Study on the Influencing Factors of the Emulsion Stability of a Polymeric Surfactant Based on a New Emulsification Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
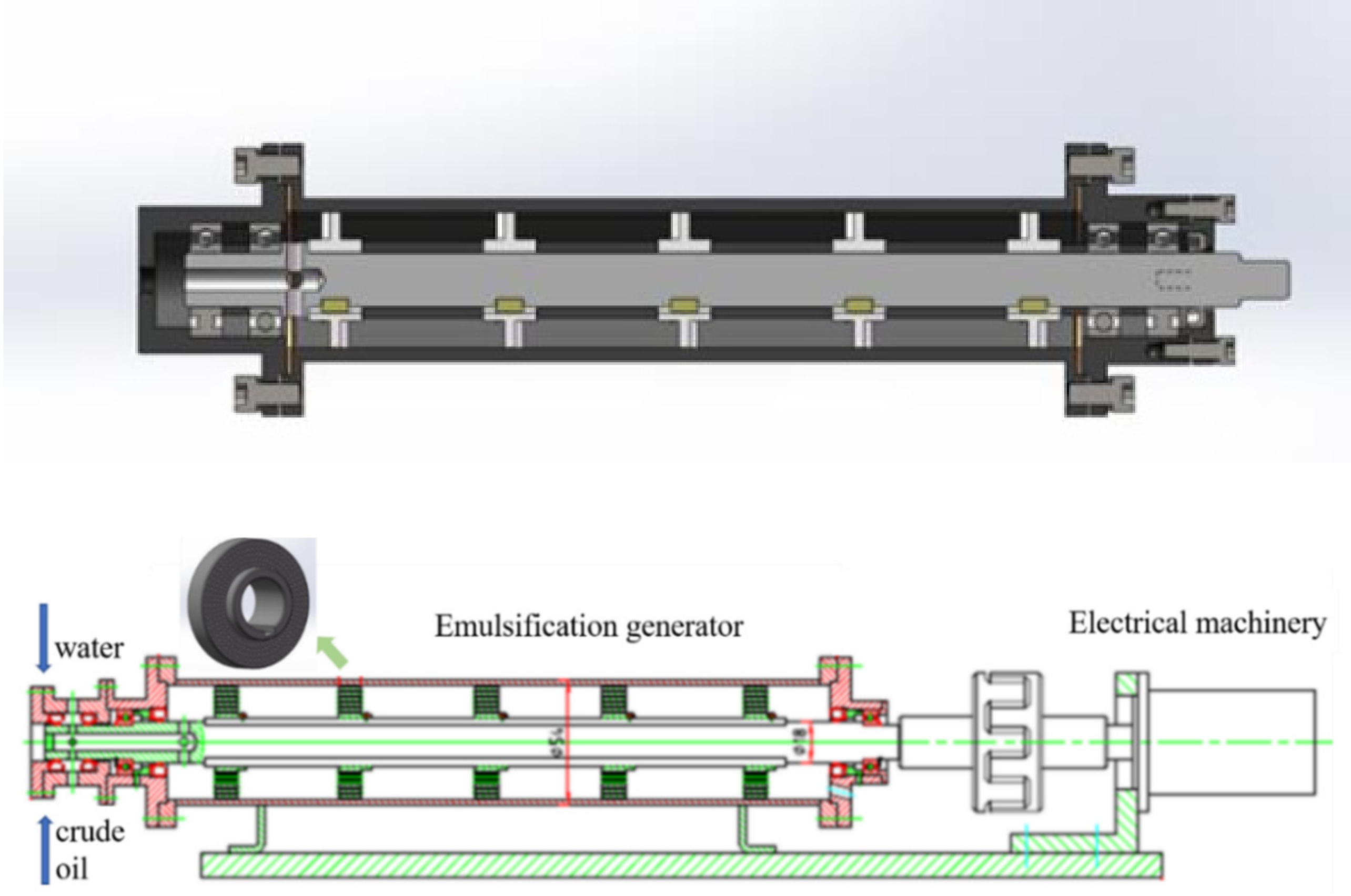
2.2.1. Preparation of the Emulsion by the Sieve Plate Rotary Emulsification Device
2.2.2. Determination of the Water Separation Rate
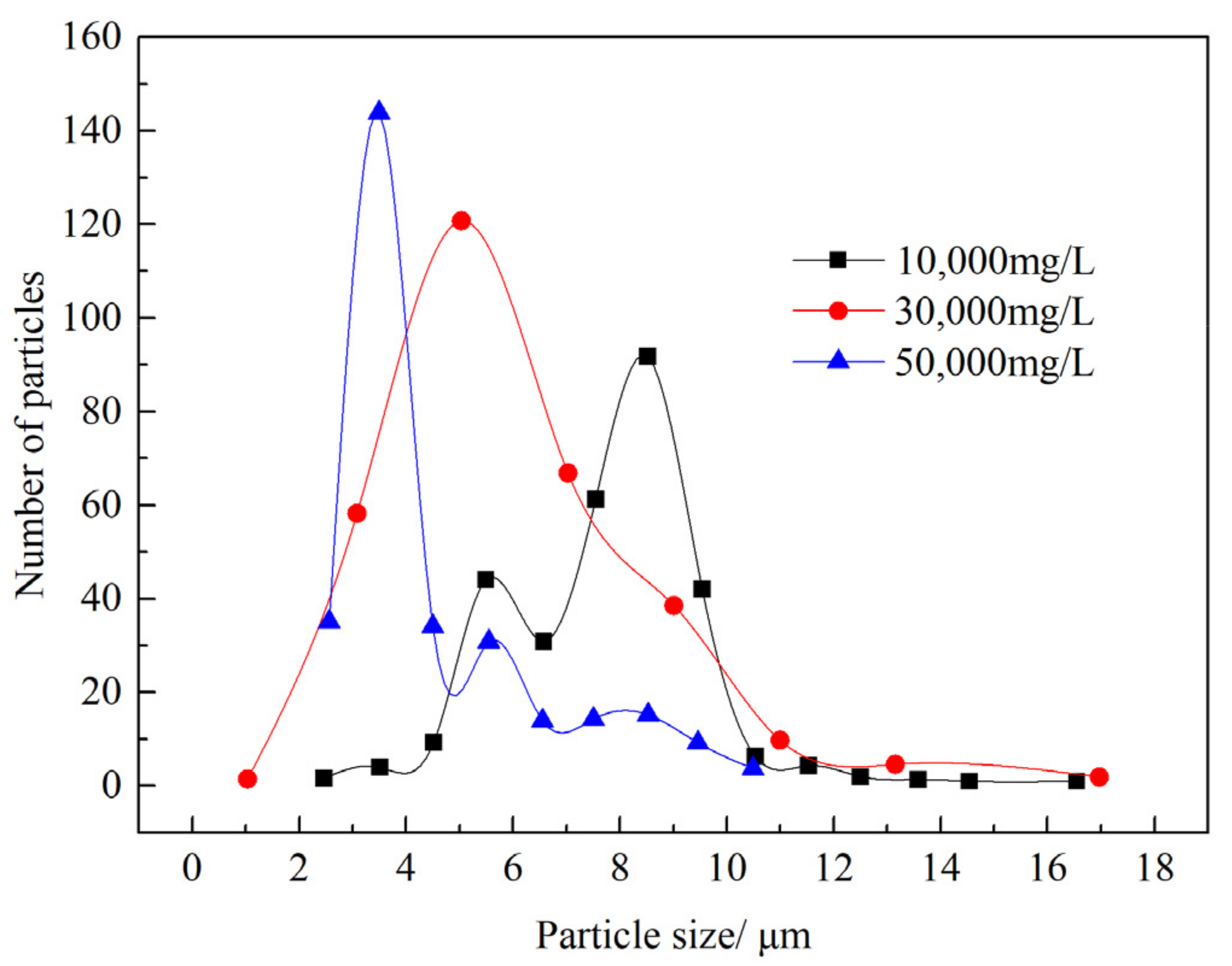
2.2.3. Measurement of Particle Size
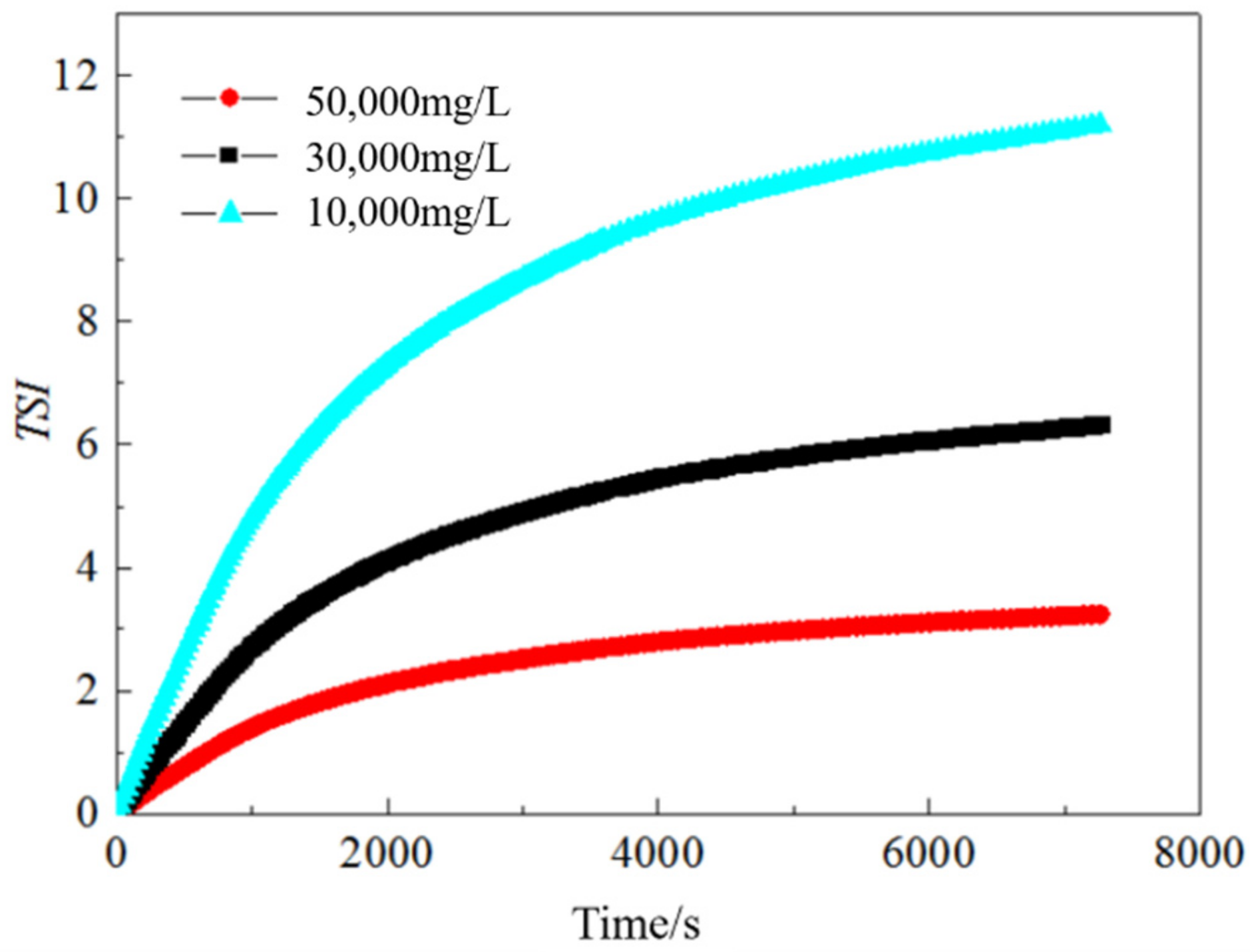
2.2.4. Stability Analysis of the Emulsion
3. Results and Discussion
3.1. Parameter Optimization of the Sieve Plate Rotary Emulsification Device
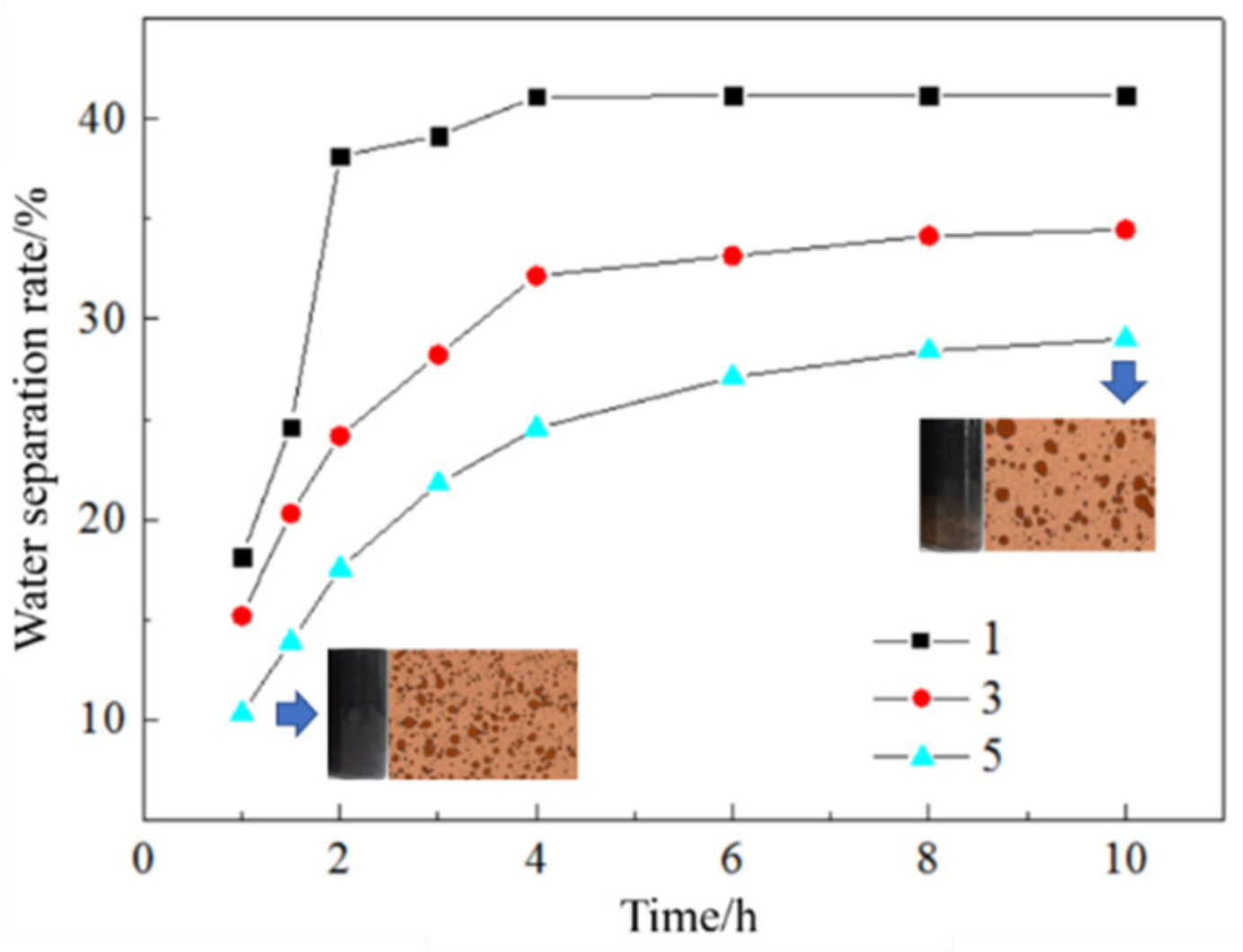
3.1.1. Number of Sieve Plates
3.1.2. Diameter of Sieve Pores
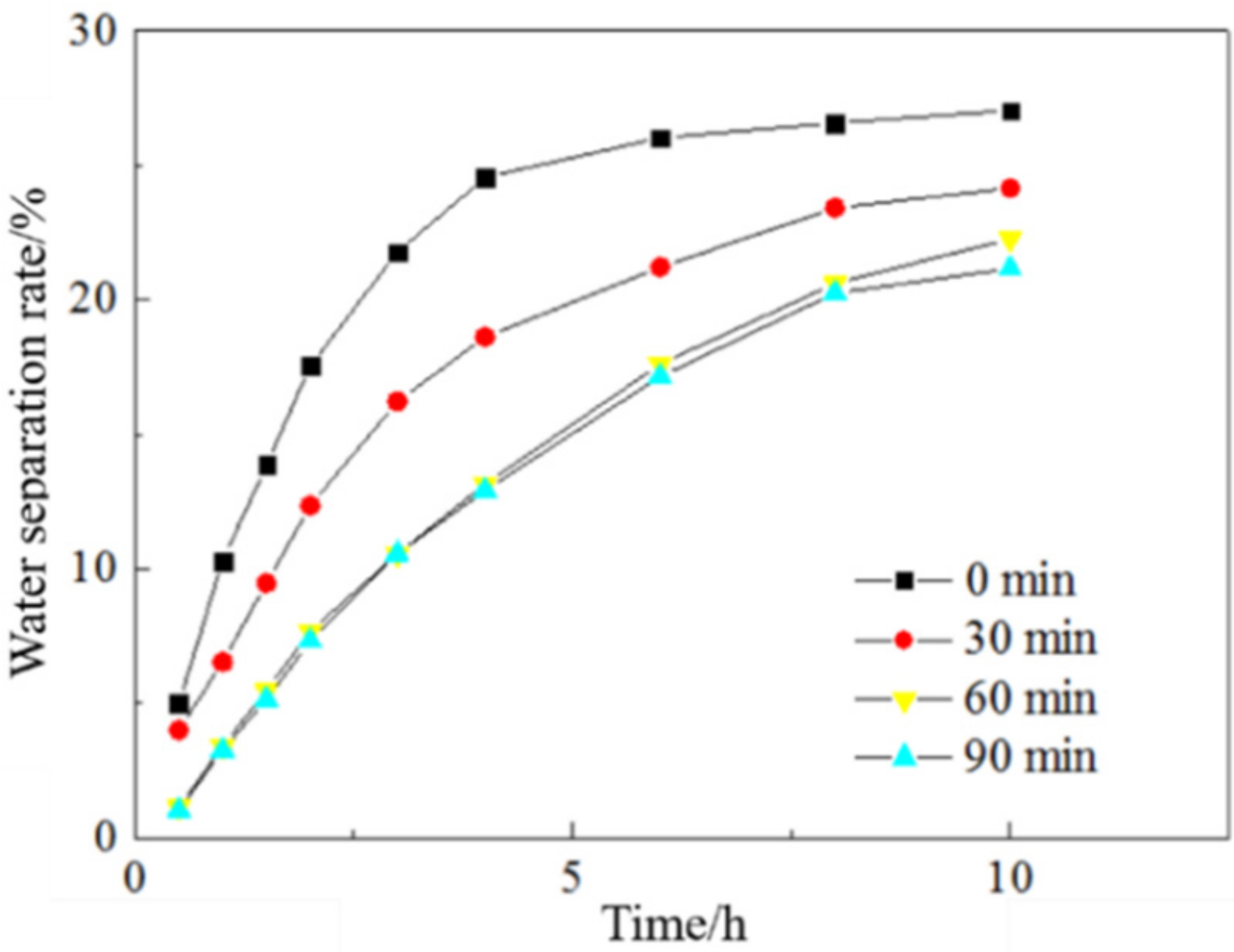
3.1.3. Emulsification Time
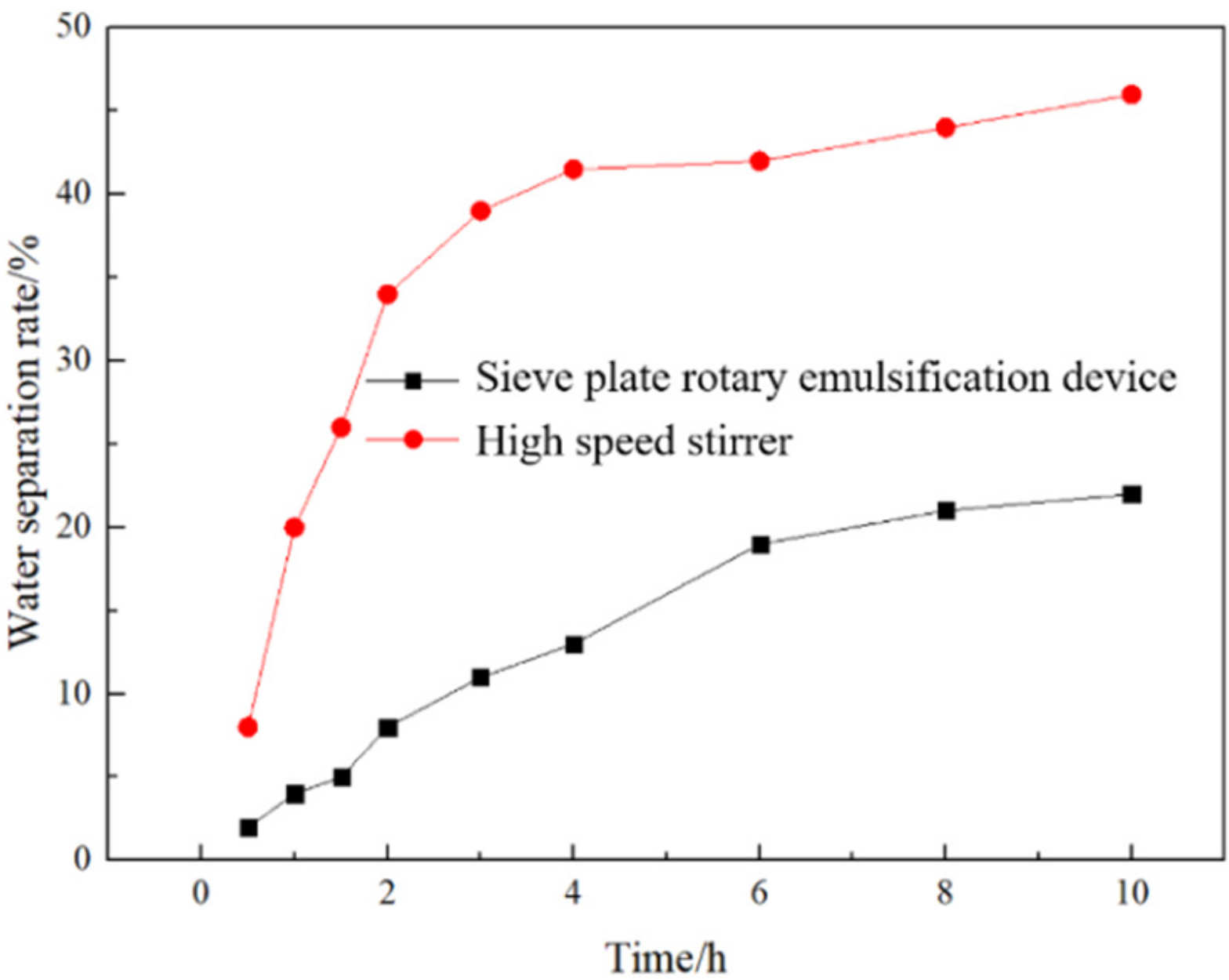
3.2. Comparison of Emulsions Prepared by Different Devices
3.3. Influencing Factors of the Stability of the Emulsion Prepared with the Polymeric Surfactant
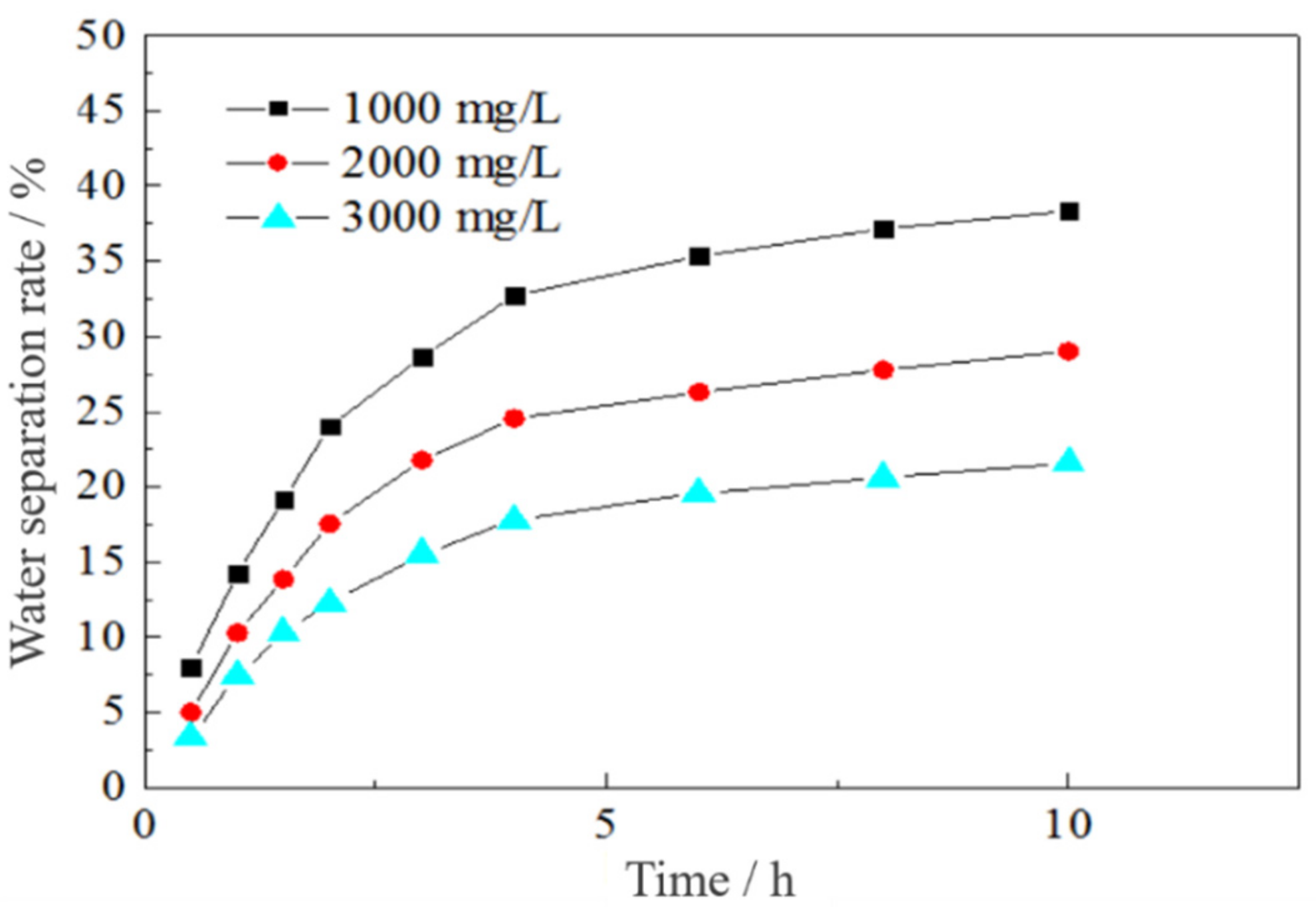
3.3.1. Effect of the Surfactant Concentration on Emulsion Stability
3.3.2. Effect of Salinity on the Emulsion Stability
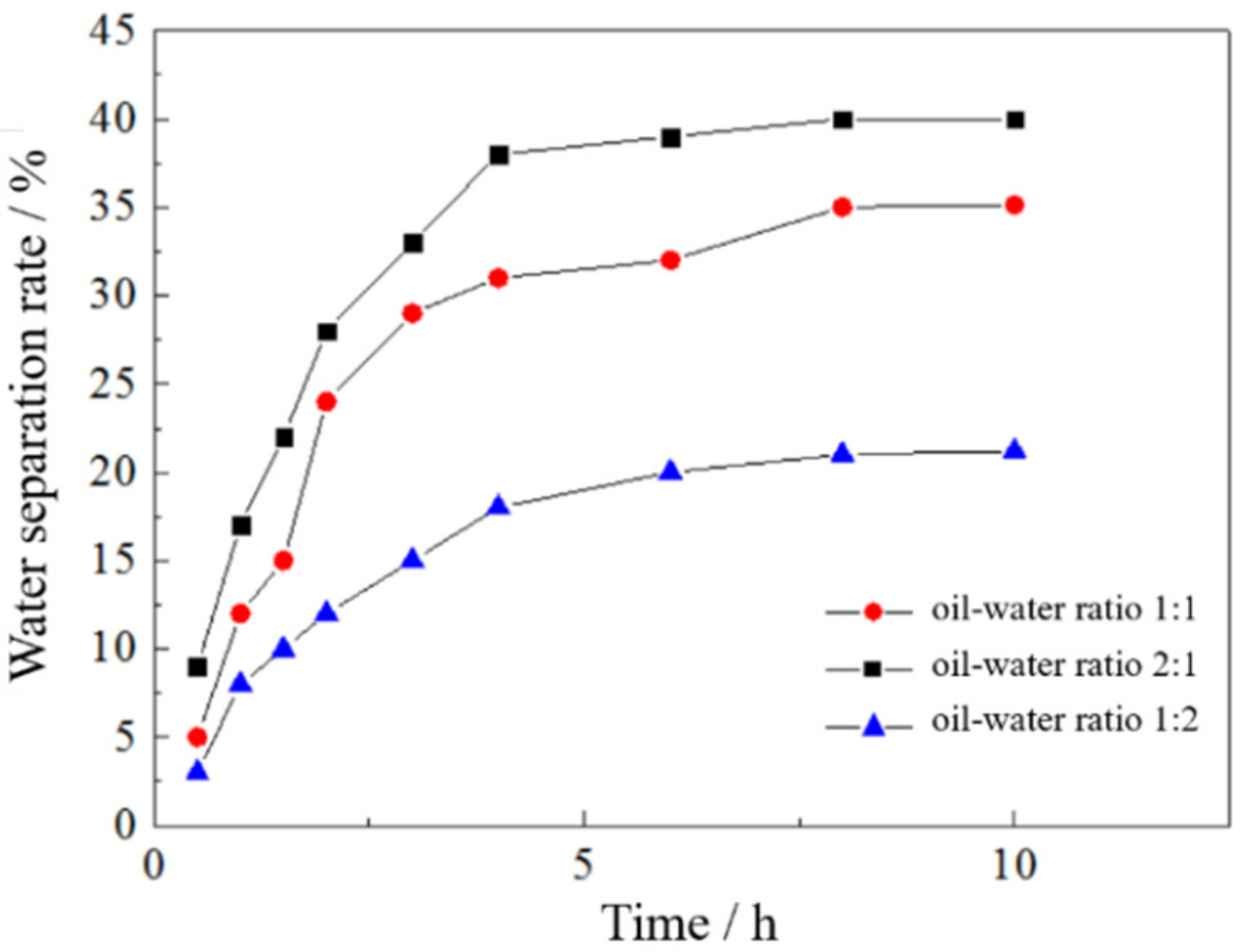
