The Influence of the Process of Sugar Beet Storage on Its Biochemical Methane Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- pH, potentiometric method, PN-EN 12176: 2004;
- total solids, mass method, PN-EN 12880: 2004;
- ignition loss (ignition residues), mass method, PN-EN 12879: 2004;
- collection of samples for chemical and physical research, PN-EN ISO 5667-13: 2011;
- biogas production, DIN 38414-S.8 [33];
- sugar content, polarisation method, methanol, ethanol, acetic acid, lactic acid and gas chromatography methods in an accredited external laboratory.
2.2. Preparation for the Research
- FSB—fresh roots;
- O—open-air container;
- H—hermetic container (airtight conditions);
- O/H4—after 4 weeks of storage;
- O/H8—after 8 weeks of storage;
- O/H16—after 16 weeks of storage;
- O/H32—after 32 weeks of storage.
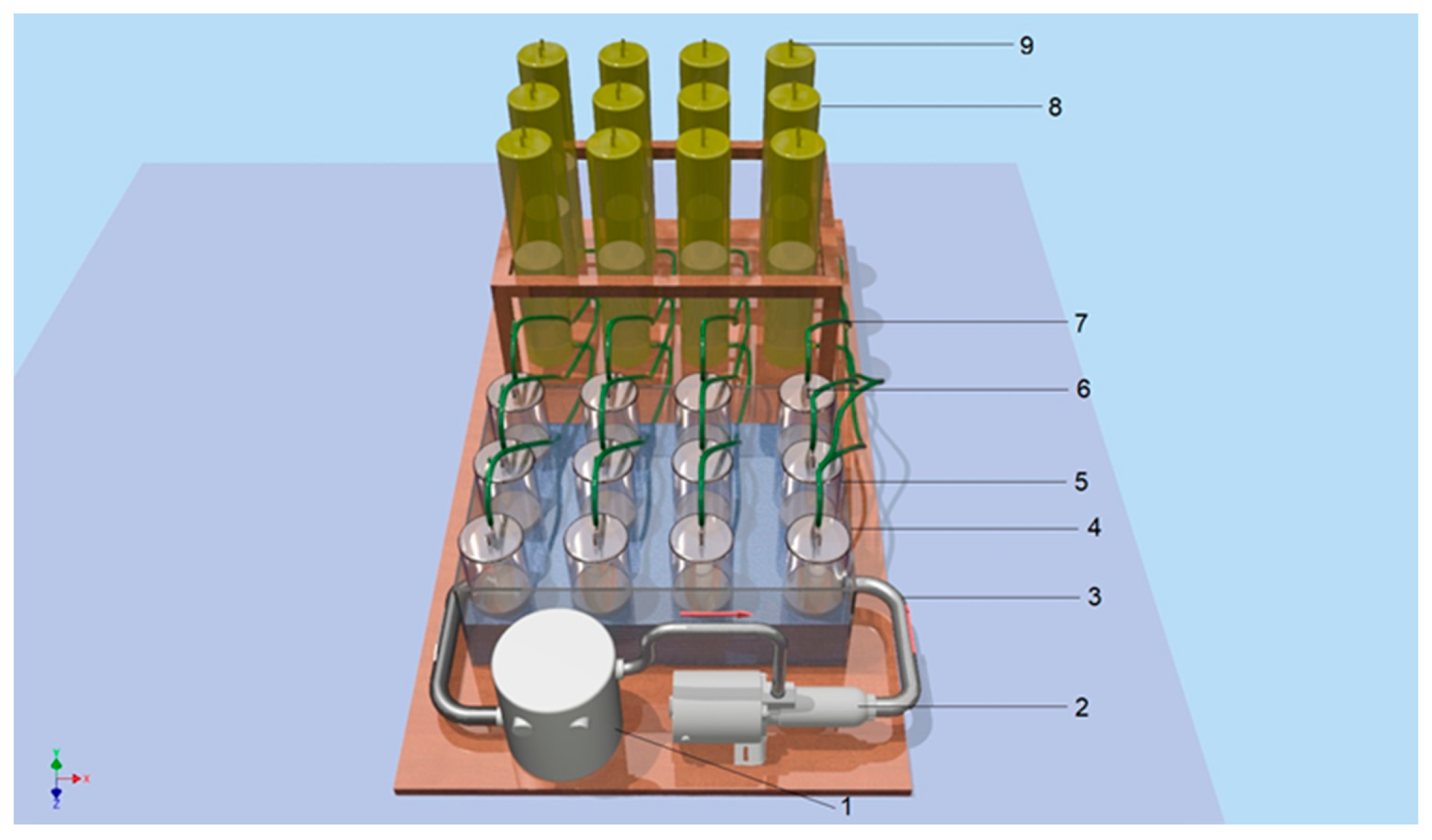
2.3. Biogas Production in Laboratory
3. Results and Discussion
4. Conclusions
- During storage, the following were observed: a decrease in the pH (higher for samples in the open-air container) and a reduction in total solids, including volatile solids—greater in the case of the open-air container;
