1. Introduction
To satisfy either the strengthened fuel economy or carbon dioxide emission standards, many studies have been conducted to improve the internal combustion (IC) engine efficiency [
1,
2,
3]. At the same time, studies are being conducted to analyze the inefficient factors in an IC engine. The analysis method of an IC engine can be divided into an energy analysis and an exergy analysis. The energy analysis is a method of analyzing how the lower heating value of the fuel is converted into work, heat, and exhaust energy [
4,
5]. However, the energy analysis has a drawback in that the quality difference between work and heat cannot be considered. To overcome these drawbacks, analysis using the exergy concept is being performed. Exergy is the maximum work that can be achieved by an interaction between the system and the environment [
6]. Thus, the exergy can represent the quality difference between the work and heat. For example, even if the same amount of heat is transferred into the system, the increment of exergy is affected by the temperature of the system [
7]. Therefore, some studies simultaneously perform the exergy analysis to discuss the available work in the IC engine operation, in addition to the energy analysis [
7,
8,
9,
10].
Exergy can be destroyed through an irreversible process, which means the available work of the system can decrease. There are several intrinsic irreversible processes in a spark-ignition (SI) engine, such as fuel and air mixing, combustion [
6,
11,
12], and heat transfer [
13]. Many previous studies have shown that the exergy destruction of IC engines occurs mainly during the combustion process. Approximately 20% of the chemical exergy of fuel is destroyed during the combustion process [
7,
11,
12], and studies have been conducted to suggest strategies to reduce the exergy destruction during the combustion process since this exergy destruction leads to a reduction in the system’s available work [
14,
15]. These previous studies suggest increasing the temperature during the combustion process in order to reduce the exergy destruction by combustion. Increasing the temperature at which combustion occurs reduces the exergy destruction by the intermolecular heat transfer occurring at finite temperature differences during the combustion process [
16]. However, strategies to reduce the exergy destruction do not necessarily lead to an increase in the IC engine efficiency [
10,
17,
18]. This is because exergy represents the maximum work that can be obtained by a theoretical interaction between the system and environment; it does not consider the specific work extraction methods. In a real system, it is not always possible to turn a thermodynamic state of the system into an environment state through a reversible process.
In an IC engine, the sensible energy of the in-cylinder mixture is increased by the combustion process and then converted to the work through the expansion process. In this system, the maximum work that can be obtained is the pressure-volume work through the adiabatic expansion process [
19,
20]. Since the process to obtain the maximum work of the system is limited, the thermodynamic state of the mixture cannot be converted to the environmental thermodynamic state in a given architecture. This leads to the important points that it is not possible to convert all the thermomechanical exergy to a pressure-volume work and, therefore, there is no one-to-one relationship between the exergy destruction and the efficiency in an IC engine operation. As a result, a strategy for reducing the exergy destruction during the combustion process presented in previous studies may not necessarily lead to an efficiency increase. In addition, these studies did not explain how reducing the exergy destruction during the combustion process affects the efficiency.
This study aims to analyze the factors that reduce the exergy destruction and the factors that can increase the pressure-volume work in the simplified IC engine system. Then, the relationship between exergy destruction and pressure-volume work will be discussed in various initial thermodynamic state and compression ratio conditions. Especially, the analysis regarding asymmetric compression and expansion ratio is related to the Miller cycle or Atkinson cycle, which is applied in a modern IC engine, and will provide an explanation on its efficiency benefit and exergy destruction characteristic, as compared to those of the Otto cycle at the same compression ratio.
The main body of the paper is written in the following order. In
Section 2, we introduce a simplified system to model and analyze the IC engine. Subsequently, in
Section 3, the exergy destruction and pressure-volume work of the system are expressed analytically. Then, in
Section 4, the effect of the initial thermodynamic states and compression ratio on exergy destruction and pressure-volume work is derived of an analytic form. Finally, in
Section 5, the change in the exergy destruction and pressure-volume work according to the various work input method to the initial mixture are calculated through the simulation using a specific fuel.
4. Exergy Destruction and System’s Net Work Sensitivity
In
Section 3, the exergy destruction by combustion and the system’s net work are analytically expressed. The exergy destruction and system’s net work derived in
Section 3 are affected by the type of fuel, the constant pressure specific heat capacity of the mixture, and the temperature and pressure before and after combustion. This study aims to discuss the exergy destruction and pressure-volume work that typical IC engine operation. The results in
Section 3 show that the temperature and pressure at before combustion timing affect exergy destruction and pressure-volume work for certain fuels. These thermodynamic conditions depend on the temperature and pressure of state 1 and the compression ratio. Therefore, in this section, we analyzed the effect of state 1 temperature, pressure, and compression ratio on exergy destruction and system’s net work in an analytic method.
4.1. Initial Temperature
In this section, the change in the exergy destruction and the system’s net work caused by the combustion process when the temperature of the initial state changes is examined. The detailed derivation is described in
Appendix C. The effects of the initial temperature on the exergy destruction and system’s net work is shown in Equations (21) and (22), respectively.
We have seen how the exergy destruction changes as the initial temperature changes. If the number of moles does not change before and after the combustion, Equation (21) can be written as follows:
In a typical IC engine, the temperature increases during the combustion process, so increasing the initial temperature reduces the exergy destruction. If the number of moles before and after the reaction changes, the exergy destruction decreases as the initial temperature increases in the reaction, which satisfies the Inequality (24).
Therefore, in reactions with a reduced number of moles, such as a hydrogen-air mixture, exergy destruction always decreases with increasing initial temperature. Even if the number of moles increases, the exergy destruction still decreases with increasing initial temperature if the condition in the Inequality (24) is satisfied.
