3.1. Mass Balance of Hydrocracking
The summary of the yields of the gaseous and liquid products of the hydrocracking and the fractional composition of the obtained liquid products are shown in
Table 5. The reported yields are related to the feedstock used for the hydrocracking. In total, nine primary gaseous and liquid hydrocracking products were obtained, whereas the reaction temperature and the feedstock composition were variable. While the products obtained from the HVGO were labelled in the following way: reaction temperature in °C/HVGO, the products obtained from the blends of the HVGO and FT wax were labelled in the following way: reaction temperature in °C/ratio HVGO:FT wax. The conversion, i.e., the transformation of the fraction boiling above 400 °C into products boiling below 400 °C, was calculated for each of the reaction conditions and feedstock types from the results of the liquid products fractionation (the column distillation) and is reported in
Table 5 as well.
The yields of the liquid products ranged between 67.8 and 94.5 wt.% and were affected by both the reaction temperature of the hydrocracking and the composition of the feedstock. The increasing reaction temperature and the FT wax content in the feedstock were reflected by the decrease in the liquid products’ yields and the increase in the gaseous products’ yields. This phenomenon was especially more obvious for the feedstocks containing FT wax. It can be attributed to the higher content of easily crackable n-alkanes. The influence of the FT wax addition to the feedstock on the liquid product yield was opposite to the trend observed by Xing et al. [
19], where the addition of the FT wax up to 50 wt.% was reflected by the increase in the liquid product yield.
The utilisation of the higher reaction temperatures enhanced the cracking rate which resulted in a higher conversion and, thus, in a lower yield in the fraction boiling above 400 °C and a higher yield in the lighter fractions, especially the fraction boiling up to 200 °C (naphtha fraction). The addition of the FT wax to the feedstock increased the naphtha fraction’s yield as well. Moreover, the addition of the FT wax resulted in a significant increase in the gas yield, this effect was magnified at higher reaction temperatures. The products obtained using the feedstocks containing the FT wax and the reaction temperatures of 400 and 410 °C contained only fractions boiling up to 400 °C, which means that the full conversion was reached at these conditions. It can be again associated with the higher n-alkanes content in these feedstocks and their high reactivity during the hydrocracking. While the yield of the middle distillates fraction from the processing of the feedstocks containing the FT wax decreased with the increasing reaction temperature, the opposite effect was observed when only the HVGO was utilised, which is in compliance with the results achieved by Xing et al. [
19].
The conversion of the feedstocks containing the FT wax hydrocracked at 390 °C was 67 % on average. Although Xing et al. [
19] reached higher conversions (80 % on average) while using similar FT wax concentrations in the feedstocks and a lower reaction temperature, it can be mainly attributed to the different reactor setup resulting in the different contact time of the feedstock and the catalyst. The difference between the HVGO utilised in this study and the HVGO used by Xing et al. [
19] could also affect the conversion. On the other hand, the products of the hydrocracking of the FT wax containing the feedstocks obtained at 400 and 410 °C did not contain particles of the unreacted FT wax which were observed in the products obtained by Xing et al. [
19] when the feedstocks containing 50 wt.% or less of FT wax were hydrocracked. This can be attributed to the application of the higher reaction temperatures in this work.
