Expression of VHb Improved Lipid Production in Rhodosporidium toruloides
Abstract
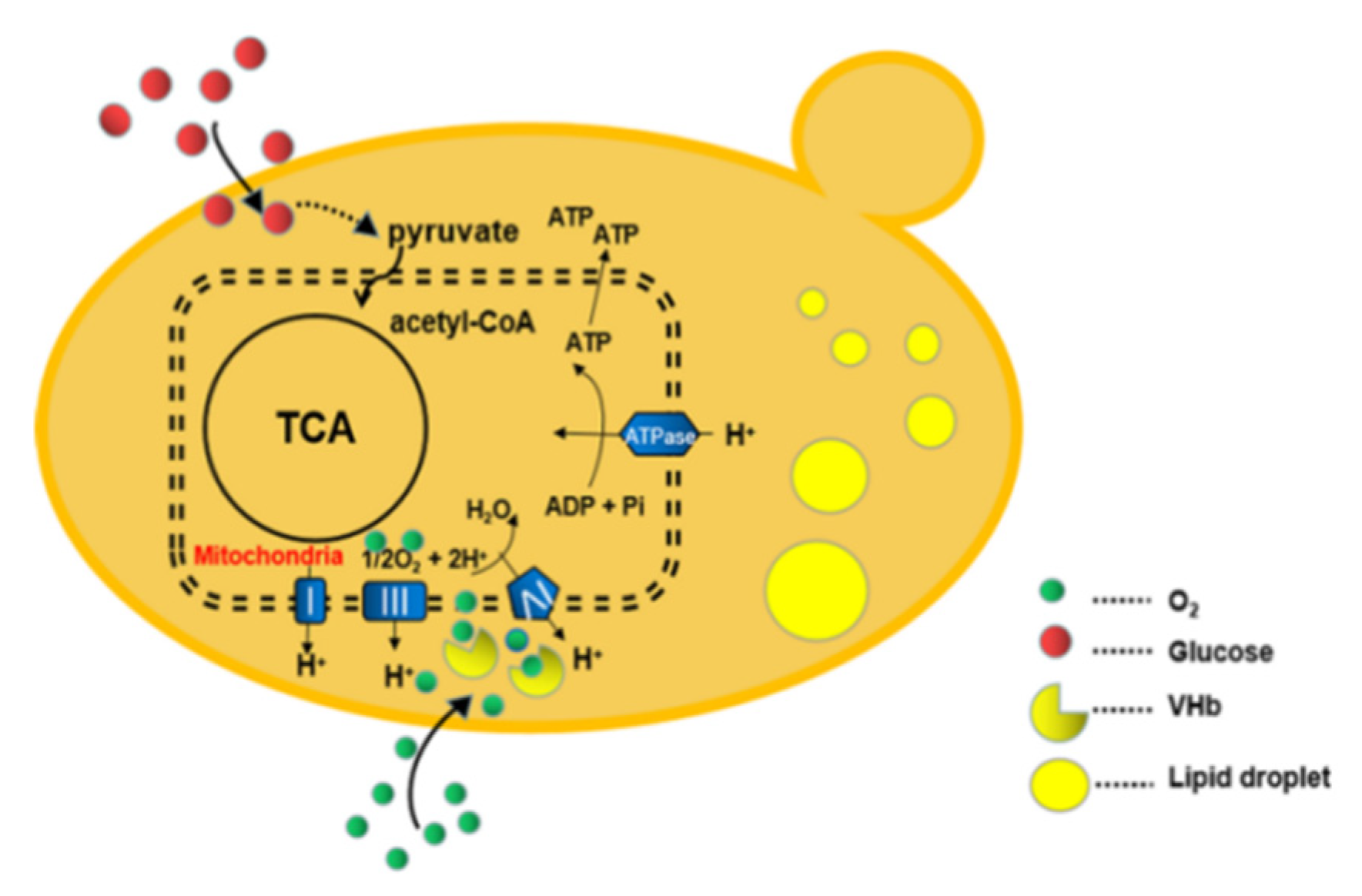
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmid, and Medium
2.2. Reagents
