Abstract
Biological desulfurization of biogas from a field-scale anaerobic digester in Peru was tested using air injection (microaeration) in separate duplicate vessels and chemical desulfurization using duplicate iron filters to compare hydrogen sulfide (H2S) reduction, feasibility, and cost. Microaeration was tested after biogas retention times of 2 and 4 h after a single injection of ambient air at 2 L/min. The microaeration vessels contained digester sludge to seed sulfur-oxidizing bacteria and facilitate H2S removal. The average H2S removal efficiency using iron filters was 32.91%, with a maximum of 70.21%. The average H2S removal efficiency by iron filters was significantly lower than microaeration after 2 and 4 h retention times (91.5% and 99.8%, respectively). The longer retention time (4 h) resulted in a higher average removal efficiency (99.8%) compared to 2 h (91.5%). The sulfur concentration in the microaeration treatment vessel was 493% higher after 50 days of treatments, indicating that the bacterial community present in the liquid phase of the vessels effectively sequestered the sulfur compounds from the biogas. The H2S removal cost for microaeration (2 h: $29/m3 H2S removed; and 4 h: $27/m3 H2S removed) was an order of magnitude lower than for the iron filter ($382/m3 H2S removed). In the small-scale anaerobic digestion system in Peru, microaeration was more efficient and cost effective for desulfurizing the biogas than the use of iron filters.
1. Introduction
Anaerobic digestion (AD) reduces organic pollution while creating renewable energy in the form of methane (CH4)-enriched biogas. However, the hydrogen sulfide (H2S) concentration in biogas can be high, ranging from 100 to 30,000 ppm [1,2]. Sulfate-rich feedstocks, such as swine manure, can produce biogas with high H2S concentrations, which affects the use of the biogas after digestion [3]. H2S is originated by the microbial breakdown of organic material during the anaerobic digestion [4]. The presence of H2S in biogas can corrode biogas system components (pipes, compressors, gas storage tanks, engine generator sets (EGS) for electricity production, and boilers) through the formation of corrosive sulfuric acid when water vapor is present [5,6]. The presence of H2S can compromise the functions of EGS, produce odor prior to biogas utilization, and is toxic at high concentrations [7]. In addition, H2S contaminants in advanced energy conversion equipment, such as fuel cells, can permanently foul the equipment [8]. According to the US Occupational Safety and Health Administration (OSHA), 0.01–1.5 ppm H2S is the odor threshold (characteristic rotten egg smell), while 2–5 ppm H2S may cause nausea and headaches, and 1000–2000 ppm H2S can cause death, depending on the length of exposure.
Biogas purification has been researched in recent years to protect health and biogas utilization equipment [9]. While biogas desulfurization can be achieved using multiple methods, generally, H2S removal methods can be divided into two groups: physical-chemical removal and biological removal using sulfur-oxidizing bacteria (SOB) [10]. Physical-chemical methods include adsorption, absorption, scrubbing, membrane separation, and precipitation, where chemicals are used to either bind and capture sulfur prior to H2S formation or capture the H2S molecule after formation [11]. The iron sponge process was implemented during the 19th century for H2S removal and has been in use in Europe and the United States for over 100 years [12]. In Peru and areas with other low-cost digestion systems, a simple iron filtration system consists of a tank, usually a cylindrical tank or pipe, containing iron oxide in the form of iron sponges located between the digester and biogas utilization system [13]. The chemical bond between sulfur in the biogas and iron oxide occurs within an iron filter system at ambient temperature. Alkaline conditions are needed, with a pH value greater than 7.5 [5]. Shelford et al. (2019) asserted that for each kg of iron oxide (Fe2O3) present in the system, 0.56 kg of sulfide can be removed from the biogas as elemental sulfur, as shown below in Equation (1). Iron oxide can be regenerated by adding air (O2), which prolongs the life of the media, as shown in Equation (2) [5]. However, after each regeneration, the filters lose approximately 30% of their effectiveness due to the obstruction by elemental sulfur, which reduces the volume of biogas that can be treated between filter changes [5,14]. Saturated iron sponge material (when H2S is no longer removed and/or when the wood bark has deteriorated) can be burned (where permitted), landfilled, or spread on agricultural land [5].
Formation of iron sulfide: Fe2O3 + 3H2S → Fe2S3 + 3H2O
Regeneration and formation of elemental sulfur: Fe2S3 + 3/2O2 →Fe2O3 + 3S
There are varying operational and maintenance costs associated with iron filter systems. Generally, physical-chemical removal methods have higher costs due to chemical acquisition, energy use associated with the regeneration of saturated materials, and disposal of secondary pollutants produced [15]. For example, the cost of using iron filters for H2S removal (without regeneration) have been shown to vary from $2.93/d for 3000 ppm H2S removed at a 13.42 m3/h biogas production rate from the manure of 100 lactating cows, to $117.77/d for 3000 ppm H2S removed at a 536.89 m3/h biogas production rate from the manure of 4000 lactating cows [5]. When the adsorbent materials are saturated, the materials must be properly disposed of, which poses health and environmental risks [16]. The cost of iron filter use and disposal will vary based on the cost of the fresh material, revenue for any sulfur extracted, disposal costs of processed adsorbent, and transportation distance [17].
Schiavon et al. (2017) determined that a commercial reagent powder containing Fe-EDTA resulted in a high H2S removal efficiency (99%), forming elemental sulfur through absorption [18]. Metallic wastes rich in Fe or metallic waste products, such as mining wastes or fly ash from thermo-electrical plants, have also been proposed as sulfide removal materials [19]. A recent study showed that corn stover and maple biochar impregnated with Fe added into an anaerobic digester significantly reduced H2S production by up to 93.3% without affecting CH4 production [20]. Electrochemically-assisted scrubbing and stripping of H2S has also been shown [21]. However, many of these H2S removal techniques may not be available for small-scale digester operators due to the high costs and the need for specialized knowledge and access to equipment and absorbent materials [15], which reinforces the need for low-cost and easy-to-operate H2S removal techniques for small-scale digester operators.