3.2. Composition of the Gaseous Products
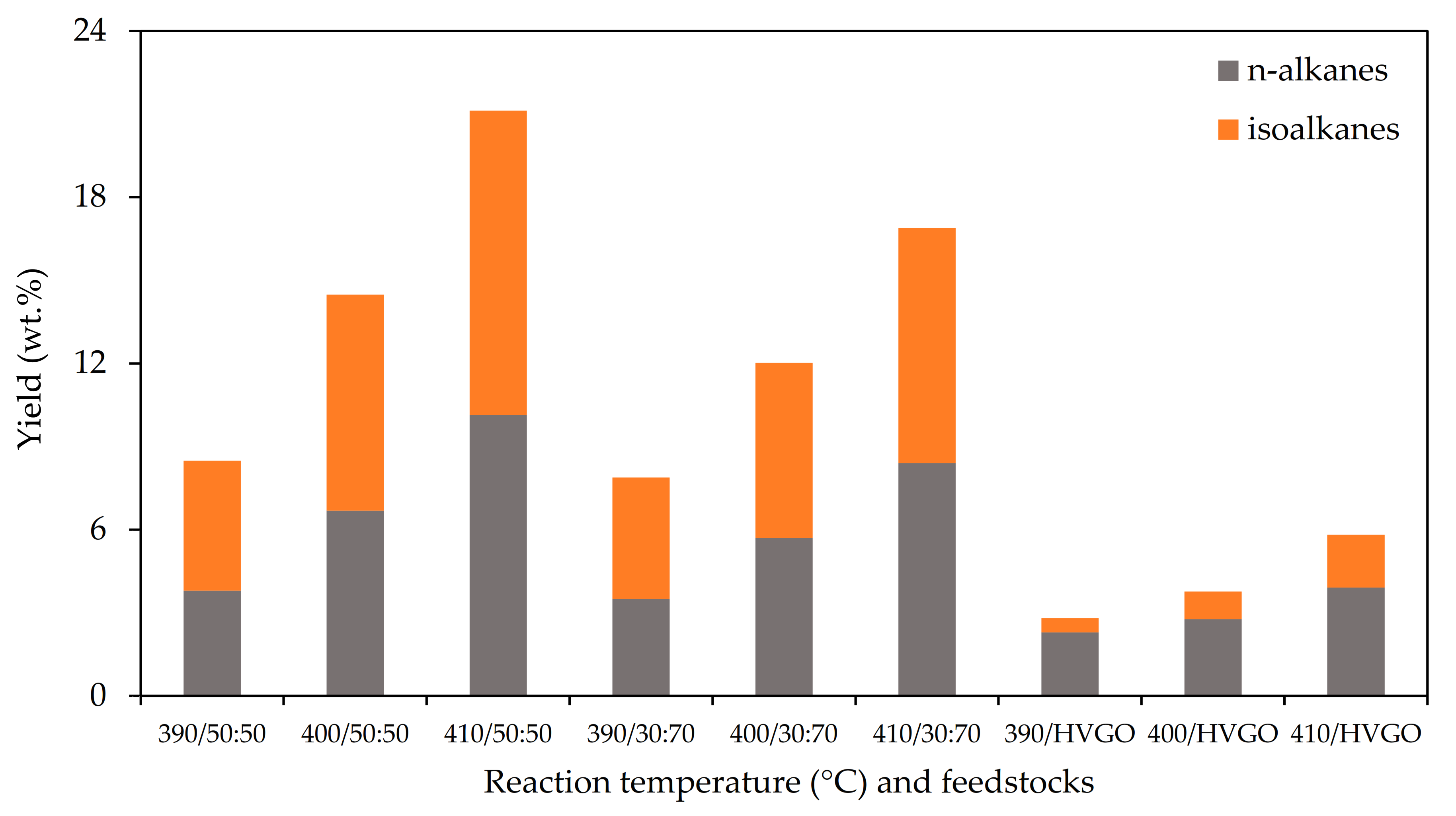
The gaseous products of the hydrocracking were predominantly composed of n-alkanes and isoalkanes. The content of the other hydrocarbon groups was negligible. The increasing reaction temperature of the hydrocracking was reflected by an increase in the yields of both major hydrocarbon groups. The yields also increased with an increasing FT wax content in the feedstock (see
Figure 2). This phenomenon was especially observed when the yields from the neat HVGO and feedstock containing 30 wt.% of the FT wax were compared. The increase in the FT wax content from 30 to 50 wt.% was followed only by the less pronounced increase in the gaseous products yields.
While the ratio of n-alkanes and isoalkanes in the gaseous products obtained from the feedstocks containing the FT wax was close to one, the gaseous products from the neat HVGO hydrocracking were mostly composed of n-alkanes. Propane, n-butane and n-pentane were the dominant n-alkanes detected in the gaseous products of the hydrocracking of the FT wax containing feedstocks. The gaseous products obtained from the hydrocracking of the neat HVGO also contained a significant amount of methane and ethane. The branched hydrocarbons were predominantly represented by the C4–C6 isoalkanes in all the gaseous products.