2.3. Plasmid Construction
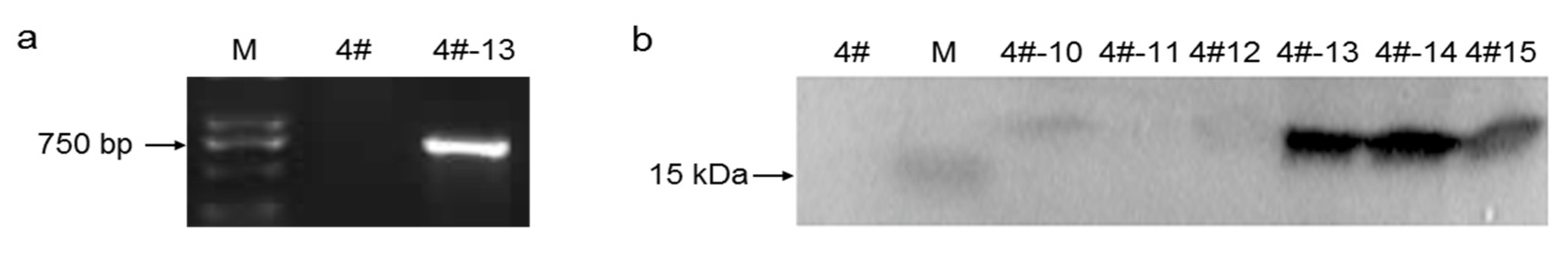
2.4. R. toruloides Transformation and Screening of Clones
2.5. Western Blot Analysis
2.6. VHb Activity Detection
2.7. Shaking Flask Cultures