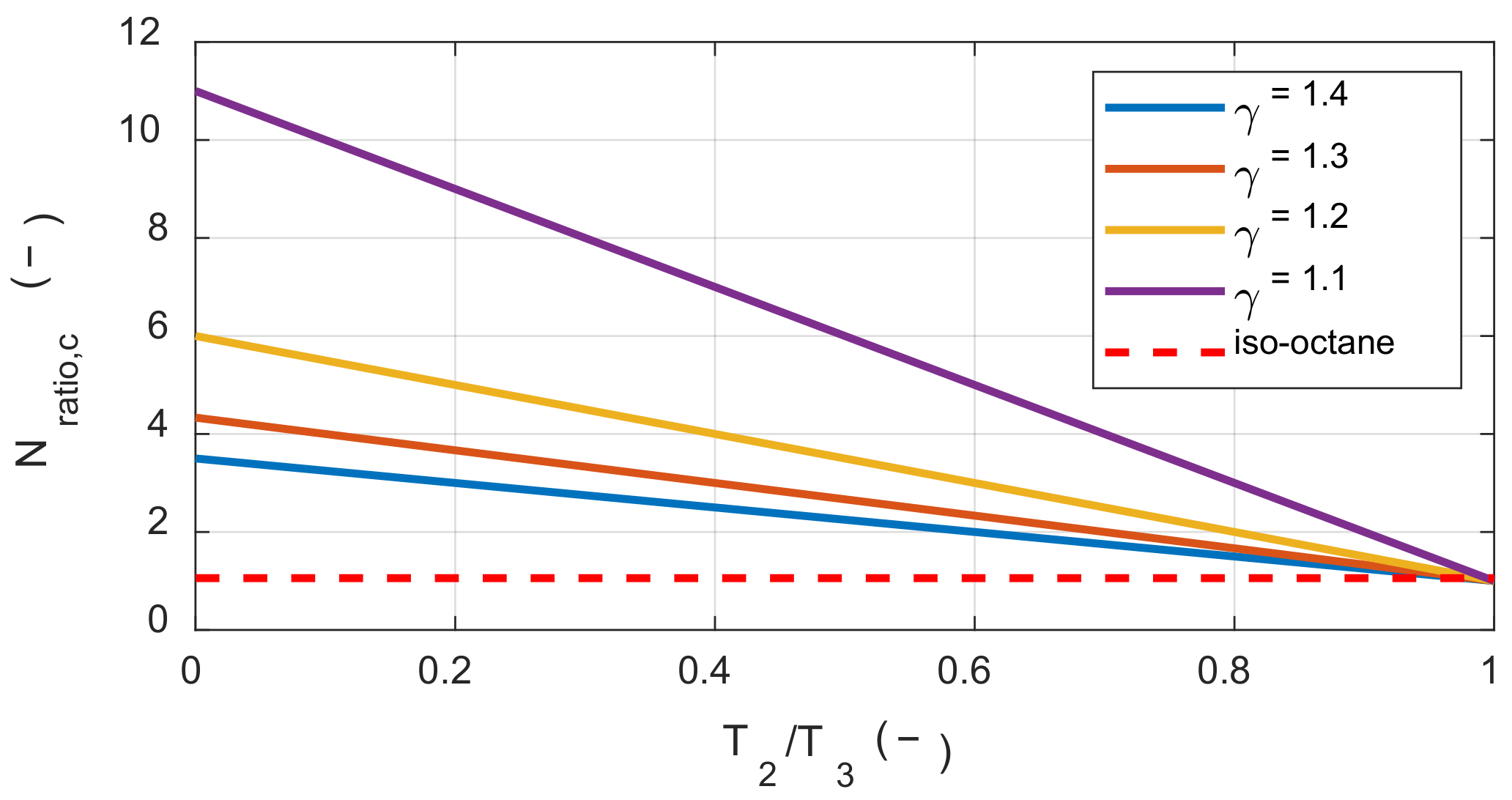
Figure 3 shows the above conditions according to
γr(T1) and the temperature ratio before and after combustion. Here, if
Nratio is greater than
Nratio,c then initial temperature increases could rather lead to an increase in exergy destruction during the combustion process.
The stoichiometric mixture of iso-octane and air had a molar ratio of approximately 1.05 before and after the reaction. Therefore, when the temperature ratio before and after the reaction was approximately 0.98 or greater, the effect of the initial temperature on the exergy destruction was changed. However, it was not possible to achieve this temperature ratio by combustion of the stoichiometric mixture under typical IC engine operating conditions. This explains why the exergy destruction decreased under typical IC engine operating conditions with increased initial temperature.
Next, the effect of the initial temperature on the system’s net work was investigated. Equation (22) is summarized as shown in Inequality (25). The detailed procedure is shown in
Appendix C.
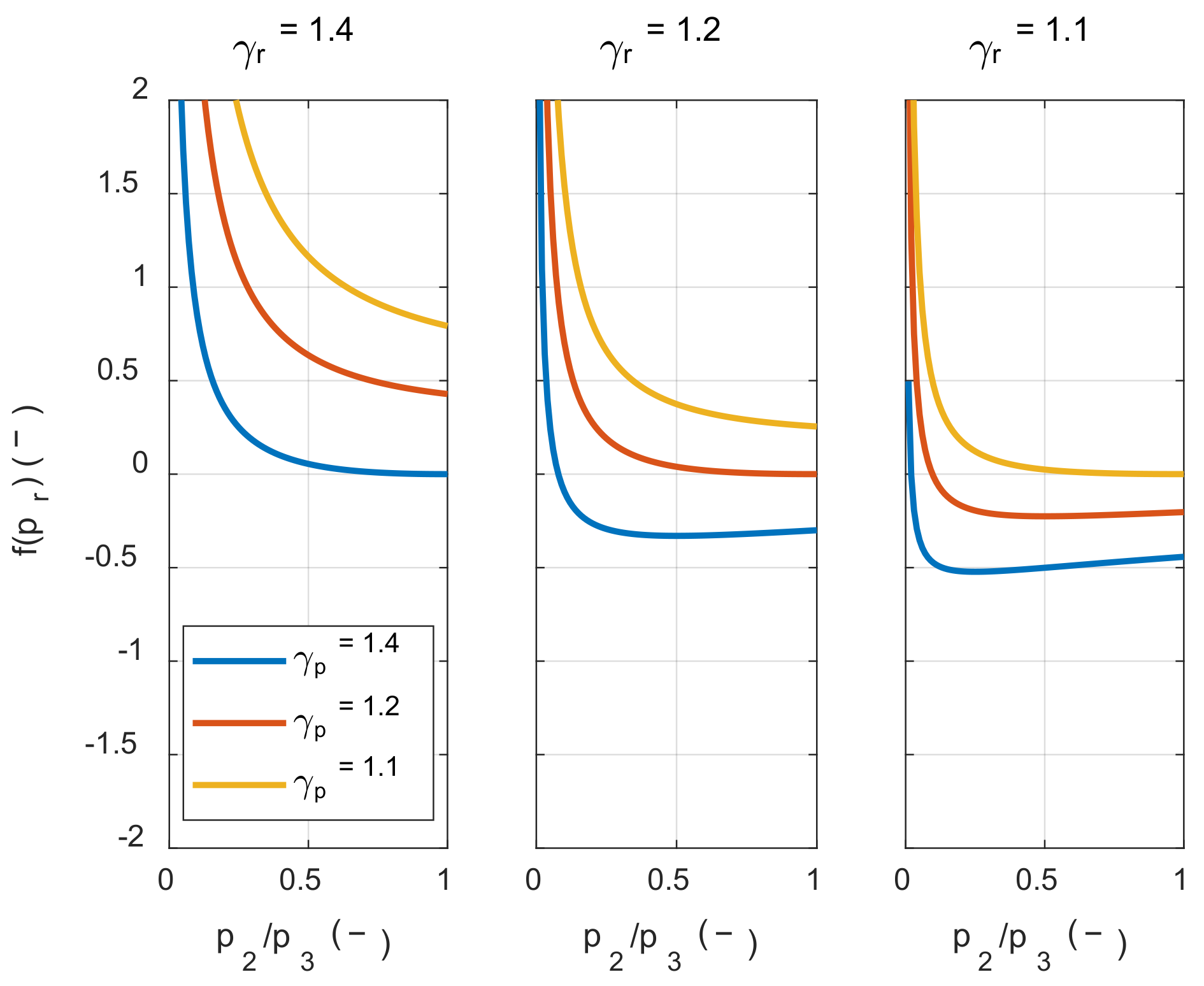
If the value inside the square bracket on the right side of Inequality (25) was positive, the system’s net work decreased as the initial temperature increased. Therefore, in order to examine the condition in which the value of
f(
pr) is positive, the value of
f(
pr) was calculated along with the pressure ratio, as shown in
Figure 4.