Biological-based H2S removal methods include biotrickling filters (BTF), biofiltration, bioscrubbers, and air injection [10,22]. Bio-desulfurization is based on the inoculation of microorganisms, mainly from the families of Thiobacillus, Thiomonas, Paracoccus, Acidithiobacillus, Sulfurimonas or Halothiobacillus, which oxidize H2S to elemental sulfur, sulfate, or thiosulfate through bacterial activity [9,16,23]. Biological-based methods have some advantages over the physical-chemical techniques, such as less intensive operational conditions, and thus, lower operational costs, energy consumption, and emissions of secondary pollutants [24,25]. Biological-based techniques can be divided into two categories: internal and external desulfurization [20]. An internal desulfurization system applies microaerobic conditions directly into the anaerobic digester headspace. External desulfurization includes bioscrubbers, biotrickling filters (BTF), biofiltration, and external microaeration, where the micro-aerated environment is separate from the digestion environment, with H2S removal occurring in a separate biogas vessel between the digester and the biogas utilization equipment [26].
According to Wu et al. (2020), BTF is currently the most widely used method for cost-effective H2S removal [25]. It has been suggested that the biological process should be monitored and controlled to reduce issues, such as bed clogging [27]. Additionally, with high H2S concentrations in the biogas, a nitrate source may be needed to support high biological growth rates [24,25]. Using a BTF system, Zhang et al. (2020) obtained more than 95% H2S removal, and Dupnock and Deshusses (2020) showed more than 97% H2S removal from biogas [10,12]. Wu et al. (2020) showed 100% removal of H2S using a slightly alkaline BTF with polypropylene rings as the packing material and air injection to provide the O2 used as the electron acceptor [25]. Khanongnuch et al. (2019a, 2019b) used Thiobacillus sp. as a sulfur-oxidizing and nitrate-reducing bacterium in a BTF to achieve >99% H2S removal from a gas stream while simultaneously removing NO3- [28,29].
While biofiltration is less common than BTF, biofiltration has also been shown to be effective for H2S removal [27]. A biogas upgrading system based on the microalgae Chlorella sorokiniana provided 100% H2S removal, with oxidation of H2S to sulfate [30]. Haosagul et al. (2020) showed 100% H2S removal using a bioscrubber with Pseudomonas, Leucobacter, Thioalkalimicrobium, and Brevundimonas present [31]. Enrichment of SOB inoculum using Na2S has been shown to increase H2S removal by stabilizing and increasing the pH. Cheng et al. (2018) found a stable H2S removal, up to 95%, when a pH of 7.5 to 8.2 was maintained in the sludge [32].
Microaeration consists of dosing very small amounts of oxygen into an anaerobic digester, converting H2S to elemental sulfur, sulfate or thiosulfate [33]. Microaeration has shown a great potential for H2S removal due to lower costs, yet there are still challenges that need to be addressed, such as the need for periodic maintenance to avoid clogging problems in the microaeration pump, and the contamination with N2 with air injection [34]. Large-scale digesters have shown from 80 to 100% H2S reduction using microaeration [6].
Alternative H2S reduction methods have also been investigated. Adding gummy vitamin waste to dairy manure digesters reduced the H2S content in the biogas by 66 to 83% [7]. Waste-derived adsorbent materials (wood-derived biochar, sludge-derived activated carbon, and activated ash) were used for H2S removal, with the activated ash having the highest H2S removal efficiency (3.2 mg H2S/g of adsorption capacity) and the sludge-derived activated carbon having the lowest efficiency (2.2 mg H2S/g of adsorption capacity) [35]. While biochar impregnated with Fe had 100% H2S reduction in diary manure digesters, and unmodified biochar resulted in 90.5% H2S removal [20].
Farmers in rural Latin America operate over 1100 small-scale agricultural digesters compared to over 350 in the US and over 1100 in Europe [36,37]. Many small farmers in Latin America use digesters to manage their manure. In Peru, the most common technique to remove H2S is filtration using iron chips or iron sponges, which is used in ecological education centers, such as Casa Blanca in Pachacamac, Peru. Microaeration has been applied mainly in large-scale digesters. Most small farmers in Latin America have not used microaeration to remove H2S from biogas and rely on iron filtration. This study aims to demonstrate how microaeration compares to iron filtration in terms of H2S removal efficiency and cost for small-scale digesters. This research compared two low-cost techniques: traditional iron filters used in Latin America and microaeration in removing H2S from biogas produced in a field-scale digester in Peru. The objectives of this research were to (1) test the efficiency of duplicate microaeration vessels using two retention times to reduce H2S from biogas, (2) compare the efficiency of microaeration to the use of iron filters in duplicate, and (3) conduct a cost analysis based on the quantity of H2S removed. The results can be used to understand how efficiency, cost, and accessibility affect H2S removal techniques used by thousands of small-scale anaerobic digester operators.
2. Materials and Methods
2.1. Digestion Reactor
A field-scale digestion system using the Taiwanese-model was constructed using high density polyethylene (HDPE) at the Model Centre for Solid Waste Treatment (CEMTRAR) in La Molina, Lima, Perú. The reactor capacity was 8.5 m3, with a 6.37 m3 liquid volume and a 2.13 m3 biogas headspace (Figure 1). The reactor was loaded daily with swine manure from the Porcine Experimental Unit at La Molina National Agrarian University (UNALM) in Lima, Peru, using an average organic loading rate of 1.13 kg VS/m3 d. The manure had 31.6% total solids (TS), with 0.73% sulfur (S). During the study, the temperature and pH of the effluent were measured daily, with an average temperature of 21 ± 2 °C in the digester (ambient temperature conditions), an average pH of 7.44, and an average retention time of 50 days. Analyses of the liquid manure entering the digester were conducted by the UNALM Laboratory for the Analysis of Water, Soils, Plants and Fertilizers. These analyses included percentage of carbon, using the modified Walkley and Black method [38], oxidizing organic carbon with potassium dichromate, and percentage of nitrogen according to the Kjeldahl method [39]. The TS (total solids) and VS (volatile solids) of the swine manure were measured according to Standard Methods [40], and the pH using the electrometric procedure according to US EPA Method 9045D. The percentage of sulfur was measured using a turbidimetric method using barium chloride, and percentage of sodium using atomic absorption spectrophotometry, according to Method 3111 [41]. The digester had operated for seven years prior to this experiment, with no specific desulfurization method utilized during prior operation.