The gaseous products contained a significant portion of C5+ hydrocarbons (22–41 wt.%) which can be attributed to the setup of the samples collection system during the hydrocracking. This loss of highly volatile components via the gaseous products strongly affected the composition and the properties of the naphtha fractions which will be discussed in the following paragraphs.
Gaseous products can be used in the same way as similar petroleum-derived products. They can be used for LPG (liquefied petroleum gas) production or as a feedstock for steam cracking. Although the FT wax used in this work was not produced from a biomass, similar results can be expected with a bio-based feedstock. Such gaseous products can, thus, contribute not only to the production of a renewable LPG fuel with low GHG (greenhouse gas) emissions, but also to the production of renewable (“green”) monomers for the petrochemical industry.
3.4. Composition and Properties of the Naphtha Fractions
The group-type composition of the naphtha fractions is shown in
Table 6. While the naphtha fractions obtained from the hydrocracking of the neat HVGO were dominantly composed of cycloalkanes and aromatics (especially C
7–C
9), isoalkanes (majorly C
6–C
10) were the dominant group in the naphtha fractions obtained from the processing of the FT wax containing feedstocks.
It is evident that the increasing reaction temperature led to an increase in the n-alkanes and isoalkanes content. The content of the aromatic hydrocarbons and cycloalkanes decreased with an increasing reaction temperature and an increasing FT wax content in the feedstock. This phenomenon was especially observed from the comparison of the naphtha fractions obtained from the neat HVGO and from the feedstock containing 30 wt.% of the FT wax. The increase in the FT wax content in the feedstock from 30 to 50 wt.% only caused a minor reduction in the aromatics content in the naphtha fractions.
The total aromatics content in all the obtained naphtha fractions ranged from ca. 9 to 30 wt.%. Considering the density of the obtained naphtha fractions (Table 8) and the average density of the aromatics present in these samples (i.e., 870 kg·m−3), all the samples can be considered as complying with the requirement of EN 228 limiting the aromatics content (35 vol.%). Since the highest determined content of benzene was 0.9 wt.%, the same conclusion can also be made for the benzene content, i.e., all the naphtha fractions fulfilled the limit for benzene (max. 1 vol.%) required by the standard EN 228.
The olefins content in all the naphtha fractions was below 0.1 wt.%, so all the samples fulfilled the limit for olefin content (max. 18 vol.%) by a large margin.
While the content of the n-alkanes in the naphtha fractions obtained from the neat HVGO was not affected by the reaction temperature, an increase in their content with an increasing reaction temperature was observed in the naphtha fractions obtained from the feedstocks containing the FT wax.
The content of the i-alkanes was affected by both the reaction temperature and the FT wax content in the feedstock. The increasing reaction temperature and increasing FT wax content in the feedstock were reflected by an increasing i-alkanes content in the naphtha fractions. A significant increase was especially observed in the content of the isopentanes and isohexanes.