As shown in
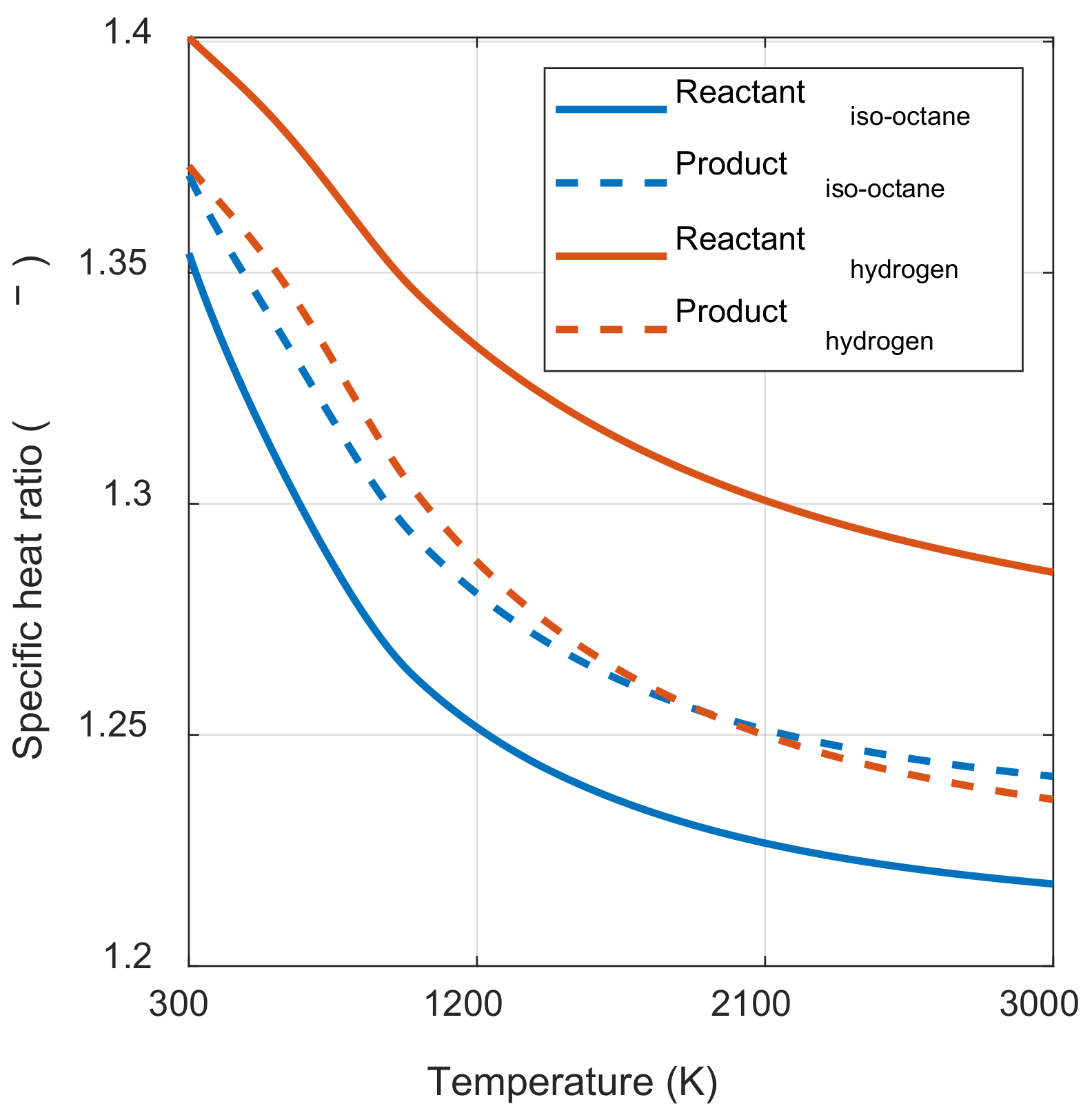
Figure 4, the value inside the square bracket was positive when the specific heat ratio of the product in state 3 was smaller than the reactant in state 1 m, regardless of the pressure ratio before and after combustion. In hydrocarbon fuels, which are mainly used in the IC engine, the specific heat ratio of the product was typically greater than that of the reactants at the same temperature. However, as the temperature increased, the specific heat ratio decreased by activating the vibrational energy mode of each molecule. Therefore, as shown in
Figure 5,
γp(
T3) was smaller than
γr(
T1m) when comparing the specific heat ratio of the product at the temperature after combustion and the specific heat ratio of the reactant at the temperature before combustion. As a result, the system’s net work decreased as the initial temperature increased.