2.8. Two-Stage Fed-Batch Flask Cultures
2.9. Fed-Batch Fermenter Culture
2.10. Lipid Extraction and Quantification
2.11. Microscopy
3. Results
3.1. Construction and Confirmation of VHb Expression in R. toruloides
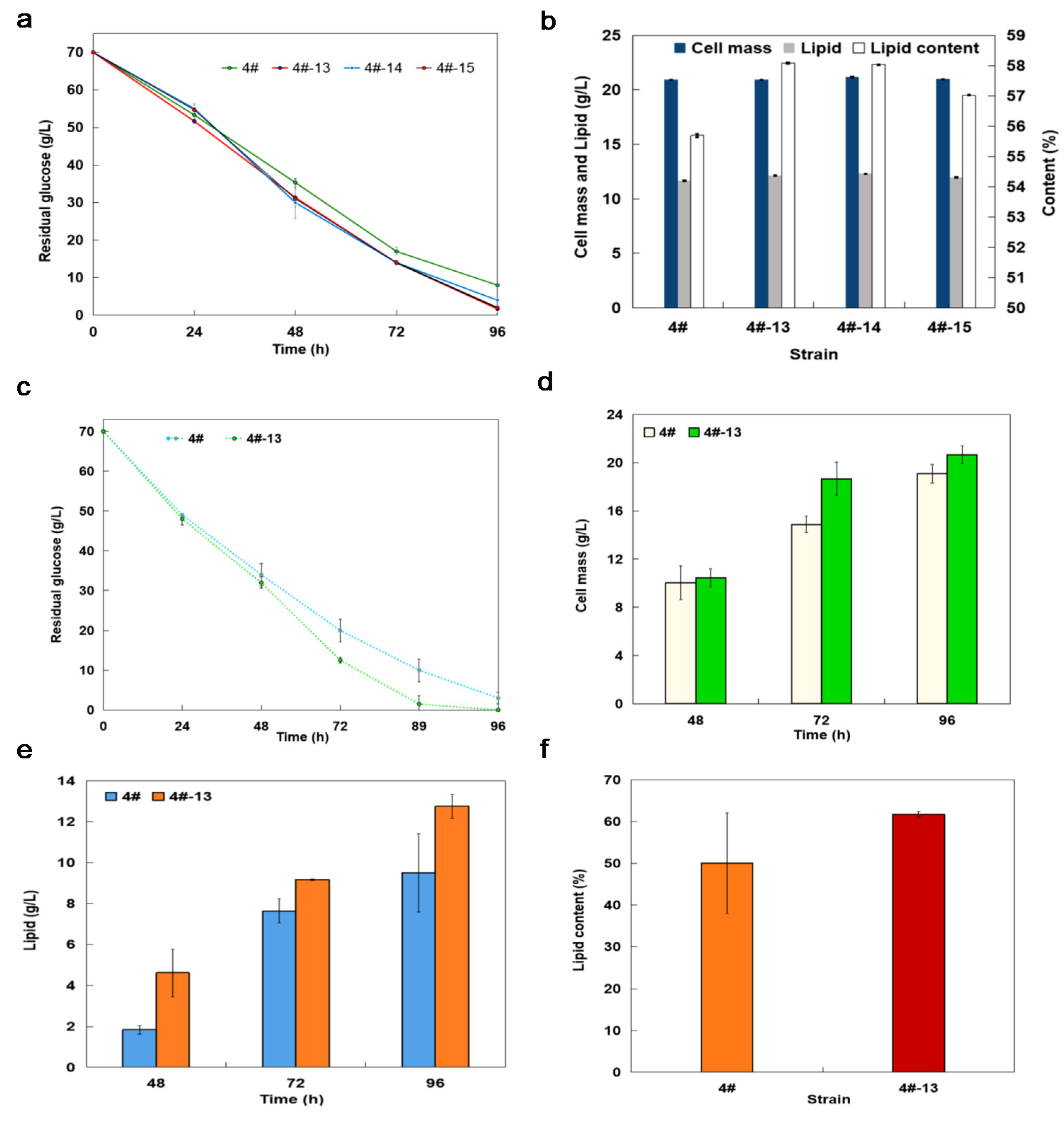