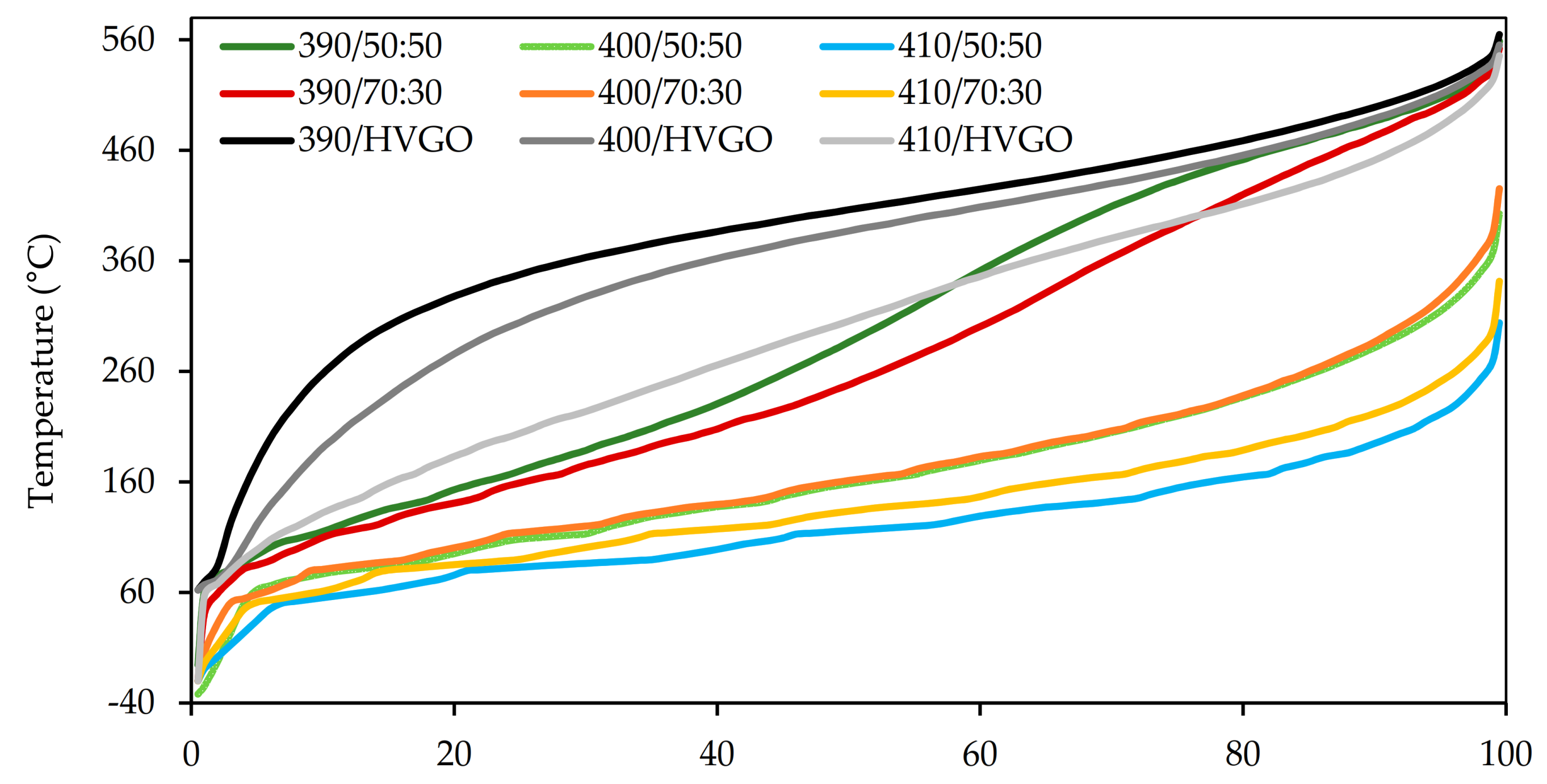
The distillation curves of the naphtha fractions are shown in
Figure 4. Since the yields of the naphtha fractions of several products were not sufficient to provide the required volume of the sample (i.e., 100 mL) for the standard distillation test according to EN ISO 3405, the standard distillation test was applied only to four samples (
Figure 4A). The distillation profiles of the remaining five samples were determined by the simulated distillation only (
Figure 4B).
The distillation profiles obtained via the simulated distillation did not show significant differences among the analysed naphtha fractions. On the other hand, the standard distillation test showed a shift in the distillation curves of the naphtha fractions obtained from the feedstocks containing the FT wax at the reaction temperature of 410 °C to lower boiling points. Nevertheless, the naphtha fractions were mostly composed of components having a boiling point above 80 °C.
