Analysing the Material Suitability and Concentration Ratio of a Solar-Powered Parabolic trough Collector (PTC) Using Computational Fluid Dynamics
Abstract
1. Introduction
2. Materials and Methods
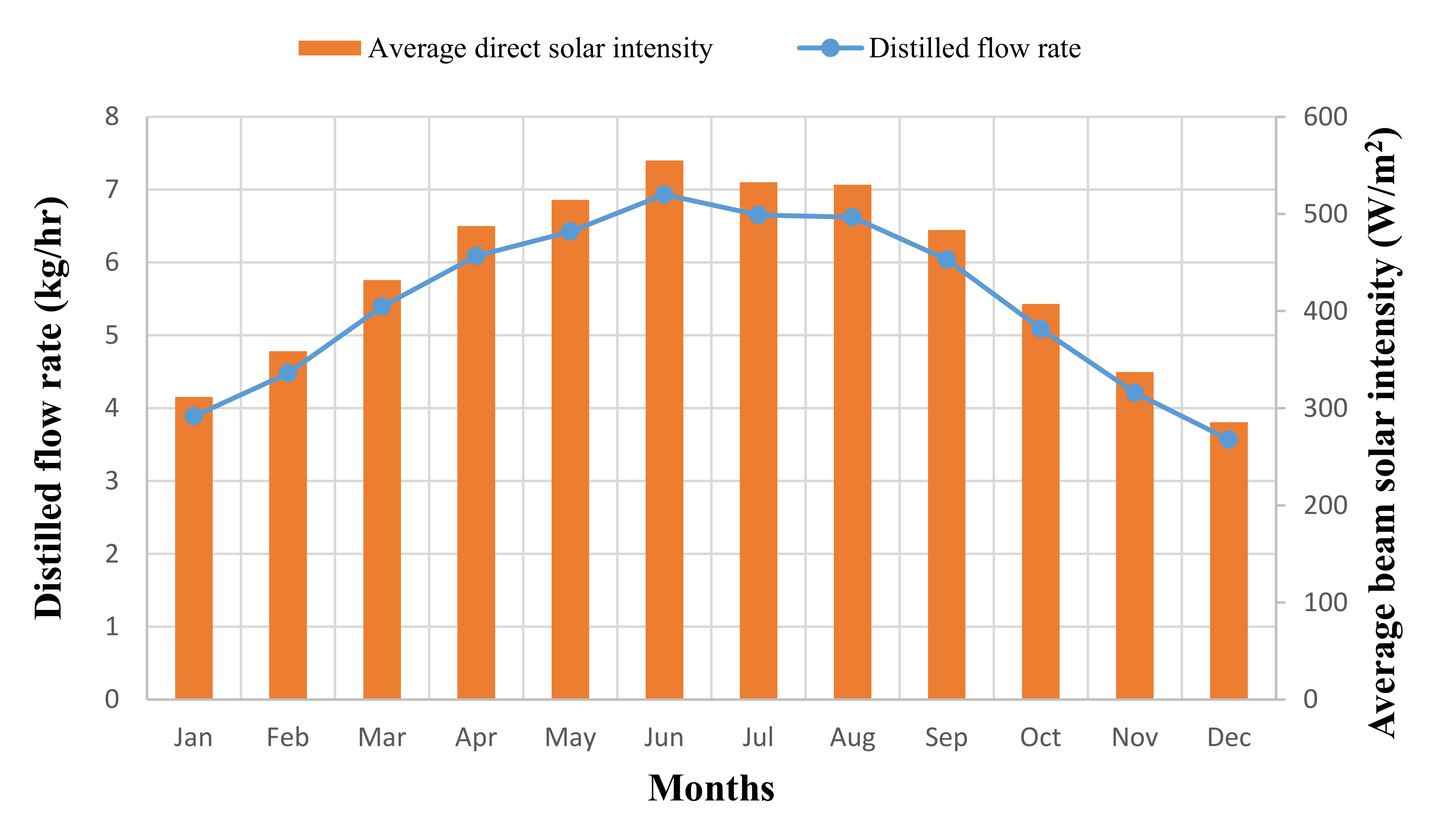
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Saline water density (kg/m3) | |
| Saline water velocity (m/s) | |
| Receiver tube inner diameter (m) | |
| Dynamic viscosity of saline water (kg/m·s) | |
| Specific heat capacity (J/K) | |
| Saline water thermal conductivity (W/m K) | |
| Surface area of the collector (m2) | |
| Surface area of the receiver tube (m) | |
| Pressure (Pa) | |
| External forces (N) | |
| External forces (N) | |
| External forces (N) | |
| Dissipation function to describe viscous stresses effects | |
| External forces (N) | |
| Mass transfer from the liquid phase to the vapour phase (kg/s/m3) | |
| Mass transfer intensity factor (s−1) | |
| Phase volume fraction | |
| Density of the fluid (kg/m3) | |
| Temperature of the liquid (°C) |
References
- Allan, T. The Middle East Water Question: Hydropolitics and the Global Economy; Bloomsbury Publishing: London, UK, 2012. [Google Scholar]
- Al-Karaghouli, A.A.; Kazmerski, L. Renewable energy opportunities in water desalination. In Desalination, Trends and Technologies; 2011; pp. 149–184. Available online: https://www.intechopen.com/books/desalination-trends-and-technologies/renewable-energy-opportunities-in-water-desalination (accessed on 10 May 2020).