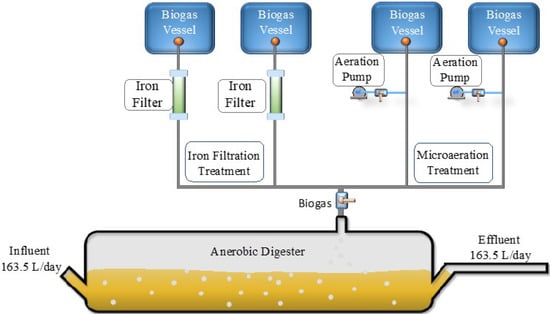
Figure 1.
Schematic design of duplicate treatments for the desulfurization of biogas using iron filtration and microaeration.
Upon exiting the digester and before entering the biogas storage vessels, the untreated biogas was analyzed for CH4, CO2, O2, H2, and H2S using a Multitec 545 gas analyzer (Sewerin, Gütersloh, Germany). The biogas was stored in four separate biogas vessels, with two vessels storing the biogas after duplicate microaeration injection, and two vessels storing the treated biogas after passing through the duplicate iron filters. The composition of the treated biogas in each vessel was analyzed daily using the Multitec 545 gas analyzer. The biogas production rate was measured with a flowmeter (Pa 2-25l Flow Meter, Barry Century, Beijing, China) once a day while the digester was loaded with manure.
2.2. Micro-Aeration
Biogas microaeration was conducted using duplicate biogas vessels composed of a thick Low Density Polyethylene (LDPE) (0.208 m3 each). The biogas holding vessels were loaded and unloaded daily to renew the biogas and simulate daily use of the biogas. After each vessel was loaded with biogas, ambient air was pumped into the headspace of the biogas vessels for approximately one minute using an air pump (Venus AP 208, Shanghai, China), with a minimum and maximum flow rate of 2 and 3 L/min, respectively. Approximately 2.08 L of air were injected daily (1% of the vessel volume). Measurements were taken daily at 2 and 4 h after the single air injection to test the effect of two retention times on the biological oxidation process.
Each microaeration vessel was inoculated with SOB using 10 L of sludge from the anaerobic digester outlet. This sludge was characterized after the end of the experiment for total S and sulfate. The microaeration treatment experiment began over three stages. Initially, microaeration tests were conducted before inoculum introduction. Inoculum was then introduced and a start-up period of 3 days was given for adaption before the microaeration treatment began. The monitoring period was 50 days from 28 June to 16 August 2018.
2.3. Sulfur-Oxidizing Bacteria (SOB) Observation and Isolation
The SOB observation and isolation technique was based on the methodology from the Open and Distance National University [42] in Bogotá, Colombia. The H2S-oxidizing microorganisms were grown using 1 mL of sludge from each microaeration vessel inoculated separately in tubes consisting of an incubated culture medium at 37 °C. The criteria for identifying SOB-positive tubes were (1) visualization of the turbidity of the medium, (2) microscopic verification of the growth of the microorganism in the medium liquid using an optical microscope, and (3) observation of the presence of colonies in the solids. Due to the high bacterial density in the tubes, isolates were taken and incubated in a solid medium to obtain pure cultures. Finally, a Gram stain was used to identify the phenotypic characteristics of the isolated SOB colonies in the digester effluent samples and replicate microaeration vessels using a 100x microscope with a stereoscope.
2.4. Iron Filter
Two 0.979 L iron filters were built using two PVC tubes (0.1108 m × 0.1016 m) filled with iron sponge (domestic material used for scrubbing pots and pans), which was composed of iron oxide (Fe2O3). Prior to use, the iron sponge substrate was immersed in HCl and NaOH solutions to oxidize the material. The biogas from the digester passed through one of the two duplicate iron filters positioned inside a biogas conveyance hose. The scrubbed biogas then entered the connecting duplicate biogas vessels.
The filter substrate (iron sponge) was renewed every 10 days during the 50 day experiment. The quantity of the filter material needed for the total H2S removal was based on the average of the biogas production rate (0.5 L/min) measured prior to the experiment and average H2S concentration in the biogas (3000 ppm). The calculations were based on Equations (1) and (2) above and previous research that showed the H2S absorption limit of iron sponge is 56% (0.56 kg H2S/kg Fe2O3), resulting in 7.45 g of Fe2O3 needed for H2S removal [5,43,44]. The amount of Fe filter used in each PVC pipe was 8.94 g Fe2O3, resulting in 20% more filter added than the theoretical need.
2.5. Statistical Methods
Statistical analysis, including ANOVA and multiple regression was performed using Minitab 18, with significance defined at alpha of 0.05. A non-parametric Kruskal–Wallis test using the percentage removal of H2S was performed, with the levels based on applied treatment technology (iron filter or microaeration) and retention time (2 or 4 h), as the samples were independent of each other.
3. Results and Discussion
3.1. Digester pH and Temperature and Initial H2S Concentration
There was no significant correlation between the pH of the digester and the initial (pre-treatment) H2S concentration in the biogas (p-value = 0.178). The digester pH started high (8.14) at the beginning of the 50 day experiment, with an average of 7.44 and a range of 6.91 to 8.33 over the 50 days, with 4 of 32 data points < 7. The pH values did not correspond with the H2S concentration from the digester, which started low (2300 ppm H2S), increased to a maximum of 4000 ppm, but remained <3500 ppm after Day 33 and <3000 ppm from Days 48–50. The variation in the H2S concentration was more likely influenced by the influent S concentration and bacterial community than the pH value in the digester. According to Krayzelova et al. (2015), the concentration of H2S can increase when the pH decreases [23], influencing the distribution of sulfur in the liquid and gas phases.