4.2. Initial Pressure
By the ideal gas assumption, the specific heat capacity of the gas is not affected by the pressure. This assumption was used for calculating how the exergy destruction and system’s net work are affected by the initial pressure. Detailed procedures are described in
Appendix D.
If the number of moles before and after the combustion does not change, then the initial pressure does not affect the exergy destruction. If the number of moles before and after combustion is different, the exergy destruction is affected by the initial pressure and the ratio of the mole numbers between the product and reactant. This can be understood through Equation (A51). When the initial pressure is increased, the difference between the entropy change of reactant before combustion and the entropy change of product after combustion affects the entropy generation. When the initial pressure is increased, the decrease of entropy per reactant one mole and the decrease of entropy per product one mole are the same as shown in
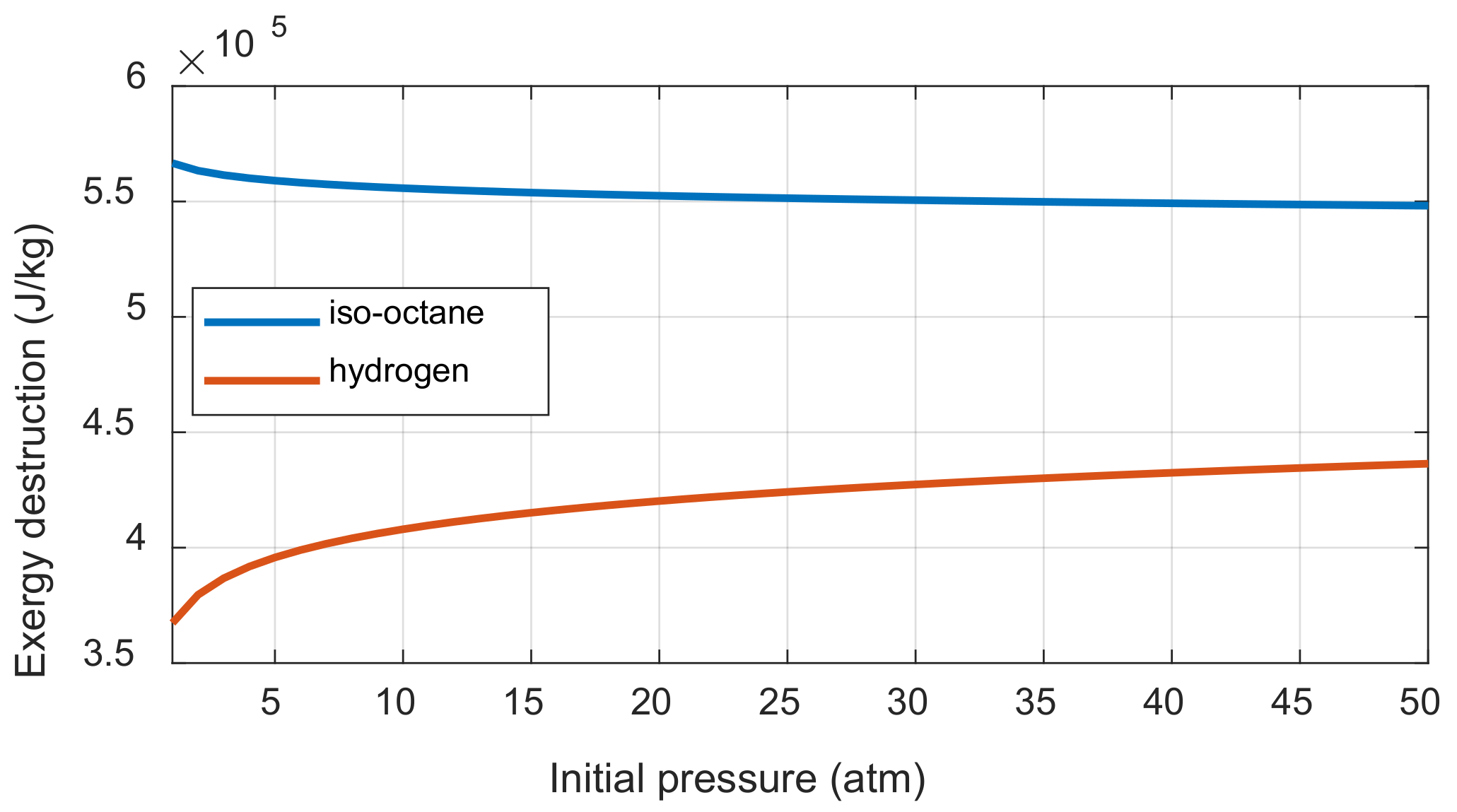
Appendix D. However, the entropy generation is affected by the initial pressure because the mole of product varies depending on the fuel when the one mole of reactant is burned. The exergy destruction was calculated when the initial pressures of iso-octane/air and hydrogen/air mixtures were increased under the complete combustion assumption, as shown in
Figure 6. The iso-octane-air mixture with increasing molar numbers after combustion decreases the exergy destruction as the initial pressure increased, while the hydrogen-air mixture shows the opposite trend.
Next, we examined the effect of the initial pressure on system’s net work. Equation (27) can be rewritten as shown in Inequality (28), with the detailed procedure described in
Appendix D. In Inequality (28), state 3s means that the gas in state 3 expands until it has the same pressure as that in state 2.
In a typical system, the pressure increases during the combustion process, so the system must expand to have the same pressure at the before combustion pressure. It means the volume at state 3s is larger than volume at state 3. Thus, the right side of Inequality (28) is greater than zero. Therefore, in a constant internal energy and volume combustion system, the system’s net work increases as the initial pressure increases. Thus, the pressure-volume work that can be additionally obtained as the constant internal energy and volume combustion system increases through the combustion process.
4.3. Compression Ratio
In the previous two cases, the effects of the mixture’s initial thermodynamic state on the exergy destruction and the system’s net work under a given geometrics condition were examined. In this section, we examined how the exergy destruction and system’s net work caused by combustion change with the compression ratio when the initial mixture state is fixed. In the same thermodynamic state of the initial mixture, the effects of the elevated temperature and pressure before combustion due to the additional work during the compression process on the exergy destruction and the system’s net work were examined. The detailed procedure was included in
Appendix E.
Since the pressure at the end of combustion is higher than that at the start of combustion when a typical hydrocarbon is burned under constant internal energy and volume combustion conditions, the exergy destruction decreases as the compression ratio increases.