- Kabeel, A.E.; Abdelgaied, M. Observational study of modified solar still coupled with oil serpentine loop from cylindrical parabolic concentrator and phase changing material under basin. Sol. Energy 2017, 144, 71–78. [Google Scholar] [CrossRef]
- Abdelrassoul, R.A. Potential for economic solar desalination in the Middle East. Renew. Energy 1998, 14, 345–349. [Google Scholar] [CrossRef]
- Moser, M.; Trieb, F.; Fichter, T. Potential of concentrating solar power plants for the combined production of water and electricity in MENA countries. J. Sustain. Dev. Energy Water Environ. Syst. 2013, 1, 122–140. [Google Scholar] [CrossRef]
- Breyer, C.; Gerlach, A.; Beckel, O.; Schmid, J. Value of solar PV electricity in MENA region. In Proceedings of the 2010 IEEE International Energy Conference, Manama, Bahrain, 18–22 December 2010. [Google Scholar]
- Tsikalakis, A.; Tomtsi, T.; Hatziargyriou, N.; Poullikkas, A.; Malamatenios, C.; Giakoumelos, E.; Jaouad, O.C.; Chenak, A.; Fayek, A.; Matar, T. Review of best practices of solar electricity resources applications in selected Middle East and North Africa (MENA) countries. Renew. Sustain. Energy Rev. 2011, 15, 2838–2849. [Google Scholar] [CrossRef]
- Fath, H.E.S.; Ghazy, A. Solar desalination using humidification—Dehumidification technology. Desalination 2002, 142, 119–133. [Google Scholar] [CrossRef]
- Mosleh, H.J.; Mamouri, S.J.; Shafii, M.; Sima, A.H. A new desalination system using a combination of heat pipe, evacuated tube and parabolic trough collector. Energy Convers. Manag. 2015, 99, 141–150. [Google Scholar] [CrossRef]
- Nafey, A.S.; Fath, H.E.S.; El-Helaby, S.O.; Soliman, A. Solar desalination using humidification–dehumidification processes. Part II. An experimental investigation. Energy Convers. Manag. 2004, 45, 1263–1277. [Google Scholar] [CrossRef]
- Qtaishat, M.R.; Banat, F. Desalination by solar powered membrane distillation systems. Desalination 2013, 308, 186–197. [Google Scholar] [CrossRef]
- AlMadani, H. Water desalination by solar powered electrodialysis process. Renew. Energy 2003, 28, 1915–1924. [Google Scholar] [CrossRef]
- Tong, X.; Liu, S.; Chen, Y.; Crittenden, J. Thermodynamic analysis of a solar thermal facilitated membrane seawater desalination process. J. Clean. Prod. 2020, 256, 120398. [Google Scholar] [CrossRef]
- Santosh, R.; Arunkumar, T.; Velraj, R.; Kumaresan, G. Technological advancements in solar energy driven humidification-dehumidification desalination systems—A review. J. Clean. Prod. 2019, 207, 826–845. [Google Scholar] [CrossRef]
- Sharon, H.; Reddy, K. A review of solar energy driven desalination technologies. Renew. Sustain. Energy Rev. 2015, 41, 1080–1118. [Google Scholar] [CrossRef]
- Fath, H.E. Solar distillation: A promising alternative for water provision with free energy, simple technology and a clean environment. Desalination 1998, 116, 45–56. [Google Scholar] [CrossRef]
- Ullah, I.; Rasul, M.G. Recent Developments in Solar Thermal Desalination Technologies: A Review. Energies 2019, 12, 119. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Wang, H.; Chen, X. Calculation of carbon footprints for water diversion and desalination projects. Energy Procedia 2015, 75, 2483–2494. [Google Scholar] [CrossRef]
- Qiblawey, H.M.; Banat, F. Solar thermal desalination technologies. Desalination 2008, 220, 633–644. [Google Scholar] [CrossRef]
- Thomson, A.M. Reverse-Osmosis Desalination of Seawater Powered by Photovoltaics without Batteries. Ph.D. Thesis, Loughborough University, Loughborough, UK, 2003. [Google Scholar]
- Lacroix, C.; Perier-Muzet, M.; Stitou, D. Dynamic Modeling and Preliminary Performance Analysis of a New Solar Thermal Reverse Osmosis Desalination Process. Energies 2019, 12, 4015. [Google Scholar] [CrossRef]
- Kalogirou, S. Use of parabolic trough solar energy collectors for sea-water desalination. Appl. Energy 1998, 60, 65–88. [Google Scholar] [CrossRef]
- Porteous, A. Saline Water Distillation Processes; Longman: London, UK, 1975. [Google Scholar]
- Zarza, E.; Ajona, J.; León, J.; Gregorzewski, A.; Genthner, K. Solar thermal desalination project at the Plataforma Solar de Almeria. Sol. Energy Mater. 1991, 24, 608–622. [Google Scholar] [CrossRef]
- Al-Othman, A.; Tawalbeh, M.; Assad, M.E.H.; Alkayyali, T.; Eisa, A. Novel multi-stage flash (MSF) desalination plant driven by parabolic trough collectors and a solar pond: A simulation study in UAE. Desalination 2018, 443, 237–244. [Google Scholar] [CrossRef]
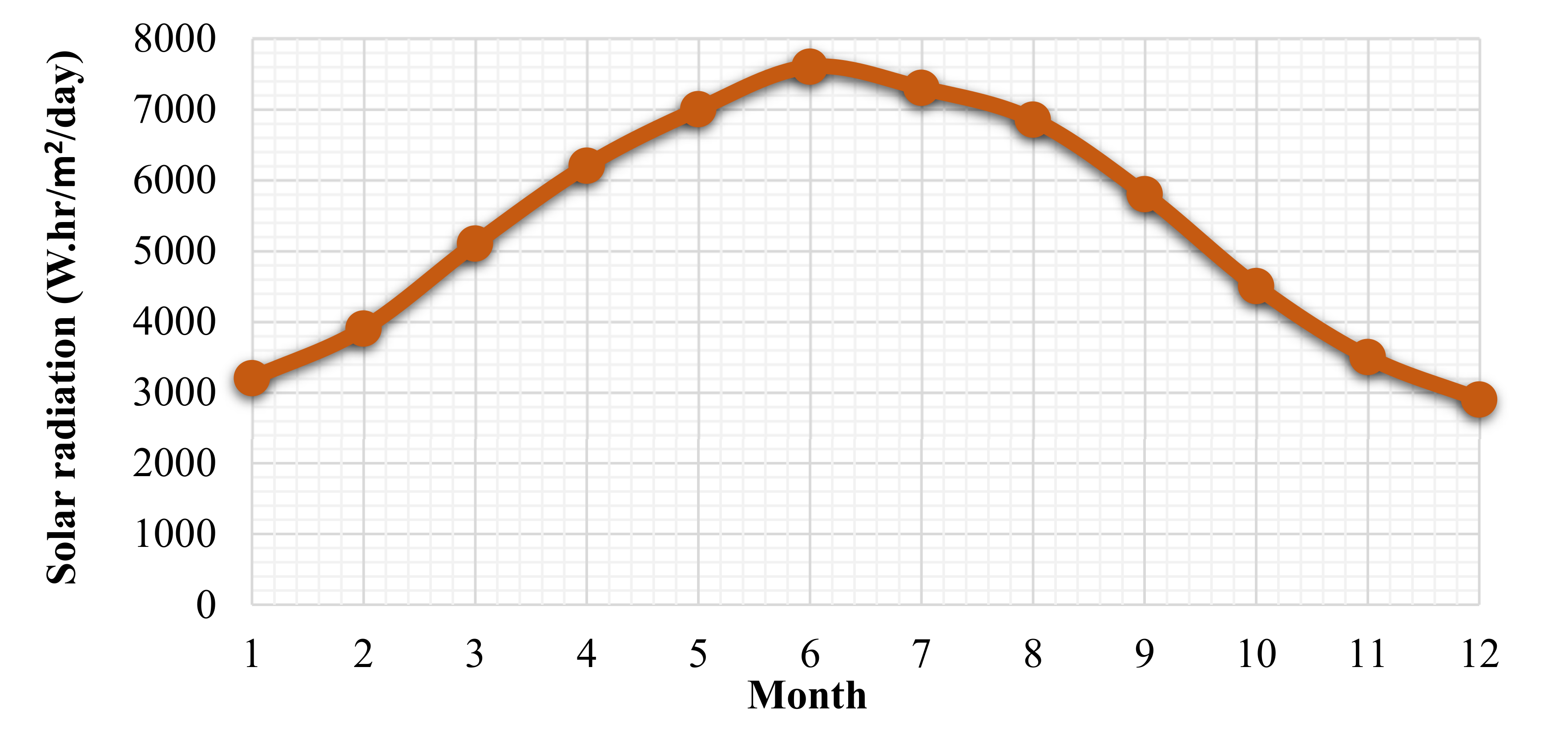