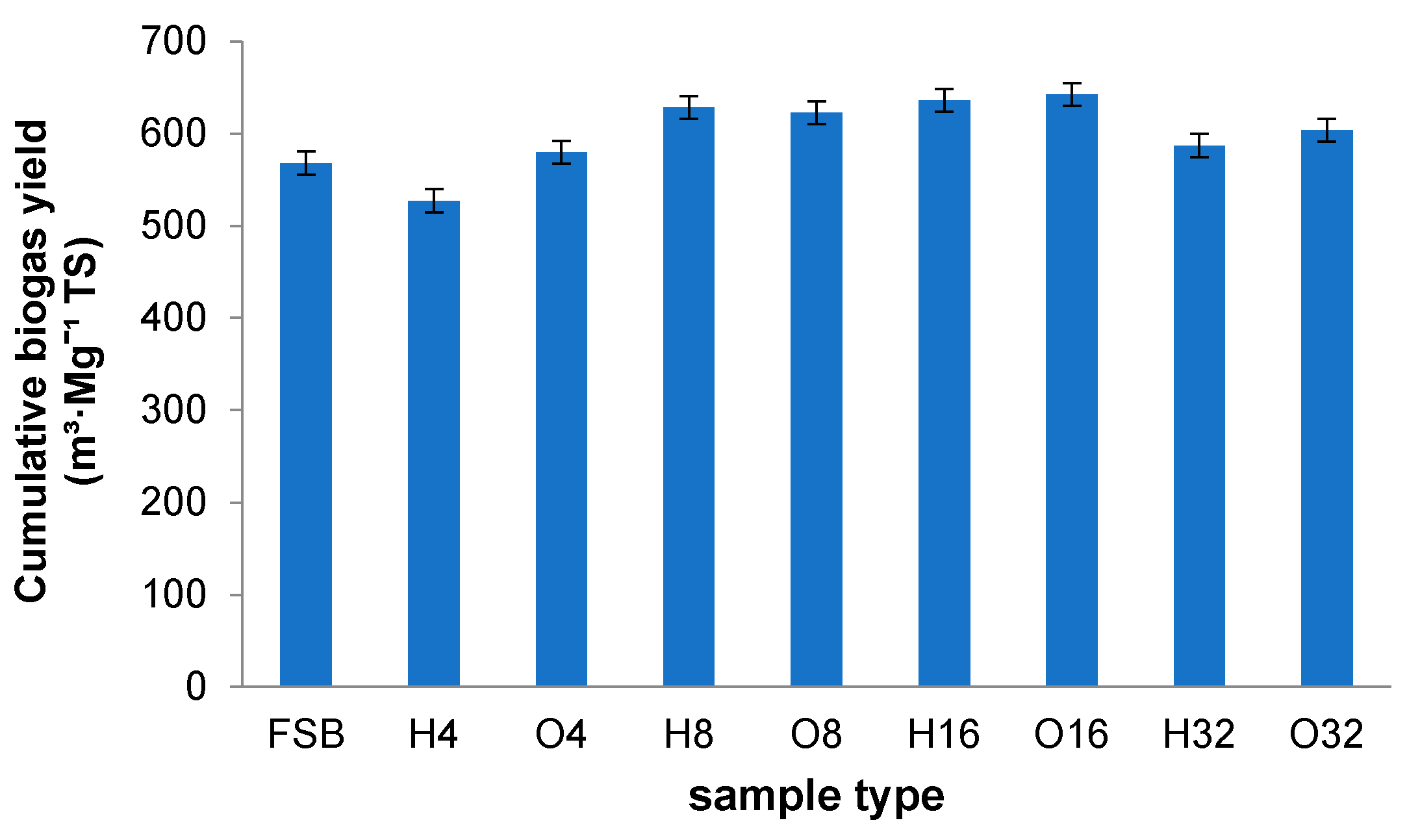
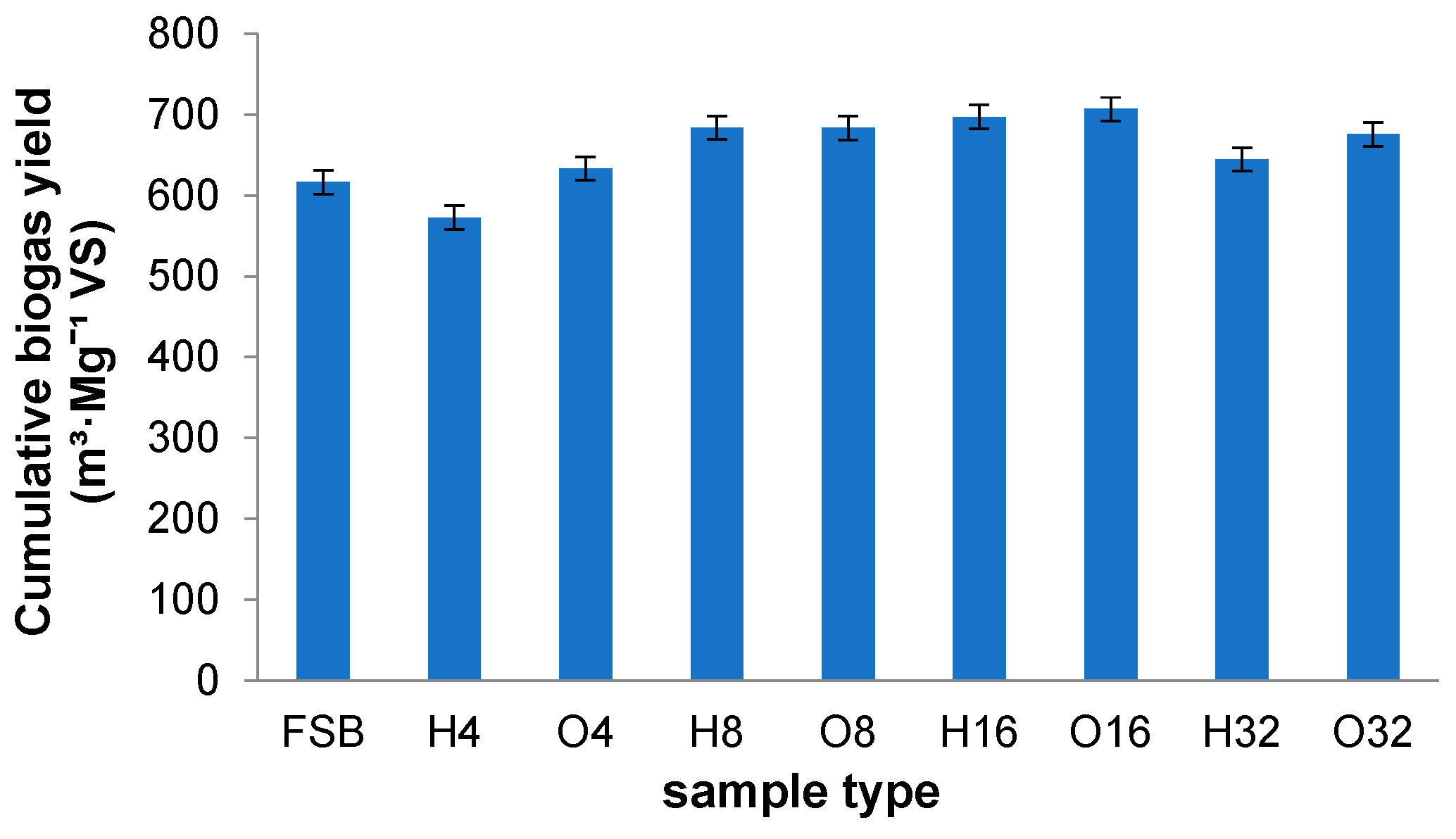
- The method of storage of sugar beets had little impact on the differentiation of the BMP of the samples. The highest biogas volume was obtained for fresh sugar beets—148.23 mL·g−1 FM (with 52.36% methane). Similar (and slightly lower in airtight conditions) values were recorded in the 8th and 16th weeks of storage—139.35 mL·g−1 FM (H) and 144.14 mL·g−1 FM (O), and 147.58 mL·g−1 FM (H) and 148.22 mL·g−1 FM (O), respectively.
