On the Investigation of Microstructured Charcoal as an ANFO Blasting Enhancer
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructured Charcoal Characteristics
3.2. ANFO and Non-Ideal Explosive Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Biessikirski, A.; Kuterasiński, Ł. Research on Morphology and Topology of ANFO Based on Various Types of Oxygen Component; Wydawnictwa AGH: Kraków, Poland, 2018. [Google Scholar]
- Miyake, A.; Takahara, K.; Ogawa, T.; Ogata, Y.; Wada, Y.; Arai, H. Influence of physical properties of ammonium nitrate on the detonation behavior of ANFO. J. Loss. Prev. Process Ind. 2001, 14, 533–538. [Google Scholar] [CrossRef]
- Zygmunt, B. Detonation Parameters of Mixtures Containing Ammonium Nitrate and Aluminium. Cent. Eur. J. Energ. Mater. 2009, 6, 57–66. [Google Scholar]
- Oxley, J.C.; Smith, J.L.; Rogers, E.; Yu, M. Ammonium Nitrate: Thermal Stability and Explosivity Modifiers. Thermochim. Acta 2002, 384, 23–45. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Wang, W. Compatibility of Ammonium Nitrate with Monomolecular Explosives 2. Nitroarenes12. J. Phys. Chem. 1994, 98, 3901. [Google Scholar] [CrossRef]
- Brower, K.R.; Oxley, J.C.; Tewari, M. Evidence for Homolytic Decomposition of Ammonium Nitrate at High Temperature. J. Phys. Chem. 1989, 93, 4029. [Google Scholar] [CrossRef]
- Wada, Y.; Hori, K.; Arai, M. Combustion Mechanism of Mixtures of Guanidine Nitrate, Ammonium Nitrate and Basic Copper Oxide. Sci. Technol. Energ. Mater. 2010, 71, 83. [Google Scholar]
- Marshall, M.; Jimmie, C.O. (Eds.) Aspects of Explosives Detection; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Sinditskii, V.P.; Egorshev, V.Y.; Levshenkov, A.I.; Serushkin, V.V. Ammonium Nitrate: Combustion Mechanism and the Role of Additives. Propellants Explos. Pyrotech. 2005, 30, 269–280. [Google Scholar] [CrossRef]
- Gunawan, R.; Zhang, D. Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite. J. Hazard. Mater. 2009, 165, 751–758. [Google Scholar] [CrossRef]
- Deribas, A.A.; Kudinov, V.M.; Matveenkov, F.I.; Simonov, V.A. Effect of initial parameters on the process of wave formation in explosive welding. Combust. Explos. Shock Waves 1967, 3, 344–348. [Google Scholar] [CrossRef]
- Miyake, A.; Ohtagaki, Y.; Abe, T.; Wada, Y.; Nakayama, Y.; Ogawa, T. Experimental Determination of the Detonation Front Curvature of Non-Ideal Explosive ANFO. Mater. Sci. Forum 2004, 465, 181–184. [Google Scholar] [CrossRef]
- Maranda, A.; Gałęzowski, D.; Papliński, A. Investigations on detonation and thermochemical parameters of aluminized ANFO. J. Energ. Mater. 2003, 21, 1–14. [Google Scholar] [CrossRef]
- Maranda, A.; Paszula, J.; Zawadzka-Małota, I.; Kuczyńska, B.; Witkowski, W.; Nikolczuk, K.; Wilk, Z. Aluminum powder influence on ANFO detonation parameters. Cent. Eur. J. Energ. Mater. 2011, 8, 279–293. [Google Scholar]
- Babrauskas, V.; Leggett, D. Thermal decomposition of ammonium nitrate. Fire Mater. 2020, 44, 250–268. [Google Scholar] [CrossRef]
- Reiset, J.; Millon, E. Ueber die durch Contact bewirkten chemischen Erscheinungen. J. Prakt. Chem. 1843, 29, 365–371. [Google Scholar] [CrossRef]
- Izato, Y.; Miyake, A.; Date, S. Combustion characteristics of ammonium nitrate and carbon mixtures based on a thermal decomposition mechanism. Propell. Explos. Pyrotech. 2013, 38, 129–135. [Google Scholar] [CrossRef]
- Hussain, G.; Rees, G.J. Combustion of NH4NO3 and carbon based mixtures. Fuel 1993, 72, 1475–1479. [Google Scholar] [CrossRef]
- Lurie, B.A.; Chang, L. Kinetics and mechanism of thermal decomposition of ammonium nitrate powder under the action of carbon black. Combust. Explos. Shock Waves 2000, 36, 607–617. [Google Scholar] [CrossRef]