3.3.3. Effect of the Oil–Water Ratio on the Emulsion Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shiran, B.S.; Skauge, A. Enhanced oil recovery (EOR) by combined low salinity water/polymer flooding. Energy Fuels 2013, 27, 1223–1235. [Google Scholar] [CrossRef]
- Urbissinova, T.S.; Trivedi, J.J.; Kuru, E. Effect of elasticity during viscoelastic polymer flooding: A possible mechanism of increasing the sweep efficiency. J. Can. Pet. Technol. 2010, 49, 49–56. [Google Scholar] [CrossRef]
- Jamaloei, B.Y.; Kharrat, R.; Torabi, F. Analysis and correlations of viscous fingering in low-tension polymer flooding in heavy oil reservoirs. Energy Fuels 2010, 24, 6384–6392. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Zheng, W.; Zhou, B.; Zhao, H.; Li, X.; Zhang, L.; Zhu, Z.; Kang, W.; Ketova, Y.A.; et al. Effect of hydrophobic group content on the properties of betaine-type binary amphiphilic polymer. J. Mol. Liq. 2020, 311, 113358–113365. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, J.; Wu, J.; Wang, G. Application of polymer flooding technology in Daqing Oilfield. Acta Pet. Sin. 2005, 26, 74–78. [Google Scholar] [CrossRef]
- Seright, R.S.; Zhang, G.; Akanni, O.O.; Wang, D. A comparison of polymer flooding with in-depth profile modification. J. Can. Pet. Technol. 2012, 51, 393–402. [Google Scholar] [CrossRef]
