In the last ten years, the main contribution to the increase of greenhouse gas (GHG) emissions has been the growing energy demand. In 2017, the annual global greenhouse gas emissions (excluding emissions from climate change in land use) reached a record 49.2 Gton CO
2eq, that is, 1.1% more than the previous year [
1]. In the reference scenarios evaluated in the Fifth Evaluation Report that was conducted by the IPCC, the projections indicate that direct CO
2 emissions from the energy supply sector will almost double or could even triple in 2050, when compared to the concentration of CO
2 in 2010 [
2].
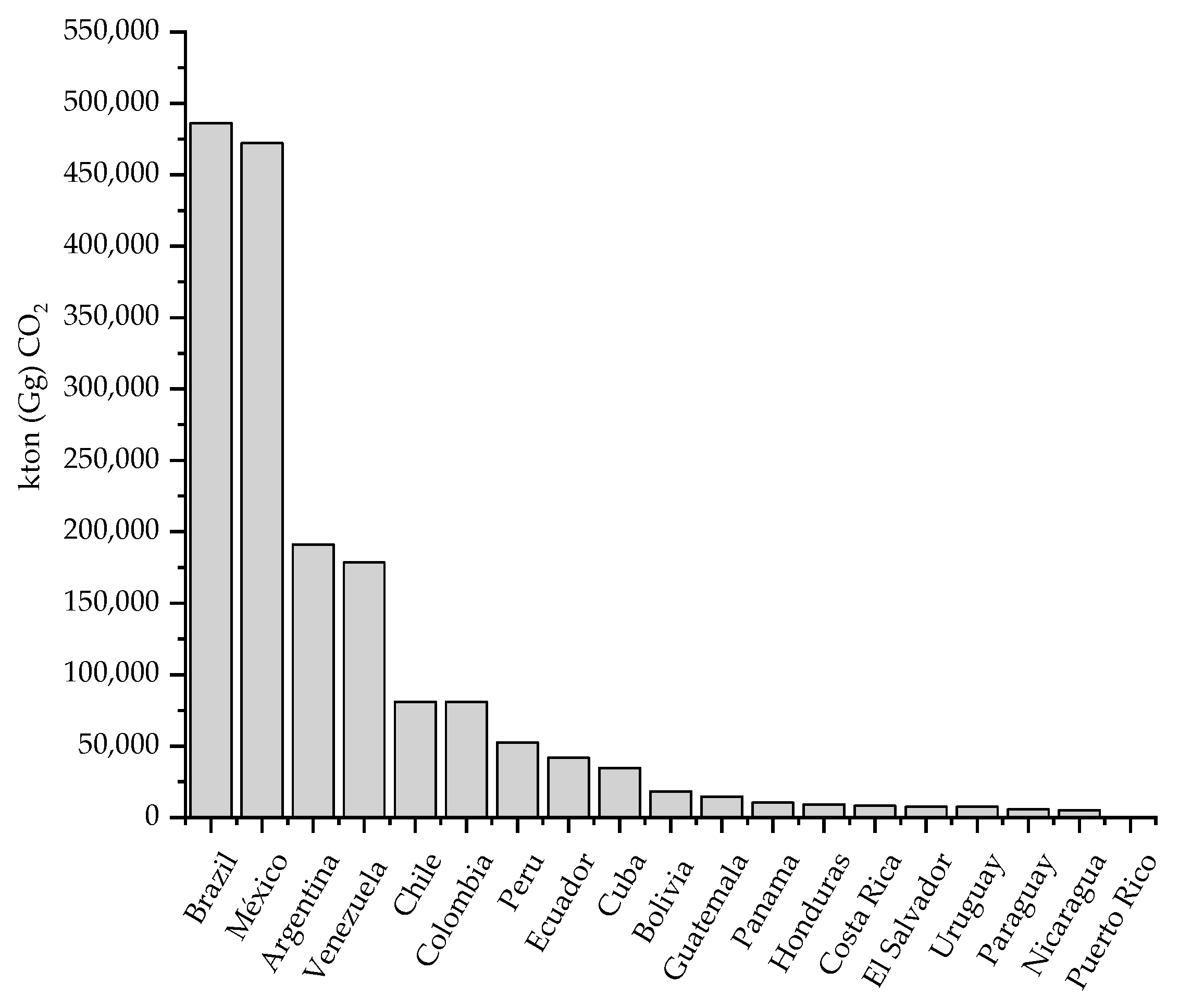
Brazil, Mexico, Argentina, and Venezuela (
Figure 1) are those responsible for most of the emissions in Latin America, contributing 78% of the total emissions in this region. Regarding Mexico, CO
2 emissions have increased considerably from 1970 to 2015. According to the National Inventory of Gas Emissions and Greenhouse Compounds (INEGyCEI), of the National Institute of Ecology and Climate Change (INECC), in 2015 emissions of 537 Mton (Megatons) of CO
2 were recorded, of which 125 Mton were coming from the electric power production industry (from conventional technologies), representing 23.38% of the total CO
2 emissions in the country, being the second activity with greater impact on the environment after mobile sources [
3].