- Brill, T.B.; Brush, P.J.; Patil, D.G. Thermal decomposition of energetic materials 58. Chemistry of ammonium nitrate and ammonium dinitramide near the burning surface temperature. Combust. Flame 1993, 92, 186. [Google Scholar]
- Glazkova, A.P.; Kazarova, Y.A.; Savel’ev, A.V. Oxidation of carbon by nitrites and nitrates. Combust. Explos. Shock Waves 1983, 19, 308–314. [Google Scholar] [CrossRef]
- Miyake, A.; Kobayashi, H.; Echigoya, H.; Ogawa, T. Combustion and ignition properties of ammonium nitrate and activated carbon mixtures. Int. J. Energ. Mater. Chem. Propuls. 2009, 8, 411–419. [Google Scholar] [CrossRef]
- Izato, Y.I.; Miyake, A.; Echigoya, H. Influence of physical properties of carbon on the detonation behaviour of ammonium nitrate and carbon mixtures. In Proceedings of the Seventh International Symposium on Hazards, Prevention, and Mitigation of Industrial Explosions, St. Petersburg, Russia, 7–11 July 2008; pp. 255–259. [Google Scholar]
- Biessikirski, A.; Wądrzyk, M.; Janus, R.; Biegańska, J.; Jodłowski, G.; Kuterasiński, Ł. Study on fuel oils used in ammonium nitrate-based explosives. Przem. Chem. 2018, 97, 457–462. [Google Scholar]
- BN-80/6091-42. Górnicze Materiały Wybuchowe. Obliczanie Parametrów Użytkowych; Zakłady Tworzyw Sztucznych ERG: Tychy-Bieruń Stary, Poland, 1981. [Google Scholar]
- Wang, L.; Ago, M.; Borghei, M.; Ishaq, A.; Papageorgiou, A.C.; Lundahl, M.; Rojas, O.J. Conductive Carbon Microfibers Derived from Wet-Spun Lignin/ Nanocellulose Hydrogels. ACS Sustain. Chem. Eng. 2019, 7, 6013–6022. [Google Scholar] [CrossRef]
- Hurtado, R.B.; Calderón-Ayala, G.; Cortez-Valadez, M.; López Torres, R.; Berrellez-Reyes, F.; Flores-Acosta, M. Efficient synthesis of carbon microtubes–gold nanoparticles composite: Optical and micro-analytical study. Appl. Phys. A 2019, 125, 844. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liua, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.M.; Moutinho, M.V.; Stavale, F.; Lucchese, M.; Capaz, R.B.; Achete, C.; Jorio, A. Evolution of the Raman spectra from single-, few-, and many-layer graphene with increasing disorder. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 82, 125429. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A. Chemical Analysis of Graphene Oxide Films after Heat and Chemical Treatments by X-ray Photoelectron and Micro-Raman Spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Z.; Wang, Y.; Yang, R.; Shi, D.; Zhang, G. Catalyst-free growth of nanographene films on various substrates. Nano Res. 2011, 4, 315–321. [Google Scholar] [CrossRef]
- Zhu, H.; Kuang, T.; Zhu, B.; Lei, S.; Liu, Z.; Ringer, S.P. Flame synthesis of carbon nanostructures on Ni-plated hardmetal substrates. Nanoscale Res. Lett. 2011, 6, 331. [Google Scholar] [CrossRef]
- Gentoiu, M.; Betancourt-Riera, R.; Vizireanu, S.; Burducea, I.; Marascu, V.; Stoica, S.D.; Bita, B.I.; Dinescu, G.; Riera, R. Morphology, Microstructure, and Hydrogen Content of Carbon Nanostructures Obtained by PECVD at Various Temperatures. J. Nanomater. 2017, 1–8. [Google Scholar] [CrossRef]
- Nasri-Nasrabadi, B.; Kaynak, A.; Komeily-Nia, Z.; Adams, S.D.; Li, J.; Kouzani, A.Z. Surface nanogrooving of carbon microtubes. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Wu, H.B.; Chan, M.N.; Chan, C.K. FTIR Characterization of Polymorphic Transformation of Ammonium Nitrate. Aerosol Sci. Technol. 2007, 41, 581–588. [Google Scholar] [CrossRef]
- Available online: https://webbook.nist.gov/cgi/cbook.cgi?Name=ammonium+nitrate&Units=SI&cIR=on#Refs (accessed on 1 January 2020).