- Akiyama, E.; Kashimoto, A.; Hotta, H.; Kitsuki, T. Mechanism of oil-in-water emulsification using a water-soluble amphiphilic polymer and lipophilic surfactant. J. Colloid Interface Sci. 2006, 300, 141–148. [Google Scholar] [CrossRef]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef]
- Qiang, T.; Zhang, G.; Wang, X. Research developments on amphiphilic hyperbranched polymers. Chem. Ind. Eng. Prog. 2013, 32, 666–670. [Google Scholar] [CrossRef]
- Wei, D.; Song, Y.; Jin, Y.; Sun, J. Research Progress in Amphiphilic Polymer Used for Enhanced Oil Recovery. Fine Chem. 2004, 21, 662–666. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, H.; Meng, Z.; Jiang, J.; Jin, Y.; Deng, Z.; Su, W.; Li, Z.; Kang, W. Salt effect on hydrophobically modified polyacrylamide-containing crude oil emulsions: Stability and rheology study. Colloid Polym. Sci. 2018, 296, 515–527. [Google Scholar] [CrossRef]
- Aman, Z.M.; Dieker, L.E.; Aspenes, G.; Sum, A.K. Influence of model oil with surfactants and amphiphilic polymers on cyclopentane hydrate adhesion forces. Energy Fuels 2010, 24, 5441–5445. [Google Scholar] [CrossRef]
- Qiu, J. Stability of the oil-water emulsion formed during amphiphilic polymer flooding. Pet. Sci. Technol. 2013, 31, 142–147. [Google Scholar] [CrossRef]
