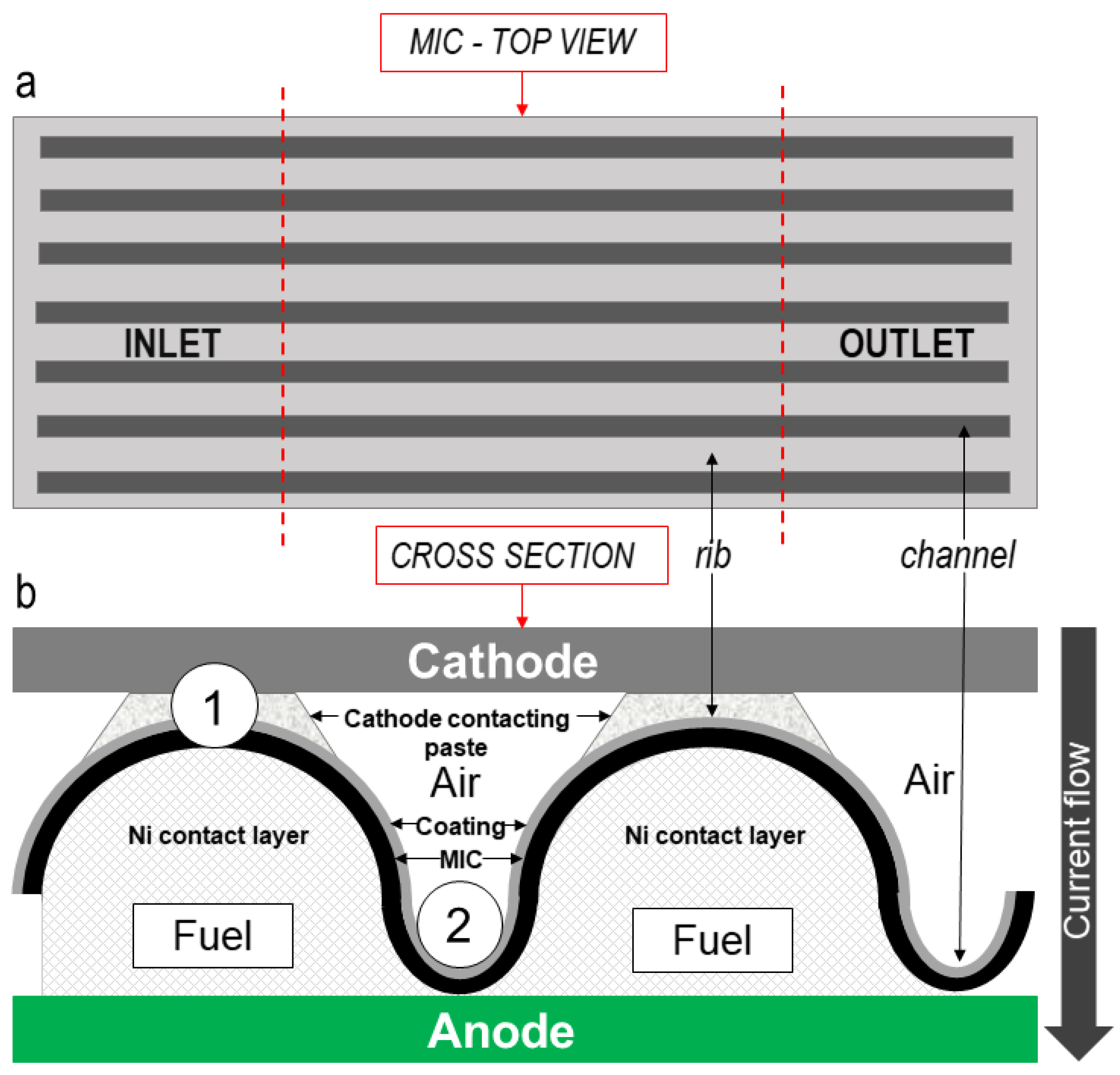
3.2.1. Choice of the Sites of Analysis
- Air/rib (position 1): area subjected to the electric current flow where the MCO coated MIC is interfaced with the LSCM cathode contact layer;
- Air/channel (position 2): area at the bottom between two ribs at air side, subjected only to the air flow, coated with MCO but not in contact with other materials.