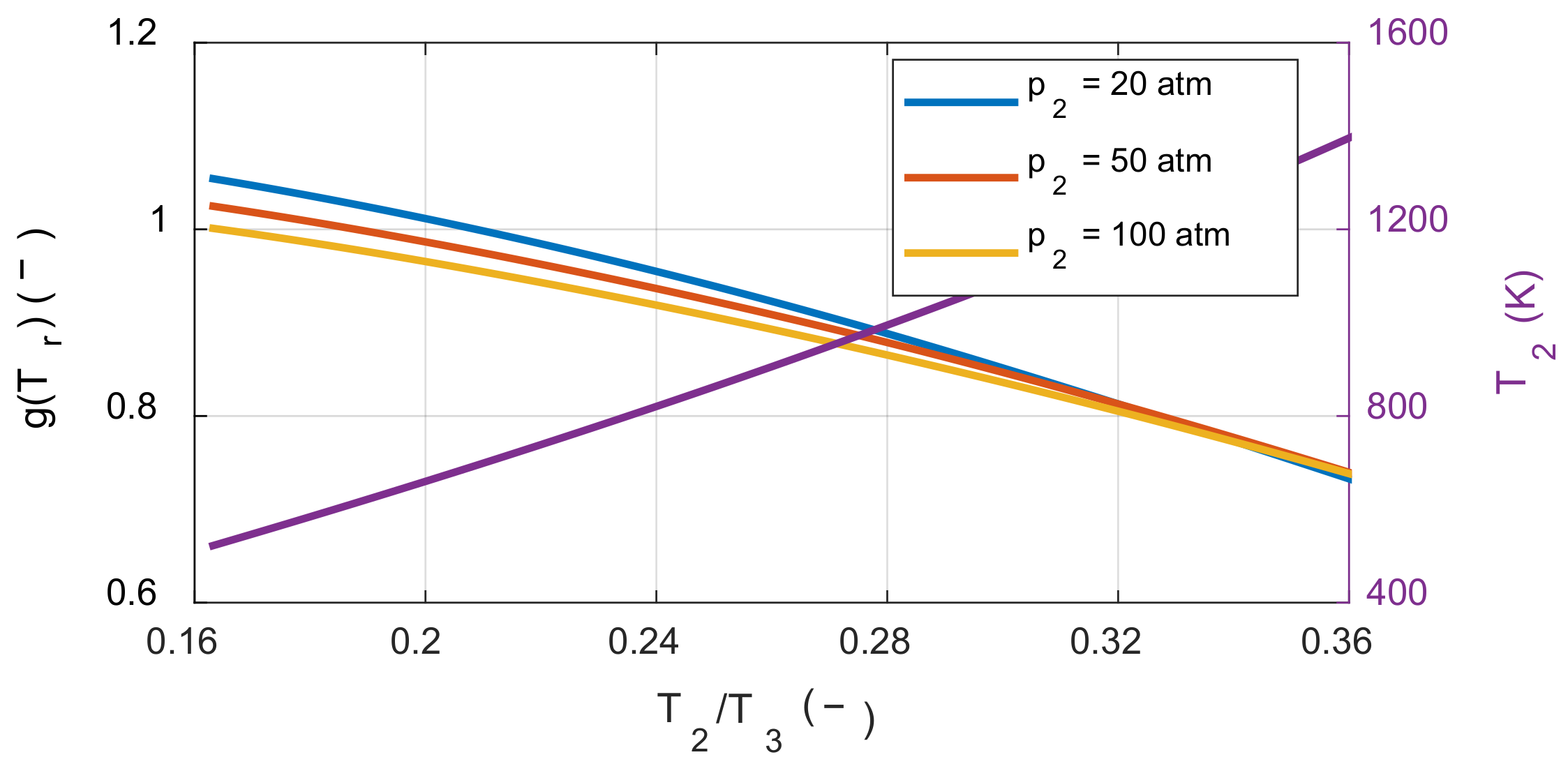
The effect of the compression ratio on the system’s net work depends on the molar ratio before and after combustion. If the moles of the product are greater than or equal to the moles of the reactant, the system’s net work always increases as the compression ratio increases, which is the case with typical hydrocarbon fuels. However, if the moles of the product are less than the moles of the reactant, the tendency now depends on the temperature ratio before and after combustion. To understand this latter effect, the sensitivity of the compression ratio in the hydrogen-air mixture was also examined. On the right side of Equation (30), we investigated how the value of
g(Tr) was affected by the temperature before combustion as shown in
Figure 7.
When the values of g(Tr) were calculated by varying the temperature and pressure before combustion, the values in the square bracket were greater than zero under all the calculated conditions. Therefore, even when using fuels for which molar number decreased after the combustion, such as hydrogen, the system’s net work increased as the compression ratio increased under typical IC engine operating conditions. As a result, under typical IC engine operating conditions, increasing the compression ratio reduced the exergy destruction during the combustion process as well as increased the system’s net work.
5. System Simulation
In
Section 4, we analytically investigated the effect of the initial thermodynamic state and compression ratio on the exergy destruction and the system’s net work. In this section, we examined how the exergy destruction and the system’s net work changed when the thermodynamic state of the initial mixture changes, using the fuel-air mixture typically used for an IC engine modeling. When changing the thermodynamic state of the initial mixture with isobaric heating, isothermal compression, and isentropic compression, the exergy destruction, and the system’s net work were examined along with the work input to change the thermodynamic state. It was assumed that the initial mixture is obtained by supplying the energy to the mixture at a thermomechanical dead state. The minimum work required to change the thermodynamics state of the initial mixture in three ways is the same as the thermomechanical exergy of state 1. The thermodynamic state and species composition of the environment required to calculate the exergy are shown in
Table 1. The fuel used in this simulation was iso-octane, and the properties were taken from the information provided by Lawrence Livermore National Laboratory [
31].