The energy sector in Mexico has an electricity production capacity of 68,044 MW (registered in 2015), of which 71.7% comes from conventional power plants and the rest is generated with clean technologies [
5]. Thermoelectric plants only represent 18.7% of the total installed capacity in the country and produce on average 680 kg of CO
2 per MWh generated within conventional energy production technologies, becoming one of the largest emitting sources. The emissions of the largest installed power plant in Mexico have an average of 10.75 Mton of annual CO
2. The typical composition of these emissions not only contains large amounts of CO
2, but also NOx and SOx, and its treatment is important in reducing the negative effects of these emissions into the atmosphere.
In general, and not only in the energy sector, worldwide the strategies that have been followed to mitigate CO
2 emissions include the need for: greater energy efficiency, that is, a decrease in the use of energy per unit of product, process, or service; increased use of clean fossil energy, this is the use of fossil fuels by coupling CO
2 capture from flue gases; and, greater use of renewable energies and the development of neutral energy resources in CO
2 [
6,
7,
8]. The capture of CO
2 as a source of carbon in the production of microalgae biomass has also been considered to be an option for reducing these emissions. Due to their high photosynthetic capacity, as compared to terrestrial plants [
9], they are able to capture up to 1.7 kg of CO
2 per kilogram of biomass produced [
10]. Microalgae accumulate significant amounts of fats, carbohydrates, proteins, and other compounds of great economic importance, such as pigments and vitamins [
11,
12,
13].
1.1. Microalgae
Microalgae are distinguished due to their high performance in terms of oil productivity per area, as compared to biomass that is derived from other sources, being approximately 10 times higher than that obtained with other oil crops [
14,
15]. Another advantage of microalgae biomass is that it does not belong to edible crops and does not compete for the use of arable or forested land, since they do not require fertile land for cultivation, guaranteeing food security and land use [
16,
17]. Microalgae have high growth rates, they can double their biomass in periods as short as 3.5 h and a greater amount can be produced continuously compared to land crops [
18]. On the other hand, important aspects must be considered before selecting a species for its use, it must be evaluated based on certain parameters, such as being resistant to environmental changes, withstanding high concentrations of CO
2, NOx, and SOx in the cultivation stage (capture them if the purpose is their use as bioremediation), a high growth rate, a high content, and good quality of oils.
One of the most promising species for the capture of CO
2 from combustion gases is
Chlorella. It has been reported that certain
Chlorella species could grow in an atmosphere that contains up to 40% (v/v) CO
2 [
6], with a CO
2 fixation rate between 0.73 to 2.22 g/L/day [
19]. In addition, it has been found that other compounds present in the fed CO
2 stream, for example, NOx and SOx, do not affect the production of microalgal biomass from
Chlorella [
19,
20,
21]. Kao et al. [
22] and Duarte et al. [
23] analyzed the cultivation of the
Chlorella species as a bioremediation option not only for CO
2 in combustion gases from power plants, but also for other components, finding that the NOx and SOx content in these currents is also reduced. Both of the authors agree that part of the NOx dissolves in water forming nitric acid or nitrous acid and, thus, the microalgae can use it in their metabolism, contributing to the saving of nutrients during cultivation. This shows the great potential of microalgae (especially the
Chlorella species) as a bioremediation option, not only for CO
2, but also for other greenhouse gases.
The content of lipid oils in microalgae varies from 2% to 75% by weight of dry biomass; however, species that reach high lipid content have low productivity (for example,
Botryococcus braunii). In contrast, species, such as
Chlorella, Crypthecodinium, Cylindrotheca, Dunaliella, Isochrysis, Nannochloris, and
Nannochloropsis have oil levels between 20 and 50%, but they are more productive [
11]; that is why the latter are mostly chosen for the production of oils [
18]. Lipid productivity considers both the concentration of lipids within cells and the biomass produced by these cells; therefore, it is a more useful indicator for the evaluation of potential costs in the production of liquid biofuels or other uses of lipids. The constitution of microalgal lipids is like vegetable oils; these are mainly triglycerides, where their chemical structure consists of three hydroxyl groups that are esterified with carboxyl groups of straight chain fatty acids. Regarding the composition of fatty acids, this differs in each species of microalgae; this is another important factor, since it provides the quality of lipids [
24]; for example, in the production of biodiesel, species that produce mostly neutral lipids must be selected [
25].
1.2. Production of Microalgal
There are different types of microalgae production, including photoautotrophic, heterotrophic, and myxotrophic culture. Photoautotrophic production is an autotrophic photosynthesis, where the carbon source is CO
2, heterotrophic production requires organic substances (for example, glucose) to stimulate growth, some strains while of algae can combine autotrophic photosynthesis and heterotrophic assimilation of organic compounds in a mixotrophic process. Currently, photoautotrophic production is the most common form of microalgae culture and the only method that is technically and economically viable for large-scale microalgae biomass production, since carbon consumption costs are low due to the use of CO
2 from of waste effluents from various industrial processes [
6,
26]. The cultivation stage consumes more process time; it can be operated in continuous or batch mode. As for the systems for the cultivation of microalgae, they can be open systems (system of channels or ponds), closed systems (photobioreactors), or a combination of the above. Several factors strongly influence the option for one or the other alternative, such as the species of microalgae in question, the desired metabolic regime, temperature, and the compound of interest.