- Kinsinger, M.I.; Sun, B.; Abbott, N.L.; Lynn, D.M. Reversible control of ordering transitions at aqueous/liquid crystal interfaces using functional amphiphilic polymers. Adv. Mater. 2007, 19, 4208–4212. [Google Scholar] [CrossRef]
- Hevus, I.; Kohut, A.; Voronov, A. Interfacial micellar phase transfer using amphiphilic invertible polymers. Polym. Chem. 2011, 2, 2767–2770. [Google Scholar] [CrossRef]
- Kang, W.; Wei, S.; Ji, Y.; Zhao, H.; Zhao, J.; Yang, R. Characterization of amphiphilic polymer O/W emulsion through porous media. Polym. Mater. Sci. Eng. 2014, 30, 76–80. [Google Scholar] [CrossRef]
- Sarkar, B.; Venugopal, V.; Bodratti, A.M.; Tsianou, M. Nanoparticle surface modification by amphiphilic polymers in aqueous media: Role of polar organic solvents. J. Colloid Interface Sci. 2013, 397, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Zhu, Z.; Wang, P.; Yin, X.; Cao, C.; Guo, S.; Kang, W. Effect of different molecular weight amphiphilic polymers on emulsifying behavior. Pet. Sci. Technol. 2018, 36, 1544–1551. [Google Scholar] [CrossRef]
- Sun, J.; Xu, X.; Wang, J.; Zhang, W.; Yang, H.; Jing, X.; Shi, X. Synthesis and emulsification properties of an amphiphilic polymer for enhanced oil recovery. J. Dispers. Sci. Technol. 2010, 31, 931–935. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, F.; Kang, X.; Zhao, J.; Cui, Y.; Zhao, W. Experimental study of heavy oil percolation in porous medium by amphiphilic polymer flooding. China Offshore Oil Gas 2016, 28, 61–65. [Google Scholar] [CrossRef]