- Large differences were observed in the content of selected organic compounds in individual samples, i.e., sugar, methanol, ethanol, lactic acid and acetic acid, which affect the quality of the stored product. The high content of sugar, methanol, ethanol and the remaining chemical compounds in the materials stored in the open-air container confirmed the decomposition of the organic matter at the stage of hydrolysis and acidogenesis, occurring under the influence of easily accessible microorganisms. In light of the above, it is more reasonable to store whole sugar beet roots in hermetic conditions rather than pulp in open storage lagoons.
Author Contributions
Funding
Conflicts of Interest
References
- Pach-Gurgul, A. Renewable energy of the European Union in the conditions of economic crisis. Works Com. Ind. Geogr. Pol. Geogr. Soc. 2014, 27, 130–147. [Google Scholar]
- Redlarski, G.; Piechocki, J.; Dąbkowski, M. Reducing air pollutant emissions from the residential sector by switching to alternative energy sources in single-family homes. Pol. J. Environ. Stud. 2013, 22, 197–203. [Google Scholar]
- Błażejewska, K. Legal aspects of agricultural biogas production and utilization in Poland. Agric. Law Rev. 2010, 1, 97–120. [Google Scholar]
- Pääakkönen, A.; Tolvanen, H.; Rintala, J. Techno-economic analysis of a power to biogas system operated based on fluctuating electricity. Renew. Energy 2018, 117, 166–174. [Google Scholar] [CrossRef]
- Czapiewska, G. Creating of sustainable rural areas development based on the agricultural biogas plants. Sci. J. Facult. Econom. Sci. 2014, 18, 11–25. [Google Scholar]
- Stańczyk, K.; Ludwik, M. Cultivation of energy crops—Possibilities of developing wastelands and arable lands where agricultural production is unprofitable. Min. Environ. 2003, 3, 71–81. [Google Scholar]
- Sajnóg, N.; Wójcik, J. Possibilities of developing degraded and uncultivated lands in consolidation. Infrastruct. Ecol. Rural Areas 2013, 2, 155–166. [Google Scholar]
- Ruszel, M. Evaluation of the security of natural gas supplies to Poland: The present state and the 2025 perspective. Energy Policy J. 2017, 20, 1–5. [Google Scholar]
- Luz, F.C.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Anaerobic digestion of coffee grounds soluble fraction at laboratory scale: Evaluation of the biomethane potential. Appl. Energy 2017, 207, 166–175. [Google Scholar] [CrossRef]
- Luz, F.C.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar characteristics and early applications in anaerobic digestion—A review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar]
- European Union (EU). Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009. Off. J. Eur. Union 2009, L140, 16–62. [Google Scholar]
- Pilarski, K.; Pilarska, A.A. Production efficiency of Poland farm-scale biogas plants: A case study. In Proceedings of the Renewable Energy Sources—6th International Conference, ICoRES 2019, Krynica, Poland, 12–14 June 2019. E3S Web of Conf. 2020, 154, 2002. [Google Scholar] [CrossRef]
- Pilarski, K.; Pilarska, A.A.; Boniecki, P.; Niedbała, G.; Durczak, K.; Witaszek, K.; Mioduszewska, N.; Kowalik, K. The Efficiency of Industrial and Laboratory Anaerobic Digesters of Organic Substrates: The Use of the Biochemical Methane Potential Correction Coefficient. Energies 2020, 13, 1280. [Google Scholar] [CrossRef]
- Nevens, F.; Reheul, D. Crop rotation versus monoculture; yield, N yield and ear fraction of silage maize at different levels of mineral N fertilization. Neth. J. Agric. Sci. 2001, 49, 405–425. [Google Scholar] [CrossRef]
- Sieling, K.; Herrmann, A.; Wienforth, B.; Taube, F.; Ohl, S.; Hartung, E.; Kage, H. Biogas cropping systems: Short term response of yield performance and N use efficiency to biogas residue application. Eur. J. Agron. 2013, 47, 44–54. [Google Scholar] [CrossRef]