The quantity and quality of oils from microalgae vary according to species, cultivation techniques, and oil extraction technologies. Obtaining lipid oils from microalgae for the production of biofuels on a commercial scale is currently not economically viable [
27,
28]. The high energy consumption in the processes of harvesting and extraction of oils, the low growth efficiency of microalgae, and the consumption of nutrients for their cultivation are some of the limitations. Studies have reported that the harvest stage can represent approximately 20–33% of total production costs [
6,
25,
29]. One of the reasons why this occurs is because the concentration of the cells in the culture is low, typically around 0.1 to 8 g/L in dry weight. In addition, they are cells of very small size (1–10 µm in diameter), and with a density that is similar to that of the culture medium (1020 kg/m
3), which can make the harvest even more difficult [
28,
30]. Some technologies for this stage are flocculation, flotation, sedimentation, filtration, and centrifugation, although, in some, their energy requirement is high. The selection of dewatering technology is crucial for the economical production of microalgae biomass [
31], although the selection of suitable strains is also an important consideration, since certain species of microalgae are much easier to harvest. The collection technique depends on the characteristics of the microalgae, such as size, cell density, value, and specifications of the products obtained from this biomass; however, an optimal collection method should be independent of the species, use less chemicals and energy, and, if possible, it should also release intracellular materials [
29].
The biomass collected (approximate concentration of biomass of 200 g/L and with an approximate content of 80% humidity) is perishable and must undergo additional processes for storage or convert it to other products immediately [
32]. A drying or dehydration stage is necessary [
33], which must reach a concentration of approximately 90% in dry solids of biomass, to increase the stability of the biomass and obtain greater efficiency in the extraction of oils (or any other use). The above can be achieved by drying in the sun, in a drum dryer, spray drying, freeze drying, or any other technique that allows for moisture to be removed from the biomass [
6,
28]. Drying in the sun requires large areas, a lot of time, and there is a risk of material loss; spray drying is very expensive, which only makes it viable for high value products; lyophilization is equally expensive, especially for large-scale operations, but it facilitates the extraction of oils. When the bulk microalgae biomass is not the desired product, it must undergo further processing to obtain one or more cell fractions. Some microalgae have a rigid cell wall, which leads to cell breakdown being necessary for releasing internal compounds, such as lipids and pigments that are contained in the cytoplasm and carbohydrates stored in the cell wall [
34]. For the extraction of microalgal oil, there are mechanical (pressing), chemical (solvent extraction), and supercritical fluid extraction methods [
17,
25]. It has been reported that the combination of methods can increase the yield of microalgae oil extraction [
35].
Several reports analyze the possible technological routes to produce microalgae oil; however, several of these analyzes include the evaluation of technologies with little viability to be implemented on an industrial scale. Some of these technologies are highly efficient, however, their energy consumption is high, which considerably increases the operating costs and decreasing the technical and economic viability of the process. Other reports only include the evaluation of a single process unit, focusing on energy efficiency and demand [
32,
36,
37,
38].
On the other hand, recent researches to improve the cost of obtaining microalgae has focused on the use of waste and integrating the co-production of high-value compounds [
39]. CO
2 sequestration using a biorefinery approach, through interconnection with the cultivation of microalgae, is an interesting idea, since the waste generated from power plants or other industrial facilities is used [
34,
40]. The residual microalgae biomass, which was rich in protein and carbohydrates, could still be used as a carbon source for anaerobic fermentation in the production of volatile fatty acids, biohydrogen, CO
2, among others. The resulting CO
2 and volatile fatty acids can be reused to produce bioplastics, bioelectricity, and biohydrogen [
41]. The biorefinery models analyzed in this context could open new avenues to convert CO
2 into several valuable products, materials, and fuels, which could help to close the carbon cycle and contribute to the bioeconomy of the process [
42,
43].
Recently, there is a growing need for energy and materials, which forces humanity to move from a linear economy that is based on fossil sources to a sustainable circular bioeconomy [
44]. The production of energy or valuable products from microalgae biomass is an example of circular bio-economics, since CO
2 emissions can be used as a carbon source for biomass production, contributing to mitigating sources of greenhouse gases. Electric power generating plants, one of the industries with the highest CO
2 emissions, could well consider installing a microalgae cultivation plant at or near their facilities and, thus, feed their effluents both gaseous and wastewater. On the other hand, the biomass obtained (rich in carbohydrates, lipids and proteins) could well be a raw material for the production of bioenergetics, biolubricant oils and other by-products [
11,
45,
46,
47], which can be consumed by themselves or commercialized. These reasons increase the interest in cultivating microalgae as a raw material for obtaining valuable products, providing alternative methods for the use of carbon dioxide emitted by power plants.