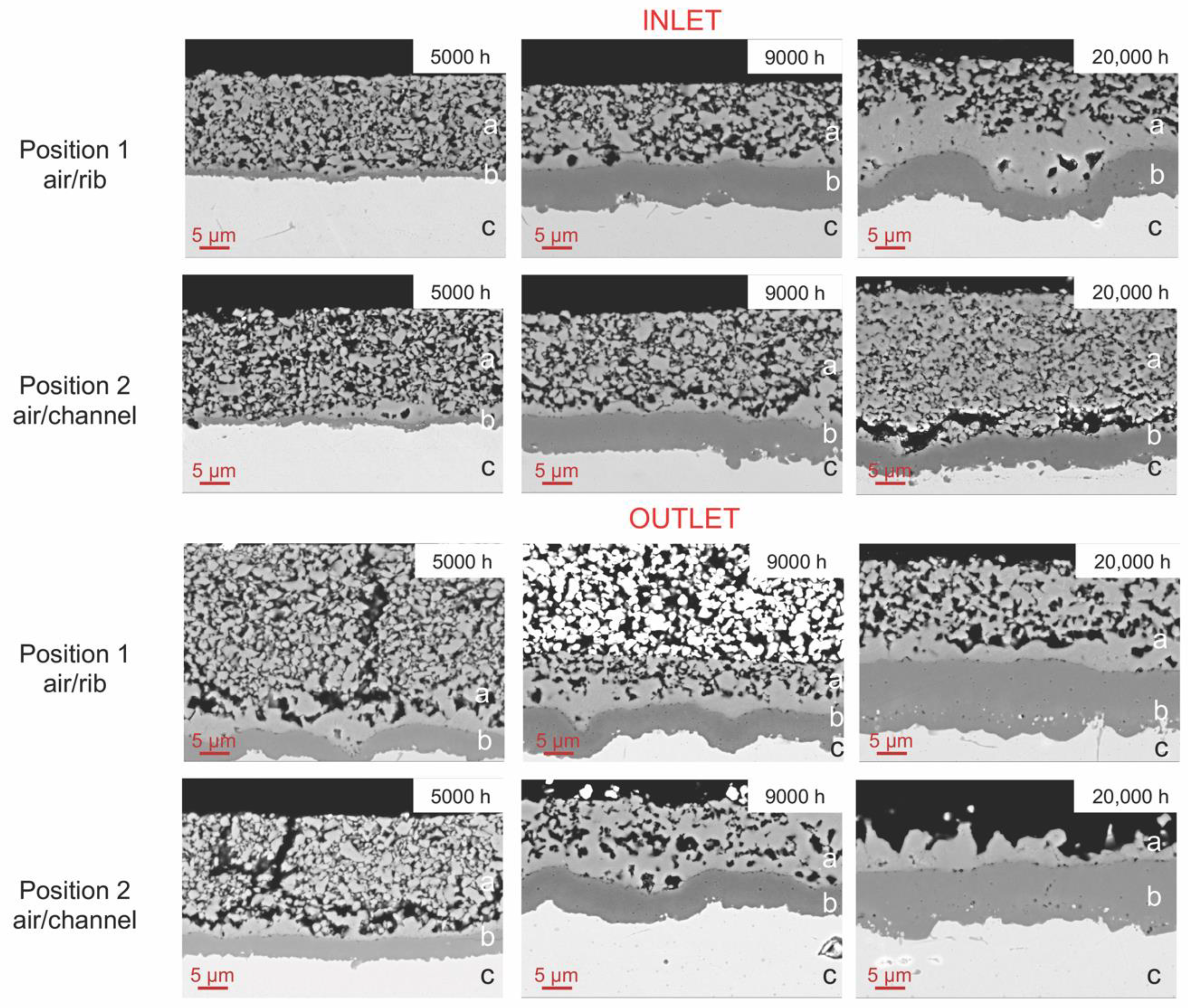
3.2.2. Morphological Results
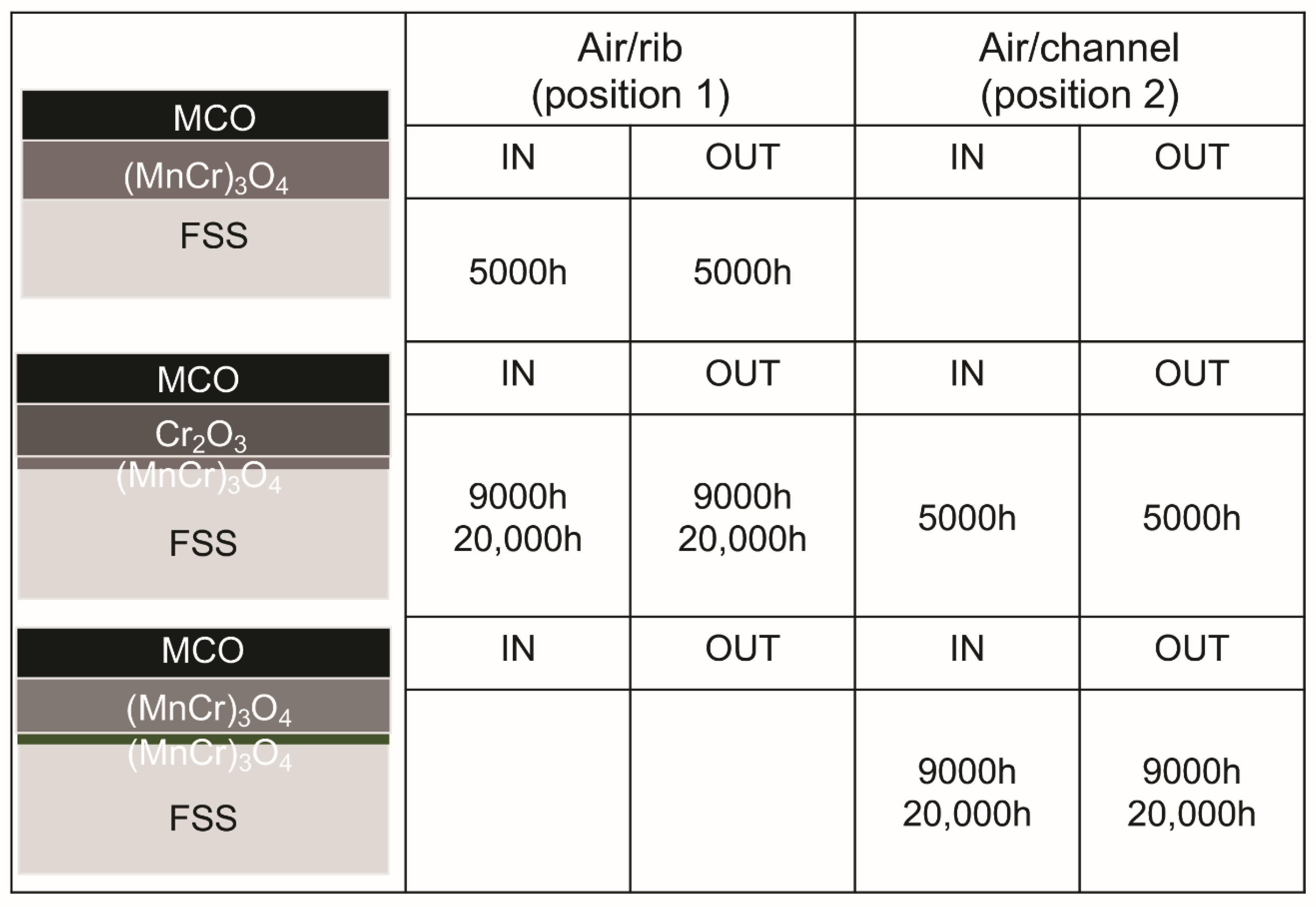
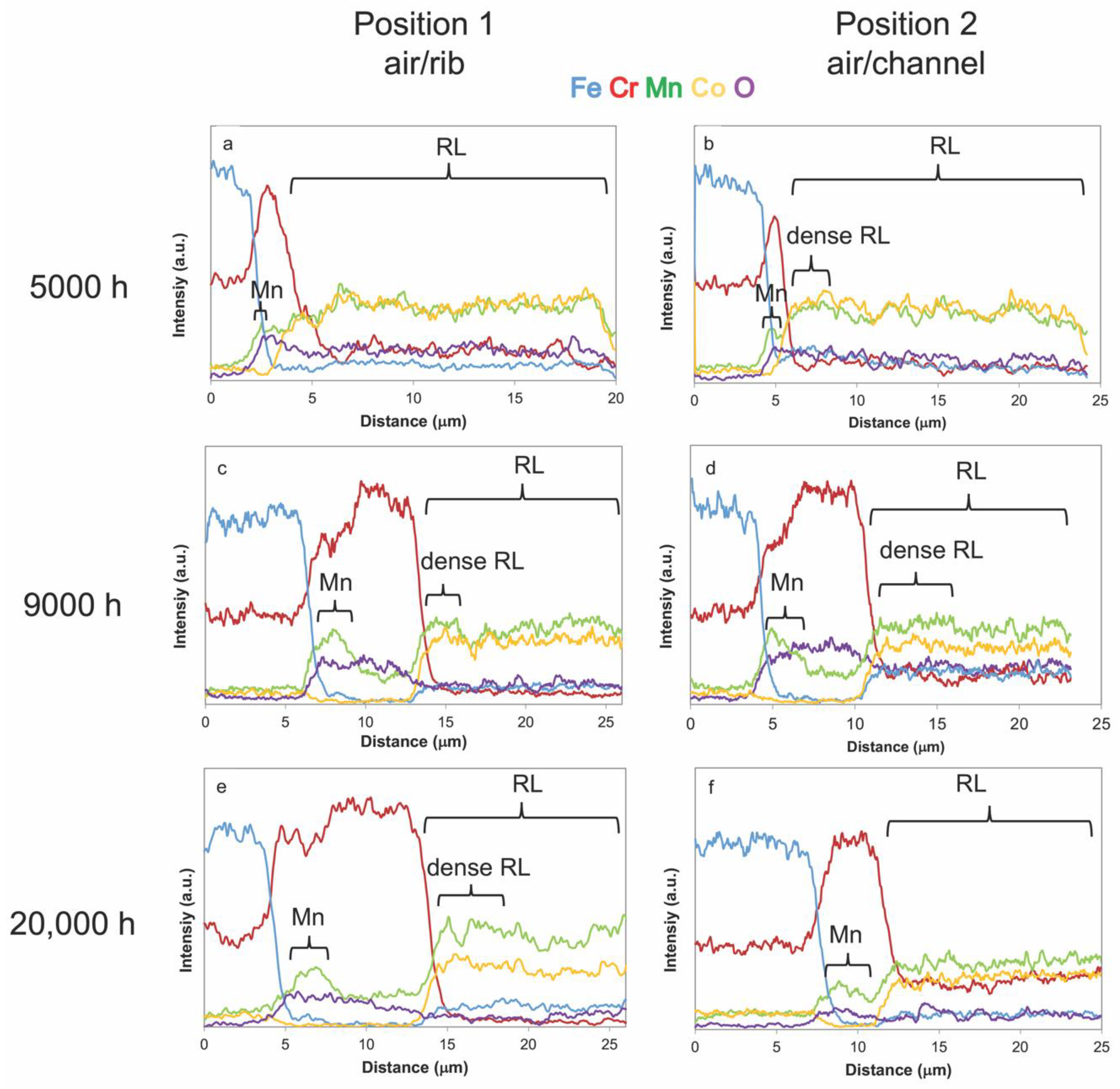
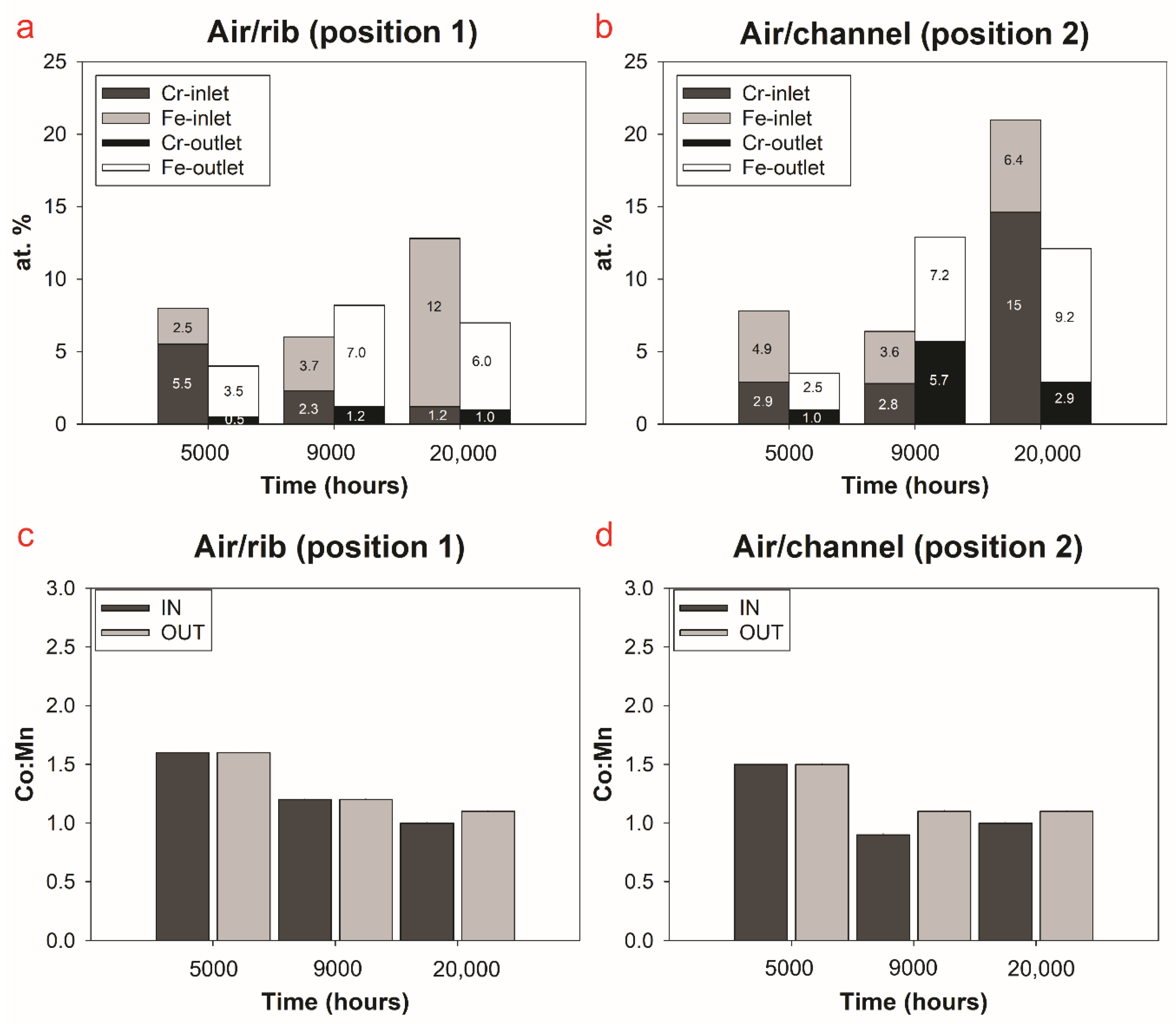
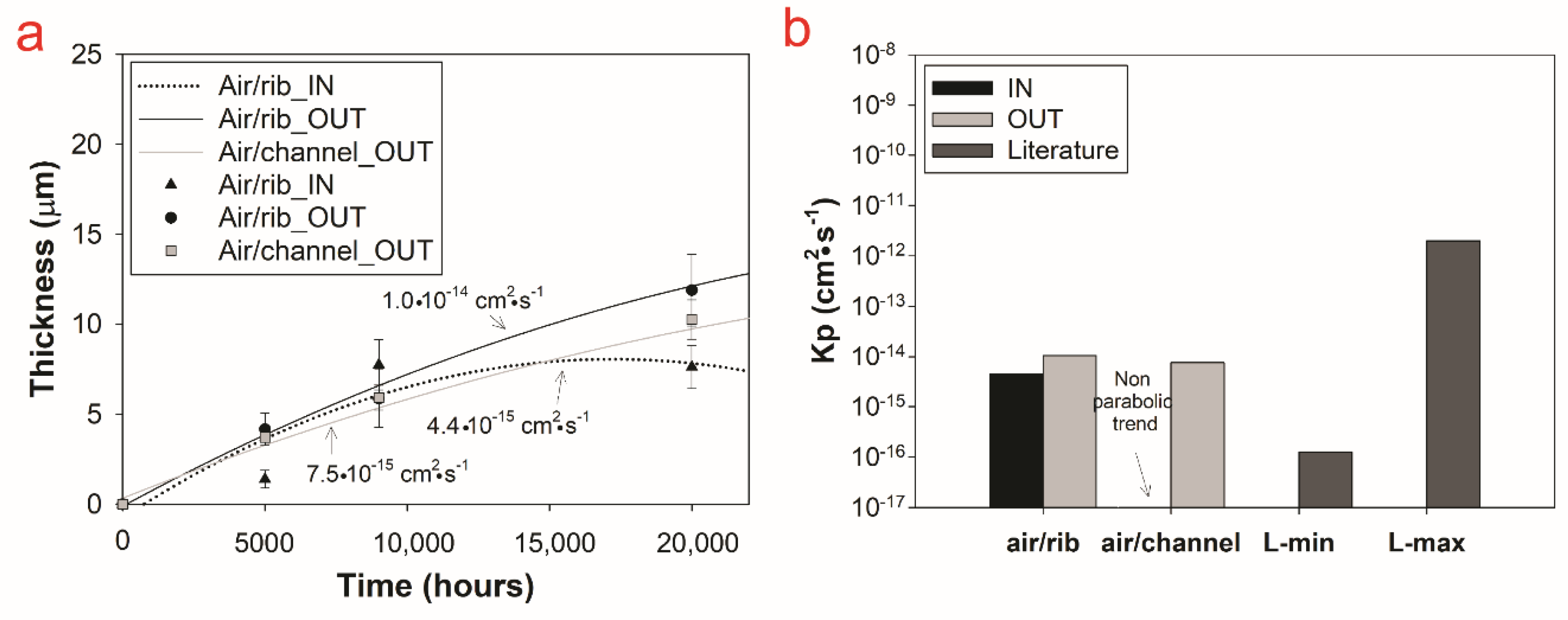
3.2.3. Compositional Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Damo, U.M.; Ferrari, M.L.; Turan, A.; Massardo, A.F. Solid oxide fuel cell hybrid system: A detailed review of an environmentally clean and efficient source of energy. Energy 2019, 168, 235–246. [Google Scholar] [CrossRef]
- Fernandes, M.D.; Bistritzki, V.; Domingues, R.Z.; Matencio, T.; Rapini, M.; Sinisterra, R.D. Solid oxide fuel cell technology paths: National innovation system contributions from Japan and the United States. Renew. Sustain. Energy Rev. 2020, 127, 109879. [Google Scholar] [CrossRef]
- Öztürk, B.; Topcu, A.; Öztürk, S.; Cora, Ö.N. Oxidation, electrical and mechanical properties of Crofer®22 solid oxide fuel cell metallic interconnects manufactured through powder metallurgy. Int. J. Hydrogen Energy 2018, 43, 10822. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, L.; Liu, Y.; Huang, Z. Oxidation resistance optimization of TiC/astelloy composites by adding Ta element applied for intermediate temperature solid oxide fuel cell interconnects. J. Power Sources 2018, 401, 1–5. [Google Scholar] [CrossRef]
- Mah, J.C.W.; Muchtara, A.; Somalu, M.R.; Ghazali, M.J. Metallic interconnects for solid oxide fuel cell: A review on protective coating and deposition techniques. Int. J. Hydrogen Energy 2019, 42, 9219–9229. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X. Recent Development of SOFC Metallic Interconnect. J. Mater. Sci. Technol. 2010, 26, 293–305. [Google Scholar] [CrossRef]
- Sreedhar, I.; Agarwal, B.; Goyal, P.; Agarwal, A. An overview of degradation in solid oxide fuel cells-potential clean power sources. J. Solid State Electrochem. 2020, 24, 1239–1270. [Google Scholar] [CrossRef]
- Swaminathan, S.; Ko, Y.S.; Lee, Y.-S.; Kim, D.-I. Oxidation behavior and area specific resistance of La, Cu and B alloyed Fe-22Cr ferritic steels for solid oxide fuel cell interconnects. J. Power Sources 2017, 369, 13–26. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, B.; Kang, J.; Lee, S.; Bae, J. Evaluation of Ag-doped (MnCo)(3)O-4 spinel as a solid oxide fuel cell metallic interconnect coating material. Int. J. Hydrogen Energy 2017, 42, 29511–29517. [Google Scholar] [CrossRef]