The summary of the selected parameters of the distillation test and the limits required by the EN 228 standard (class A) for naphtha fractions obtained from the feedstocks containing the FT wax at reaction temperatures of 400 and 410 °C is shown in
Table 7. None of the samples met the requirement put on E70. The content of high-volatile compounds was generally quite low in all the naphtha fractions. It can be attributed to the collection system of the liquid products during the hydrocracking in the high and low-pressure separators. The most volatile components of the naphtha fractions left the hydrocracking unit as a part of the gaseous product. It can be supported by the rather large yields of C
5–C
6 hydrocarbons detected in the gaseous products. While only one sample (410/50:50) met the requirement put on E100, three samples complied with the E150 limit. Only the sample 400/50:50 was slightly below the limit. All the samples met the requirements put on the final boiling point and distillation residue. Generally, the best distillation profile was obtained for the naphtha fraction of the product hydrocracking at 410 °C using the feedstock containing 50 wt.% of the FT wax.
The results of the determination of the other physicochemical properties, i.e., the density and vapour pressure, are shown in
Table 8. The increasing reaction temperature and FT wax content in the feedstock were reflected by the decreasing density in the naphtha fractions. It can be mainly attributed to the decreasing content of the cycloalkanes and aromatic hydrocarbons which generally have a higher density when compared to acyclic hydrocarbons. While the naphtha fractions of the products obtained via the hydrocracking of the feedstocks containing the FT wax at 390 and 400 °C met the requirements put on the density, the utilisation of the highest temperature caused a decrease in the density below the limit. The naphtha fractions obtained from the processing of the neat HVGO were on the other edge of the required density range. The fractions of the products 390/HVGO and 400/HVGO were actually above the density limit.
The vapour pressure of the naphtha fraction is dependent on the content of the low-boiling compounds which were lost during the liquid product collection. In spite of the fact that the increasing reaction temperature led to an increase in the vapour pressure of the naphtha fractions obtained from the feedstock containing the FT wax. The vapour pressure of the samples obtained at the lowest reaction temperature, i.e., 390 °C, was significantly below the limit. The increase in the reaction temperature to 400 °C caused a large increase in the naphtha fractions’ vapour pressure. Nevertheless, the vapour pressure of the samples obtained at this reaction temperature was still below the limit. Only the naphtha fractions of the products from the FT wax containing feedstocks obtained at the highest reaction temperature met the requirement of EN 228.
The naphtha fraction yields of the neat HVGO processing at temperatures of 390 and 400 °C were not sufficient enough to measure the vapour pressure. Nevertheless, it can be expected that the result of these samples will be very low.
It can be concluded that both the reaction temperature and the content of the FT wax in the feedstock significantly affected both the chemical composition and physicochemical properties of the naphtha fraction. None of the analysed samples fully met the requirements of the EN 228 standard for automotive gasoline which can be at least partly associated with the loss of the volatile components. This issue could be resolved by using a different setup for the samples’ collection.
Although the naphtha fractions obtained from the feedstocks containing the FT wax contained a rather high portion of isoalkanes, a low knocking resistance (octane number) can be expected. Partly due to the fact that the isoalkanes were majorly represented by mono methyl alkanes and partly due to the high content of n-alkanes and cycloalkanes and the rather low content of aromatic compounds. The direct utilisation of the naphtha fractions as automotive gasoline components would be, therefore, quite limited. Nevertheless, the naphtha fractions could be easily utilised in the petrochemical industry (e.g., steam cracking) or as feedstock for common refinery processes like catalytic reforming and isomerisation.
3.5. Composition and Properties of the Middle Distillates
The results of the group composition of the middle distillates measured by the GC-FID and HPLC analysis are shown in
Table 9.
The middle distillates were predominantly composed of saturated hydrocarbons. Their content increased with the increasing content of the FT wax in the feedstock. Cycloalkanes and isoalkanes were the dominant groups of hydrocarbons found in all the middle distillates. While the content of the n-alkanes in the middle distillates obtained from the feedstocks containing the FT wax slightly increased with the increasing reaction temperature, the opposite trend was observed for the middle distillates obtained from the neat HVGO.
