Characteristics of Gel Time and Dielectric Strength of Epoxy Composite According to the Mixing Ratio of Micro-Fillers
Abstract
1. Introduction
2. Composition of Epoxy Composites
2.1. Epoxy Resin Composition
2.2. Hardener Composition
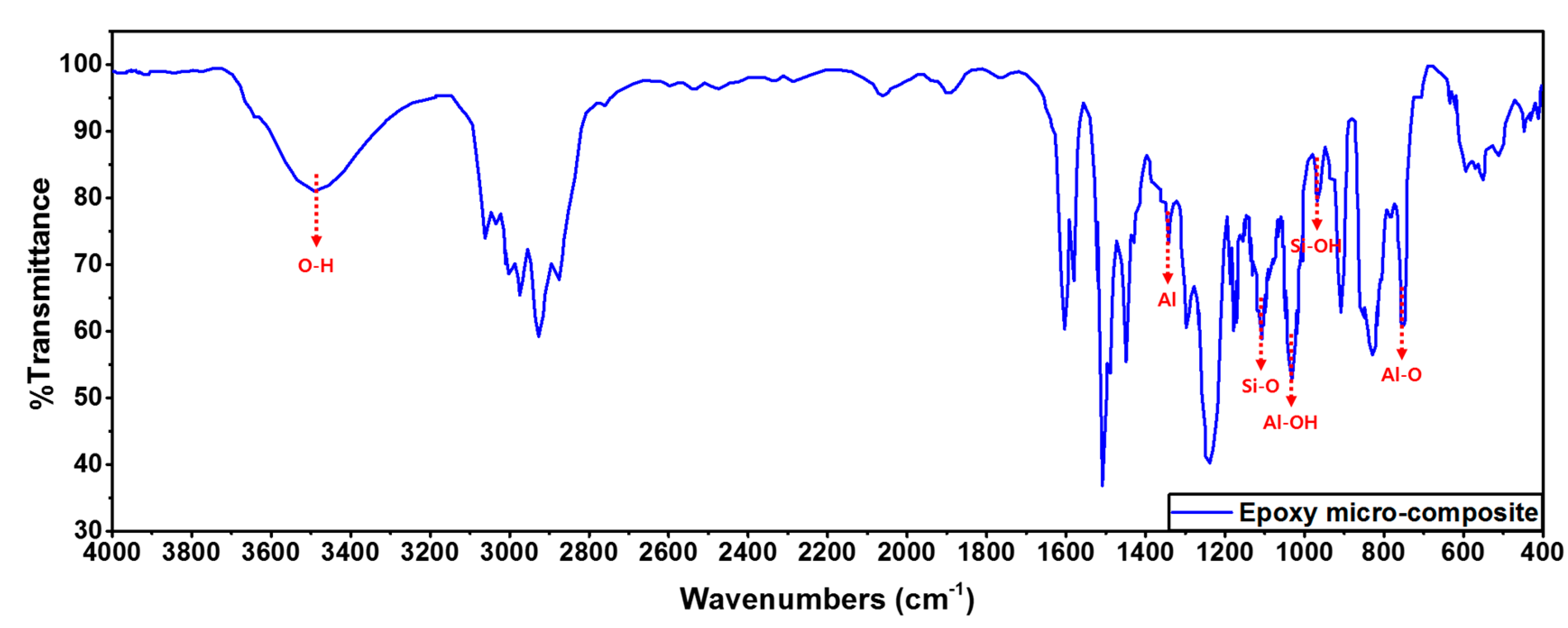
2.3. Micro-Fillers
3. Fabrication of Epoxy Micro-Composite
3.1. Preparation of Epoxy Resin Composition and Combination with Micro-Filler
3.2. Preparation of Hardener Composition and Combination with Micro-Filler
3.3. Manufacturing Process of Epoxy Micro-Composite
4. Material Characteristic Evaluation of Epoxy Micro-composite
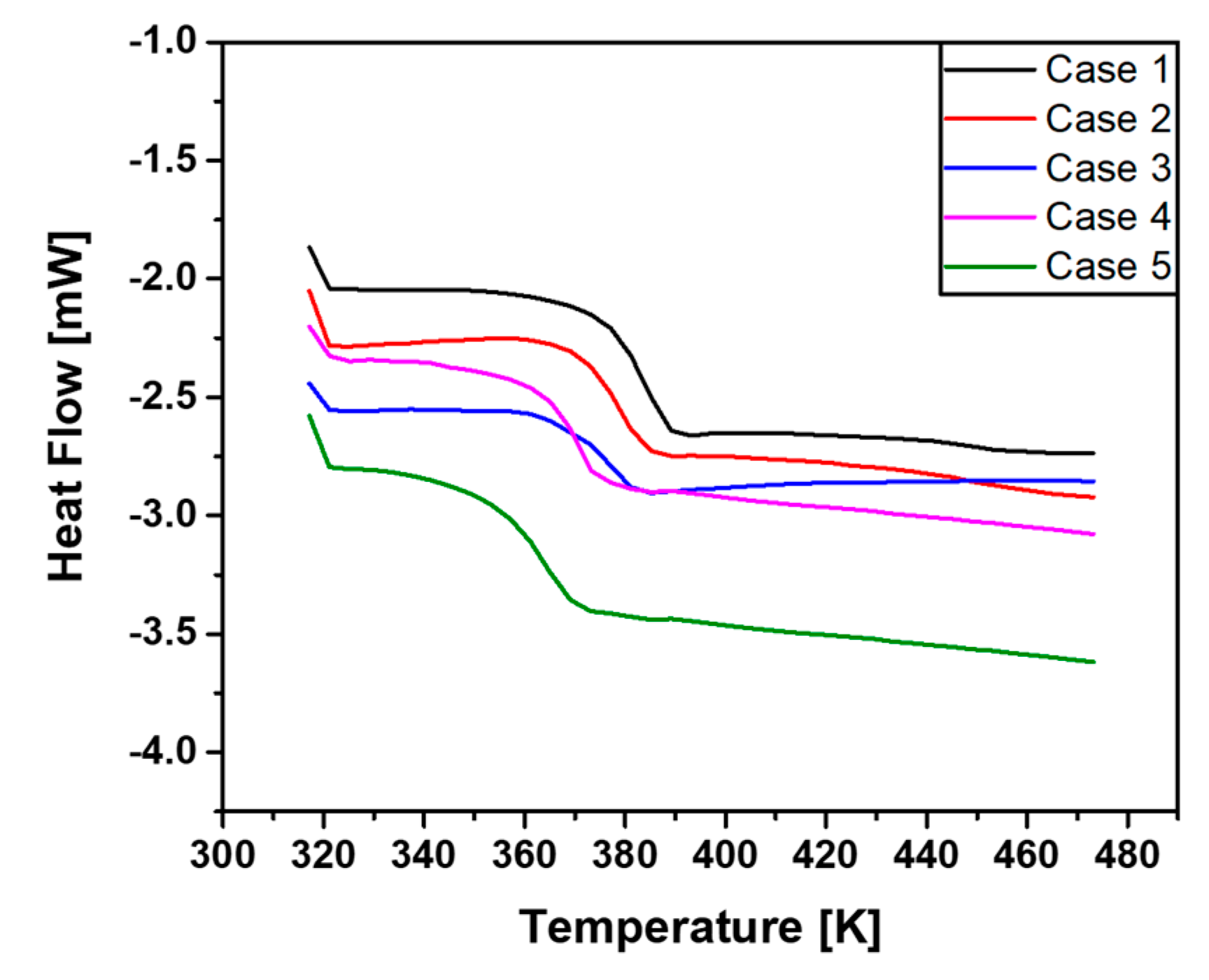
4.1. Measurement of Gel Time and Glass Transition Temperature
4.2. Measurement of Relative Permittivity and Dielectric Loss Tangent
5. Evaluation of Dielectric Breakdown Characteristic
5.1. Dielectric Breakdown Test System