The temperature of the digester was kept in the lower portion of the mesophilic temperature range (15–35 °C), ranging from 17.8 to 29.9 °C during the 50 day experiment. There was no significant correlation between the temperature of the digester and the initial (pre-treatment) H2S concentration in the biogas (p-value = 0.703). The digester and the biogas holding vessels were maintained at ambient temperature, with some fluctuations between day and night temperatures.
3.2. Biological Desulfurization Using Microaeration
Prior to inoculation of the biogas vessels with SOB, a microaeration environment was maintained through injecting ambient air into the empty biogas vessels, with a H2S removal efficiency ranging from 29.4 to 72.1%, with an average of 55.2 ± 7.1%. Without the relevant bacteria in the inoculum, the sulfur could only be oxidized through reacting with the O2 in the air. According to van der Zee et al. (2007), this process is not as efficient as bacterial oxidation [45].
The 10 L of sludge added for microaeration was based on the study of Ramos et al. (2013) [46], which suggested a volumetric ratio of 1:10 inoculum to desulfurization unit volume. During the three day inoculum stabilization period, the H2S reduction efficiency increased from 59.8% on Day 2 to 74.9% on Day 3. By Day 4, 100% H2S removal was achieved, indicating that a bacterial adjustment period of three days was needed for optimal removal efficiency. The replicate average of the H2S removal 2 h after air injection was 91.5 ± 1.87%, increasing to 99.8 ± 0.04% after 4 h (Figure 2), with 4 h having a significantly higher H2S removal efficiency than 2 h (p-value < 0.001). In five of the 50 days, efficiencies of 100% were achieved for 2 and 4 h. Adding up to 3% air in the microaeration reactor completely removed the H2S content (around 4000 ppm) in the biogas for these five days. The H2S removal efficiency was significantly affected by the initial H2S concentration (p-value = 0.026). The duplicate microaeration treatments were not significantly different during the 50 day experiment (p-values = 0.995 for 2 h retention time and 0.308 for 4 h retention time).
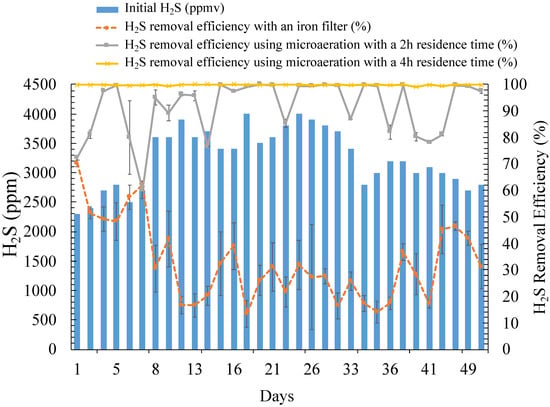
Figure 2.
Relationship between initial H2S (ppm) and H2S removal efficiency (%) using iron filter and microaeration treatments, with gas analysis at 2 h and 4 h after initial air injection for microaeration.
Similar results were obtained by Ramos et al. (2013), with high removal efficiencies obtained at a maximum H2S content of 0.35% v/v (3500 ppm) [46]. Krayzelova et al. (2015) also found residence time (RT) to be a key factor in microaeration, with efficiencies > 97% H2S removal commonly achieved with a RT > 5 h [23]. According to Schneider et al. (2002), 88% H2S removal efficiency was found with a residence time of 2.5 h, while it was less than 40% if the time was <1.25 h [47]. Ramos et al. (2014) had a 2.5 h RT [48], with 88% H2S removal, and Köchermann et al. (2015) achieved 99% H2S removal in 4.0–5.5 h [49]. Montalvo et al. (2020) demonstrated H2S removal exceeding 90% in most cases with similar RTs (2.8–4.8 h), while maintaining flammable biogas concentrations [19].
The CH4 and CO2 concentrations in the microaeration vessels decreased with ambient air injection due to the presence of nitrogen gas (N2) in ambient air diluting the biogas [27,33] (Figure 3). When there was 3% residual O2 in the biogas after microaeration, more than 3000 ppm of H2S was removed, indicating that the air injection was higher than the rate needed for H2S removal. When the O2 level was <3%, the CH4 concentration values were not significantly different before and after microaeration (p-value = 0.58). When the O2 concentration in the biogas after microaeration was >3%, the CH4 was significantly lower after treatment (p-value = 0.004). The average CH4 concentration prior to microaeration was 56.6%, which is equivalent to 22,555 kJ/m3 of biogas. Two hours after microaeration, the average CH4 concentration decreased to 53.8% (21,404 kJ/m3 of biogas), decreasing to 52.8% CH4 (20,982 kJ/m3 of biogas) 4 h after microaeration. In Mulbry et al. (2017), there was no apparent trend between aeration and percentage of CH4 with O2 concentrations ≤ 1% [33]. Díaz et al. (2011) showed that the concentrations of CO2 and CH4 remained stable during microaeration with O2 direct (not the air injection) [50]. According to Köchermann et al. (2015), lower O2 concentrations would be expected at higher concentrations of H2S [51]. Giordano et al. (2019) achieved nearly 100% H2S reduction in a full-scale thermophilic digester using microaerobic conditions with residual oxygen ranging from 0.2 to 2.0% O2 [52].
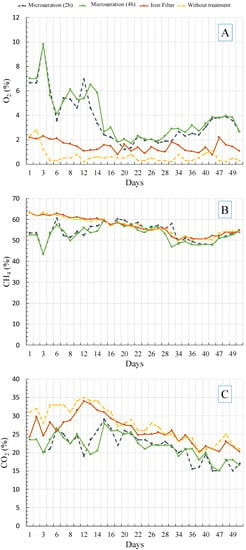
Figure 3.
The concentrations of O2 (A), CH4 (B), and CO2 (C) in the biogas over 50 days showing concentrations pre-treatment, with iron filter H2S removal and microaeration.