- Ryter, J.; Amendola, R.; McCleary, M.; Shong, W.-J.; Liu, C.-K.; Spotorno, R.; Piccardo, P. Effect of electrical current on the oxidation behavior of electroless nickel-plated ferritic stainless steel in solid oxide fuel cell operating conditions. Int. J. Hydrogen Energy 2018, 43, 426–434. [Google Scholar] [CrossRef]
- Park, M.; Shin, J.-S.; Lee, S.; Kim, H.-J.; An, H.; Ji, H.-I.; Kim, H.; Son, J.-W.; Lee, J.-H.; Kim, B.-K.; et al. Thermal degradation mechanism of ferritic alloy (Crofer 22 APU). Corros. Sci. 2018, 134, 17–22. [Google Scholar] [CrossRef]
- Sachitanand, R.; Sattari, M.; Svensson, J.-E.; Froitzheim, J. Evaluation of the oxidation and Cr evaporation properties of selected FeCr alloys used as SOFC interconnects. Int. J. Hydrogen Energy 2013, 38, 15328–15334. [Google Scholar] [CrossRef]
- Talic, B.; Molin, S.; Hendriksen, P.V.; Lein, H.L. Effect of pre-oxidation on the oxidation resistance of Crofer 22 APU. Corros. Sci. 2018, 138, 189–199. [Google Scholar] [CrossRef]
- Hosseini, N.; Karimzadeh, F.; Abbasi, M.H.; Choi, G.M. Correlation between microstructure and electrical properties of Cu1.3Mn1.7O4/La2O3 composite-coated ferritic stainless steel interconnects. J. Alloys Compd. 2016, 673, 249–257. [Google Scholar] [CrossRef]
- Goebel, C.; Alnegren, P.; Faust, R.; Svensson, J.-E.; Froitzheim, J. The effect of pre-oxidation parameters on the corrosion behavior of AISI 441 in dual atmosphere. Int. J. Hydrogen Energy 2018, 43, 14665–14674. [Google Scholar] [CrossRef]
- Alnegren, P.; Sattari, M.; Svensson, J.-E.; Froitzheim, J. Severe dual atmosphere effect at 600 °C for stainless steel 441. J. Power Sources 2016, 301, 170–178. [Google Scholar] [CrossRef]
- Chandra-Ambhorn, S.; Wouters, Y.; Antoni, L.; Toscan, F.; Galerie, A. Adhesion of oxide scales grown on ferritic stainless steels in solid oxide fuel cells temperature and atmosphere conditions. J. Power Sources 2007, 171, 688–695. [Google Scholar] [CrossRef]
- Young, D.J.; Zurek, J.; Singheiser, L.; Quadakkers, W.J. Temperature dependence of oxide scale formation on high-Cr ferritic steels in Ar–H2–H2O. Corros. Sci. 2011, 53, 2131–2141. [Google Scholar] [CrossRef]
- Bongiorno, V.; Piccardo, P.; Anelli, S.; Spotorno, R. Influence of Surface Finishing on High-Temperature Oxidation of AISI Type 444 Ferritic Stainless Steel Used in SOFC Stacks. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 697–711. [Google Scholar] [CrossRef]