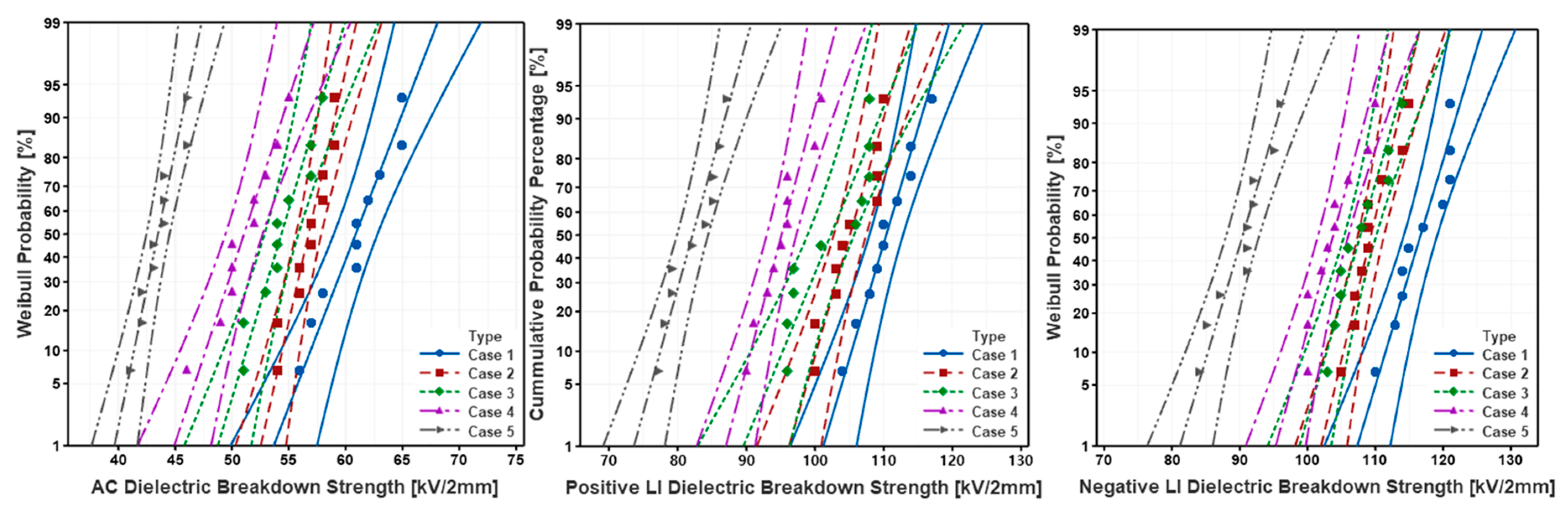
5.2. AC/LI Dielectric Breakdown Test Result
6. Discussion
- As the mixing ratio of SiO2 increased, the gel time decreased due to strengthening of the hydrogen bonding by -OH groups present in the SiO2 and epoxy resin molecular structure.
- As the curing temperature increased, the increased temperature accelerated the mixing reaction of the epoxy resin, curing agent and additives, which reduces the gel time.
- As the mixing ratio of SiO2 increased, the Tg was increased, but the change of Tg was insignificant when the mixing ratio of SiO2 and Al2O3 exceeded 3:1.
- As the mixing ratio of SiO2 increased, hydrogen bonding by -OH groups present on the surface of SiO2 was decreased, and eventually the bonding force at the interface of the epoxy composite was strengthened. Therefore, the relative permittivity was decreased and the dielectric loss tangent was increased.
- As the mixing ratio of SiO2 increased, the number of molecules of micro-SiO2 relative to the same weight was increased by the difference in specific gravity, and eventually the AC/+LI/-LI dielectric strength was improved due to the increased cross-link density.
- As the mixing ratio of SiO2 increased, hydrogen bonding by -OH groups present in the molecular structure of SiO2 and epoxy resin was strengthened, and eventually the AC/+LI/-LI dielectric strength was improved due to the strengthened interfacial bonding strength.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The Intergovernmental Panel on Climate Change (IPCC). Greenhouse Gas Protocol Report. Available online: https://www.ghgprotocol.org/sites/default/files/ghgp/Global-Warming-Potential-Values%20%28Feb%2016%202016%29_1.pdf (accessed on 14 September 2020).