- Zhao, Y.; Ke, Y.; Hu, X.; Peng, F. Synthesis, characterization and emulsification properties of an amphiphilic copolymer for enhanced oil recovery. In Proceedings of the 2nd International Conference on Frontiers of Materials Synthesis and Processing, Sanya, China, 10–11 November 2018. [Google Scholar] [CrossRef]
- Yang, H.; Shao, S.; Zhu, T.; Chen, C.; Liu, S.; Zhou, B.; Hou, X.; Zhang, Y.; Kang, W. Shear resistance performance of low elastic polymer microspheres used for conformance control treatment. J. Ind. Eng. Chem. 2019, 79, 295–306. [Google Scholar] [CrossRef]
- Sawada, H.; Umedo, M.; Kawase, T.; Tomita, T. Synthesis and properties of fluoroalkylated end-capped betaine polymers. Eur. Polym. J. 1999, 35, 1611–1617. [Google Scholar] [CrossRef]
- Favresse, P.; Laschewsky, A.; Emmermann, C.; Gros, L. Synthesis and free radical copolymerization of new zwitterionic monomers: Amphiphilic carbobetaines based on isobutylene. Eur. Polym. J. 2001, 37, 877–885. [Google Scholar] [CrossRef]
- Murugaboopathy, S.; Matsuoka, H. Salt-dependent surface activity and micellization behaviour of zwitterionic amphiphilic diblock copolymers having carboxybetaine. Colloid Polym. Sci. 2015, 293, 1317–1328. [Google Scholar] [CrossRef]