The content of aromatic hydrocarbons decreased with the increasing FT wax content in the feedstock. The increasing reaction temperature was reflected by a decrease in the total content of the aromatic hydrocarbons in the middle distillates obtained from the neat HVGO and the feedstock containing 30 wt.% of the FT wax. The minimum content of the aromatic hydrocarbons in the middle distillates obtained from the feedstock containing 50 wt.% was reached at 400 °C. Monocyclic aromatic hydrocarbons were the major group of the aromatic hydrocarbons. The sum of the dicyclic and polycyclic aromatic hydrocarbons in all of the middle distillates did not exceed the limit of EN 590 for diesel fuel, i.e., 8 wt.%. The content of these groups in the middle distillates obtained from the neat HVGO was, however, very close to the limit.
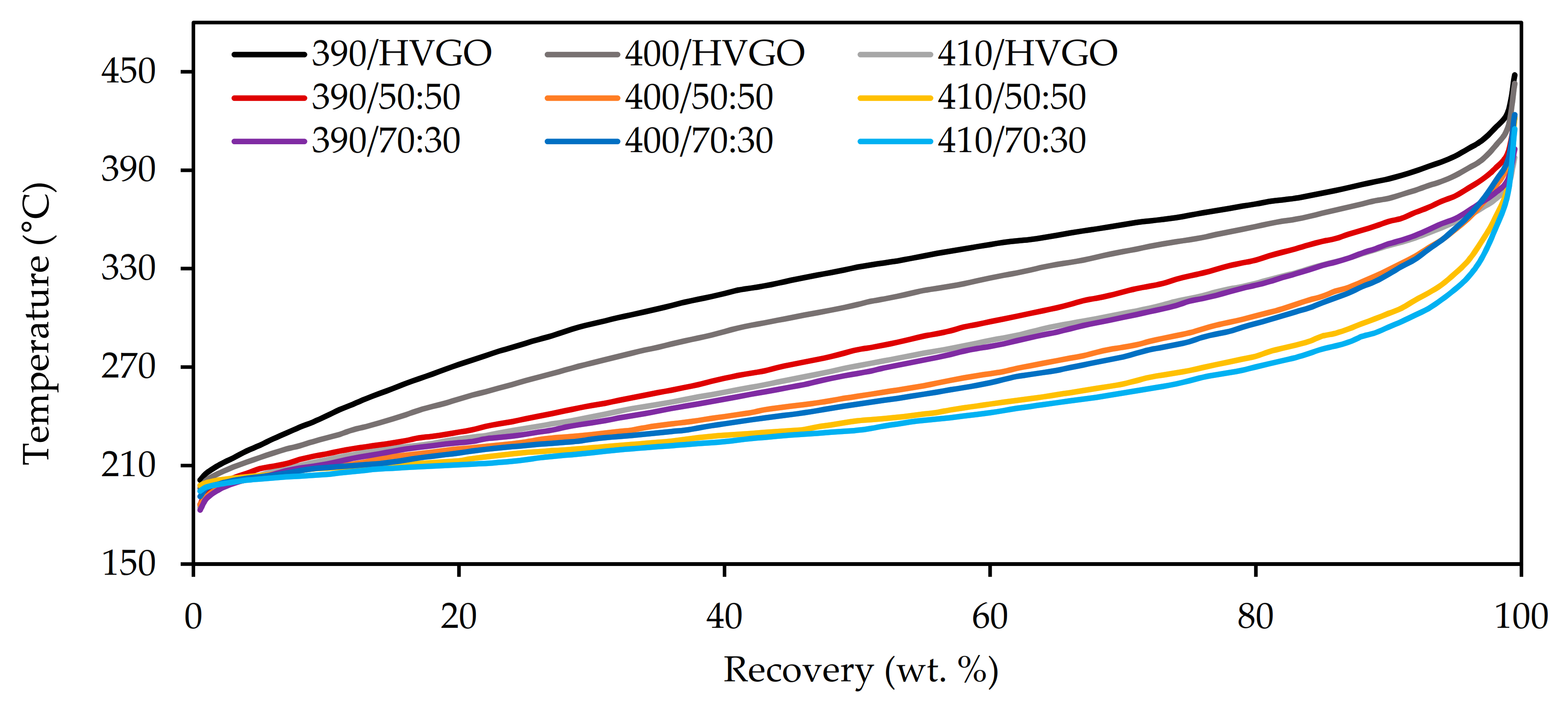
The SIMDIST distillation profiles of all the middle distillates are displayed in
Figure 5. Both the increasing reaction temperature and the increasing content in the FT wax in the feedstock were reflected by a shift in the distillation curves to lower boiling points.