There was no significant relationship between the microaeration vessel temperature and H2S removal efficiency (p-value = 0.323). Ramos et al. (2013) showed higher H2S removal efficiencies with higher temperatures, concluding that temperature does affect the process [46]. According to de Arespacochaga et al. (2014), increasing the temperature from 10 to 30 °C increased H2S removal efficiency, with the highest H2S removal using the bacterial consortium at 35 °C [8]. However, in our field-based study, the reactor temperature was based on ambient air temperature, and thus it fluctuated throughout the day and across days, resulting in no clear relationship.
Sulfide Oxidation during Microaeration
The microaeration inoculum source added to the biogas reactors had an initial elemental sulfur (S) concentration of 259 mg/L and a sulfate concentration of 771 mg/L. At the end of the experiment, the microaeration biomass had an elemental sulfur concentration of 1536 mg/L, and a sulfate concentration of 4606 mg/L. The influent (164 L/day) contained 42,347 mg sulfur and 126,059 mg sulfate, while the effluent contained 251,136 mg sulfur and 753,081 mg sulfate (Figure 4), a 493% and 497% increase in concentration, respectively. The average daily H2S removed was 4.52 mg/L after a 4 h retention, and 1.49 mg/L after the iron filter (Figure 4). At the end of the experiment, neither sulfate nor sulfur clusters were visualized, but the results showed a large increase in elemental sulfur and sulfate in the microaeration biomass, indicating that H2S in the biogas was oxidized by SOB in greater amounts than the amount of sulfur that settled in the biomass.
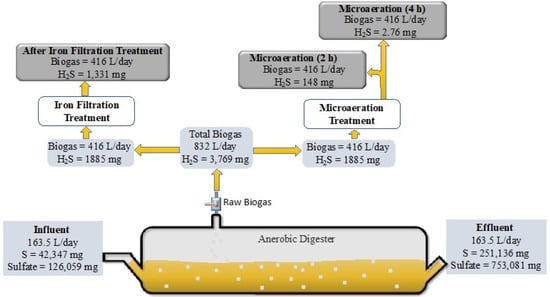
Figure 4.
Schematic diagram of the sulfur mass balance.
Sulfide oxidation with O2 during microaeration can form polysulfides (S2-n), which are protonated to form elemental sulfur [45,53,54], and can then form more oxidized species of sulfur, such as thiosulfate, sulfate, and sulfite [45,54,55]. Ramos et al. (2013) observed precipitated sulfur in the sludge near the liquid surface. This observation was not seen in this study, possibly due to the length of time: 50 days in our study compared to their 91 day study [46]. Mahdy et al. (2020) showed slight sulfate accumulation (<330 mg L−1) inside the microaerated digesters and higher sulfate concentrations in the effluents of microaerated digesters than in the control [56].
At the end of the study, the sludges from the digester and microaeration vessels were examined to enumerate and characterize the SOB bacteria. There were >11 × 104 MPN/g of bacteria in the fresh sludge added to each of the microaeration vessels. The bacteria in the microaeration biomass from one of the vessels included wavy-edged, creamy-surfaced, white-colored, and irregular-shaped bacteria, while the other vessel included whole-edged, creamy-surfaced, irregular-shaped, and translucent-colored bacteria. In one microaeration vessel, the bacteria average size was 0.85 mm and they were Gram-negative, with short and thick coccobacilli, characteristics that are consistent with Thiobacillus sulfooxidans. The second microaeration vessel had bacteria that were 2.73 mm and included short and thick coccobacilli, which could also have been in the Thiobacillus group, but this is not conclusive [42]. Hurtado and Salamanca (2017) analyzed the phenotypic characteristics of oxidizing strains of sulfur bacteria and described the characteristics as 5 mm diameter, long and thin bacilli and Gram-negative, which is generally consistent with the bacteria found in the digester effluent inside both microaeration vessels.
3.3. Desulfurization Using the Iron Filter System
The average H2S removal efficiency using iron filters was 32.91%, with a maximum of 70.21% and a minimum of 13.39% (Figure 2). H2S removal efficiency by iron filters was significantly lower than for microaeration (p-value < 0.001). While McKinsey (2003) indicated theoretical efficiencies of up to 85% (0.56 kg H2S/kg Fe2O3) in batch mode, the maximum H2S removal efficiency was reached on the first day of this experiment, with significant reductions in H2S removal efficiency over time (p-value < 0.001) [44]. The H2S efficiency was significantly affected by the pre-treatment H2S concentration (p-value = 0.026), with decreases in removal efficiency with higher pre-scrubber H2S concentration. Figueroa (2019) [57] stated that an iron filter can remove approximately 50% of H2S and is the most common method utilized in countries like Peru, where small-scale digesters are prevalent. Neither CH4 nor CO2 were affected by the iron filter, with no significant differences between CH4 (p-value = 0.119) and CO2 (p-value = 0.986) concentrations before and after iron filter treatment. In addition, no relationship was determined between the temperature of the digester and the H2S removal efficiency when using the iron filter (p-value = 0.301).
3.4. Economic Analysis of Low-Cost H2S Removal Technologies
Operational costs, infrastructure costs (packing material, equipment, pumps, iron filters, and piping), energy costs, and maintenance costs were evaluated. The energy cost to run the microaeration pump was $0.26/year for microaeration with an air retention time of 4 h based on $0.07kWh−1 (ENEL Peruvian company website). There were no energy costs for the iron filters. The construction and material cost for iron filters ($31.0/year) was higher than for microaeration ($6.5/year). After 4 h, microaeration removed 0.25 m3 H2S/year, which was much more efficient than using iron filters (0.06 m3 H2S removed/year). The operational costs were $27/m3 H2S removed/year for 4 h microaeration and $382/m3 H2S removed/year for iron filters. The operational cost of iron filters was higher due to adding new iron material 36 times/year due to the material wear, lost efficiency, and oxidation.