- Biessikirski, A.; Kuterasiński, Ł.; Pyra, J.; Dworzak, M. Comparison of properties of ammonium nitrates used for production of fertilizers and explosive materials. Przem. Chem. 2016, 95, 1381–1384. [Google Scholar]
- Steele, B.A.; Oleynik, I.I. New crystal phase ammonium nitrate: First-principles prediction and characterization. In Proceedings of the AIP Conference Proceedings, Tampa Bay, FL, USA, 14–19 June 2015; Volume 1793, p. 130008. [Google Scholar]
- Chien, W.M.; Chandra, D.; Franklin, J.; Rawn, C.J.; Helmy, A.K. X-ray Diffractometry studies and lattice parameter calculation on KNO3-NH4NO3 solid solutions. In Proceedings of the International Centre for Diffraction Data, Philadelphia, PA, USA, 20–25 June 2004. [Google Scholar]
- Ferg, E.; Masalova, I. Using PXRD to Investigate the Crystallization of Highly Concentrated Emulsions of NH4NO3. S. Afr. J. Chem. 2011, 64, 7–16. [Google Scholar]
- Xu, Z.X.; Fu, X.Q.; Wang, Q. Phase Stability of Ammonium Nitrate with Organic Potassium Salts. Cent. Eur. J. Energ. Mater. 2016, 13, 736–754. [Google Scholar] [CrossRef]