- Picco, A.S.; Silbestri, G.F.; Dario Falcone, R.; Azzaroni, O.; Ceolin, M.; Mariano Correa, N. Probing the microenvironment of unimicelles constituted of amphiphilic hyperbranched polyethyleneimine using 1-methyl-8-oxyquinolinium betaine. Phys. Chem. Chem. Phys. 2014, 16, 13458–13464. [Google Scholar] [CrossRef]
- Zhu, Z.; Kang, W.; Yang, H.; Wang, P.; Zhang, X.; Yin, X.; Lashari, Z.A. Study on salt thickening mechanism of the amphiphilic polymer with betaine zwitterionic group by beta-cyclodextrin inclusion method. Colloid Polym. Sci. 2017, 295, 1887–1895. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, W.; Meng, L.; Wang, Y.; Guo, L. Emulsion Characteristics of the Hydrophobically Associating Polyacrylamid Used for Oilflooding. Acta Pet. Sin. Pet. Process. Sect. 2010, 26, 628–634. [Google Scholar] [CrossRef]
- Zhu, Z.; Kang, W.; Sarsenbekuly, B.; Yang, H.; Dai, C.; Yang, R.; Fan, H. Preparation and solution performance for the amphiphilic polymers with different hydrophobic groups. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Hao, H.; Wu, X.; Sun, j.; Guo, D. Mixing Charactistiss of Liquid-Liquid Dispersions in a Central Tornado Flow Stirring Vessel. Petrochem. Technol. 2003, 32, 33–36. [Google Scholar] [CrossRef]