- Lenka, R.K.; Patro, P.K.; Sharma, J.; Mahat, T.; Sinha, P.K. Evaluation of La0.75Sr0.25Cr0.5Mn0.5O3 protective coating on ferritic stainless steel interconnect for SOFC application. Int. J. Hydrogen. Energy 2016, 41, 20365–20372. [Google Scholar] [CrossRef]
- Hua, B.; Kong, Y.; Zhang, W.; Pu, J.; Chi, B.; Jian, L. The effect of Mn on the oxidation behavior and electrical conductivity of Fe–17Cralloys in solid oxide fuel cell cathode atmosphere. J. Power Sources 2011, 196, 7627–7638. [Google Scholar] [CrossRef]
- Hua, B.; Pu, J.; Lu, F.; Zhang, J.; Chi, B.; Jian, L. Development of a Fe–Cr alloy for interconnect application in intermediate temperature solid oxide fuel cells. J. Power Sources 2010, 195, 2782–2788. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Z.; Pal, U.B.; Gopalan, S.; Basu, S.N. Mitigation of chromium poisoning of cathodes in solid oxide fuel cells employing CuMn1.8O4spinel coating on metallic interconnect. J. Power Sources 2018, 376, 100–110. [Google Scholar] [CrossRef]
- Chou, Y.-S.; Stevenson, J.W.; Choi, J.-P. Long-term evaluation of solid oxide fuel cell candidate materials in a 3-cell generic stack test fixture, part III: Stability and microstructure of Ce-(Mn,Co)-spinel coating, AISI441 interconnect, alumina coating, cathode and anode. J. Power Sources 2014, 257, 444–453. [Google Scholar] [CrossRef]
- Menzler, N.H.; Sebold, D.; Guillon, O. Post-test characterization of a solid oxide fuel cell stack operated for more than 30,000 hours: The cell. J. Power Sources 2018, 374, 69–76. [Google Scholar] [CrossRef]
- Yang, J.J.; Yan, D.; Huang, W.; Li, J.; Pu, J.; Chi, B.; Jian, L. Improvement on durability and thermal cycle performance for solid oxide fuel cell stack with external manifold structure. Energy 2018, 149, 903–913. [Google Scholar] [CrossRef]
- Sun, Z.; Gopalan, S.; Pal, U.B.; Basu, S.N. Cu1.3Mn1.7O4 spinel coatings deposited by electrophoretic deposition on Crofer 22 APU substrates for solid oxide fuel cell applications. Surf. Coat. Technol. 2017, 323, 49–57. [Google Scholar] [CrossRef]
- Zhang, H.; Zhan, Z.; Liu, X. Electrophoretic deposition of (Mn,Co)3O4 spinel coating for solid oxide fuel cell interconnects. J. Power Sources 2011, 196, 8041–8047. [Google Scholar] [CrossRef]