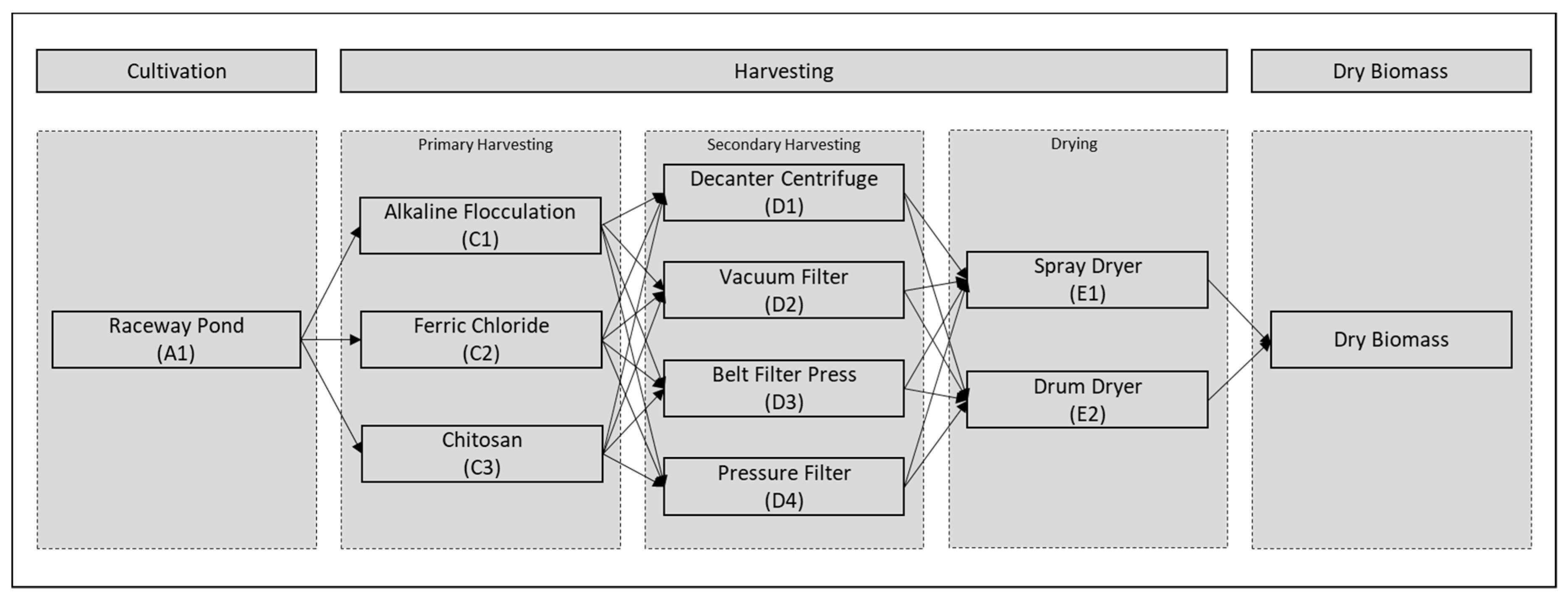
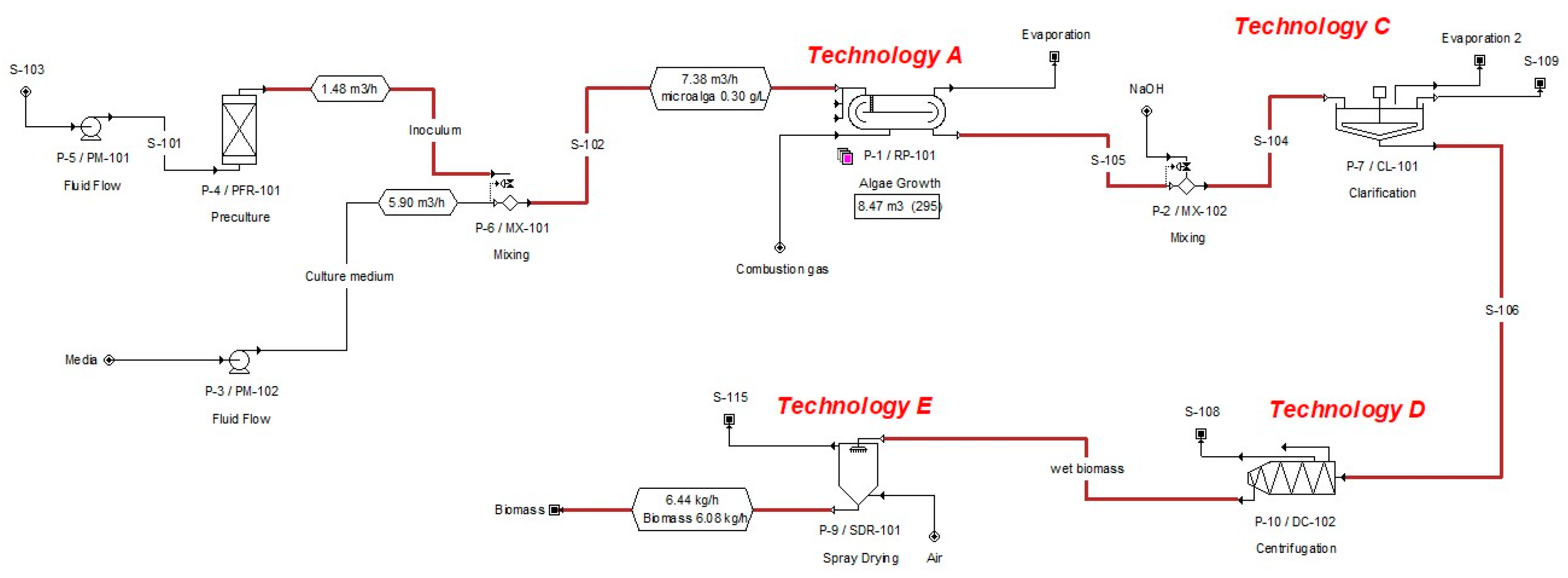
Given the great potential of use that microalgae have, the objective of this work is the techno-economic evaluation of biomass production from these, considering only production scenarios