The parameters of the distillation test, like R250; R350 and T95, were calculated using the SIMDIST data and the correlation described in the ASTM D2887 standard. The results are displayed in
Table 10. As already mentioned above, the increasing reaction temperature led to a decrease in the middle distillates‘ boiling points which was reflected by an increase in both R250 and R350. The same phenomenon also led to a decrease in the temperature at which 95 vol.% (T95) of the sample was recovered. All the middle distillates fractions obtained from the neat HVGO fulfilled the requirements put on the R250 parameter. The addition of the FT wax to the processed feedstock was reflected by the increase in R250. While the middle distillates obtained from the hydrocracking of the FT wax containing the feedstocks at 390 and 400 °C complied with the specification, the R250 parameter of the middle distillates obtained at 410 °C was above the limit. All the analysed middle distillates, except the sample obtained from the neat HVGO at 390 °C, fulfilled the requirement of the EN590 standard put on the R350 parameter. While all the middle distillates obtained from the feedstocks containing the FT wax met the specification in the case of the T95 parameter, only the sample obtained from the neat HVGO at 410 °C was slightly above the maximum value.
The other physicochemical parameters of the middle distillates are summarised in
Table 11. The increasing reaction temperature of the hydrocracking was reflected by the decreasing density and kinematic viscosity of the middle distillate fractions. The same phenomenon was also observed for the increasing FT wax content in the feedstocks. The density of the middle distillates obtained from the FT wax containing the feedstocks was below the lower specification limit. On the other hand, the density of the middle distillates obtained from the neat HVGO processing was above the upper limit. The influence of the FT wax content was more significant than the influence of the reaction temperature. The density decrease can be predominantly attributed to the aromatics content decrease discussed above. While the addition of 30 wt.% of the FT wax to the feedstock was reflected by a significant decrease in the middle distillates’ viscosity, the higher content of the FT wax in the feedstock only caused a mild change. The required range of the kinematic viscosity was fulfilled only by the middle distillates obtained from the hydrocracking of the feedstocks containing the FT wax at 390 and 400 °C and of the neat HVGO at 410 °C. The kinematic viscosity of the fractions obtained at 410 °C from the feedstock containing the FT wax was slightly below the limit. On the other hand, the viscosity of the fractions obtained from the neat HVGO at 390 and 400 °C was above the limit.
Although the oxidation stability of the middle distillates was not measured via the standard method (EN 15751), but only via the rapid method based on EN 16091, the middle distillates obtained from the FT wax containing the feedstocks can be considered as very stable. The results, obtained on the basis of correlation between the EN 16091 method (PetroOxy) and the standard EN 15751 method (Rancimat), were significantly higher than the limit value of EN 590.
The cetane index of the middle distillates was calculated using density and the data obtained via the simulated distillation correlated to the standard distillation test. All the samples, except the middle distillate obtained from the hydrocracking of the neat HVGO at 410 °C, complied with the requirement of the EN 590 standard put on the cetane index. Nevertheless, the cetane index of the non-complying sample mentioned above was only slightly below the lower specification limit. The increasing FT wax content in the feedstock was reflected by an increase in the cetane index. The cetane indexes of the middle distillates obtained from the FT wax containing the feedstocks were, thus, significantly above the minimum value required by EN 590.
Although the middle distillate fractions obtained from the hydrocracking of the neat HVGO and the HVGO containing the FT wax could not be utilised as diesel fuel in a pure form, they can be used at least as a blending component. Due to the low aromatics content and high of iso- and cycloalkanes content, the middle distillates from the FT wax containing the feedstocks could also be used as low-aromatic industrial solvents. The distillation cut of 180–275 °C could be, additionally, used for the production of high-quality jet fuel.