- Assi, A.; Jama, M. Estimating global solar radiation on horizontal from sunshine hours in Abu Dhabi–UAE. In Proceedings of the Advances in Energy Planning, Environmental Education and Renewable Energy Sources, Proceedings of the 4th WSEAS International Conference on Renewable Energy Sources, Kantaoui, Sousse, Tunisia, 3–6 May 2010. [Google Scholar]
- Nafaa, H.; Farhat, M.; Lassaad, S. A PV water desalination system using backstepping approach. In Proceedings of the 2017 International Conference on Green Energy Conversion Systems (GECS), Hammamet, Tunisia, 23–25 March 2017. [Google Scholar]
- Kalogirou, S.A. Solar Energy Engineering: Processes and Systems; Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Parsa, S.M.; Rahbar, A.; Javadi, Y.D.; Koleini, M.; Afrand, M.; Amidpour, M. Energy-matrices, exergy, economic, environmental, exergoeconomic, enviroeconomic, and heat transfer (6E/HT) analysis of two passive/active solar still water desalination nearly 4000m: Altitude concept. J. Clean. Prod. 2020, 261, 121243. [Google Scholar] [CrossRef]
- Salah, A.H.; Hassan, G.E.; Fath, H.; Elhelw, M.; Elsherbiny, S. Analytical investigation of different operational scenarios of a novel greenhouse combined with solar stills. Appl. Therm. Eng. 2017, 122, 297–310. [Google Scholar] [CrossRef]
- Akrami, M.; Salah, A.H.; Dibaj, M.; Porcheron, M.; Javadi, A.A.; Farmani, R.; Fath, H.E.; Negm, A. A Zero-Liquid Discharge Model for a Transient Solar-Powered Desalination System for Greenhouse. Water 2020, 12, 1440. [Google Scholar] [CrossRef]
- Behnam, P.; Ghasempour, R. Examination of a solar desalination system equipped with an air bubble column humidifier, evacuated tube collectors and thermosyphon heat pipes. Desalination 2016, 397, 30–37. [Google Scholar] [CrossRef]
- Ahmad, F. Valuation of solar power generating potential in Iran desert areas. J. Appl. Sci. Environ. Manag. 2018, 22, 967. [Google Scholar] [CrossRef][Green Version]
- Schwarzer, K.; Da Silva, E.V.; Hoffschmidt, B.; Schwarzer, T. A new solar desalination system with heat recovery for decentralised drinking water production. Desalination 2009, 248, 204–211. [Google Scholar] [CrossRef]
- Saettone, E.A. Desalination using a parabolic-trough concentrator. Appl. Sol. Energy 2012, 48, 254–259. [Google Scholar] [CrossRef]
- Arun, C.; Sreekumar, P. Modeling and performance evaluation of parabolic trough solar collector desalination system. Mater. Today Proc. 2018, 5, 780–788. [Google Scholar] [CrossRef]
- Narayanan, S.R.; Vijay, S. Desalination of water using parabolic trough collector. Mater. Today Proc. 2020, 21, 375–379. [Google Scholar] [CrossRef]
- Chaouchi, B.; Zrelli, A.; Gabsi, S. Desalination of brackish water by means of a parabolic solar concentrator. Desalination 2007, 217, 118–126. [Google Scholar] [CrossRef]
- Panahi, R.; Khanjanpour, M.H.; Javadi, A.A.; Akrami, M.; Rahnama, M.; Ameri, M. Analysis of the thermal efficiency of a compound parabolic Integrated Collector Storage solar water heater in Kerman, Iran. Sustain. Energy Technol. Assess. 2019, 36, 100564. [Google Scholar] [CrossRef]
- Jamali, H. Investigation and review of mirrors reflectance in parabolic trough solar collectors (PTSCs). Energy Rep. 2019, 5, 145–158. [Google Scholar] [CrossRef]
- Gad, H.; El-Gayar, S. Absorptance of Different Local Coated Surfaces to Global Solar Radiation in Egypt. J. Sol. Energy Res. 2016, 1, 18–24. [Google Scholar]
- Zheng, H. Chapter 2—Solar Energy Utilization and Its Collection Devices. In Solar Energy Desalination Technology; Zheng, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–171. [Google Scholar]
- Bellos, E.; Tzivanidis, C. Thermal analysis of parabolic trough collector operating with mono and hybrid nanofluids. Sustain. Energy Technol. Assess. 2018, 26, 105–115. [Google Scholar] [CrossRef]
- Alarcón, J.A.; Hortúa, J.E.; Lopez, A. Design and construction of a solar collector parabolic dish for rural zones in Colombia. Tecciencia 2013, 7, 14–22. [Google Scholar] [CrossRef]
- Harris, J.A.; Lenz, T.G. Thermal performance of solar concentrator/cavity receiver systems. Sol. Energy 1985, 34, 135–142. [Google Scholar] [CrossRef]
- Andersson, B. Computational Fluid Dynamics For Engineers; Cambridge University Press: Cambridge, UK, 2012; pp. 89–100. [Google Scholar]
- Versteeg, H.; Malalasekera, W. An Introduction to Computational Fluid Dynamics; Pearson Education Ltd.: Harlow, UK, 2007; pp. 91–92. [Google Scholar]
- Akrami, M.; Gilbert, S.J.; Dibaj, M.; Javadi, A.A.; Farmani, R.; Salah, A.H.; Fath, H.E.S.; Negm, A. Decarbonisation Using Hybrid Energy Solution: Case Study of Zagazig, Egypt. Energies 2020, 13, 4680. [Google Scholar] [CrossRef]
- Global Solar Atlas. Available online: https://globalsolaratlas.info/map (accessed on 10 May 2020).