- Kevork, M.; Ozcan, K.; Orhan, E.; Ergun, C. AC Breakdown Strength of N2, SF6 and a Mixture of N2+SF6 containing a Small Amount of SF6. IEEE Inter. Symp. Electr. Insul. 1996, 763–765. [Google Scholar]
- Malik, N.H.; Qureshi, A.H. A Review of Electrical Breakdown in Mixtures of SF6 and Other Gases. IEEE Trans. Electr. Insul. 1979, E1-14, 1–13. [Google Scholar] [CrossRef]
- Sato, J.; Sakaguchi, O.; Kubota, N.; Makishima, S.; Kinoshita, S.; Shioiri, T.; Yoshida, T.; Miyagawa, M.; Homma, M.; Kaneko, E. New technology for medium voltage solid insulated switchgear. In Proceedings of the IEEE Asia Pacific Transmission and Distribution Conference and Exhibition, Yokohama, Japan, 6–10 October 2002; pp. 1791–1796. [Google Scholar]
- Shimizu, T.; Kinoshita, S.; Makishima, S.; Sato, J.; Sakaguchi, O. Material and simulation technology for solid insulated switchgear. In Proceedings of the IEEE 7th International Conference Properties and Application of Dielectric Materials (ICPADM), Nagoya, Japan, 1–5 June 2003; pp. 1194–1197. [Google Scholar]
- Ellis, B. Chemistry and Technology of Epoxy Resins; Springer: Cham, Switzerland, 1993; p. 29. [Google Scholar]
- Wetzel, B.; Haupert, F.; Zhang, M.Q. Epoxy nanocomposites with high mechanical and tribological performance. Compos. Sci. Technol. 2003, 63, 2055–2067. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Maga. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Fessler, H.; Perla, M. Precision casting of epoxy-resin photoelastic models. J. Strain Anal. Eng. Design. 1973. [Google Scholar] [CrossRef]
- John, A.M. Interfacial effects in composites. Pure Appl. Chem. 1985, 57, 1667–1678. [Google Scholar]
- Imai, T.; Sawa, F.; Nakano, T.; Ozaki, T.; Shimizu, T. Effects of Nano- and Micro-filler Mixture on Electrical Insulation Properties of Epoxy Based Composites. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 319–326. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, C.H.; Lee, J.Y.; Kim, H.D. Preparation of Epoxy/Micro-and NanoComposites by Electric Field Dispersion Process and Its Mechanical and Electrical Properties. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 667–674. [Google Scholar] [CrossRef]
- Tanaka, T.; Montanari, G.C.; Mülhaupt, R. Polymer nanocomposites as dielectrics and electrical insulation-perspectives for processing technologies, material characterization and future applications. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 763–784. [Google Scholar] [CrossRef]
- Lewis, T.J. Nanometric dielectrics. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 812–825. [Google Scholar] [CrossRef]
- Kochetov, R.; Andritsch, T.; Morshuis, P.H.F.; Smit, J.J. Thermal and Electrical Behaviour of Epoxy-based Microcomposites Filled with Al2O3 and SiO2 Particles. In IEEE International Symposium on Electrical Insulation, Proceedings of the 2010 IEEE International Symposium on Electrical Insulation (ISEI 2010), San Diego, CA, USA, 6–9 June 2010; IEEE: New York, NY, USA, 2010; pp. 1–5. [Google Scholar]
- Andritsch, T.; Kochetov, R.; Gebrekiros, Y.T.; Morshuis, P.H.F.; Smit, J.J. Short term DC breakdown strength in epoxy based BN nano- and microcomposites. In Proceedings of the IEEE International Conference on Solid Dielectrics (ICSD), Potsdam, Germany, 4–9 July 2010; pp. 179–182. [Google Scholar]
- Castellon, J.; Nguyen, H.N.; Agnel, S.; Toureille, A.; Frechette, M.; Savoie, S.; Krivda, A.; Schmidt, L.E. Electrical Properties Analysis of Micro and Nano Composite Epoxy Resin Materials. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 651–658. [Google Scholar] [CrossRef]
- Danikas, M.G.; Tanaka, T. Nanocomposites-a review of electrical treeing and breakdown. IEEE Electr. Insul. Maga. 2009, 25, 19–24. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, C.; Ai, T.; Wu, K.; Zhao, F.; Gu, H. A novel fiber-reinforced polyethylene composite with added silicon nitride particles for enhanced thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2019, 40, 830–836. [Google Scholar] [CrossRef]
- Amin, M. Methods for Preparation of Nano-Composites for Outdoor Insulation Applications. Rev. Adv. Mater. Sci. 2013, 173–184. [Google Scholar]
- SIBELCO. Coating. Available online: https://coatings.sibelcotools.com/mineral/quartz/ (accessed on 16 September 2020).
- EUROQUARZ. Available online: https://www.euroquarz.de/uploads/media/TDS_Silverbond_1.pdf (accessed on 16 September 2020).
- SHOWA DENKO. Available online: https://showadenko.us/wp-content/uploads/2019/06/Showa-Denko-Thermal-Filler-Brochure-2019.pdf (accessed on 16 September 2020).
