Numerical Predictions of a Swirl Combustor Using Complex Chemistry Fueled with Ammonia/Hydrogen Blends
Abstract
1. Introduction
2. Materials and Methods
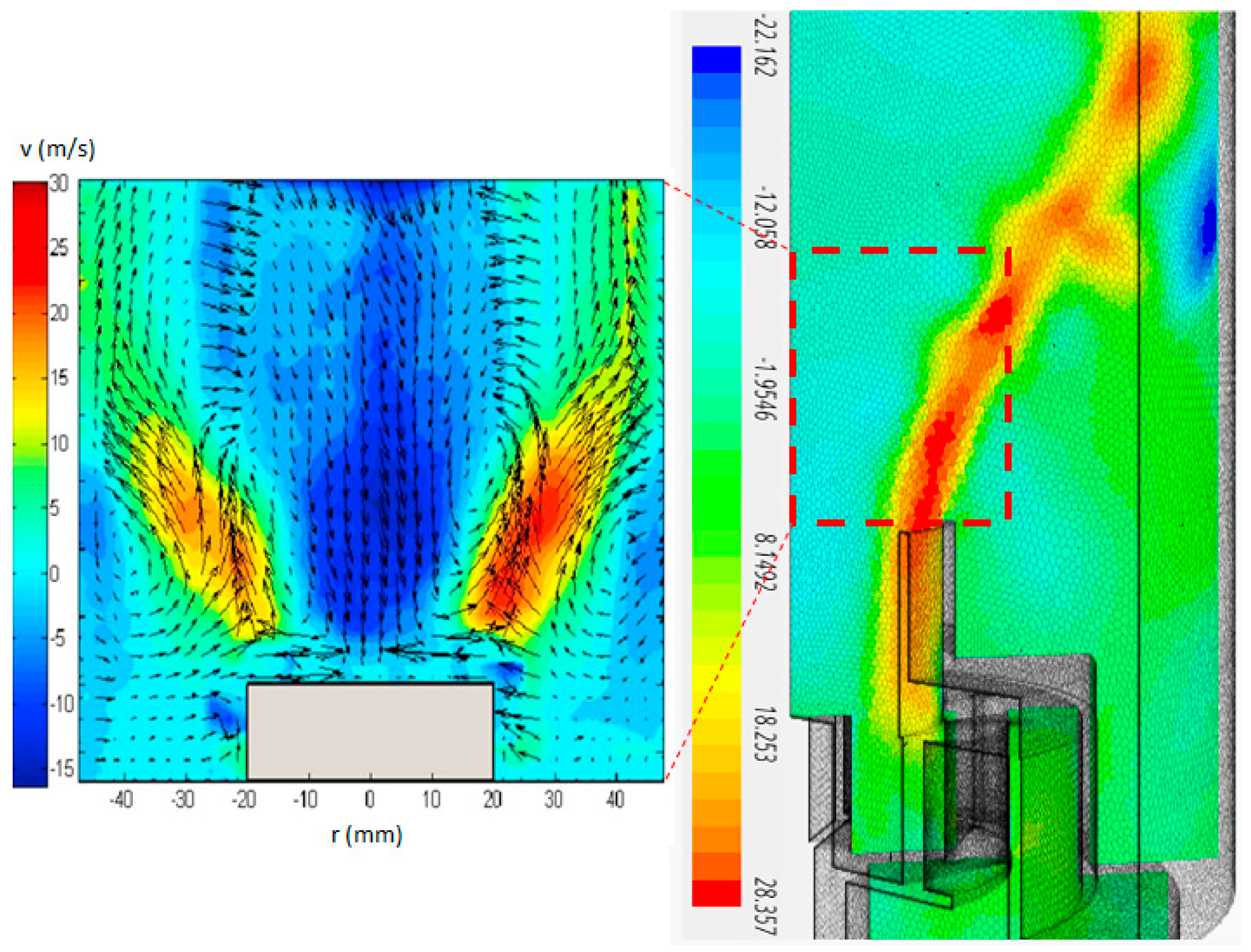
3. Results
4. Conclusions
- The reaction model has a good prediction for the consumption/production of ammonia under atmospheric conditions. However, hydrogen reactivity is still not fully solved, with nitrogen oxides also being over-estimated.
- Nitrogen oxides remain at similar levels in most calculations, a consequence of a combined effect between the higher reactivity of the reactants at higher confinement temperatures and the increased reactivity of NO with unburned ammonia along the wall.
- Higher temperatures across the post-flame region (e.g., reduced heat losses through the boundary condition) show an increase in reactivity of ammonia for the formation of hydrogen. Ammonia, whose reactivity remains relatively the same at different conditions across the flame, also produces nitrogen oxides (probably as a result of the production of NHO and NH).