- Compere, C.; Le Bozec, N. Behaviour of stainless steel in natural seawater. In Proceedings of the First Stainless Steel Congress in Thailand, Bangkok, Thailand, 15–17 December 1997. [Google Scholar]
- Heiser, J.H.; Poo, S. Corrosion of Barrier Materials in Seawater Environments. 1995, Brookhaven National Lab. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:27032332 (accessed on 10 May 2020).
- Al-Moubaraki, A.H.; Al-Rushud, H.H. The red sea as a corrosive environment: Corrosion rates and corrosion mechanism of aluminum alloys 7075, 2024, and 6061. Int. J. Corros. 2018. [Google Scholar] [CrossRef]
- Tulhill, A. Guidelines for the use of copper alloys in seawater 11. Mater. Perform. 1987, 26, 13–22. [Google Scholar]
- Diaconu, A.; Solomon, C.; Benea, L.; Dumitraşcu, V.; Mardare, L. Corrosion Resistance of Zinc Coated Steel in Sea Water Environment. Ann. Dunarea Jos Univ. Galati Fascicle IX Metall. Mater. Sci. 2015, 38, 34–39. [Google Scholar]
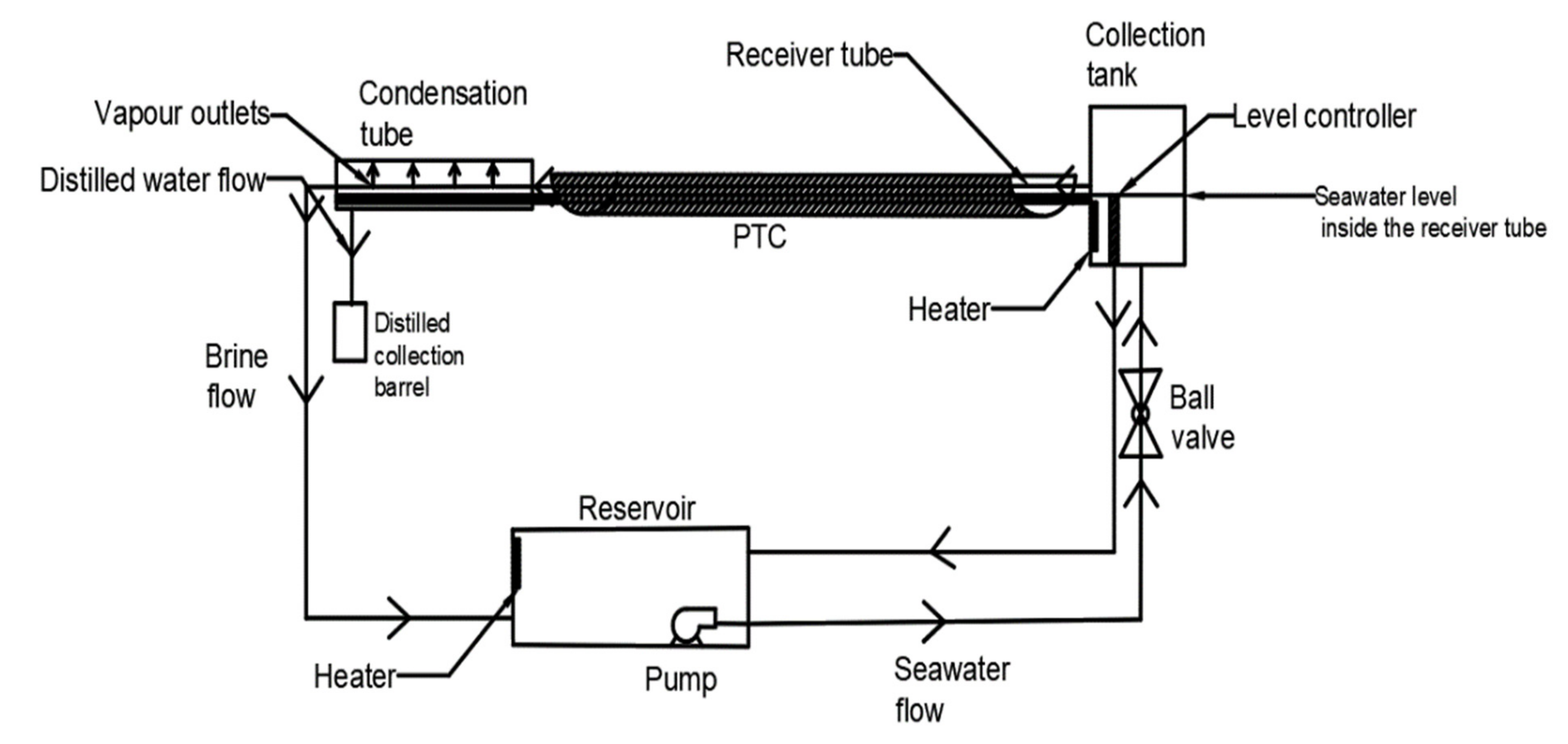
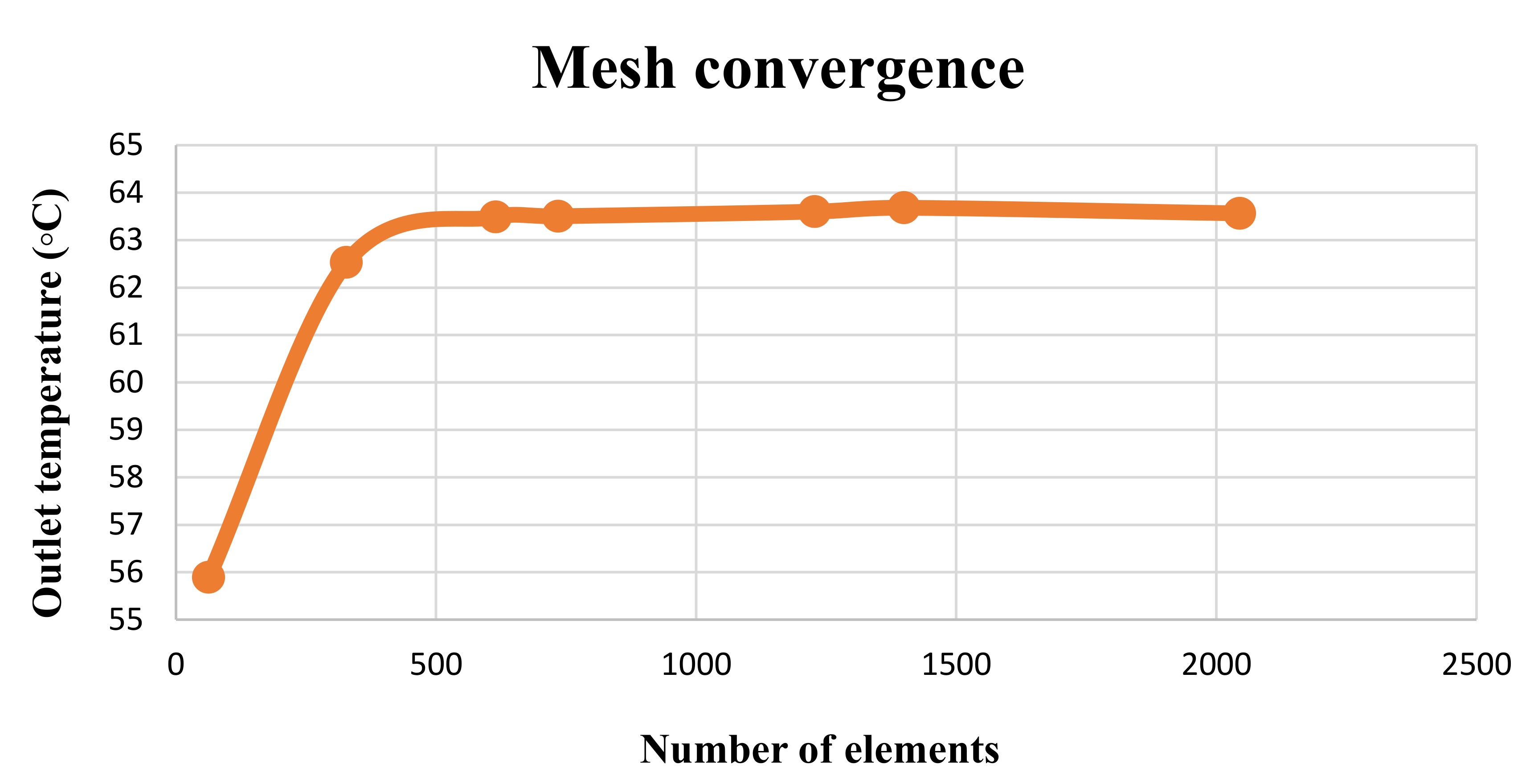
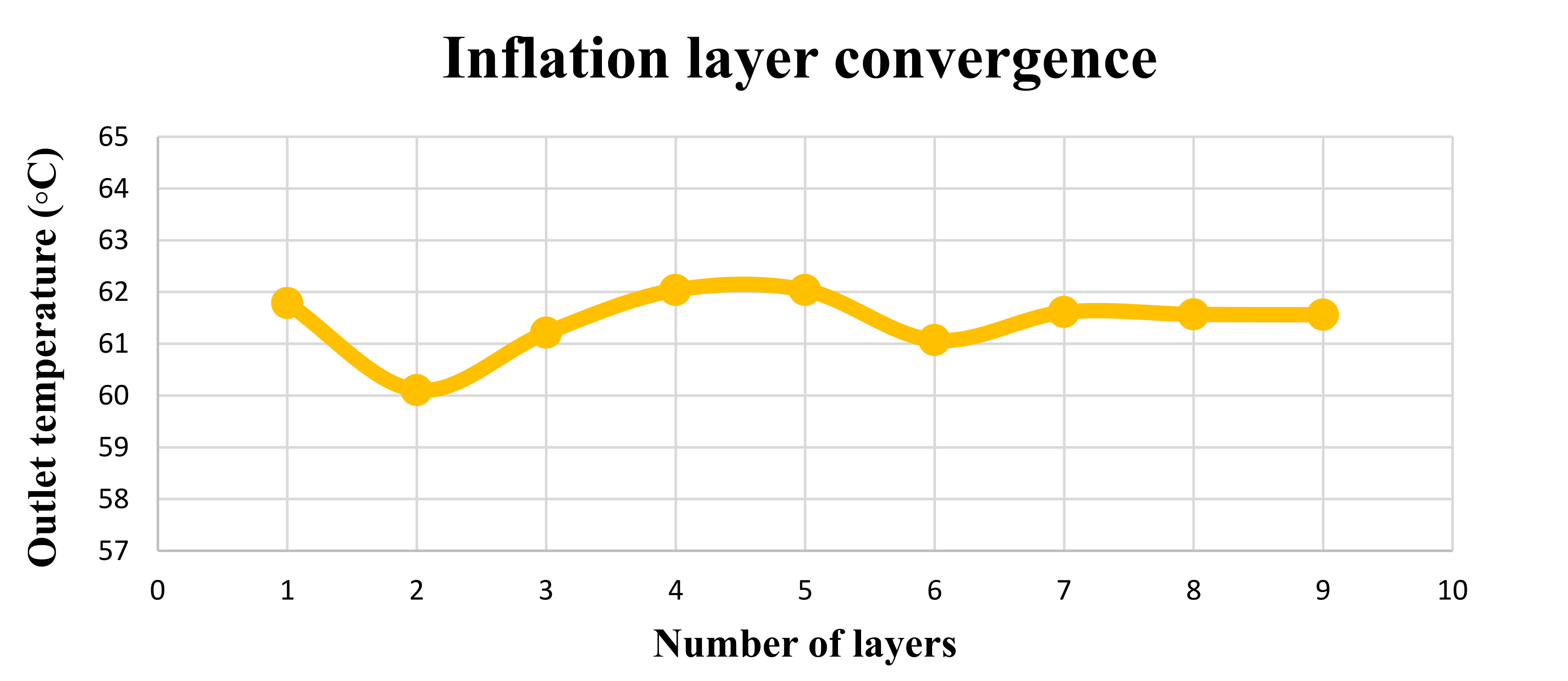
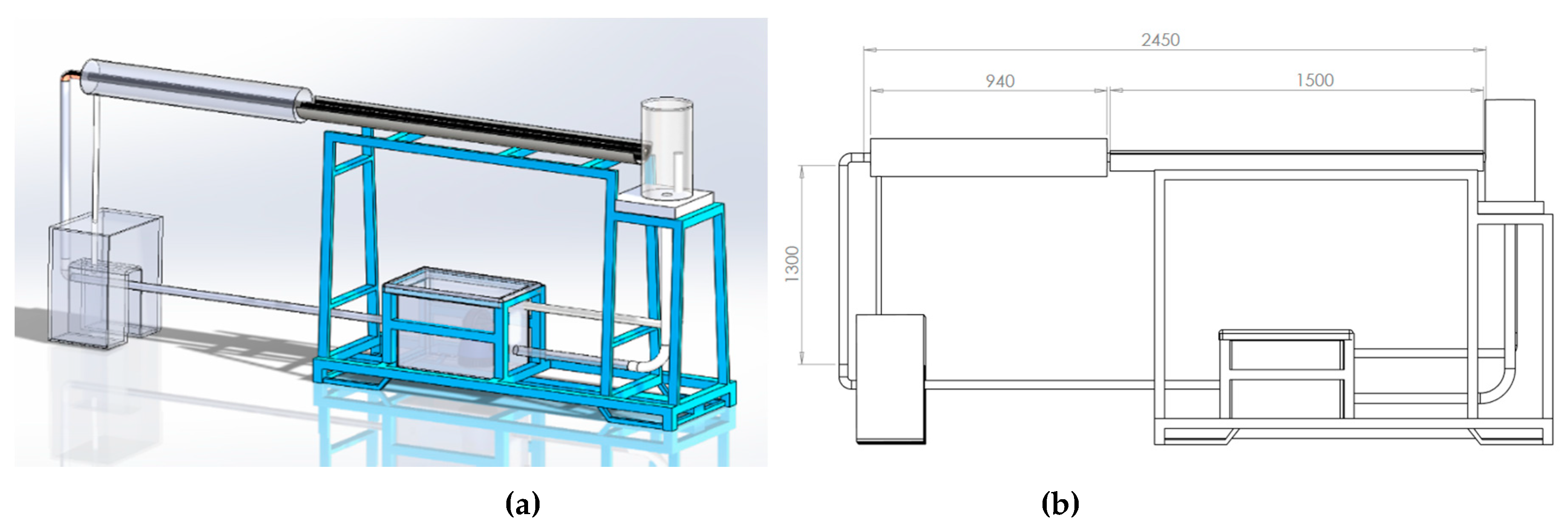

| Feature/Parameter | (m2) | (m2) | Receiver Tube Size | (maximum) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Value | 0.36 | 0.15 m2 | Outer diameter: 32 mm, thickness: 4 mm | 2.4 | 0.9 [40] | 0.886 [41] | 1 | 0.58 | 0.47 |
| Parameter | Value |
|---|---|
| (at 34 °C) | 1020 kg/m3 |
| (at 34 °C) | 4011 kJ/kgK |
| (at 34 °C) | 0.00087 Ns/m2 |
| 1765.82 W/m2K | |
| 334 kJ/kg |
| Multiphase Model | Volume Fraction Parameters (Formulation) | Body Force Formulation | VOF Sub-Models | Interface Modelling | Surface Tension Coefficient (n/m) |