- Park, J.J.; Lee, C.H. The Properties of DSC and DMA for Epoxy Nano-and-Micro Mixture Composites. Trans. Electr. Electron. Mater. 2011, 11, 69–72. [Google Scholar]
- WIKIPEDIA-InInfrared Spectroscopy Correlation Table. Available online: https://en.wikipedia.org/wiki/Infrared_spectroscopy_correlation_table (accessed on 20 September 2020).
- Klapiszewski, L.; Jamrozik, A.; Strzemiecka, B.; Koltsov, I.; Borek, B.; Matykiewicz, D.; Voelkel, A.; Jesionowski, T. Characteristics of Multifunctional, Eco-Friendly Lignin-Al2O3 Hybrid Fillers and Their Influence on the Properties of Composites for Abrasive Tools. Molecules 2017, 22, 1920. [Google Scholar] [CrossRef] [PubMed]
- Beganskienė, A.; Sirutkaitis, V.; Kurtinaitienė, M.; Juškėnas, R.; Kareiva, A. FTIR, TEM and NMR Iinvestigations of Stöber Silica Nanopartic. Mater. Sci. 2004, 10. [Google Scholar]
- Teo, J.K.J.; Teo, K.C.; Pan, B.; Xiao, Y.; Lu, X. Epoxy/polyhedral oligomeric silsesquioxane (POSS) hybrid networks cured with an anhydride: Cure kinetics and thermal properties. Polymer 2007, 48, 5671–5680. [Google Scholar]
- IEC. IEC 62631-2-1: Dielectric and Resistive Properties of Solid Insulating Materials-Part 2-1: Relative Permittivity and Dissipation Factor-Technical Frequencies (0.1 Hz-10 MHz)-AC Methods; IEC: Geneva, Switzerland, 2018. [Google Scholar]
- Iyer, G.; Gorur, R.S.; Richert, R.; Krivda, A.; Schmidt, L.E. Dielectric properties of epoxy based nanocomposites for high voltage insulation. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 659–666. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Y.; Jia, Z.; Tanaka, T.; Wu, J.; Cheng, Y.H. Study on Electrical Properties of Micro-nano Structured Epoxy Composites. In Proceedings of the 2014 International Symposium on Electrical Insulating Materials, Niigata, Japan, 1–5 June 2014; pp. 441–444. [Google Scholar]
- Klampar, M.; Spohner, M.; Skarvada, P.; Dallaeva, D.; Kobrtck, J.; Liedermann, K. Dielectric properties of epoxy resins with oxide nanofillers and their accelerated ageing. In Proceedings of the IEEE Electrical Insulation Conference (EIC), Ottawa, ON, Canada, 2–5 June 2013; pp. 286–290. [Google Scholar]
- IEC. IEC 60243-1: Electric Strength of Insulating Materials-Test Methods-Part 1: Tests at Power Frequencies; IEC: Geneva, Switzerland, 2013. [Google Scholar]
- IEC. IEC 60060-1: High-Voltage Test Techniques-Part 1: General Definitions and Test Requirements; IEC: Geneva, Switzerland, 2010. [Google Scholar]
- Ovsik, M.; Fiala, T.; Manas, M.; Dockal, A. Ultra-Nano Indentation Properties of Crosslinked PBT. In Proceedings of the MATEC Web of Conferences; 2010. Available online: https://www.matec-conferences.org/articles/matecconf/pdf/2018/69/matecconf_cscc2018_02038.pdf (accessed on 16 September 2020).