- On the other hand, hydrogen consumption through the flame is dependent on confinement temperatures, with an increase of the molecule caused by the decomposition of ammonia downstream the flame.
- Larger quantities of water and nitrogen are produced at higher temperatures through the complete reaction of intermediate molecules. Hydrogen and ammonia remain at the same concentrations at the exhaust. Therefore, the energy provided by the change in confinement temperature will have a direct impact on hydrogen through the flame and radical formation post-combustion.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Birol, F. Hydrogen: Accelerating & Expanding Deployment. Available online: https://www.nedo.go.jp/content/100885441.pdf (accessed on 10 September 2019).
- YARA. YARA Fertilizer Industry Handbook; Yara: Oslo, Norway, 2018. [Google Scholar]
- Porter, D.H. The Life and Times of Sir Goldsworthy Gurney: Gentleman Scientist and Inventor, 1793-1875; Lehigh University Press: Bethlehem, PA, USA, 1998. [Google Scholar]
- Krock, E. Ammonia—A Fuel for Motor Busses. J. Inst. Pet. 1945, 31, 213–223. [Google Scholar]
- Verkamp, F.J.; Hardin, M.C.; Williams, J.R. Ammonia Combustion Properties and Performance in Gas-Turbine Burners. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1967; Volume 11, pp. 985–992. [Google Scholar]
- Pratt, D.T. Performance of Ammonia-Fired Gas-Turbine Combustors; DTIC Document, University of California: Los Angeles, CA, USA, 1967. [Google Scholar]
- HI Solar. Development of an Ammonia-Burning Gas Turbine Engine. 1966. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/6716 (accessed on 19 October 2019).
- Kailos, N.C. Utilization of Ammonia as an Alternative Fuel in Army Aircraft Engines: USAAVLABS Technical Report 66-52; U.S. Army, USA, 1966. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/638360.pdf.chemical (accessed on 5 January 2020).
- Fenimore, C.P.; Jones, G.W. Oxidation of Ammonia in Flames. J. Phys. Chem. 1961, 65, 298–303. [Google Scholar] [CrossRef]
- Maclean, D.I.; Wagner, H.G. The Structure of the Reaction Zones of Ammonia-Oxygen and Hydrazine-Decomposition Flames. Symp. Combust. 1967, 11, 871–878. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowman, C.T. Mechanism and Modeling of Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Lindstedt, R.; Lockwood, F.C.; Selim, M.A. Detailed Kinetic Modelling of Chemistry and Temperature Effects on Ammonia Oxidation. Combust. Sci. Technol. 1994, 99, 253–276. [Google Scholar] [CrossRef]
- Skreiberg, Ø.; Kilpinen, P.; Glarborg, P. Ammonia Chemistry below 1400 K under Fuel-Rich Conditions in a Flow Reactor. Combust. Flame 2004, 136, 501–518. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Jeanmart, H.; Vandooren, J. Ammonia Combustion at Elevated Pressure and Temperature Conditions. Fuel 2010, 89, 3540–3545. [Google Scholar] [CrossRef]
- Konnov, A.A. Detailed Reaction Mechanism for Small Hydrocarbons Combustion. Release 0.5 2000; Lund University: Lund, Sweden, 2000. [Google Scholar]
- Tian, Z.; Li, Y.; Zhang, L.; Glarborg, P.; Qi, F. An Experimental and Kinetic Modeling Study of Premixed NH3/CH4/O2/Ar Flames at Low Pressure. Combust. Flame 2009, 156, 1413–1426. [Google Scholar] [CrossRef]
- Mendiara, T.; Glarborg, P. Ammonia Chemistry in Oxy-Fuel Combustion of Methane. Combust. Flame 2009, 156, 1937–1949. [Google Scholar] [CrossRef]
- Klippenstein, S.J.; Harding, L.B.; Glarborg, P.; Miller, J.A. The Role of NNH in NO Formation and Control. Combust. Flame 2011, 158, 774–789. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A. Chemical Kinetic Mechanism Study on Premixed Combustion of Ammonia/Hydrogen Fuels for Gas Turbine Use. J. Eng. Gas Turbines Power 2017, 139. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Modeling Combustion of Ammonia/Hydrogen Fuel Blends under Gas Turbine Conditions. Energy Fuels 2017, 31, 8631–8642. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and Modeling Study on the High-Temperature Oxidation of Ammonia and Related NOx Chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Guteša Božo, M.; Valera-Medina, A.; Syred, N.; Bowen, P.J. Fuel quality impact analysis for practical implementation of corn cob gasification gas in conventional gas turbine power plants. Biomass Bioenergy 2019, 122, 221–230. [Google Scholar]
- Guteša Božo, M.; Vigueras-Zuniga, M.; Buffi, M.; Seljak, T.; Valera-Medina, A. Fuel Rich Ammonia-Hydrogen Injection for Humidified Gas Turbines. Appl. Energy 2019, 251, 113334. [Google Scholar] [CrossRef]
- Gutesa-Bozo, M.; Valera-Medina, A. Novel Humidified Ammonia/Hydrogen Gas Turbine Cycles. SMARTCATS Conference. COST Action, EU. 2019. Available online: https://www.smartcats.eu/ (accessed on 5 January 2020).