|---|---|---|---|---|---|
| Volume of Fluid (VOF) | Implicit | Implicit body force | Open channel flow | Sharp | 0.07 [48] |
| Turbulence Model | Turbulence Intensity (%) | Turbulence Viscosity Ratio |
|---|---|---|
| k-omega SST | 5 | 10 |
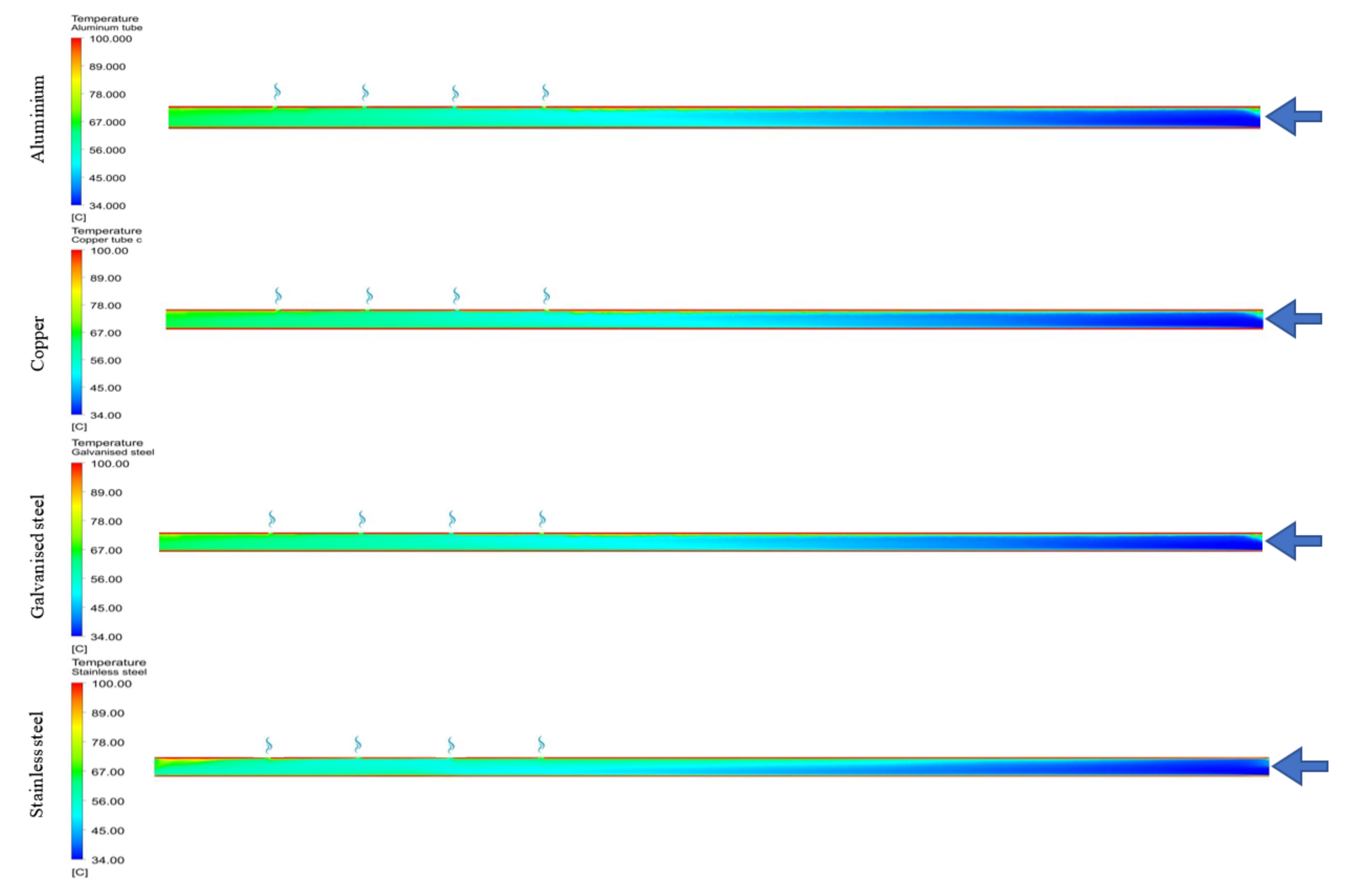
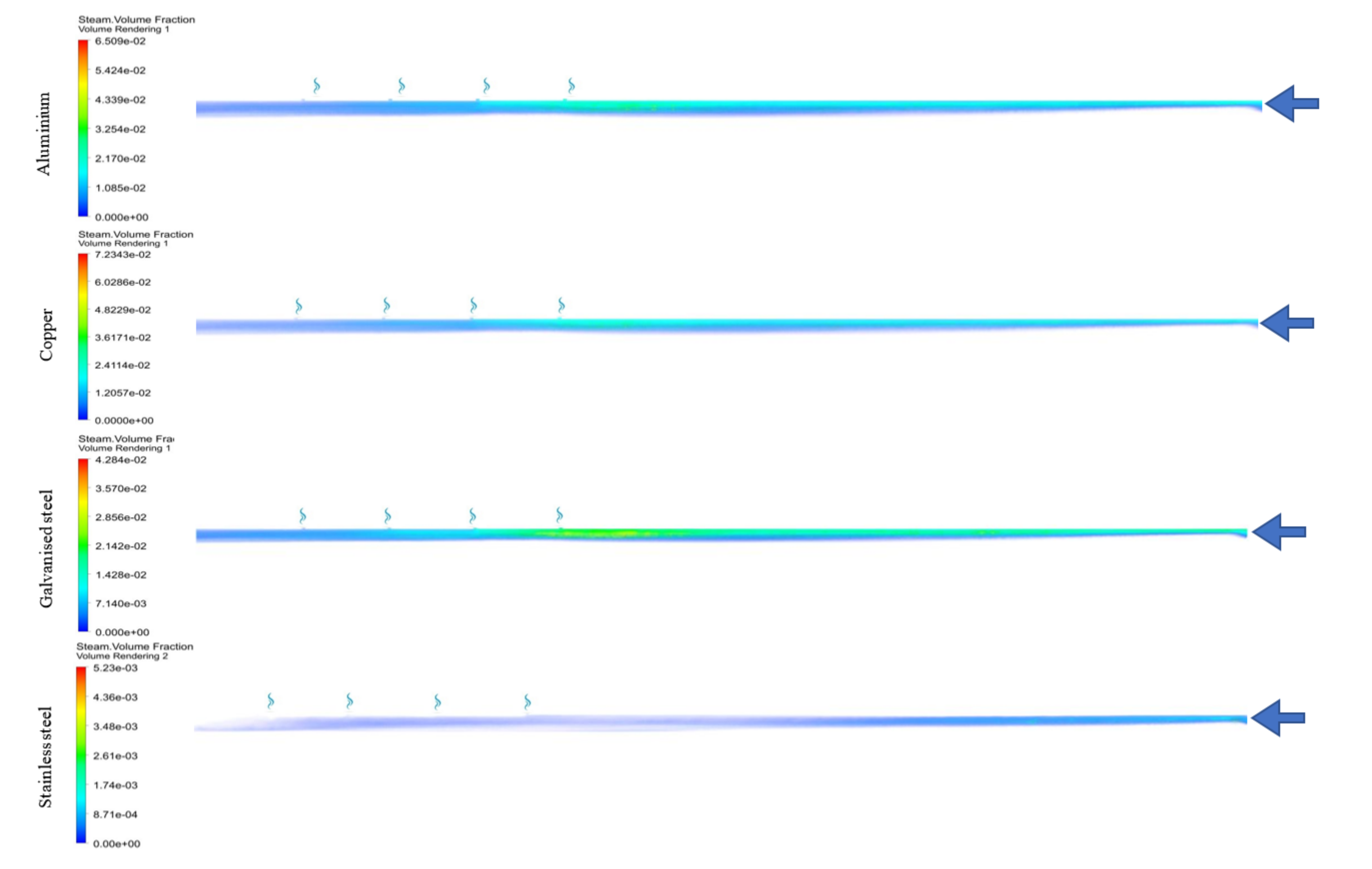
| Material | Brine Outlet Temperature (°C) | Maximum Vapour Volume Fraction | Corrosion Potential (mV) | Corrosion Rate (miles/year) | Receiver Tube Cost ($) |
|---|---|---|---|---|---|
| Stainless steel (grade 316) | 62.5 | 0.00523 | −180 [50] | 0.59 [51] | 149.38 |
| Aluminium (Al 6061) | 67.7 | 0.065 | −1035 [52] | 3.1496 [51] | 23.55 |
| Copper | 67.74 | 0.072 | −330 [53] | 1 [53] | 33.38 |