- Spotorno, R.; Piccardo, P.; Perrozzi, F.; Valente, S.; Viviani, M.; Ansar, A. Microstructural and Electrical Characterization of Plasma Sprayed Cu-Mn Oxide Spinels as Coating on Metallic Interconnects for Stacking Solid Oxide Fuel Cells. Fuel Cells 2015, 15, 728–734. [Google Scholar] [CrossRef]
- Spotorno, R.; Piccardo, P.; Schiller, G. Effect of Cathode Contacting on Anode Supported Cell Performances. J. Electrochem. Soc. 2016, 163, F872–F876. [Google Scholar] [CrossRef]
- Spotorno, R.; Piccardo, P.; Perrozzi, F. Interaction between Crofer 22 APU Current Collector and LSCF Cathode Contacting Layer under Cell Operation. ECS Trans. 2015, 68, 1633–1640. [Google Scholar] [CrossRef]
- Chou, P.-Y.; Ciou, C.-J.; Lee, Y.-C.; Hung, I.-M. Effect of La0.1Sr0.9Co0.5Mn0.5O3−δ protective coating layer on the performance of La0.6Sr0.4Co0.8Fe0.2O3−δ solid oxide fuel cell cathode. J. Power Sources 2012, 197, 12–19. [Google Scholar] [CrossRef]
- Shaikh, S.P.S.; Muchtar, A.; Somalu, M.R. A review on the selection of anode materials for solid-oxide fuel cells. Renew. Sustain. Energy Rev. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Qu, J.; Julião, P.S.B.; Shao, Z. Recent Advances in the Development of Anode Materials for Solid Oxide Fuel Cells Utilizing Liquid Oxygenated Hydrocarbon Fuels: A Mini Review. Energy Technol. 2019, 7, 33–44. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Progrog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Bianco, M.; Ouweltjes, J.P.; Van herle, J. Degradation analysis of commercial interconnect materials for solid oxide fuel cells in stacks operated up to 18,000 hours. Int. J. Hydrogen Energy 2019, 44, 31406–31422. [Google Scholar] [CrossRef]
- Bianco, M.; Caliandro, P.; Diethelm, S.; Yang, S.; Dellai, A.; Steinberger-Wilckens, R. In-situ experimental benchmarking of solid oxide fuel cell metal interconnect solutions. J. Power Sources 2020, 461, 228163. [Google Scholar] [CrossRef]
- ThyssenKrupp VDM, Crofer 22 APU—Material, Data Sheet No. 4046. 2010. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj0sc6_i77tAhViNKYKHfPFAsQQFjAAegQIAxAC&url=https%3A%2F%2Fwww.vdm-metals.com%2Ffileadmin%2Fuser_upload%2FDownloads%2FData_Sheets%2FData_Sheet_VDM_Crofer_22_APU.pdf&usg=AOvVaw1V79Q-iR8EsCt_ZbvNhTe5 (accessed on 2 November 2020).