with scaling potential at the industrial level. In addition, the capture of CO
2 from a thermoelectric plant is analyzed as a carbon source for the cultivation of microalgae. The production scenarios that were analyzed include several technologies in the harvest and drying stage. A bioprocess simulation tool was used to carry out the evaluation of the production models (SuperPro Designer v10®). The
Chlorella microalgae was selected as a reference species to establish the operating conditions for each technology involved in the process. The stage of cultivation in the raceway pond was evaluated, while the harvest was divided into two stages, as a strategy to reduce costs [
48]; subsequently, two technologies for biomass drying were included.
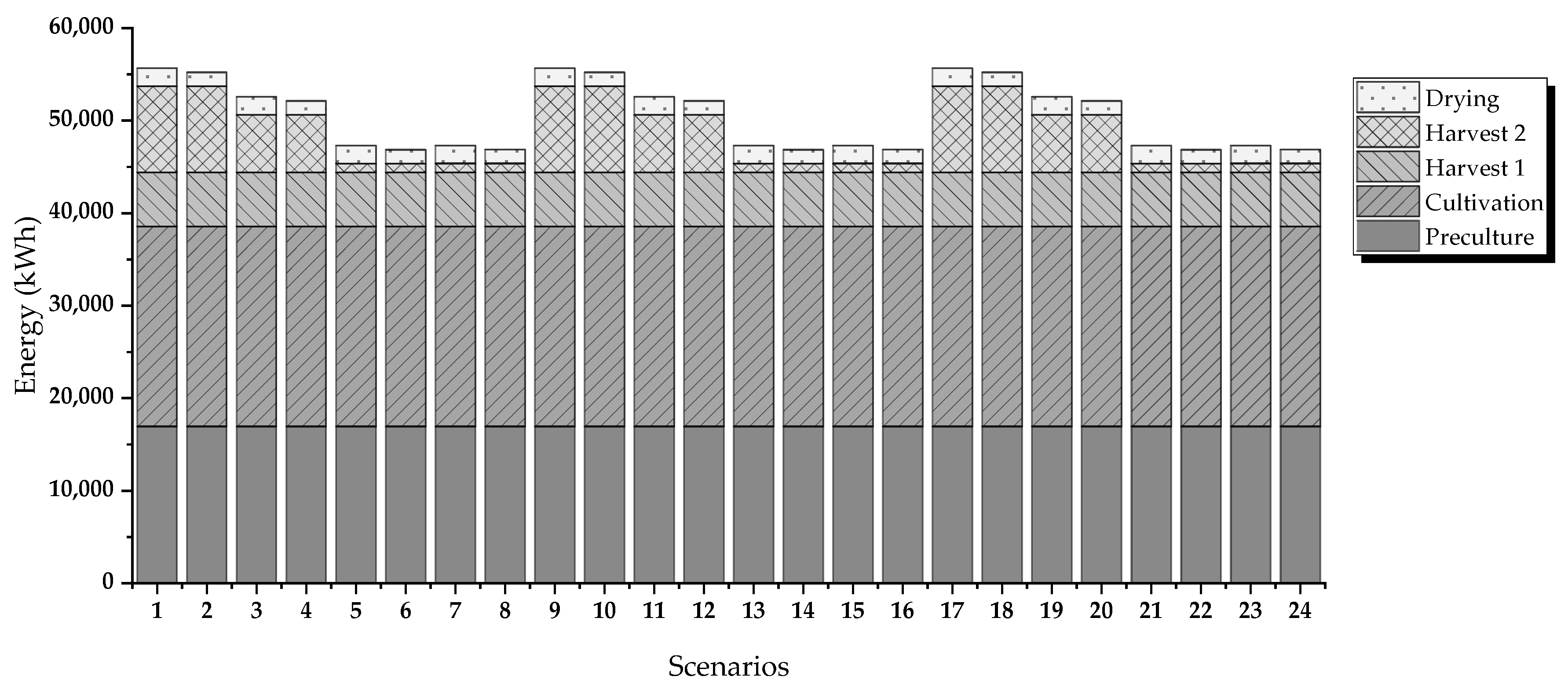
