Thermal Efficiency of Oxyhydrogen Gas Burner
Abstract
1. Introduction
2. Materials and Methods
2.1. H2 and OH2G Gas Properties
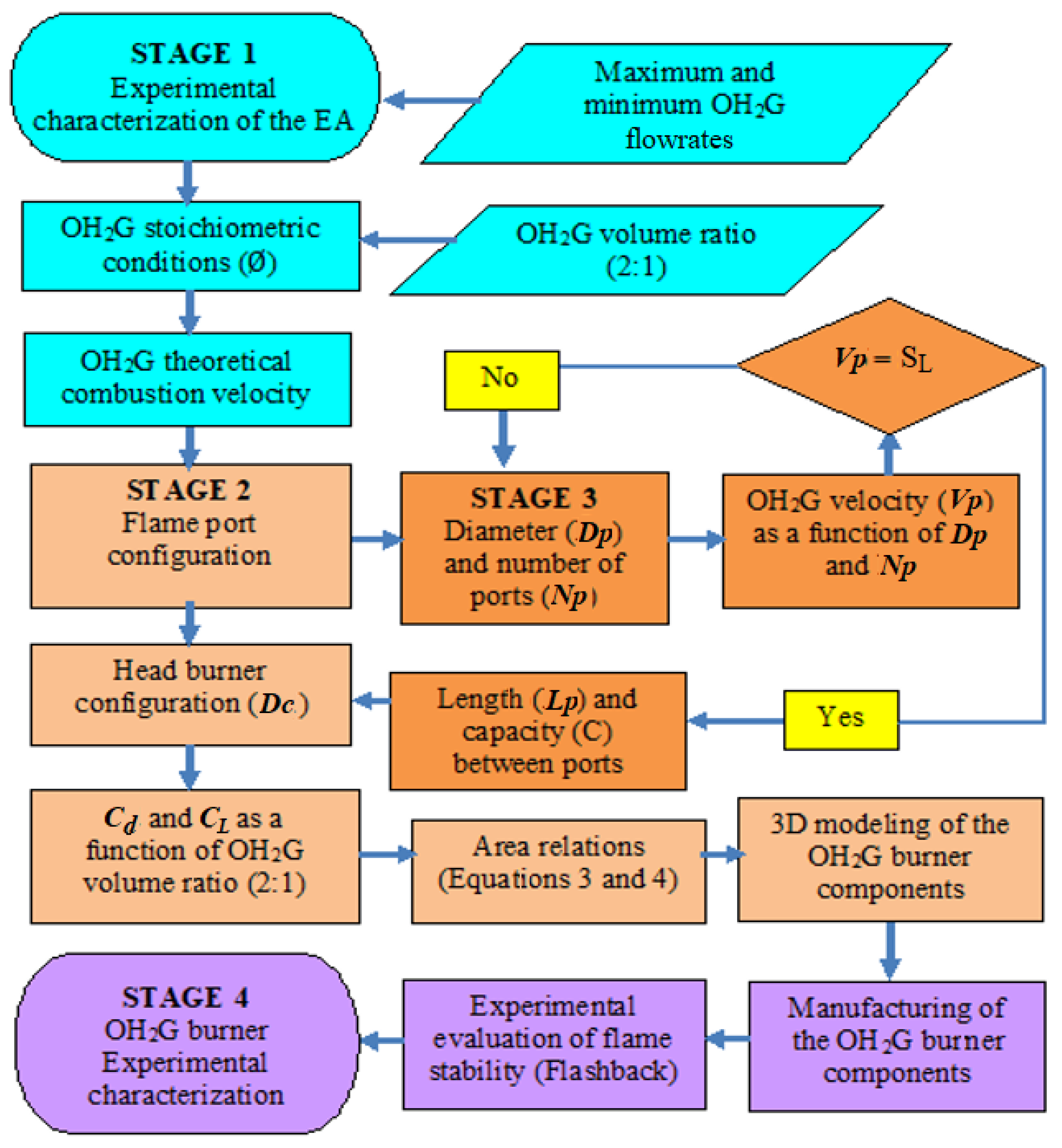
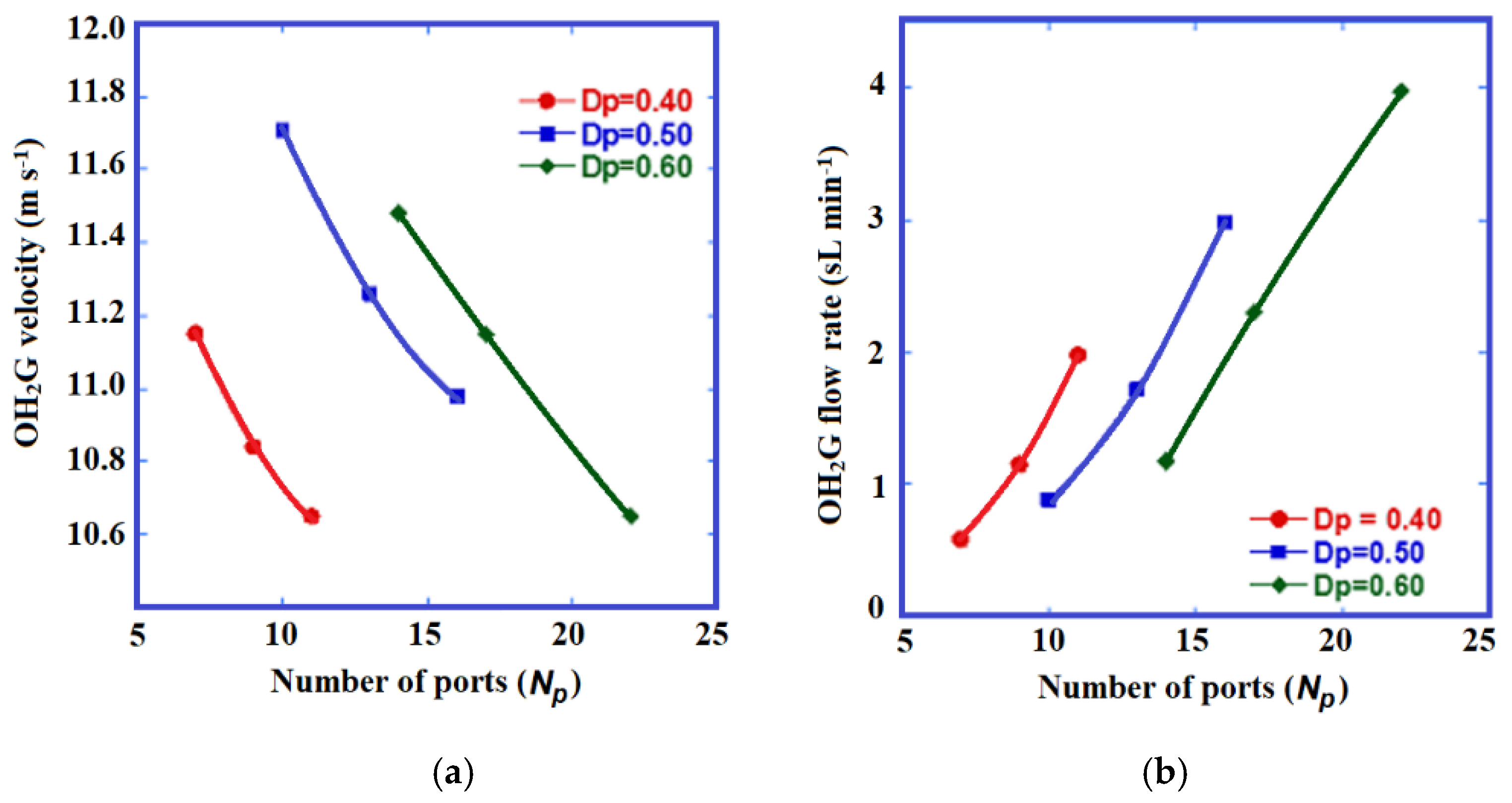
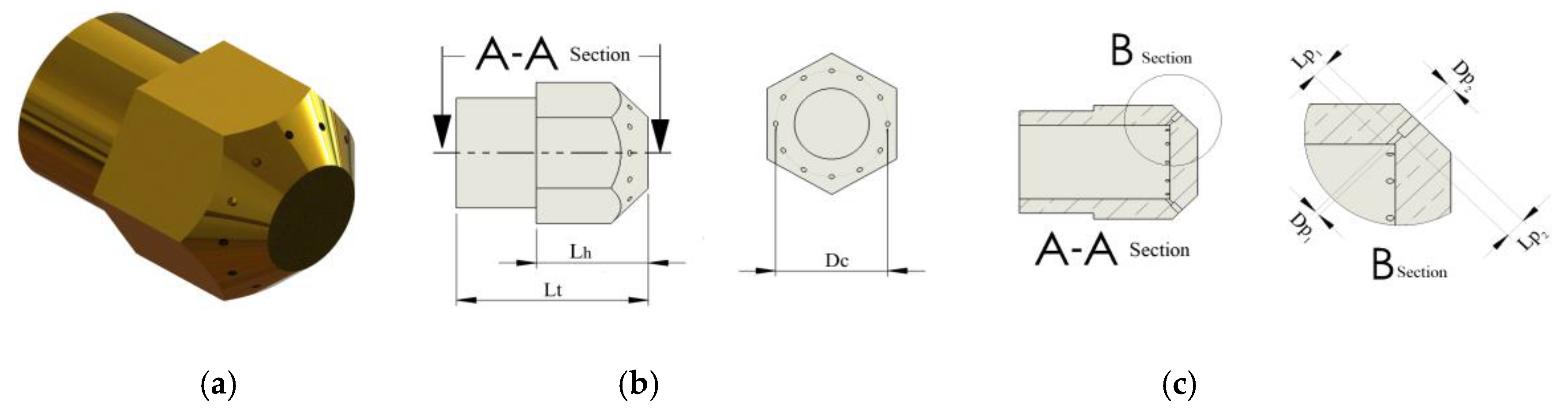
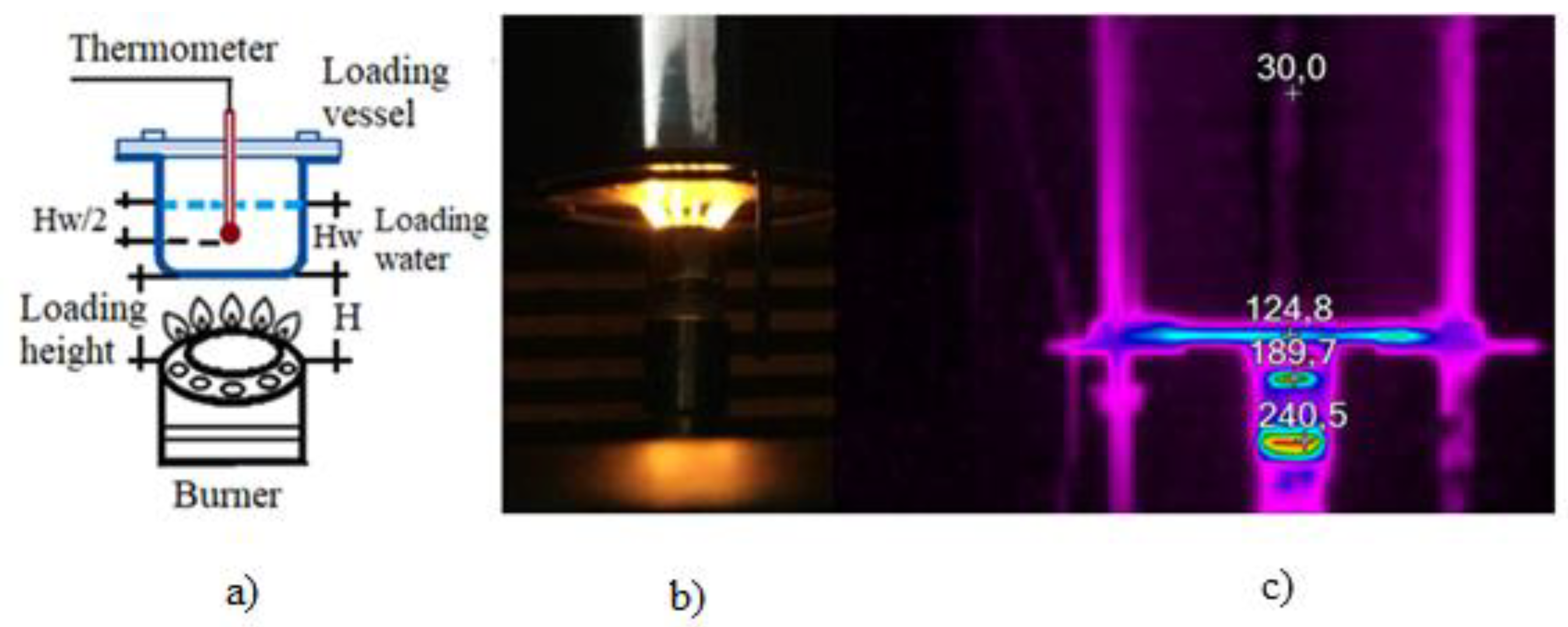
2.2. Procedure to Establish Dimensions of the Burner
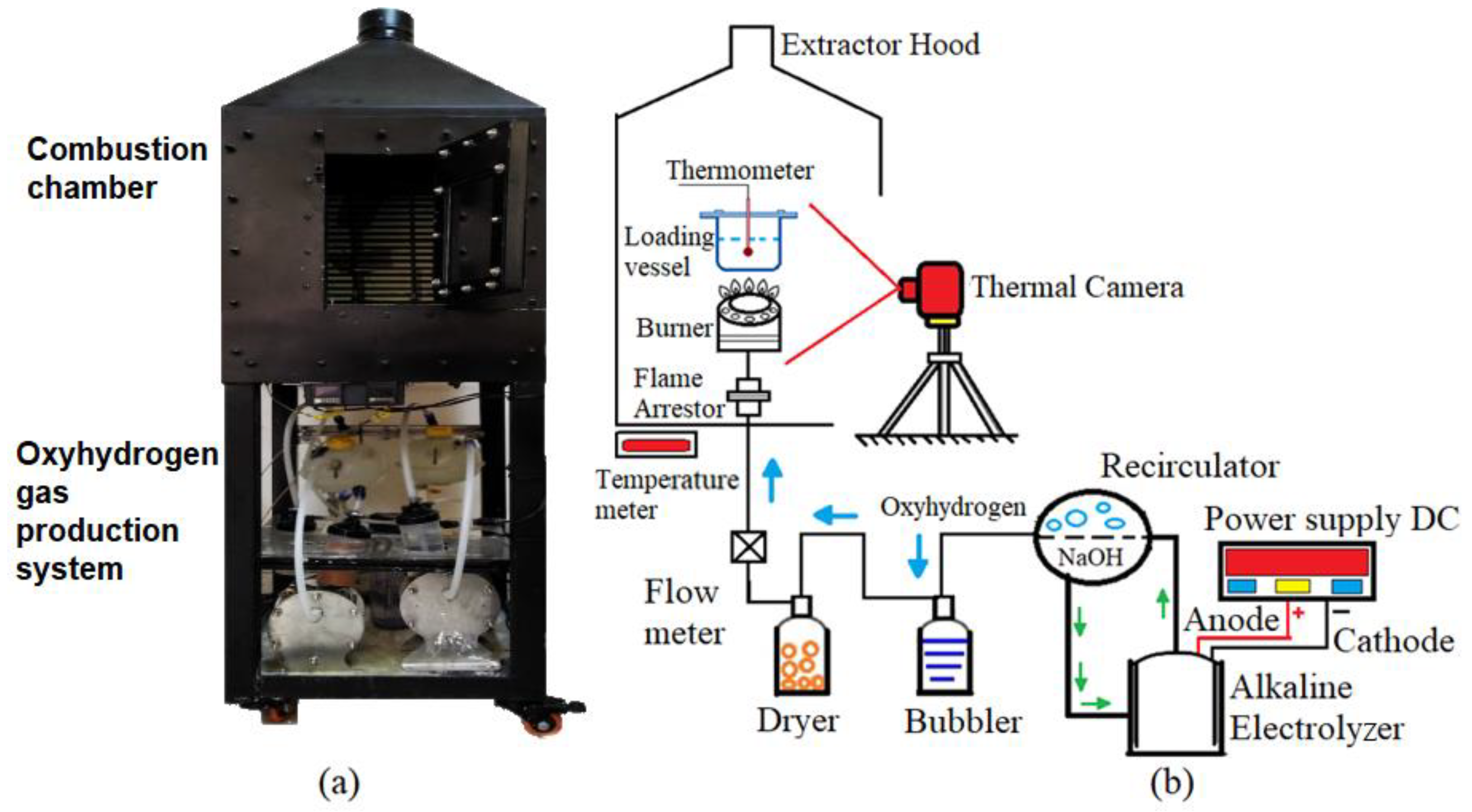
2.3. Burner Evaluation in the Experimental Test Module
3. Results and Discussion
3.1. Efficiency Tests
3.2. Analysis of Flames as a Function of the WAE Operating Time
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cámara de Diputados del H. Congreso de la Unión. Ley de la Regulación de la Industria Eléctrica; Diario Oficial de la Nación; Cámara de Diputados del H. Congreso de la Unión: Mexico City, Mexico, 2014. [Google Scholar]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Subramanian, B.; Ismail, S. Production and use of HHO gas in IC engines. Int. J. Hydrogen Energy 2018, 43, 7140–7154. [Google Scholar] [CrossRef]
- Yilmaz, A.C.; Uludamar, E.; Aydin, K. Effect of hydroxy (HHO) gas addition on performance and exhaust emissions in compression ignition engines. Int. J. Hydrogen Energy 2010, 35, 11366–11372. [Google Scholar] [CrossRef]