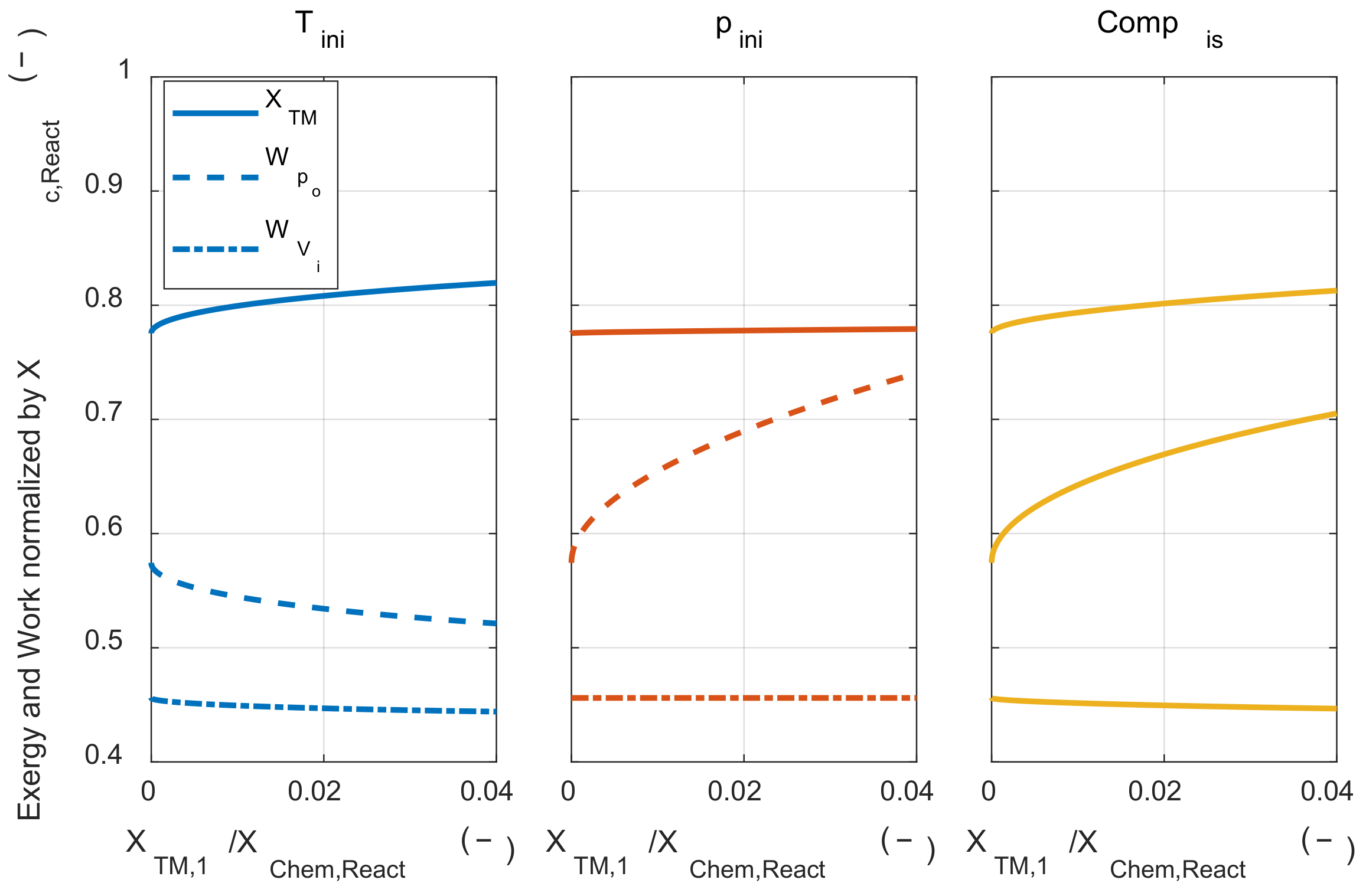
Figure 8 shows how the pressure and temperature of the initial mixture change with respect to the work input. Each marker indicated the work input at intervals of 0.01 for the chemical exergy of the initial mixture.
Figure 8 shows how the thermodynamic state of the initial mixture changes through each process when the same work input is applied. If there is also another process that changes the thermodynamic state of the initial mixture, it can be understood as the sum of the initial pressure change effect and the initial temperature change effect.
First, the effect of the initial mixture thermodynamic state change process on the exergy destruction during the combustion process was investigated.
Figure 9 shows how the exergy destruction via combustion changes for the minimum energy required us to change the initial mixture’s thermodynamic state. When the thermodynamic state of the initial mixture was altered in three ways, the isobaric heating process was the most effective way to reduce exergy destruction for the same work input. The next effective method was the adiabatic compression process and changing the state of the initial mixture with isothermal compression has little effect on the exergy destruction. Increasing the compression ratio reduced the exergy destruction during the combustion process for all initial mixture preparation methods. These results show that the temperature before combustion had a major effect on exergy destruction, as discussed in an analytical form in
Section 4. Therefore, the exergy destruction during the combustion process was greatly reduced in the case of isobaric heating, adiabatic compression, and high compression ratio, which could increase the temperature before combustion.