- Vargeese, A.A.; Joshi, S.S.; Krishnamurthy, V.N. Effect of method of crystallization on the IV-III and IV-II polymorphic transitions of ammonium nitrate. J. Hazard. Mater. 2009, 161, 373–379. [Google Scholar] [CrossRef]
- Lotspeich, E.; Petr, V. The characterization of ammonium nitrate mini-prills. In Dynamic Behavior of Materials Proceedings of the 2014 Annual Conference on Experimental and Applied Mechanics; Song, B., Casem, D., Kimberley, J., Eds.; Springer: Cham, Switzerland, 2015; Volume 1, pp. 319–325. [Google Scholar]
- Biessikirski, A.; Kuterasiński, Ł.; Dworzak, M.; Pyra, J.; Twardosz, M. Comparison of structure, morphology and topography of fertilizer-based explosives applied in the mining industry. Microchem. J. 2019, 144, 39–44. [Google Scholar] [CrossRef]
- Viktorov, S.D.; Frantov, A.E.; Lapikov, I.N.; Andreev, V.V.; Starshinov, A.V. Effect of microstructure of ammonium nitrate granules on the detonability of composite propellants based on it. Combust. Explos. Shock Waves 2016, 52, 727–731. [Google Scholar] [CrossRef]
- Maranda, A. Influence of aluminium chemical activity on detonation parameters of composite explosives containing aluminium powders (CX-AL). Wiad. Chem. 2001, 55, 353–375. [Google Scholar]
- Salzano, E.; Basco, A. Comparision of the explosion thermodynamics of TNT and black powder using Le Chatelier diagrams. Propellants Explos. Pyrotech. 2012, 37, 724–731. [Google Scholar] [CrossRef]
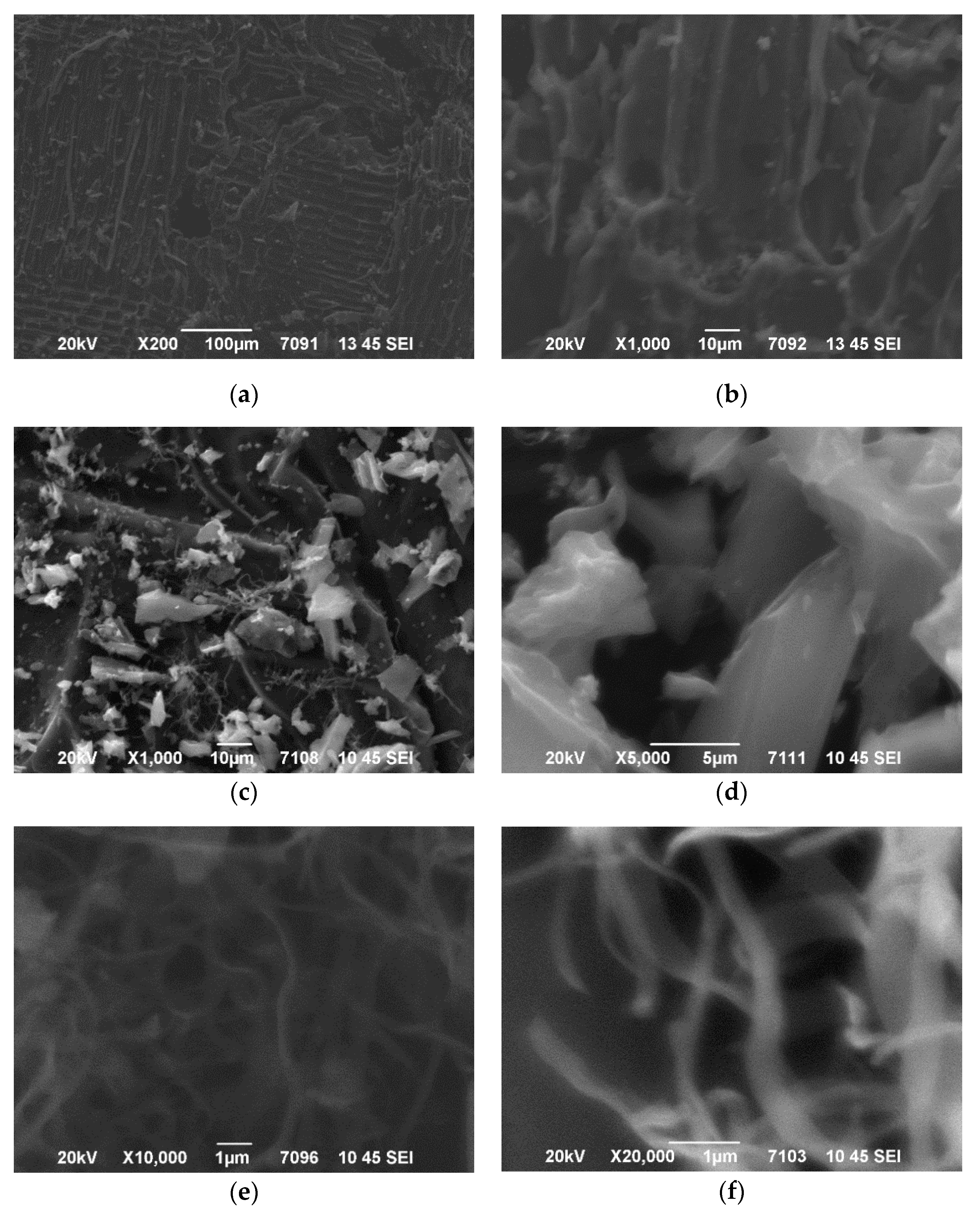
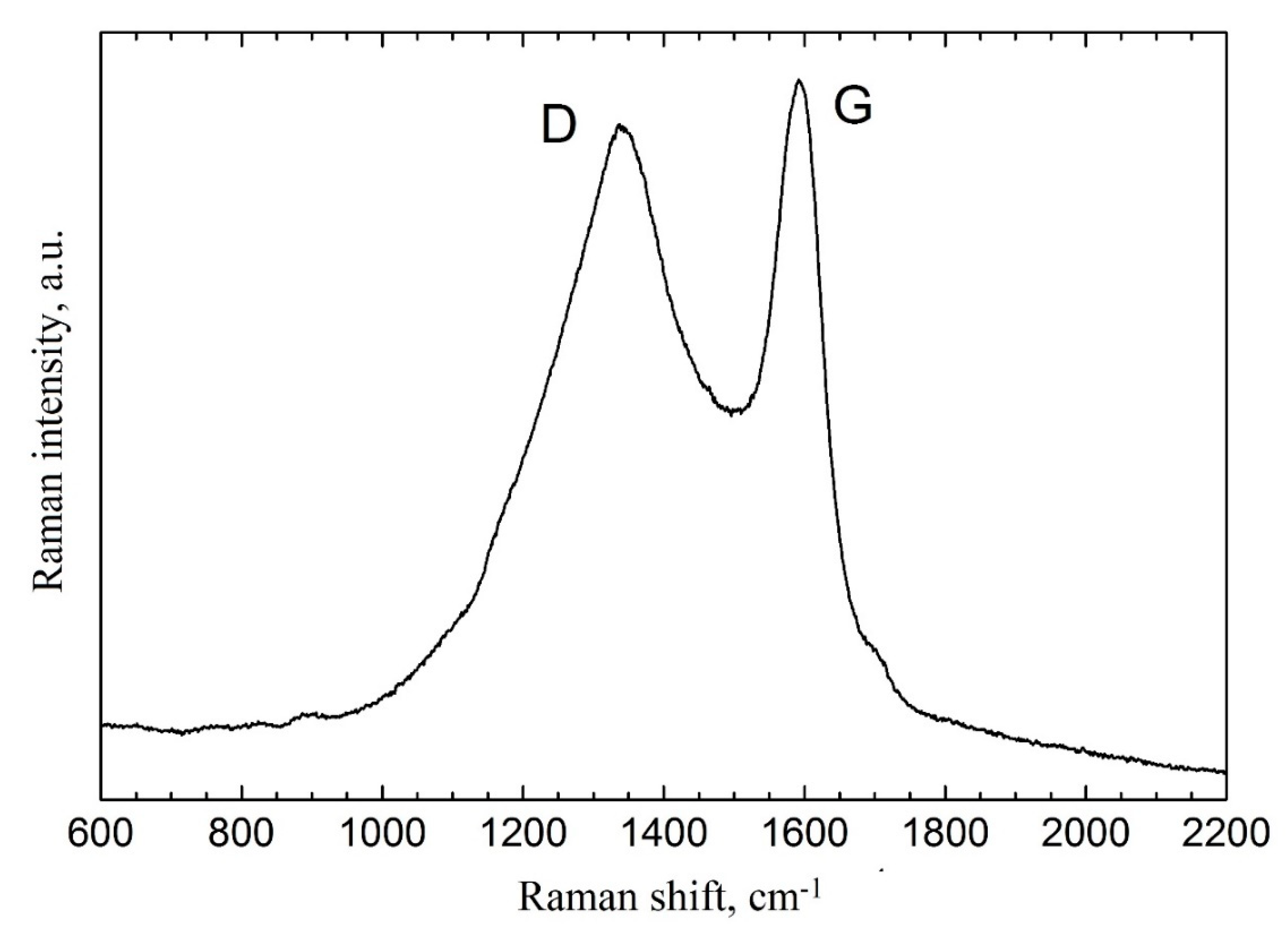


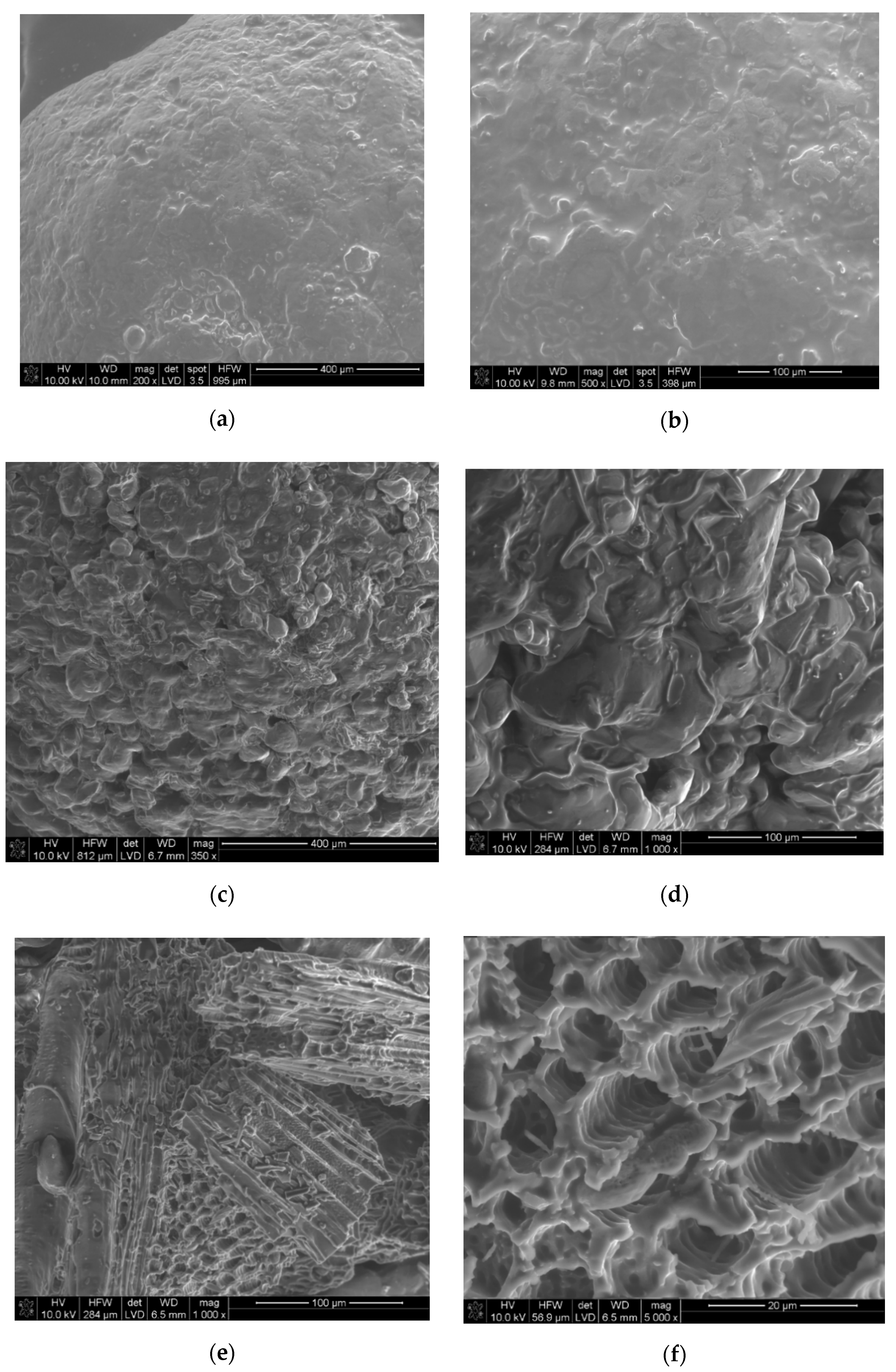
| Sample Name | AN-PP, %wt. | FO, %wt. | MC, %wt. |
|---|---|---|---|
| Sample 1 | 94.5 | 5.5 | - |
| Sample 2 | 94.5 | 4.5 | 1.0 |
| Sample 3 | 94.5 | 2.5 | 3.0 |
| Parameters | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| Enthalpy, kJ kg−1 | −4287 | −4269 | −4232 |
| Detonation pressure, MPa | 2785 | 2740 | 2799 |
| Detonation temperature, K | 2701 | 2675 | 2593 |
| Heat of explosion, kJ kg | 4012 | 3922 | 3695 |
| Strength of explosion. | 976 | 957 | 911 |
| Post-blast volume, l kg−1 | 986 | 977 | 959 |