- Trujillo-Olivares, I.; Soriano-Moranchel, F.; Álvarez-Zapata, L.A.; González-Huerta, R.d.G.; Sandoval-Pineda, J.M. Design of alkaline electrolyser for integration in diesel engines to reduce pollutants emission. Int. J. Hydrogen Energy 2019, 44, 25277–25286. [Google Scholar] [CrossRef]
- De Vries, H.; Mokhov, A.V.; Levinsky, H.B. The impact of natural gas/hydrogen mixtures on the performance of end-use equipment: Interchangeability analysis for domestic appliances. Appl. Energy 2017, 208, 1007–1019. [Google Scholar] [CrossRef]
- Zhao, Y.; McDonell, V.; Samuelsen, S. Influence of hydrogen addition to pipeline natural gas on the combustion performance of a cooktop burner. Int. J. Hydrogen Energy 2019, 44, 12239–12253. [Google Scholar] [CrossRef]
- Ge, B.; Ji, Y.; Zhang, Z.; Zang, S.; Tian, Y.; Yu, H.; Zhang, D. Experiment study on the combustion performance of hydrogen-enriched natural gas in a DLE burner. Int. J. Hydrogen Energy 2019, 44, 14023–14031. [Google Scholar] [CrossRef]
- Zhao, Y.; McDonell, V.; Samuelsen, S. Experimental assessment of the combustion performance of an oven burner operated on pipeline natural gas mixed with hydrogen. Int. J. Hydrogen Energy 2019, 44, 26049–26062. [Google Scholar] [CrossRef]
- Uykur, C.; Henshaw, P.F.; Ting, D.S.; Barron, R.M. Effects of addition of electrolysis products on methane/air premixed laminar combustion. Int. J. Hydrogen Energy 2001, 26, 265–273. [Google Scholar] [CrossRef]
- Echeverri-Uribe, C.; Amell, A.A.; Rubio-Gaviria, L.M.; Colorado, A.; Mcdonell, V. Numerical and experimental analysis of the effect of adding water electrolysis products on the laminar burning velocity and stability of lean premixed methane/air flames at sub-atmospheric pressures. Fuel 2016, 180, 565–573. [Google Scholar] [CrossRef]
- Pan, J.F.; Wu, D.; Liu, Y.X.; Zhang, H.F.; Tang, A.K.; Xue, H. Hydrogen/oxygen premixed combustion characteristics in micro porous media combustor. Appl. Energy 2015, 160, 802–807. [Google Scholar] [CrossRef]
- Tanneberger, T.; Schimek, S.; Paschereit, C.O.; Stathopoulos, P. Combustion efficiency measurements and burner characterization in a hydrogen-oxyfuel combustor. Int. J. Hydrogen Energy 2019, 44, 29752–29764. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Y.; Huang, Z. Progress in combustion investigations of hydrogen enriched hydrocarbons. Renew. Sustain. Energy Rev. 2014, 30, 195–216. [Google Scholar] [CrossRef]
- Kim, H.G.; Kwac, L.K.; Shin, J.D. Physical properties and flame characteristics of water electrolysis gas. Renew. Energy 2012, 42, 84–89. [Google Scholar] [CrossRef]
- Molnarne, M.; Schroeder, V. Hazardous properties of hydrogen and hydrogen containing fuel gases. Process. Saf. Environ. Prot. 2019, 130, 1–5. [Google Scholar] [CrossRef]
- Jones, H.R.N. The Application of Combustion Principles to Domestic Gas Burner Design; Taylor & Francis: Abingdon-on-Thames, UK, 1989; Volume 1, p. 200. [Google Scholar]
- Sutar, K.B.; Ravi, M.R.; Kohli, S. Design of a partially aerated naturally aspirated burner for producer gas. Energy 2016, 116, 773–785. [Google Scholar] [CrossRef]
- Namkhat, A.; Jugjai, S. Primary air entrainment characteristics for a self-aspirating burner: Model and experiments. Energy 2010, 35, 1701–1708. [Google Scholar] [CrossRef]
- Decker, T.; Baumgardner, M.; Prapas, J.; Bradley, T. A mixed computational and experimental approach to improved biogas burner flame port design. Energy Sustain. Dev. 2018, 44, 37–46. [Google Scholar] [CrossRef]