- Przybył, J.; Mioduszewska, N.; Dach, J.; Pilarski, K. Sugar beets used for traditional purposes and for energy. An economic comparison. Agric. Eng. 2011, 7, 131–140. [Google Scholar]
- Jacobs, A.; Auburger, S.; Bahrs, E.; Brauer-Siebrecht, W.; Christen, O.; Götze, P.; Koch, H.J.; Rücknagel, J.; Märländer, B. Greenhouse gas emission of biogas production out of silage maize and sugar beet—An assessment along the entire production chain. Appl. Energy 2017, 190, 114–121. [Google Scholar] [CrossRef]
- Dorado, C.; Cameron, R.G.; Cooper, K. Steam explosion and fermentation of sugar beets from Southern Florida and the Midwestern United States. Biocatal. Agric. Biotechnol. 2017, 11, 26–33. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Production of methane from sugar beet silage without manure addition by a single-stage anaerobic digestion process. Biomass Bioenergy 2008, 32, 203–209. [Google Scholar] [CrossRef]
- Kryvoruchko, V.; Machmuller, A.; Bodiroza, V.; Amon, B.; Amon, T. Anaerobic digestion of by-products of sugar beet and starch potato processing. Biomass Bioenergy 2009, 33, 620–627. [Google Scholar] [CrossRef]
- Vindis, P.; Mursec, B.; Rozman, C.; Janzekovic, M.; Cus, F. Mini digester and biogas production from plant biomass. J. Achiev. Mater. Manuf. Eng. 2009, 35, 191–196. [Google Scholar]
- Vindis, P.; Mursec, B.; Rozman, C.; Janzekovi, M.; Cus, F. Biogas production with the use of mini digester. J. Achiev. Mater. Manuf. Eng. 2008, 28, 99–102. [Google Scholar]
- Igliński, B.; Buczkowski, R.; Cichosz, M. Biogas production in Poland—Current state, potential and perspectives. Renew. Sust. Energy Rev. 2015, 50, 686–695. [Google Scholar] [CrossRef]
- Chodkowska-Miszczuk, J.; Szymańska, D. Agricultural biogas plants—A chance for diversification of agriculture in Poland. Renew. Sustain. Energy Rev. 2013, 20, 514–518. [Google Scholar] [CrossRef]
- Baryga, A.; Połeć, B.; Małczak, E. Technological value of raw materials from sugar beet growing area fertilized with digestate from sugar beet pulp biogas plant. Plant Soil. Environ. 2017, 63, 207–212. [Google Scholar]
- Starke, P.; Hoffmann, C. Sugar beet as a substrate for biogas production. Sugar Ind. 2011, 136, 242–250. [Google Scholar] [CrossRef]
- Mioduszewska, N.; Adamski, M.; Smurzyńska, A.; Przybył, J.; Pilarski, K. The usefulness of sugar beets for biogas production in relations of the storage time and sugar content. E3S Web of Conf. 2018, 44, 114. [Google Scholar] [CrossRef]
- Vazifehkhoran, A.H.; Triolo, J.M.; Larsen, S.U.; Stefanek, K.; Sommer, S.G. Assessment of the variability of biogas production from sugar beet silage as affected by movement and loss of the produced alcohols and organic acid. Energies 2016, 9, 368. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Panzera, M.F. Biomethane production tests on ensiled orange peel waste. Int. J. Heat Technol. 2017, 35, S130–S136. [Google Scholar] [CrossRef]
- Calabro, P.S.; Paone, E.; Komilis, D. Strategies for the sustainable management of orange peel waste through anaerobic digestion. J. Environ. Manag. 2018, 212, 462–468. [Google Scholar] [CrossRef]
- Calabro, P.S.; Fazzino, F.; Sidari, R.; Zema, D.A. Optimization of orange peel waste ensiling for sustainable anaerobic digestion. Renew. Energy 2020, 154, 849–862. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A.; Boniecki, P.; Zaborowicz, M. Use of confectionery waste in biogas production by the anaerobic digestion process. Molecules 2019, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; DIN Guideline 38 414-S8; German Institute for Standardization: Berlin, Germany, 1985.