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Lu, K.; Shen, F.; Roberts, R.; Doucette, G.; McGuire, M.; Li, W. (LaSr)xMnO3 cathode stoichiometry effects on electrochemical performance in contact with AISI 441 steel interconnect. J. Power Sources 2014, 268, 379–387. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. (Eds.) Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Talic, B.; Falk-Windisch, H.; Venkatachalam, V.; Hendriksen, P.V.; Wiik, K.; Lein, H.L. Effect of coating density on oxidation resistance and Cr vaporization from solid oxide fuel cell interconnects. J. Power Sources 2017, 354, 57–67. [Google Scholar] [CrossRef]
- Kruk, A.; Stygar, M.; Brylewski, T. Mn–Co spinel protective–conductive coating on AL453 ferritic stainless steel for IT-SOFC interconnect applications. J. Solid State Electrochem. 2013, 17, 993–1003. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Fergus, J.W. Interactions Between SOFC Interconnect Coating Materials and Chromia. J. Am. Ceram. Soc. 2011, 94, 4490–4495. [Google Scholar] [CrossRef]
- Huczkowski, P.; Christiansen, N.; Shemet, V.; Niewolak, L.; Piron-Abellan, J.; Singheiser, L.; Quadakkers, W.J. Growth Mechanisms and Electrical Conductivity of Oxide Scales on Ferritic Steels Proposed as Interconnect Materials for SOFC’s. Fuel Cells 2006, 2, 93–99. [Google Scholar] [CrossRef]
- Piccardo, P.; Spotorno, R.; Ouweltjes, J.P.; Stoynov, Z.; Vladikova, D. Parametrical Coordinates and Microsamples to Investigate Real SOFCs in Operating Stacks. ECS Trans. 2017, 78, 2087–2098. [Google Scholar] [CrossRef]
- Kofstad, P. High Temperature Corrosion; Elsevier Applied Science Publishers Ltd.: London, UK, 1988. [Google Scholar]
- Talic, B.; Molin, S.; Wiik, K.; Hendriksen, P.V.; Lein, H.L. Comparison of iron and copper doped manganese cobalt spinel oxides as protective coatings for solid oxide fuel cell interconnects. J. Power Sources 2017, 372, 145–156. [Google Scholar] [CrossRef]
- Talic, B.; Hendriksen, P.V.; Wiik, K.; Lein, H.L. Diffusion couple study of the interaction between Cr2O3 and MnCo2O4 doped with Fe and Cu. Solid State Ion. 2019, 332, 16–24. [Google Scholar] [CrossRef]
- Magdefrau, N.J.; Chen, L.; Sun, E.Y.; Yamanis, J.; Aindow, M. Formation of spinel reaction layers in manganese cobaltite e coated Crofer22 APU for solid oxide fuel cell interconnects. J. Power Sources 2013, 227, 318–326. [Google Scholar] [CrossRef]