- Fulford, D. Biogas Stove Design; Kingdom Bioenergy: Woodley, UK, 1996; pp. 1–21. [Google Scholar]
- Namkhat, A.; Jugjai, S. Prediction of Total Equivalence Ratio for a Self-Aspirating Burner. In Proceedings of the 1st TSME International Conference on Mechanical Engineering (TSME-ICoME 2010), Ubon Ratchathani, Thailand, 20–22 October 2010. [Google Scholar]
- Edmondson, H.; Heap, M.P. The burning velocity of hydrogen-air flames. Combust. Flame 1971, 16, 161–165. [Google Scholar] [CrossRef]
- Koroll, G.W.; Kumar, R.K.; Bowles, E.M. Burning velocities of hydrogen-air mixtures. Combust. Flame 1993, 94, 330–340. [Google Scholar] [CrossRef]
- Pareja, J.; Burbano, H.J.; Ogami, Y. Measurements of the laminar burning velocity of hydrogen-air premixed flames. Int. J. Hydrogen Energy 2010, 35, 1812–1818. [Google Scholar] [CrossRef]
- Stathopoulos, P.; Sleem, T.; Paschereit, C.O. Steam generation with stoichiometric combustion of H2/O2 as a way to simultaneously provide primary control reserve and energy storage. Appl. Energy 2017, 205, 692–702. [Google Scholar] [CrossRef]