| Density, kg m−1 | 823 | 821 | 818 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atlagic, S.G.; Biessikirski, A.; Kuterasiński, Ł.; Dworzak, M.; Twardosz, M.; Sorogas, N.; Arvanitidis, J. On the Investigation of Microstructured Charcoal as an ANFO Blasting Enhancer. Energies 2020, 13, 4681. https://doi.org/10.3390/en13184681
Atlagic SG, Biessikirski A, Kuterasiński Ł, Dworzak M, Twardosz M, Sorogas N, Arvanitidis J. On the Investigation of Microstructured Charcoal as an ANFO Blasting Enhancer. Energies. 2020; 13(18):4681. https://doi.org/10.3390/en13184681
Chicago/Turabian StyleAtlagic, Suzana Gotovac, Andrzej Biessikirski, Łukasz Kuterasiński, Michał Dworzak, Michał Twardosz, Niki Sorogas, and John Arvanitidis. 2020. "On the Investigation of Microstructured Charcoal as an ANFO Blasting Enhancer" Energies 13, no. 18: 4681. https://doi.org/10.3390/en13184681
APA StyleAtlagic, S. G., Biessikirski, A., Kuterasiński, Ł., Dworzak, M., Twardosz, M., Sorogas, N., & Arvanitidis, J. (2020). On the Investigation of Microstructured Charcoal as an ANFO Blasting Enhancer. Energies, 13(18), 4681. https://doi.org/10.3390/en13184681