Iron shavings or sponges can be periodically regenerated by exposing the chips to 8% O2, but this process is highly exothermic and should be carefully monitored [58]. Moreover, it has been shown that the regeneration efficiency decreases each time, with substrate replacement needed once saturation occurs [58]. In addition, each time the substrate is discarded and replaced, waste is generated, which must be disposed of properly. Moreover, a higher biogas generation rate will have a higher material cost and disposal cost due to higher replacement needs [58]. The microaeration technique has several advantages over iron filters, including simplicity, cost, and achieving an efficiency of up to 100%. In addition, it does not generate toxic waste nor are chemical reagents needed to use this technique. Microaeration can be conducted directly inside the anaerobic reactor or in a separate reactor. With either method, elemental sulfur can be produced and deposited on the walls of the digester or microaeration reactor. These sulfur deposits may need to be removed, if built up over time, but can be used as a fertilizer. Reduction in the percentage of CH4 in the biogas does occur due to the N2 dilution with air injection [59].
A previous study [60] reported a cost of $0.015/m3 biogas for biological treatment using an industrial-scale biotrickling filter, and $0.027/m3 biogas with FeCl3 chemical oxidation treatment, which are both lower than our study ($0.09/m3 biogas for microaeration and $0.41/m3 biogas for iron filter) using small-scale systems [61,62]. Active carbon use had higher costs ($0.13/m3 biogas) for removing H2S [10]. Microaeration appears to be cost competitive compared to market available, large-scale, H2S removal technologies for use in small-scale systems in countries such as Peru, and was shown to be more efficient than using iron filters.
4. Conclusions
The introduction of small amounts of ambient air (1–3%) into biogas storage allowed for the removal of more than 3000 ppm H2S daily from the produced biogas, often reaching a H2S concentration lower than the detection level (1 ppm). Microaeration H2S removal also remained stable during the 50 day experiment, while the iron filter treatment efficiency decreased over time. Efficiencies of 100% H2S removal were achieved with the microaeration method, while a maximum efficiency of only 70.2% was obtained with the iron filter system, decreasing to 13.4% H2S removal efficiency after 50 days. The costs for the desulfurization process using the iron filter system ($382/m3 H2S removed) in small-scale digesters in Peru were higher than for the microaeration method ($27/m3 H2S removed), yet most small-scale digester operators continue to use iron filter systems in Peru. This study should help illustrate the quantifiable benefits of microaeration for H2S removal compared to iron filters.
Author Contributions
Conceptualization, J.K.H. and L.Q.; methodology, J.K.H. and L.Q.; formal analysis, J.K.H., L.Q., A.H., and S.L.; investigation, J.K.H. and L.Q.; resources, J.K.H., L.Q., and S.L.; data curation, J.K.H., L.Q., A.H., and S.L.; writing—original draft preparation, J.K.H.; writing—review and editing, J.K.H., A.H., and S.L.; visualization, J.K.H. and A.H.; supervision, S.L.; funding acquisition, J.K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SNV Netherlands Development Organisation and the FondeCYT scholarship fund.
Acknowledgments
We want to thank Andreas Lemmer for the technical support in the experimental part and his valuable discussion.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Arif, S.; Liaquat, R.; Adil, M. Applications of materials as additives in anaerobic digestion technology. Renew. Sustain. Energy Rev. 2018, 97, 354–366. [Google Scholar] [CrossRef]
- Schieder, D.; Quicker, P.; Schneider, R.; Winter, H.; Prechtl, S.; Faulstich, M. Microbiological removal of hydrogen sulfide from biogas by means of a separate biofilter system: Experience with technical operation. Water Sci. Technol. 2003, 48, 209–212. [Google Scholar] [CrossRef]
- Nägele, H.J.; Steinbrenner, J.; Hermanns, G.; Holstein, V.; Haag, N.L.; Oechsner, H. Innovative additives for chemical desulphurisation in biogas processes: A comparative study on iron compound products. Biochem. Eng. J. 2017. [Google Scholar] [CrossRef]
- Wu, J.; Liu, D.; Zhou, W.; Liu, Q.; Huang, Y. High-Temperature H2S Removal from IGCC Coarse Gas; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9811068178. [Google Scholar]
- Shelford, T.; Gooch, C.; Choudhury, A.; Lansing, S. A Technical Reference Guide for Dairy-Derived Biogas Production, Treatment, and Utilization. 2019. Available online: https://go.umd.edu/FarmerBiogasHandbook (accessed on 13 September 2020).
- Choudhury, A.; Shelford, T.; Felton, G.; Gooch, C.; Lansing, S. Evaluation of hydrogen sulfide scrubbing systems for anaerobic digesters on two U.S. dairy farms. Energies 2019, 12, 4605. [Google Scholar] [CrossRef]
- Choudhury, A.; Lansing, S. Methane and hydrogen sulfide production from Co-digestion of gummy waste with a food waste, grease waste, and dairy manure mixture. Energies 2019, 12, 4464. [Google Scholar] [CrossRef]
- de Arespacochaga, N.; Valderrama, C.; Mesa, C.; Bouchy, L.; Cortina, J.L. Biogas biological desulphurisation under extremely acidic conditions for energetic valorisation in Solid Oxide Fuel Cells. Chem. Eng. J. 2014. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A review of biogas utilisation, purification and upgrading technologies. Waste Biomass Valorization 2017. [Google Scholar] [CrossRef]
- Allegue, L.B.; Hinge, J. Biogas upgrading evaluation of methods for H2S removal. Danish Technol. Inst. 2014. [Google Scholar] [CrossRef]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2019. [Google Scholar] [CrossRef]
- Speight, J.G. Natural Gas: A Basic Handbook; Gulf Professional Publishing: Houston, TX, USA, 2018; ISBN 9780128095706. [Google Scholar]
- Sifuentes, G.; de Dios, J. Empleo y Purificación del Biogás Producido en Unitrar. Use and Purification of the Biogas Produced in UNITRAR. 2003. Available online: https://alicia.concytec.gob.pe/vufind/Record/UUNI_69c27d0927f487a5461d296441b87ed2/Description#tabnav (accessed on 13 September 2020).