- Dettmann, S.; Norbert, K.; Frąś, J.; Schlegel, M. Biomass Logistics in the field of renewable energy. Res. Log. Prod. 2014, 4, 307–314. [Google Scholar]
- Van Eerd, L.L.; Congreves, K.A.; Zandstra, J.W. Sugar beet (Beta vulgaris L.) storage quality in large outdoor piles is impacted by pile management but not by nitrogen fertilizer or cultivar. Can. J. Plant Sci. 2012, 92, 129–139. [Google Scholar] [CrossRef]
- Dilek, F.B.; Yetis, U.; Gökcay, C.F. Water savings and sludge minimization in a beet-sugar factory through re-design of the wastewater treatment facility. J. Clean. Prod. 2003, 11, 327–331. [Google Scholar] [CrossRef]
- Fermentation of Organic Materials Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Norm VDI 4630; German Engineers Club: Düsseldorf, Germany, 2006.
- Pilarska, A.A.; Pilarski, K.; Witaszek, K.; Waliszewska, H.; Zborowska, M.; Waliszewska, B.; Kolasiński, M.; Szwarc-Rzepka, K. Treatment of dairy waste by anaerobic digestion with sewage sludge. Ecol. Chem. Eng. S 2016, 23, 99–115. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Ryniecki, A.; Tomaszyk, K.; Dach, J.; Wolna-Maruwka, A. Utilization of vegetable dumplings waste from industrial production by anaerobic digestion. Int. Agrophys. 2017, 31, 93–102. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K.; Janczak, D.; Przybył, K.; Gawrysiak-Witulska, M. The use of lignin as a microbial carrier in the co-digestion of cheese and wafer waste. Polymers 2019, 11, 2073. [Google Scholar] [CrossRef]
- Suhartini, S.; Heaven, S.; Banks, C.J. Comparison of mesophilic and thermophilic anaerobic digestion of sugar beet pulp: Performance, dewaterability and foam control. Bioresour. Technol. 2014, 152, 202–211. [Google Scholar] [CrossRef]
- Suhartini, S.; Heaven, S.; Zhang, Y.; Banks, C.J. Antifoam, dilution and trace element addition as foaming control strategies in mesophilic anaerobic digestion of sugar beet pulp. Int. Biodeterior. Biodegrad. 2019, 145, 104812. [Google Scholar] [CrossRef]
- Suhartini, S.; Heaven, S.; Banks, C.J. Can anaerobic digestion of sugar beet pulp support the circular economy? A study of biogas and nutrient potential. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 12048. [Google Scholar] [CrossRef]

| Samples | pH | Uncertainty of the Result (+/−) | TS (%) | Uncertainty of the Result (+/−) | VS (%) | Uncertainty of the Result (+/−) |
|---|---|---|---|---|---|---|
| Fresh sugar beets | 5.93 | 0.07 | 26.08 | 0.38 | 92.11 | 1.06 |
| H4 | 5.29 | 0.06 | 24.14 | 0.35 | 92.02 | 1.06 |
| O4 | 4.11 | 0.05 | 23.44 | 0.34 | 91.47 | 1.05 |
| H8 | 5.18 | 0.06 | 22.18 | 0.32 | 91.86 | 1.05 |
| O8 | 4.06 | 0.05 | 23.15 | 0.34 | 91.08 | 1.05 |