- Persson, Å.H.; Mikkelsen, L.; Hendriksen, P.V.; Somers, M.A.J. Interaction mechanisms between slurry coatings and solid oxide fuel cell interconnect alloys during high temperature oxidation. J. Alloys Compd. 2012, 522, 16–29. [Google Scholar] [CrossRef]
- Wagner, C. Theoretical analysis of the diffusion processes determining the oxidation rate of alloys. J. Electrochem. Soc. 1952, 99, 369–380. [Google Scholar] [CrossRef]
- Chen, X.; Hou, P.Y.; Jacobson, C.P.; Visco, S.J.; De Jonghe, L.C. Protective coating on stainless steel interconnect for SOFCs: Oxidation kinetics and electrical properties. Solid State Ion. 2005, 176, 425–433. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Basu, A.; Lu, Z.G.; Paranthaman, M.; Lee, D.F.; Payzant, E.A. LaCrO-based coatings on ferritic stainless steel for solid oxide fuel cell interconnect applications. Surf. Coat. Technol. 2004, 177–178, 65–72. [Google Scholar] [CrossRef]
- Lee, J.-W.; Park, B.-K.; Lee, S.-B.; Lim, T.-H.; Park, S.-J.; Song, R.-H. Cu- and Ni-doped Mn1.5Co1.5O4 spinel coatings on metallic interconnects for solid oxide fuel cells. Int. J. Hydrogen Energy 2013, 38, 12043–12050. [Google Scholar]
- Ardigò, M.; Popa, I.; Chevalier, S.; Parry, V.; Galerie, A.; Girardon, P.; Perry, F.; Laucournet, R.; Brevet, A.; Rigal, E. Coated interconnects development for high temperature water vapour electrolysis: Study in anode atmosphere. Int. J. Hydrogen Energy 2013, 38, 15910–15916. [Google Scholar] [CrossRef]
- Puranen, J.; Pihlatie, M.; Lagerbom, J.; Bolelli, G.; Laakso, J.; Hyvärinen, L.; Kylmälahti, M.; Himanen, O.; Kiviaho, J.; Lusvarghi, L.; et al. Post-mortem evaluation of oxidized atmospheric plasma sprayed Mn-Co-Fe oxide spinel coatings on SOFC interconnectors. Int. J. Hydrogen Energy 2014, 39, 17284–17294. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zeng, C.L. Preparation and performances of Co-Mn spinel coating on a ferritic stainless steel interconnect material for solid oxide fuel cell application. J. Power Sources 2014, 252, 122–129. [Google Scholar] [CrossRef]
- Othman, N.K.; Zhang, J.; Young, D.J. Water vapour effects on FeCr alloy oxidation. Oxid. Met. 2010, 73, 337–352. [Google Scholar] [CrossRef]
- Bowen, A.W.; Leak, G.M. Diffusion in Bcc iron base alloys. Metall. Trans. 1970, 1, 2767–2773. [Google Scholar]
| Operation Type | Total Runtime/h | Fuel gas (Co-flow) | Nominal Temperature/°C | Current Density/mA·cm−2 |