- Dupnock, T.L.; Deshusses, M.A. Biological Co-treatment of H2S and reduction of CO2 to methane in an anoxic biological trickling filter upgrading biogas. Chemosphere 2020, 256, 127078. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ghanegaonkar, P.M. Hydrogen sulfide removal from biogas using chemical absorption technique in packed column reactors. Glob. J. Environ. Sci. Manag. 2019. [Google Scholar] [CrossRef]
- Zhang, Y.; Oshita, K.; Kusakabe, T.; Takaoka, M.; Kawasaki, Y.; Minami, D.; Tanaka, T. Simultaneous removal of siloxanes and H2S from biogas using an aerobic biotrickling filter. J. Hazard. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Raabe, T.; Mehne, M.; Rasser, H.; Krause, H.; Kureti, S. Study on iron-based adsorbents for alternating removal of H2S and O2 from natural gas and biogas. Chem. Eng. J. 2019. [Google Scholar] [CrossRef]
- Schiavon Maia, D.C.; Niklevicz, R.R.; Arioli, R.; Frare, L.M.; Arroyo, P.A.; Gimenes, M.L.; Pereira, N.C. Removal of H2S and CO2 from biogas in bench scale and the pilot scale using a regenerable Fe-EDTA solution. Renew. Energy 2017. [Google Scholar] [CrossRef]
- Montalvo, S.; Huiliñir, C.; Borja, R.; Castillo, A.; Pereda, I. Anaerobic digestion of wastewater rich in sulfate and sulfide: Effects of metallic waste addition and micro-aeration on process performance and methane production. J. Environ. Sci. Heal. Part A Toxic/Hazard. Subst. Environ. Eng. 2019. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Lansing, S. Biochar addition with Fe impregnation to reduce H2S production from anaerobic digestion. Bioresour. Technol. 2020. [Google Scholar] [CrossRef]
- Verbeeck, K.; De Vrieze, J.; Biesemans, M.; Rabaey, K. Membrane electrolysis-assisted CO2 and H2S extraction as innovative pretreatment method for biological biogas upgrading. Chem. Eng. J. 2019. [Google Scholar] [CrossRef]
- Nishimura, S.; Yoda, M. Removal of hydrogen sulfide from an anaerobic biogas using a big-scrubber. Water Sci. Technol. 1997, 36, 349–356. [Google Scholar] [CrossRef]
- Krayzelova, L.; Bartacek, J.; Díaz, I.; Jeison, D.; Volcke, E.I.P.; Jenicek, P. Microaeration for hydrogen sulfide removal during anaerobic treatment: A review. Rev. Environ. Sci. Biotechnol. 2015. [Google Scholar] [CrossRef]
- Cano, P.I.; Brito, J.; Almenglo, F.; Ramírez, M.; Gómez, J.M.; Cantero, D. Influence of trickling liquid velocity, low molar ratio of nitrogen/sulfur and gas-liquid flow pattern in anoxic biotrickling filters for biogas desulfurization. Biochem. Eng. J. 2019. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, X.; Jin, Z.; Yang, S.; Zhang, J. The performance and microbial community in a slightly alkaline biotrickling filter for the removal of high concentration H2S from biogas. Chemosphere 2020. [Google Scholar] [CrossRef]
- Ángeles Torres, R.; Marín, D.; Rodero, M.d.R.; Pascual, C.; González-Sanchez, A.; de Godos Crespo, I.; Lebrero, R.; Torre, R.M. Biogas treatment for H2S, CO2, and other contaminants removal. In From Biofiltration to Promising Options in Gaseous Fluxes Biotreatment; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Vikrant, K.; Kailasa, S.K.; Tsang, D.C.W.; Lee, S.S.; Kumar, P.; Giri, B.S.; Singh, R.S.; Kim, K.H. Biofiltration of hydrogen sulfide: Trends and challenges. J. Clean. Prod. 2018. [Google Scholar] [CrossRef]
- Khanongnuch, R.; Di Capua, F.; Lakaniemi, A.M.; Rene, E.R.; Lens, P.N.L. Transient–state operation of an anoxic biotrickling filter for H2S removal. J. Hazard. Mater. 2019. [Google Scholar] [CrossRef]
- Khanongnuch, R.; Di Capua, F.; Lakaniemi, A.M.; Rene, E.R.; Lens, P.N.L. H2S removal and microbial community composition in an anoxic biotrickling filter under autotrophic and mixotrophic conditions. J. Hazard. Mater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Meier, L.; Stará, D.; Bartacek, J.; Jeison, D. Removal of H2S by a continuous microalgae-based photosynthetic biogas upgrading process. Process. Saf. Environ. Prot. 2018. [Google Scholar] [CrossRef]
- Haosagul, S.; Prommeenate, P.; Hobbs, G.; Pisutpaisal, N. Sulfide-oxidizing bacteria community in full-scale bioscrubber treating H2S in biogas from swine anaerobic digester. Renew. Energy 2020. [Google Scholar] [CrossRef]
- Cheng, Y.; Yuan, T.; Deng, Y.; Lin, C.; Zhou, J.; Lei, Z.; Shimizu, K.; Zhang, Z. Use of sulfur-oxidizing bacteria enriched from sewage sludge to biologically remove H2S from biogas at an industrial-scale biogas plant. Bioresour. Technol. Reports 2018. [Google Scholar] [CrossRef]
- Mulbry, W.; Lansing, S.; Coker, A.C. Microaeration reduces hydrogen sulfi de in biogas. Biocycle 2017, 58, 57–59. [Google Scholar]
- Khoshnevisan, B.; Tsapekos, P.; Alfaro, N.; Díaz, I.; Fdz-Polanco, M.; Rafiee, S.; Angelidaki, I. A review on prospects and challenges of biological H2S removal from biogas with focus on biotrickling filtration and microaerobic desulfurization. Biofuel Res. J. 2017. [Google Scholar] [CrossRef]
- Zhu, H.L.; Papurello, D.; Gandiglio, M.; Lanzini, A.; Akpinar, I.; Shearing, P.R.; Manos, G.; Brett, D.J.L.; Zhang, Y.S. Study of H2S removal capability from simulated biogas by using waste-derived adsorbent materials. Processes 2020, 8, 1030. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018. [Google Scholar] [CrossRef]