- Meraner, C.; Li, T.; Ditaranto, M.; Løvås, T. Effects of scaling laws on the combustion and NOx characteristics of hydrogen burners. Combust. Flame 2020, 214, 407–418. [Google Scholar] [CrossRef]

| Description | Image |
|---|---|
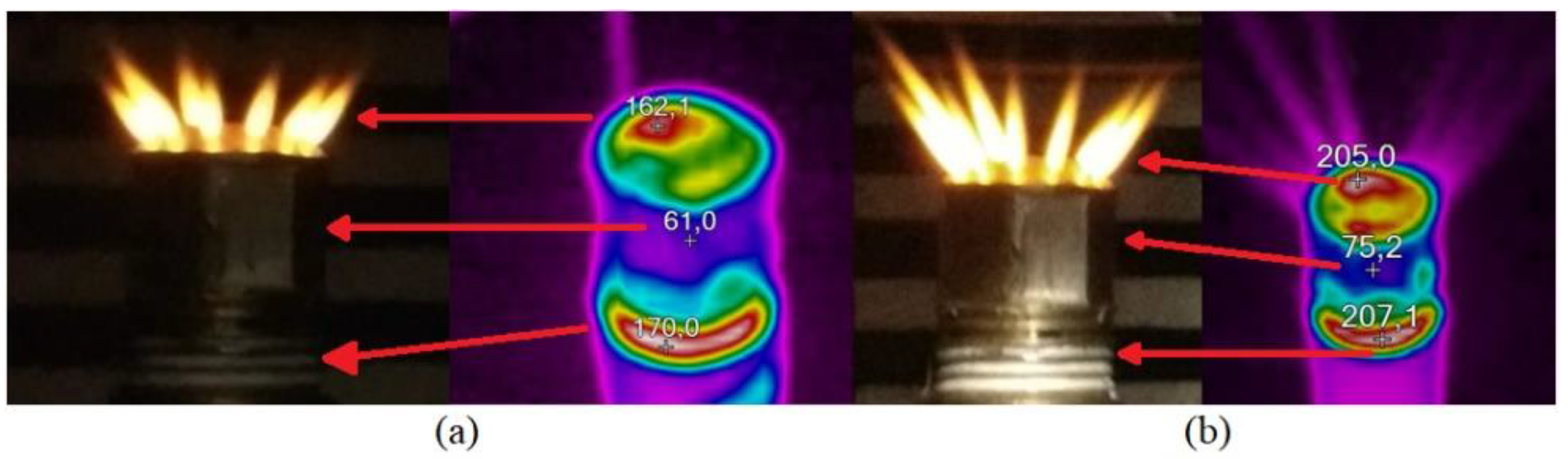
| A drastic change in the luminous zone of the flame (top to bottom) is observed as the test progresses. This is due to the initial build-up and drag of the electrolyte. |  |
| The change in the luminosity of the flame is attributed to the decrease in the volume of hydrogen and an increase in the volume of water vapor in the mixture. The increment of water vapor in the gas mixture instigates a cooling of the flame, thus causing a decrease in temperature. |  |
| A decrease in the thickness of the flames is observed, although the length of the flames shows insignificant change, which is due to the decrease in the volume of hydrogen in the gas mixture (OH2G-water vapor). Therefore, a flow rate of 2.50 sL min−1 of OH2G is the optimal operating condition. |  |