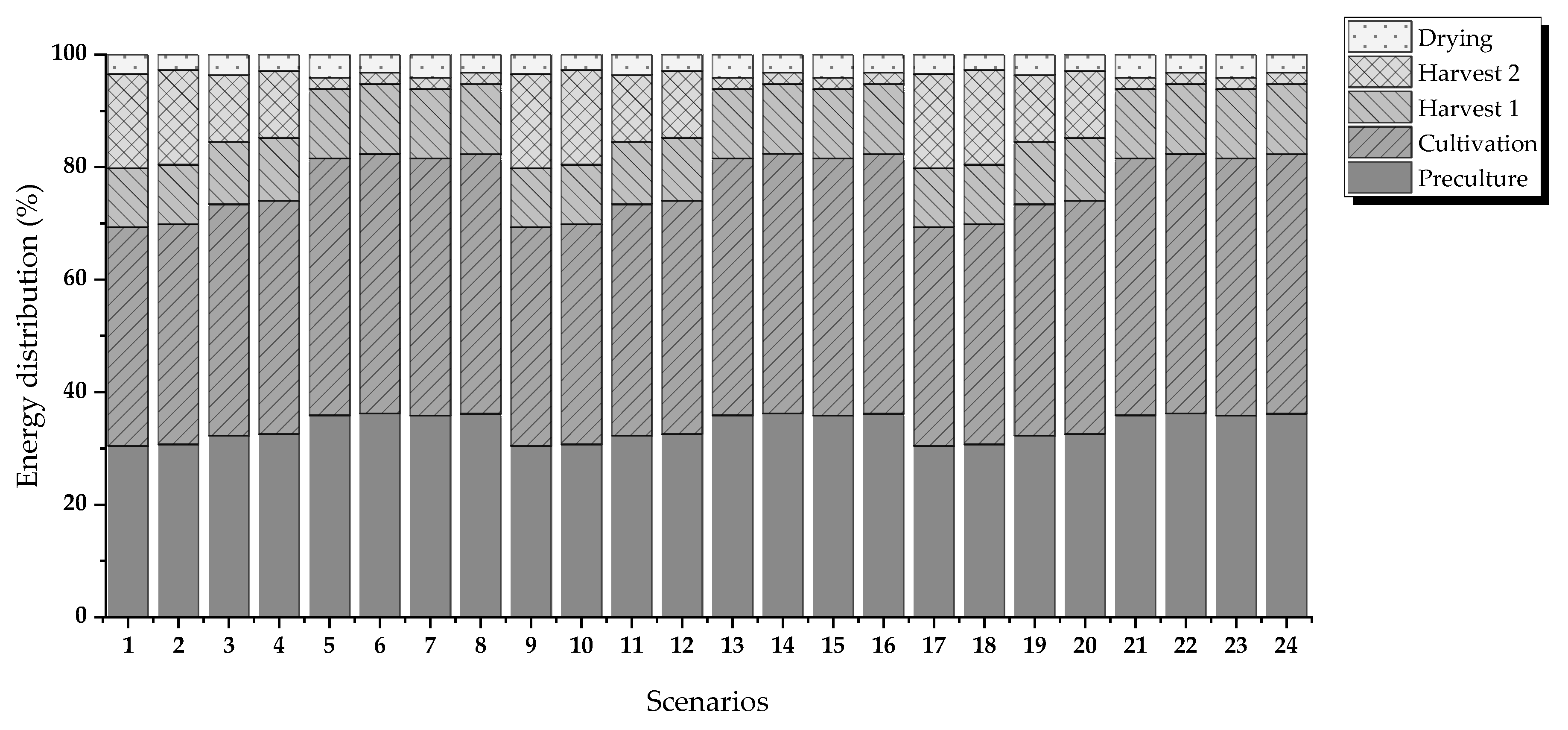
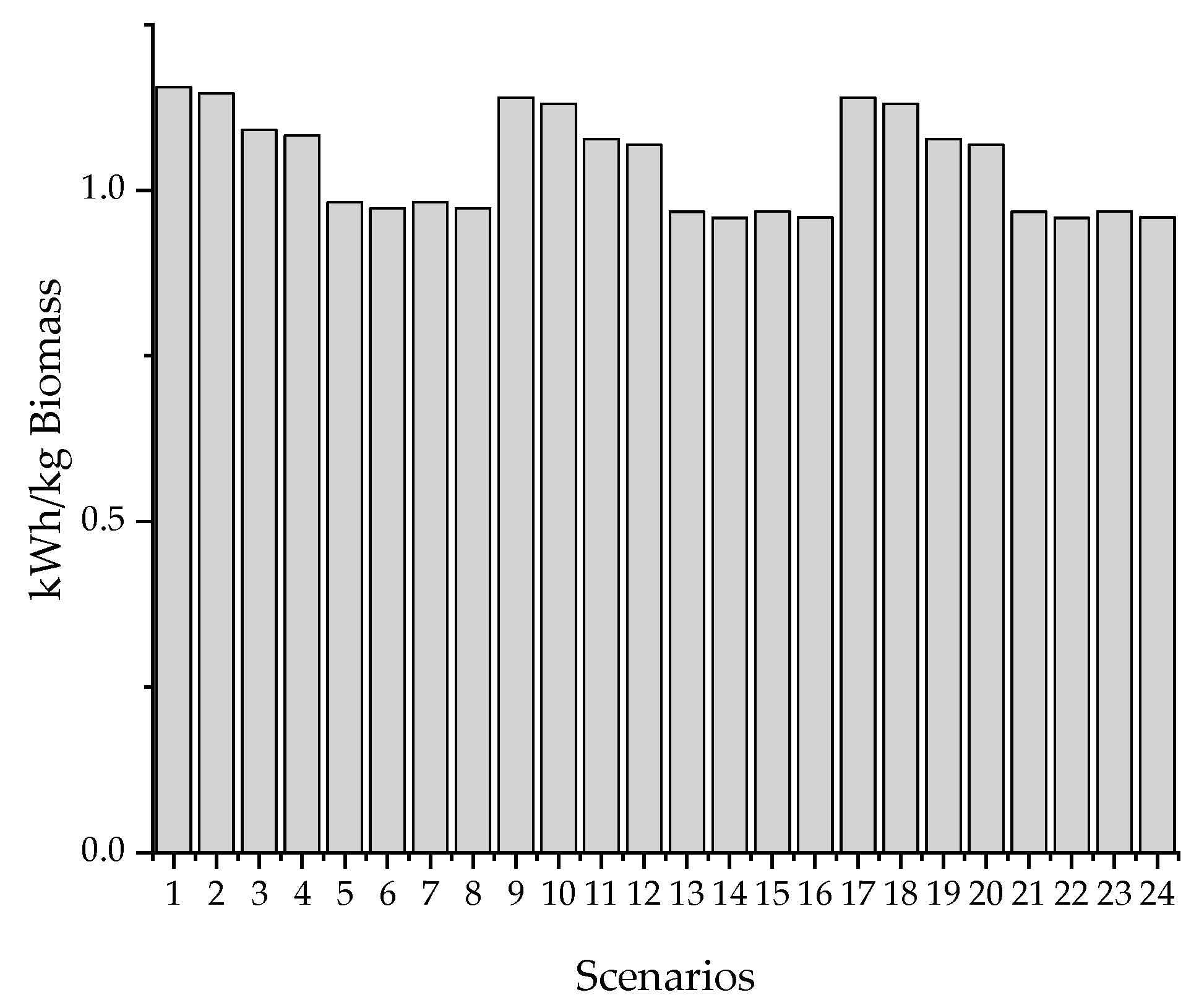
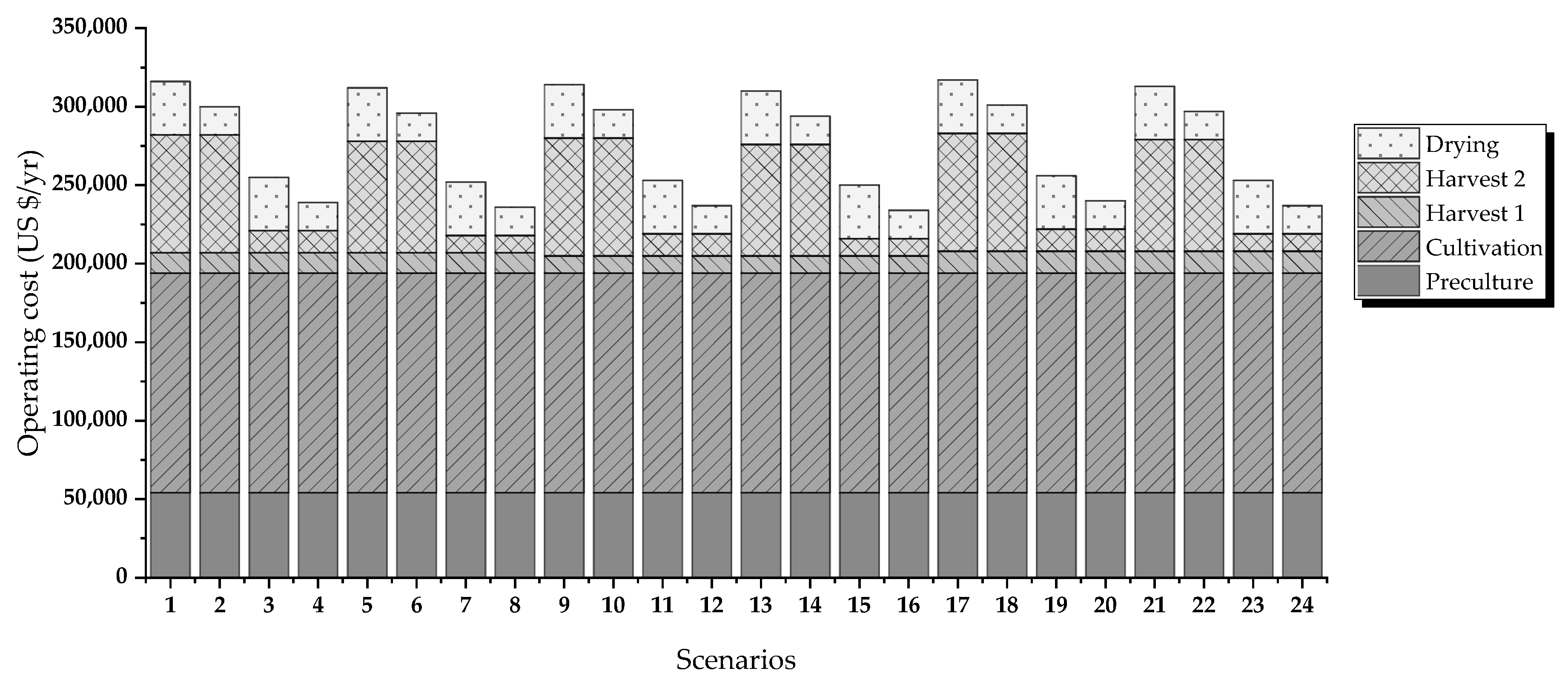
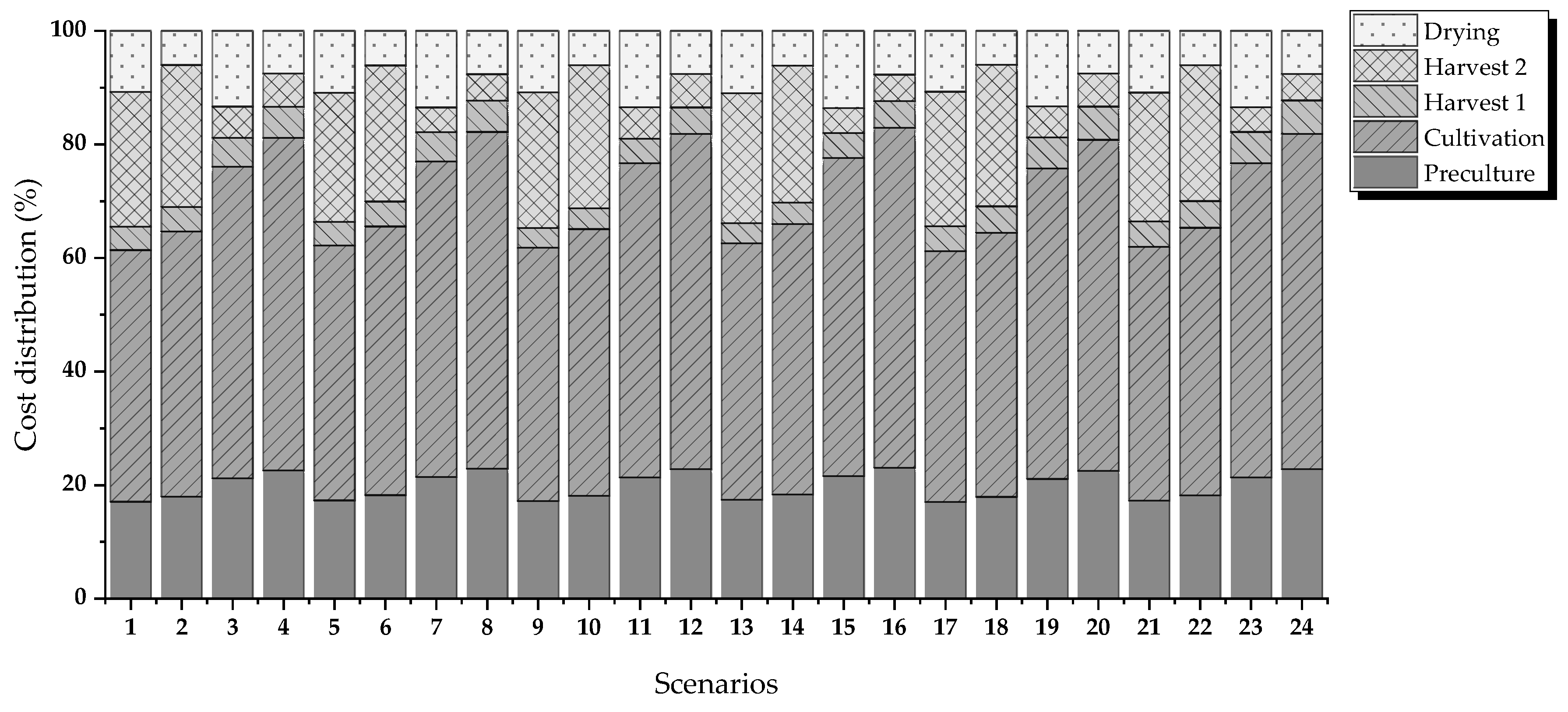
Figure 2 shows the evaluated routes. The criteria for evaluation are energy requirements and operating costs.