- Tolosa, L.-I.; Forgiarini, A.; Moreno, P.; Salager, J.-L. Combined effects of formulation and stirring on emulsion drop size in the vicinity of three-phase behavior of surfactant-oil water systems. Ind. Eng. Chem. Res. 2006, 45, 3810–3814. [Google Scholar] [CrossRef]
- Diftis, N.; Kiosseoglou, V. Stability against heat-induced aggregation of emulsions prepared with a dry-heated soy protein isolate-dextran mixture. Food Hydrocoll. 2006, 20, 787–792. [Google Scholar] [CrossRef]
- Yang, H.; Kang, W.; Wu, H.; Yu, Y.; Zhu, Z.; Wang, P.; Zhang, X.; Sarsenbekuly, B. Stability, rheological property and oil-displacement mechanism of a dispersed low-elastic microsphere system for enhanced oil recovery. RSC Adv. 2017, 7, 8118–8130. [Google Scholar] [CrossRef]
- Yang, H.; Kang, W.; Yu, Y.; Yin, X.; Wang, P.; Zhang, X. A new approach to evaluate the particle growth and sedimentation of dispersed polymer microsphere profile control system based on multiple light scattering. Powder Technol. 2017, 315, 477–485. [Google Scholar] [CrossRef]
- Yao, H. Improvement of the crude oil emulsion preparation method. Oil-Gas Field Surf. Eng. 2017, 36, 27–31. [Google Scholar] [CrossRef]








| Parameter | Value |
|---|---|