- Hjort-Gregersen, K. Market Overview Micro Scale Digesters; AgroTEch A/S: Aarhus, Denmark, 2015. [Google Scholar]
- Matus, F.J.; Escudey, M.; Förster, J.E.; Gutiérrez, M.; Chang, A.C. Is the Walkley–Black method suitable for organic carbon determination in Chilean volcanic soils? Commun. Soil Sci. Plant Anal. 2009, 40, 1862–1872. [Google Scholar] [CrossRef]
- Kjeldahl, C. A new method for the determination of nitrogen in organic matter. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005; ISBN 0875532357. [Google Scholar]
- Association, A.P.H.; Association, A.W.W.; Federation, W.P.C.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington DC, USA, 1915; Volume 2, ISBN 8755-3546. [Google Scholar]
- Hurtado, A.; Salamanca, J. Búsqueda de bacterias oxidadoras de ácido sulfhídrico para su potencial uso en la producción de biogás de alta pureza. Rev. Investig. Agrar. Ambient. 2017, 9, 295–304. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R. Gas Purification; Elsevier: Amsterdam, The Netherlands, 1997; ISBN 0080507204. [Google Scholar]
- Zicari, S.M. Removal of Hydrogen Sulfide from Biogas Using Cow-Manure Compost. Ph.D. Thesis, Cornell University, New York, NY, USA, 2003. [Google Scholar]
- van der Zee, F.P.; Villaverde, S.; García, P.A.; Fdz.-Polanco, F. Sulfide removal by moderate oxygenation of anaerobic sludge environments. Bioresour. Technol. 2007. [Google Scholar] [CrossRef]
- Ramos, I.; Pérez, R.; Fdz-Polanco, M. Microaerobic desulphurisation unit: A new biological system for the removal of H2S from biogas. Bioresour. Technol. 2013. [Google Scholar] [CrossRef]
- Schneider, R.; Quicker, P.; Anzer, T.; Prechtl, S.; Faulstich, M. Grundlegende Untersuchungen zur effektiven, Kostengünstigen Entfernung von Schwefelwasserstoff aus Biogas–Biogasanlagen: Anforderungen zur Luftreinhaltung; Bayerisches Landesamt für Umweltschutz: Augsburg, Germany, 2002; pp. 25–41. Available online: http://www.link-infos.de/Natur-Links-Energie-Biogas-Biogasanlagen-Anforderungen-Luftreinhaltung.pdf (accessed on 13 September 2020).
- Ramos, I.; Peña, M.; Fdz-Polanco, M. Where does the removal of H2S from biogas occur in microaerobic reactors? Bioresour. Technol. 2014. [Google Scholar] [CrossRef]
- Köchermann, J.; Schneider, J.; Matthischke, S.; Rönsch, S. Sorptive H2S removal by impregnated activated carbons for the production of SNG. Fuel Process. Technol. 2015. [Google Scholar] [CrossRef]
- Díaz, I.; Lopes, A.C.; Pérez, S.I.; Fdz-Polanco, M. Determination of the optimal rate for the microaerobic treatment of several H2S concentrations in biogas from sludge digesters. Water Sci. Technol. 2011. [Google Scholar] [CrossRef]
- Klok, J.B.M.; de Graaff, M.; van den Bosch, P.L.F.; Boelee, N.C.; Keesman, K.J.; Janssen, A.J.H. A physiologically based kinetic model for bacterial sulfide oxidation. Water Res. 2013. [Google Scholar] [CrossRef]
- Giordano, A.; Di Capua, F.; Esposito, G.; Pirozzi, F. Long-term biogas desulfurization under different microaerobic conditions in full-scale thermophilic digesters co-digesting high-solid sewage sludge. Int. Biodeterior. Biodegrad. 2019. [Google Scholar] [CrossRef]
- Brune, D.C. Isolation and characterization of sulfur globule proteins from chromatium vinosum and Thiocapsa roseopersicina. Arch. Microbiol. 1995. [Google Scholar] [CrossRef] [PubMed]
- Steudel, R. Mechanism for the formation of elemental sulfur from aqueous sulfide in chemical and microbiological desulfurization processes. Ind. Eng. Chem. Res. 1996. [Google Scholar] [CrossRef]
- Schlegel, H.G. General Microbiology, 7th ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Mahdy, A.; Song, Y.; Salama, A.; Qiao, W.; Dong, R. Simultaneous H2S mitigation and methanization enhancement of chicken manure through the introduction of the micro-aeration approach. Chemosphere 2020. [Google Scholar] [CrossRef]
- Fernández, M. Desulfuración de Biogás en Condiciones Anóxicas Mediante Biofiltración. Ph.D. Thesis, Universidad de Cádiz, Cádiz, Spain, 2011. [Google Scholar]
- McCollam, S.W. Viability for Oxidation of H2S Gas Using Low Concentration Solutions of H2O2 Peroxide in Applications for Biogas Purification. Master’s Thesis, University of Louisville, Kentucky, KY, USA, 2009. Available online: https://ir.library.louisville.edu/etd/937/ (accessed on 13 September 2020).
- Pokorna-Krayzelova, L.; Bartacek, J.; Vejmelkova, D.; Alvarez, A.A.; Slukova, P.; Prochazka, J.; Volcke, E.I.P.; Jenicek, P. The use of a silicone-based biomembrane for microaerobic H2S removal from biogas. Sep. Purif. Technol. 2017. [Google Scholar] [CrossRef]
- Tomàs, M.; Fortuny, M.; Lao, C.; Gabriel, D.; Lafuente, J.; Gamisans, X. Technical and economical study of a full-scale biotrickling filter for H2S removal from biogas. Water Pract. Technol. 2009. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.U.; Summers, R.S.; Cook, S.M. Environmental comparison of biochar and activated carbon for tertiary wastewater treatment. Environ. Sci. Technol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Surra, E.; Costa Nogueira, M.; Bernardo, M.; Lapa, N.; Esteves, I.; Fonseca, I. New adsorbents from maize cob wastes and anaerobic digestate for H2S removal from biogas. Waste Manag. 2019. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).