As described in many previous studies, this result shows that strategies to increase the reactant temperature would be effective in reducing the exergy destruction during the combustion process. When the exergy destruction was reduced, more of the chemical exergy of the fuel was converted to the thermomechanical exergy of the system. In the following paragraphs, we investigated whether this increased work potential could be cascaded down to the increased system’s net work in the engine architecture. The pressure-volume work obtained when the system expanded to the initial volume like Acknowledgment IC engine, which is presented as a reference. In
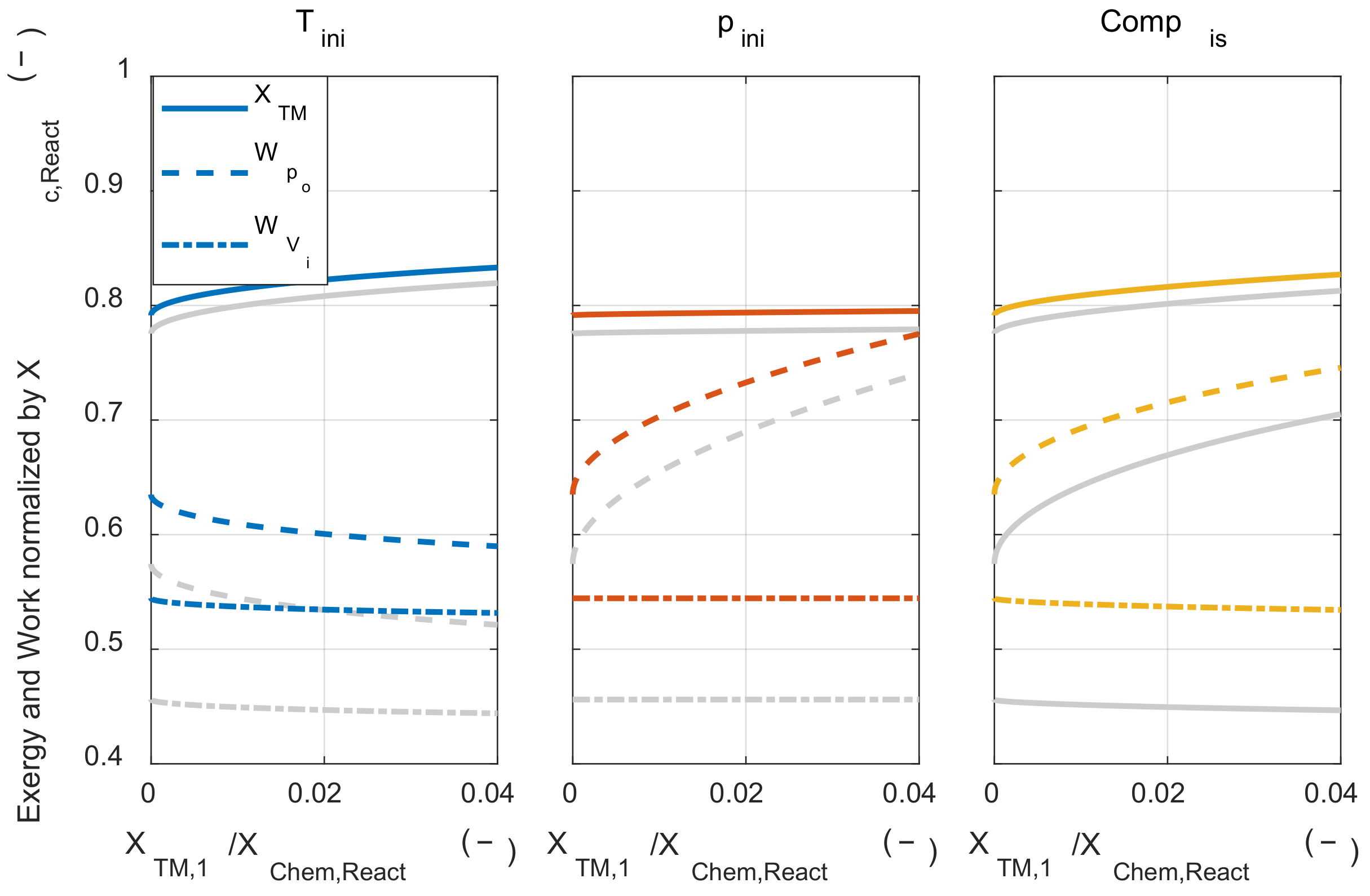
Figure 10, the solid line (―) shows that thermomechanical exergy was converted from chemical exergy by combustion, the dashed line (- -) shows the system’s net work, and alternate long and short dashed line (– -) shows that work was obtained when the system expanded to the initial volume along with the work input of each process.
As the initial temperature increases, the converted thermomechanical exergy increased due to the lower exergy destruction during the combustion process, but the system’s net work was decreased, and the work that could be obtained when expanded to the initial volume was hardly affected by the temperature change. This tendency can be explained using Equation (A40). In Equation (A40), when the change in
T3m is larger than the change in
T1m with respect to the change in initial temperature, the system’s net work was reduced as increasing the initial temperature. In
Section 4, the change in
T3m and
T1m was analytically obtained. The effect of the state 1 temperature change on state 3 m temperature could be qualitatively explained by the sum of the two effects. The first was that the state 3 temperature rose due to the state 1 temperature increase. The other was decreasing the expansion ratio when expanding to the environment pressure because the pressure decreased after the combustion. This is because the temperature ratio between the before combustion and the after combustion decreased as the temperature before combustion increases. Due to these two effects, the state 3 m temperature increase was greater than the state 1 m temperature increase when the state 1 temperature rose. Therefore, the system’s net work was decreased as increasing the initial temperature. That is, even if the chemical exergy is more converted to thermomechanical exergy by increasing the initial temperature, it does not lead to the increase of work that can be obtained in a system that obtains pressure-volume work.
Next, the effect of isothermal compression on the system’s net work was investigated. Higher initial pressure did not affect the conversion ratio from the chemical exergy to the thermomechanical exergy. However, the system’ net work increased as the initial pressure increased. As the initial pressure increased, the work that could be obtained by expansion after combustion increased, but the work that could be obtained by expansion of the initial mixture also increased. As shown in
Appendix D, the increase in the system’s net work was proportional to the temperature difference between the state 3 m and state 1 m. As mentioned in
Section 4.2, the system’s net work increased as the initial pressure increased in a typical IC engine because the temperature of state 3 m was higher than the temperature of state 1 m.
Finally, the effect of isentropic compression on the system’s net work was investigated. Isentropic compression could be understood as the sum of the effects of initial temperature change and initial pressure change. The change in the thermodynamic state of the initial mixture with isentropic compression is shown in
Figure 8. The system’s net work increased because the pressure increase effect was greater than the temperature increase effect. Although the increase in the system’s net work was lower than isothermal compression, both reduced exergy destruction and increased the system’s net work could be achieved.
Next, the effect of the initial mixture thermodynamic state on the work that could be obtained when the system expands to the initial volume like a typical IC engine was investigated. All three methods of changing the initial mixture thermodynamic state did not significantly affect system work. Under the device expanding to the initial volume, the thermodynamic state of the initial mixture did not affect the pressure-volume work. It means that the work input was discharged from the system in the form of exhaust exergy.