| Density/(g·cm−3) | 0.845 |
| Viscosity/mPa·s | 11.3 |
| w (Saturated hydrocarbon)/% | 81.32 |
| w (Aromatic hydrocarbon)/% | 14.60 |
| w (colloid)/% | 3.01 |
| w (asphaltene)/%. | 1.07 |
| Type | NaCl | MgCl2·6H2O | CaCl2 |
|---|---|---|---|
| Concentration/(mg/L) | 29,678.3 | 1467.1 | 1943.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Xiong, Y.; Guo, B.; Yang, H. Study on the Influencing Factors of the Emulsion Stability of a Polymeric Surfactant Based on a New Emulsification Device. Energies 2020, 13, 4794. https://doi.org/10.3390/en13184794
Wei Y, Xiong Y, Guo B, Yang H. Study on the Influencing Factors of the Emulsion Stability of a Polymeric Surfactant Based on a New Emulsification Device. Energies. 2020; 13(18):4794. https://doi.org/10.3390/en13184794
Chicago/Turabian StyleWei, Yusen, Youming Xiong, Bumin Guo, and Hongbin Yang. 2020. "Study on the Influencing Factors of the Emulsion Stability of a Polymeric Surfactant Based on a New Emulsification Device" Energies 13, no. 18: 4794. https://doi.org/10.3390/en13184794
APA StyleWei, Y., Xiong, Y., Guo, B., & Yang, H. (2020). Study on the Influencing Factors of the Emulsion Stability of a Polymeric Surfactant Based on a New Emulsification Device. Energies, 13(18), 4794. https://doi.org/10.3390/en13184794