| If the electrolyzer works at higher flows (3.5 sL min−1) the increment of water vapor in the gas mixture instigates a cooling of the flame, thus causing a decrease in the temperature of the flame which could result in the flame being extinguished during the test. |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Soriano, R.; Soriano-Moranchel, F.; Flores-Herrera, L.A.; Sandoval-Pineda, J.M.; González-Huerta, R.d.G. Thermal Efficiency of Oxyhydrogen Gas Burner. Energies 2020, 13, 5526. https://doi.org/10.3390/en13205526
Moreno-Soriano R, Soriano-Moranchel F, Flores-Herrera LA, Sandoval-Pineda JM, González-Huerta RdG. Thermal Efficiency of Oxyhydrogen Gas Burner. Energies. 2020; 13(20):5526. https://doi.org/10.3390/en13205526
Chicago/Turabian StyleMoreno-Soriano, Roberto, Froylan Soriano-Moranchel, Luis Armando Flores-Herrera, Juan Manuel Sandoval-Pineda, and Rosa de Guadalupe González-Huerta. 2020. "Thermal Efficiency of Oxyhydrogen Gas Burner" Energies 13, no. 20: 5526. https://doi.org/10.3390/en13205526
APA StyleMoreno-Soriano, R., Soriano-Moranchel, F., Flores-Herrera, L. A., Sandoval-Pineda, J. M., & González-Huerta, R. d. G. (2020). Thermal Efficiency of Oxyhydrogen Gas Burner. Energies, 13(20), 5526. https://doi.org/10.3390/en13205526