|---|---|---|---|---|
| SOFC (µCHP), field | 5000 | 44% N2 | 850 | 90–200 |
| 34% H2 | ||||
| 16% CO | ||||
| SOFC (µCHP), field | 9000 | 4% H2O | ||
| SOFC (µCHP), field | 20,000 | 2% CO2 |
| Wt% | Cr | Fe | C | Mn | Si | Cu | Al | S | P | Ti | La |
|---|---|---|---|---|---|---|---|---|---|---|---|
| min. | 20.0 | bal. | 0.30 | 0.03 | 0.04 | ||||||
| max. | 24.0 | 0.03 | 0.80 | 0.50 | 0.50 | 0.50 | 0.020 | 0.050 | 0.20 | 0.20 |
| Site of Interest | IN | OUT | |
|---|---|---|---|
| Time | Thickness (µm) | ||
| 5000 h | Air/rib (position 1) | 1.4 ± 0.2 | 4.2 ± 0.9 |
| 9000 h | 7.7 ± 1.4 | 5.9 ± 1.6 | |
| 20,000 h | 7.6 ± 1.2 | 11.9 ± 2 | |
| 5000 h | Air/channel (position 2) | 1.3 ± 0.2 | 3.7 ± 0.2 |
| 9000 h | 6.7 ± 1.1 | 5.9 ± 0.7 | |
| 20,000 h | 4.5 ± 1.0 | 10.3 ± 1.1 | |
| Site of Interest | IN | OUT | |
|---|---|---|---|
| Time | Porosity (%) | ||
| 5000 h | Air/rib (position 1) | 50 ± 2 | 40 ± 1 |
| 9000 h | 32 ± 3 | 35 ± 2 | |
| 20,000 h | 24 ± 1 | 23 ± 2 | |
| 5000 h | Air/channel (position 2) | 41 ± 5 | 40 ± 3 |
| 9000 h | 33 ± 3 | 25 ± 2 | |
| 20,000 h | 33 ± 1 | n.m. * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiara, G.; Piccardo, P.; Bongiorno, V.; Geipel, C.; Spotorno, R. Characterization of Metallic Interconnects Extracted from Solid Oxide Fuel Cell Stacks Operated up to 20,000 h in Real Life Conditions: The Air Side. Energies 2020, 13, 6487. https://doi.org/10.3390/en13246487
Ghiara G, Piccardo P, Bongiorno V, Geipel C, Spotorno R. Characterization of Metallic Interconnects Extracted from Solid Oxide Fuel Cell Stacks Operated up to 20,000 h in Real Life Conditions: The Air Side. Energies. 2020; 13(24):6487. https://doi.org/10.3390/en13246487
Chicago/Turabian StyleGhiara, Giorgia, Paolo Piccardo, Valeria Bongiorno, Christian Geipel, and Roberto Spotorno. 2020. "Characterization of Metallic Interconnects Extracted from Solid Oxide Fuel Cell Stacks Operated up to 20,000 h in Real Life Conditions: The Air Side" Energies 13, no. 24: 6487. https://doi.org/10.3390/en13246487
APA StyleGhiara, G., Piccardo, P., Bongiorno, V., Geipel, C., & Spotorno, R. (2020). Characterization of Metallic Interconnects Extracted from Solid Oxide Fuel Cell Stacks Operated up to 20,000 h in Real Life Conditions: The Air Side. Energies, 13(24), 6487. https://doi.org/10.3390/en13246487