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Da Rocha, R.C.; Costa, M.; Bai, X.-S. Chemical Kinetic Modelling of Ammonia/Hydrogen/Air Ignition, Premixed Flame Propagation and NO Emission. Fuel 2019, 246, 24–33. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and Numerical Study of the Laminar Burning Velocity of CH4–NH3–Air Premixed Flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- GRI-Mech, V. 3.0. Gas Research Institute, GRI-Mech, Ver. 3.0. Available online: http://www.Me.Berkely.Edu/Gri-Mech/ (accessed on 19 June 2018).
- Arif, K.; Brian, E. Fuel Conditioning System for Ammonia Fired Power Plants, NH3 Fuel Association, October 2012. Available online: Https://Nh3fuel.Files.Wordpress.Com/2012/10/Evans-Brian.Pdf (accessed on 21 November 2016).
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and Technology of Ammonia Combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.D.K.A.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Kobayashi, H. Towards the Development of an Efficient Low-NOx Ammonia Combustor for a Micro Gas Turbine. Proc. Combust. Inst. 2019, 37, 4597–4606. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Hewlett, S.G.; Valera-Medina, A.; Pugh, D.G.; Bowen, P.J. Gas Turbine Co-Firing of Steelworks Ammonia with Coke Oven Gas: A Fundamental and Cycle Analysis. In Proceedings of the ASME Turbo Expo, Phoenix, AZ, USA, 17–21 June 2019; p. GT2019-91404. [Google Scholar]
- Pugh, D.; Bowen, P.; Valera-Medina, A.; Giles, A.; Runyon, J.; Marsh, R. Influence of Steam Addition and Elevated Ambient Conditions on NOx Reduction in a Staged Premixed Swirling NH3/H2 Flame. Proc. Combust. Inst. 2019, 37, 5401–5409. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.D.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and Other Emissions in Micro Gas Turbine Combustors Fuelled with Mixtures of Methane and Ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Ito, S.; Uchida, M.; Onishi, S.; Fujimor, T.; Kobayashi, T. Performance of Ammonia–Natural Gas Co-fired Gas Turbine for Power Generation. Available online: https://nh3fuelassociation.org/wp-content/uploads/2018/12/1545-Performance-of-Ammonia–Natural-Gas-for-Power-Generation-for-Power-Generation.pdf (accessed on 24 June 2019).
- Yamagami, A.; Fujiwara, H. World’s First Successful Ammonia Synthesis Using Renewable Energy-Based Hydrogen and Power Generation. Available online: https://ammoniaindustry.com/jgc-corporation-demonstrates-worlds-first-carbon-free-ammonia-energy-cycle/ (accessed on 2 June 2019).
- Goldmann, A.; Sauter, W.; Oettinger, M.; Kluge, T.; Schröder, U.; Seume, J.; Friedrichs, J.; Dinkelacker, F. A Study on Electrofuels in Aviation. Energies 2018, 11, 392. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Gutesa, M.; Xiao, H.; Pugh, D.; Giles, A.; Goktepe, B.; Marsh, R.; Bowen, P. Premixed Ammonia/Hydrogen Swirl Combustion under Rich Fuel Conditions for Gas Turbines Operation. Int. J. Hydrogen Energy 2019, 44, 8615–8626. [Google Scholar] [CrossRef]
- Zhao, D.; Guan, Y.; Reinecke, A. Characterizing Hydrogen-Fuelled Pulsating Combustion on Thermodynamic Properties of a Combustor. Commun. Phys. 2019, 2, 44. [Google Scholar] [CrossRef]
- Terhaar, S.; Krüger, O.; Paschereit, C.O. Flow Field and Flame Dynamics of Swirling Methane and Hydrogen Flames at Dry and Steam Diluted Conditions. J. Eng. Gas Turbines Power 2015, 137. [Google Scholar] [CrossRef]
- Syred, N.; Giles, A.; Lewis, J.; Abdulsada, M.; Valera Medina, A.; Marsh, R.; Bowen, P.J.; Griffiths, A.J. Effect of Inlet and Outlet Configurations on Blow-off and Flashback with Premixed Combustion for Methane and a High Hydrogen Content Fuel in a Generic Swirl Burner. Appl. Energy 2014, 116. [Google Scholar] [CrossRef]