- Hussein, M.A.; Albeladi, H.K.; AlRomaizan, A.N. Role of Cross-Linking Process on the Performance of PMMA. Int. J. Biosens. Bioelectron. 2017, 3, 279–284. [Google Scholar] [CrossRef]



| Contents | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Epoxy resin composition | 18.6 wt% | 18.6 wt% | 18.6 wt% | 18.6 wt% | 18.6 wt% |
| Hardener composition | 15.56 wt% | 15.56 wt% | 15.56 wt% | 15.56 wt% | 15.56 wt% |
| SiO2 | 65.84 wt% | 49.38 wt% | 43.89 wt% | 32.92 wt% | - |
| Al2O3 | - | 16.46 wt% | 21.95 wt% | 32.92 wt% | 65.84 wt% |
| Mixing ratio | 100:0 | 3:1 | 2:1 | 1:1 | 0:100 |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| 353.15 K gel time | 13 h 53 min | 15 h 46 min | 17 h 23 min | 17 h 30 min | 27 h 16 min |
| 393.15 K gel time | 1 h 48 min | 2 h 42 min | 2 h 59 min | 3 h 25 min | 4 h 27 min |
| 413.15 K gel time | 37 min | 1 h 1 min | 1 h 8 min | 1 h 25 min | 1 h 52 min |
| Tg | 372.75 K | 372.56 K | 367.75 K | 364.58 K | 353.21 K |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Relative permittivity | 4.024 | 4.252 | 4.376 | 4.617 | 5.236 |
| Dielectric loss tangent | 0.027 | 0.018 | 0.016 | 0.014 | 0.004 |
| Applied Voltage | Scale Parameter (kV/2 mm); Shape Parameter | ||||
|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
| AC | 62.2; 24.1 | 57.6; 30.4 | 55.5; 26.4 | 52.2; 23.7 | 44.2; 29.1 |
| Positive LI | 112.2; 31.7 | 106.9; 33.8 | 104.8; 24.0 | 96.8; 29.8 | 83.9; 28.2 |
| Negative LI | 118.4; 36.5 | 110.9; 35.7 | 109.6; 31.1 | 105.5; 30.1 | 92.2; 28.6 |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Epoxy resin composition [wt%] | 18.6 | 18.6 | 18.6 | 18.6 | 18.6 |
| Hardener composition [wt%] | 15.56 | 15.56 | 15.56 | 15.56 | 15.56 |
| SiO2 [wt%] | 65.84 | 49.38 | 43.89 | 32.92 | - |
| Al2O3 [wt%] | - | 16.46 | 21.95 | 32.92 | 65.84 |
| Mixing ratios | 100:0 | 3:1 | 2:1 | 1:1 | 0:100 |
| 353.15 K gel time | 13 h 53 min | 15 h 46 min | 17 h 33 min | 17 h 30 min | 27 h 16 min |
| 393.15 K gel time | 1 h 48 min | 2 h 42 min | 2 h 59 min | 3 h 25 min | 4 h 27 min |
| 413.12 K gel time | 37 min | 1 h 1 min | 1 h 8 min | 1 h 25 min | 1 h 52 min |
| DSC Tg [K] | 372.75 | 372.56 | 367.75 | 364.58 | 353.21 |
| Relative permittivity | 4.024 | 4.252 | 4.376 | 4.617 | 5.236 |
| Dielectric loss tangent | 0.027 | 0.018 | 0.016 | 0.014 | 0.004 |
| AC dielectric strength (scale parameter) [kV/2 mm] | 62.2 | 57.6 | 55.5 | 52.2 | 44.2 |
| Positive LI dielectric strength (scale parameter) [kV/2 mm] | 112.2 | 106.9 | 104.8 | 96.8 | 83.9 |
| Negative LI dielectric strength (scale parameter) [kV/2 mm] | 118.4 | 110.9 | 109.6 | 105.5 | 92.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, D.-H.; Kim, H.-S.; Shim, J.-H.; Jeon, Y.-H.; Kang, D.-W.; Lee, B.-W. Characteristics of Gel Time and Dielectric Strength of Epoxy Composite According to the Mixing Ratio of Micro-Fillers. Energies 2020, 13, 5165. https://doi.org/10.3390/en13195165
Oh D-H, Kim H-S, Shim J-H, Jeon Y-H, Kang D-W, Lee B-W. Characteristics of Gel Time and Dielectric Strength of Epoxy Composite According to the Mixing Ratio of Micro-Fillers. Energies. 2020; 13(19):5165. https://doi.org/10.3390/en13195165
Chicago/Turabian StyleOh, Dong-Hun, Ho-Seung Kim, Jae-Hun Shim, Young-Ho Jeon, Da-Won Kang, and Bang-Wook Lee. 2020. "Characteristics of Gel Time and Dielectric Strength of Epoxy Composite According to the Mixing Ratio of Micro-Fillers" Energies 13, no. 19: 5165. https://doi.org/10.3390/en13195165
APA StyleOh, D.-H., Kim, H.-S., Shim, J.-H., Jeon, Y.-H., Kang, D.-W., & Lee, B.-W. (2020). Characteristics of Gel Time and Dielectric Strength of Epoxy Composite According to the Mixing Ratio of Micro-Fillers. Energies, 13(19), 5165. https://doi.org/10.3390/en13195165