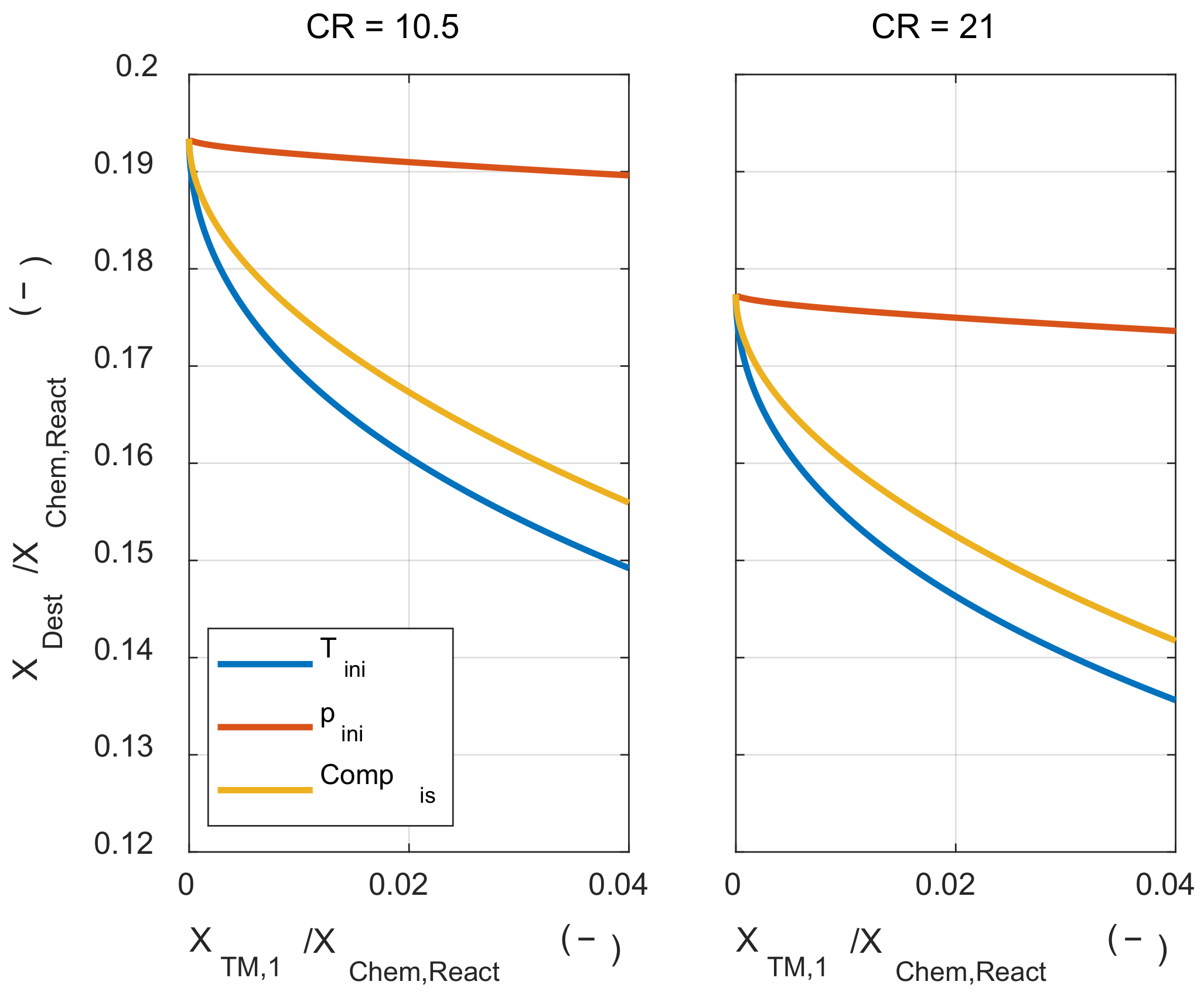
With the change in the thermodynamic state of the initial mixture, how the compression ratio affects the pressure-volume work was investigated. In
Figure 11, the exergy conversion ratio and the pressure-volume work was plotted when the compression ratio was increased from 10.5 to 21. In
Figure 11, the gray-colored line was the result when the compression ratio was 10.5. When the compression ratio was increased, the tendency of the thermodynamic state of the initial mixture to pressure-volume work was not changed. However, when the compression ratio was increased, both the exergy conversion ratio and the pressure-volume work increased because the temperature and pressure before combustion timing were increased as the compression ratio was increased. Moreover, the pressure-volume work obtained when the system expanded to the initial volume was also increased. This pressure-volume work increase can be understood as the sum of two effects. The first is the increase of the pressure-volume work that can be obtained through the expansion due to increased pressure at before combustion timing due to the high compression ratio. The other is the increase of expansion ratio from the combustion timing to the end of expansion timing.
6. Conclusions
This study aimed to improve the basic understanding of the relations between the exergy destruction during the combustion process and the efficiency in a system with a limited work extraction method, such as an IC engine. The IC engine was simplified with the adiabatic process assumption, the constant internal energy and volume combustion assumption, and the uniform distribution of thermodynamic state assumption. Under the simplified system, the maximum work that could be obtained through the expansion and the exergy destruction during the combustion could be analytically expressed using the thermodynamic states, the heat of reaction, and the entropy change in reaction.
These analysis results indicated that the factors affecting the exergy destruction and the pressure-volume work were the thermodynamic state at the before combustion timing and the fuel. Therefore, for a specific fuel, the exergy destruction during the combustion process and the pressure-volume work could be calculated using the thermodynamic state at the before combustion timing, such as the temperature and the pressure. A sensitivity analysis was performed to understand how the thermodynamic state at the before combustion timing affects the exergy destruction and the pressure-volume work. As a result, increasing the temperature of the initial mixture or the compression ratio, which could increase the temperature at the before combustion timing, could reduce the exergy destruction during the combustion process. However, these strategies that increase the temperature before combustion timing reduced the pressure-volume work of system because the pressure-volume work was affected by the pressure before combustion timing and the pressure increase through the combustion process. Increasing the pressure of the initial mixture or the compression ratio, which increases the pressure at the before combustion timing, could increase the pressure-volume work. However, when the temperature of the initial mixture increased, it was confirmed that the pressure-volume work decreased due to the decrease in the pressure rise by combustion.
Then, the simulation results showed how the exergy destruction and the efficiency change with respect to the energy input required to change the thermodynamic states of the initial mixture under the iso-octane combustion situation. For the same work input, the exergy destruction decreased when the temperature at before combustion timing increased, and the pressure-volume work increased when the pressure at before combustion timing increased. However, the strategy that minimizes the exergy destruction reduced the pressure-volume work, and the strategy that maximizes the pressure-volume work hardly affected the exergy destruction. Moreover, even when the adiabatic assumption and complete combustion assumption were removed, those tendencies were maintained as shown in
Appendix A.
A system, such as an IC engine, cannot convert all of the gas’s thermomechanical exergy into the pressure-volume work. In a system with a limited work extraction method, in order to increase efficiency, the converted exergy must be in a suitable form for the system’s work extraction method. The additional thermomechanical exergy that could be obtained by reducing the exergy destruction in an IC engine does not lead to efficiency improvement. Therefore, the strategies for reducing the exergy destruction discussed in many previous studies did not lead to efficiency improvement. Consequently, it is more important to discuss the strategies for increasing the efficiency, rather than minimizing the exergy destruction, in a system with limited work extraction methods.