- Syred, N.; Abdulsada, M.; Griffiths, A.; O’Doherty, T.; Bowen, P. The Effect of Hydrogen Containing Fuel Blends upon Flashback in Swirl Burners. Appl. Energy 2012, 89, 106–110. [Google Scholar] [CrossRef]
- Honzawa, T.; Kai, R.; Okada, A.; Valera-Medina, A.; Bowen, P.J.; Kurose, R. Predictions of NO and CO Emissions in Ammonia/Methane/Air Combustion by LES Using a Non-Adiabatic Flamelet Generated Manifold. Energy 2019, 186, 115771. [Google Scholar] [CrossRef]
- Monteiro-Davalos, R. Observations of Corrosion Product Formation and Stress Corrosion Cracking on Brass Samples Exposed to Ammonia Environments. Mater. Res. 2018, 22, e20180077. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Vigueras-Zuniga, M.O.; Baej, H.; Syred, N.; Chong, C.T.; Bowen, P.J. Outlet Geometrical Impacts on Blowoff Effects When Using Various Syngas Mixtures in Swirling Flows. Appl. Energy 2017, 207. [Google Scholar] [CrossRef]
- Baej, H.; Valera-Medina, A.; Bowen, P.; Syred, N.; O’Doherty, T.; Marsh, R. Impacts on Blowoff by a Variety of CRZs Using Various Gases for Gas Turbines. Energy Procedia 2014, 61, 1606–1609. [Google Scholar] [CrossRef][Green Version]
- Siemens. Complex Chemistry. Available online: https://documentation.thesteveportal.plm.automation.siemens.coml (accessed on 24 September 2019).
- Valera-Medina, A.; Giles, A.; Pugh, D.; Morris, S.; Pohl, M.; Ortwein, A. Investigation of Combustion of Emulated Biogas in a Gas Turbine Test Rig. J. Therm. Sci. 2018, 27, 331–340. [Google Scholar] [CrossRef]
- Runyon, J.; Marsh, R.; Bowen, P.; Pugh, D.; Giles, A.; Morris, S. Lean Methane Flame Stability in a Premixed Generic Swirl Burner: Isothermal Flow and Atmospheric Combustion Characterization. Exp. Therm. Fluid Sci. 2018, 92, 125–140. [Google Scholar] [CrossRef]












| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Quartz Temperature | 1450 K | Okafor Mechanism | 130 Reactions |
| Inlet Velocity | 3 m/s | Method | Segregated Flow |
| Inlet Temperature | 304 K | Ignition Temperature | 3000 K |
| Pressure | 101,300 Pa | Turbulence | 10% |
| Outlet Pressure | 99,274 Pa | Burner section | Symmetry (120°) |
| Walls | No-slip | Swirler walls | Adiabatic |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Quartz Temperature | 1450, 1800, 2150 K | Okafor Mechanism | 130 Reactions |
| Inlet Velocity | 20 m/s | Method | Segregated Flow |
| Inlet Temperature | 500 K | Ignition Temperature | 3000 K |
| Inlet Pressure | 0.90 MPa | Turbulence | 10% |
| Outlet Pressure | 0.88 MPa | Burner section | Symmetry (120°) |
| Walls | No-slip | Swirler walls | Adiabatic |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigueras-Zuniga, M.-O.; Tejeda-del-Cueto, M.-E.; Vasquez-Santacruz, J.-A.; Herrera-May, A.-L.; Valera-Medina, A. Numerical Predictions of a Swirl Combustor Using Complex Chemistry Fueled with Ammonia/Hydrogen Blends. Energies 2020, 13, 288. https://doi.org/10.3390/en13020288
Vigueras-Zuniga M-O, Tejeda-del-Cueto M-E, Vasquez-Santacruz J-A, Herrera-May A-L, Valera-Medina A. Numerical Predictions of a Swirl Combustor Using Complex Chemistry Fueled with Ammonia/Hydrogen Blends. Energies. 2020; 13(2):288. https://doi.org/10.3390/en13020288
Chicago/Turabian StyleVigueras-Zuniga, Marco-Osvaldo, Maria-Elena Tejeda-del-Cueto, José-Alejandro Vasquez-Santacruz, Agustín-Leobardo Herrera-May, and Agustin Valera-Medina. 2020. "Numerical Predictions of a Swirl Combustor Using Complex Chemistry Fueled with Ammonia/Hydrogen Blends" Energies 13, no. 2: 288. https://doi.org/10.3390/en13020288
APA StyleVigueras-Zuniga, M.-O., Tejeda-del-Cueto, M.-E., Vasquez-Santacruz, J.-A., Herrera-May, A.-L., & Valera-Medina, A. (2020). Numerical Predictions of a Swirl Combustor Using Complex Chemistry Fueled with Ammonia/Hydrogen Blends. Energies, 13(2), 288. https://doi.org/10.3390/en13020288