| H16 | 4.98 | 0.05 | 23.21 | 0.34 | 91.17 | 1.05 |
| O16 | 3.86 | 0.05 | 23.07 | 0.34 | 90.89 | 1.04 |
| H32 | 4.93 | 0.05 | 23.15 | 0.34 | 91.01 | 1.04 |
| O32 | 3.71 | 0.05 | 22.98 | 0.33 | 89.32 | 1.03 |
| Sample Type | Methane Level (%) | Uncertainty of the Result (+/−) | Fermentation Time (day) | Uncertainty of the Result (+/−) |
|---|---|---|---|---|
| Fresh sugar beets | 52.36 | 1.20 | 26 | 0.78 |
| H4 | 50.14 | 1.15 | 24 | 0.72 |
| O4 | 52.02 | 1.19 | 23 | 0.69 |
| H8 | 52.11 | 1.19 | 24 | 0.72 |
| O8 | 51.44 | 1.18 | 22 | 0.66 |
| H16 | 51.86 | 1.19 | 24 | 0.72 |
| O16 | 52.19 | 1.19 | 21 | 0.63 |
| H32 | 51.04 | 1.17 | 24 | 0.72 |
| O32 | 50.48 | 1.16 | 21 | 0.63 |
| Sample | Storage Method | Sugar (g∙kg−1 TS) | Uncertainty of the Result (+/−) | Methanol (g∙kg−1 TS) | Uncertainty of the Result (+/−) | Ethanol (g∙kg−1 TS) | Uncertainty of the Result (+/−) | Acetic Acid (g∙kg−1 TS) | Uncertainty of the Result (+/−) | Lactic Acid (g∙kg−1 TS) | Uncertainty of the Result (+/−) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh sugar beets | ˗ | 44.1 | 1.28 | ND | ˗ | ND | ˗ | ND | ˗ | ND | ˗ |
| 4 | H | 44.1 | 1.28 | 0.36 | 0.01 | 1.29 | 0.04 | 2.29 | 0.02 | 2.18 | 0.03 |
| O | 43.9 | 1.27 | 1.45 | 0.03 | 38.45 | 1.23 | 36.32 | 0.36 | 18.19 | 0.29 | |
| 8 | H | 43.9 | 1.27 | 0.39 | 0.01 | 2.23 | 0.07 | 2.36 | 0.02 | 2.29 | 0.04 |
| O | 42.2 | 1.22 | 1.66 | 0.03 | 42.63 | 1.37 | 38.12 | 0.37 | 22.41 | 0.36 | |
| 16 | H | 43.7 | 1.26 | 0.44 | 0.01 | 2.86 | 0.09 | 2.45 | 0.02 | 2.72 | 0.04 |
| O | 39.1 | 1.13 | 1.69 | 0.03 | 46.44 | 1.49 | 41.45 | 0.41 | 23.45 | 0.37 | |
| 32 | H | 43.4 | 1.26 | 0.49 | 0.01 | 2.95 | 0.09 | 2.84 | 0.03 | 3.11 | 0.05 |
| O | 37.6 | 1.09 | 1.72 | 0.03 | 42.61 | 1.37 | 42.16 | 0.41 | 21.54 | 0.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mioduszewska, N.; Pilarska, A.A.; Pilarski, K.; Adamski, M. The Influence of the Process of Sugar Beet Storage on Its Biochemical Methane Potential. Energies 2020, 13, 5104. https://doi.org/10.3390/en13195104
Mioduszewska N, Pilarska AA, Pilarski K, Adamski M. The Influence of the Process of Sugar Beet Storage on Its Biochemical Methane Potential. Energies. 2020; 13(19):5104. https://doi.org/10.3390/en13195104
Chicago/Turabian StyleMioduszewska, Natalia, Agnieszka A. Pilarska, Krzysztof Pilarski, and Mariusz Adamski. 2020. "The Influence of the Process of Sugar Beet Storage on Its Biochemical Methane Potential" Energies 13, no. 19: 5104. https://doi.org/10.3390/en13195104
APA StyleMioduszewska, N., Pilarska, A. A., Pilarski, K., & Adamski, M. (2020). The Influence of the Process of Sugar Beet Storage on Its Biochemical Methane Potential. Energies, 13(19), 5104. https://doi.org/10.3390/en13195104