| Galvanised steel | 67.42 | 0.0428 | −1055.9 [54] | 7.6 [54] | 14.48 |
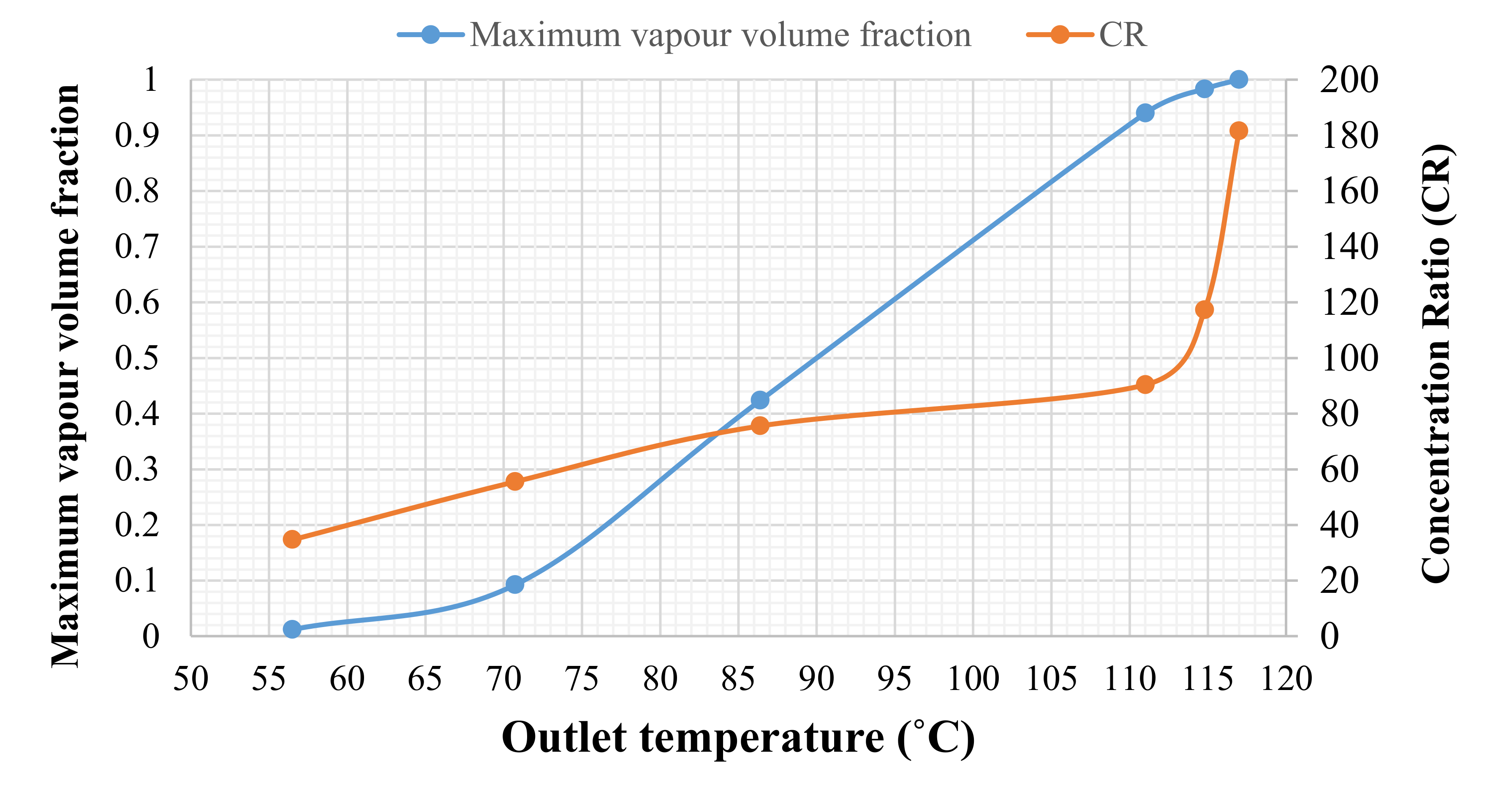
| Outer Surface Temperature (°C) | Required CR in Zagazig |
|---|---|
| 75 | 34.67 |
| 100 | 55.54 |
| 125 | 75.56 |
| 150 | 90.36 |
| 175 | 117.30 |
| 200 | 181.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akrami, M.; Alsari, H.; Javadi, A.A.; Dibaj, M.; Farmani, R.; Fath, H.E.S.; Salah, A.H.; Negm, A. Analysing the Material Suitability and Concentration Ratio of a Solar-Powered Parabolic trough Collector (PTC) Using Computational Fluid Dynamics. Energies 2020, 13, 5479. https://doi.org/10.3390/en13205479
Akrami M, Alsari H, Javadi AA, Dibaj M, Farmani R, Fath HES, Salah AH, Negm A. Analysing the Material Suitability and Concentration Ratio of a Solar-Powered Parabolic trough Collector (PTC) Using Computational Fluid Dynamics. Energies. 2020; 13(20):5479. https://doi.org/10.3390/en13205479
Chicago/Turabian StyleAkrami, Mohammad, Husain Alsari, Akbar A. Javadi, Mahdieh Dibaj, Raziyeh Farmani, Hassan E.S. Fath, Alaa H. Salah, and Abdelazim Negm. 2020. "Analysing the Material Suitability and Concentration Ratio of a Solar-Powered Parabolic trough Collector (PTC) Using Computational Fluid Dynamics" Energies 13, no. 20: 5479. https://doi.org/10.3390/en13205479
APA StyleAkrami, M., Alsari, H., Javadi, A. A., Dibaj, M., Farmani, R., Fath, H. E. S., Salah, A. H., & Negm, A. (2020). Analysing the Material Suitability and Concentration Ratio of a Solar-Powered Parabolic trough Collector (PTC) Using Computational Fluid Dynamics. Energies, 13(20), 5479. https://doi.org/10.3390/en13205479