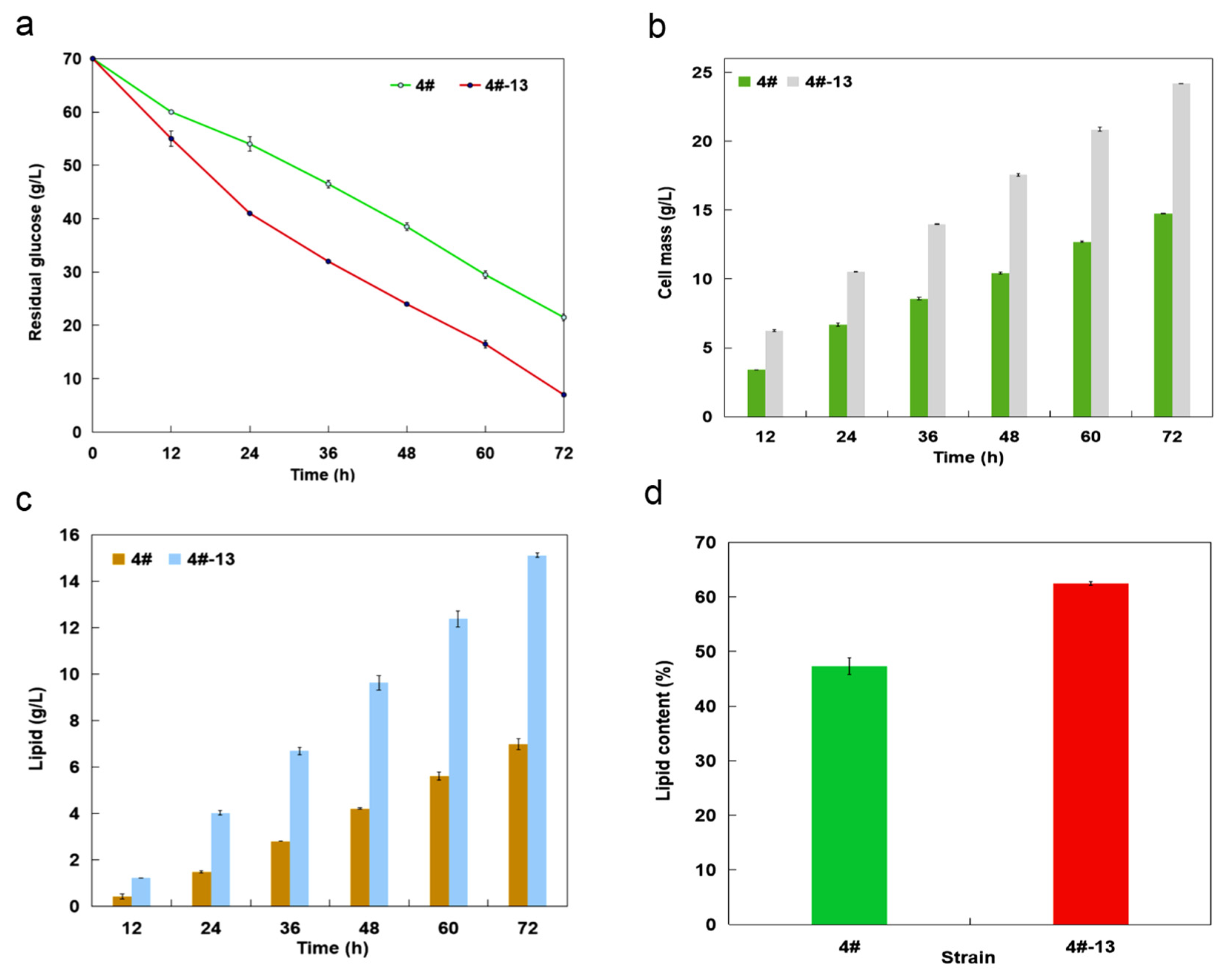
3.2. Lipid Production with Shaking Flask Cultures
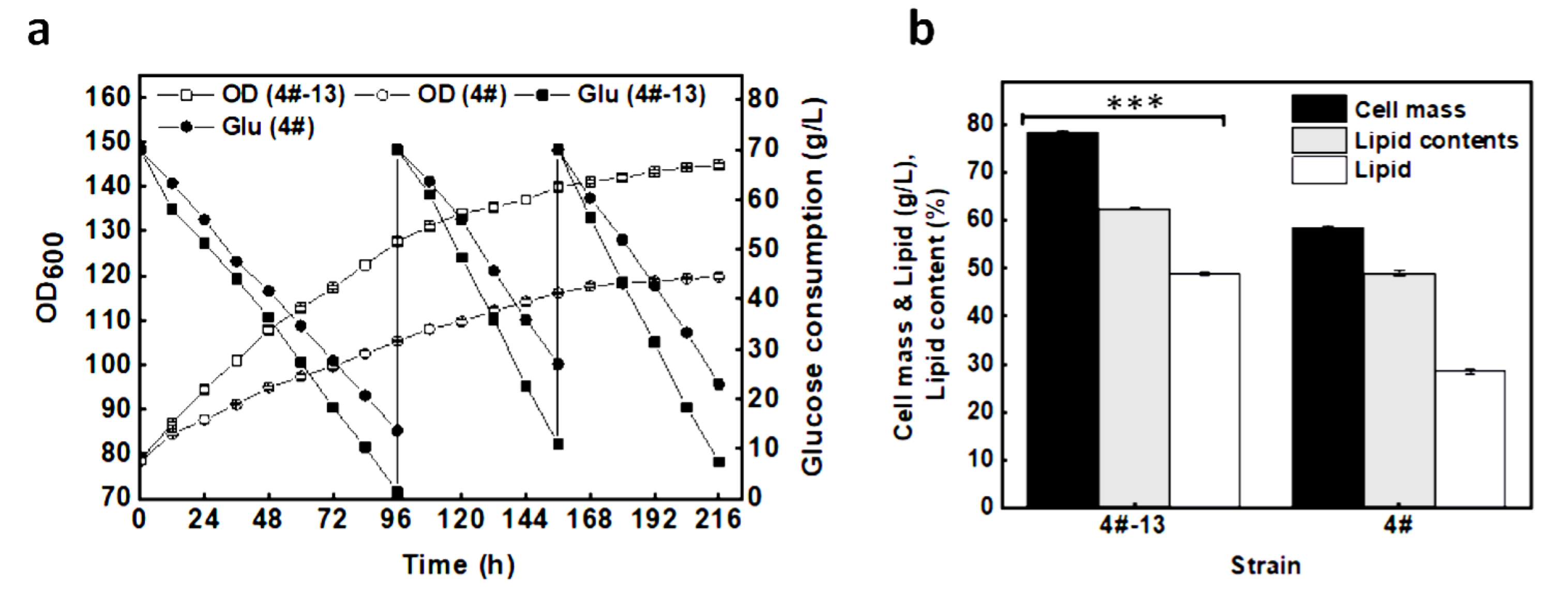
3.3. Lipid Production in 3 L Fermenter under a Reduced DO Level
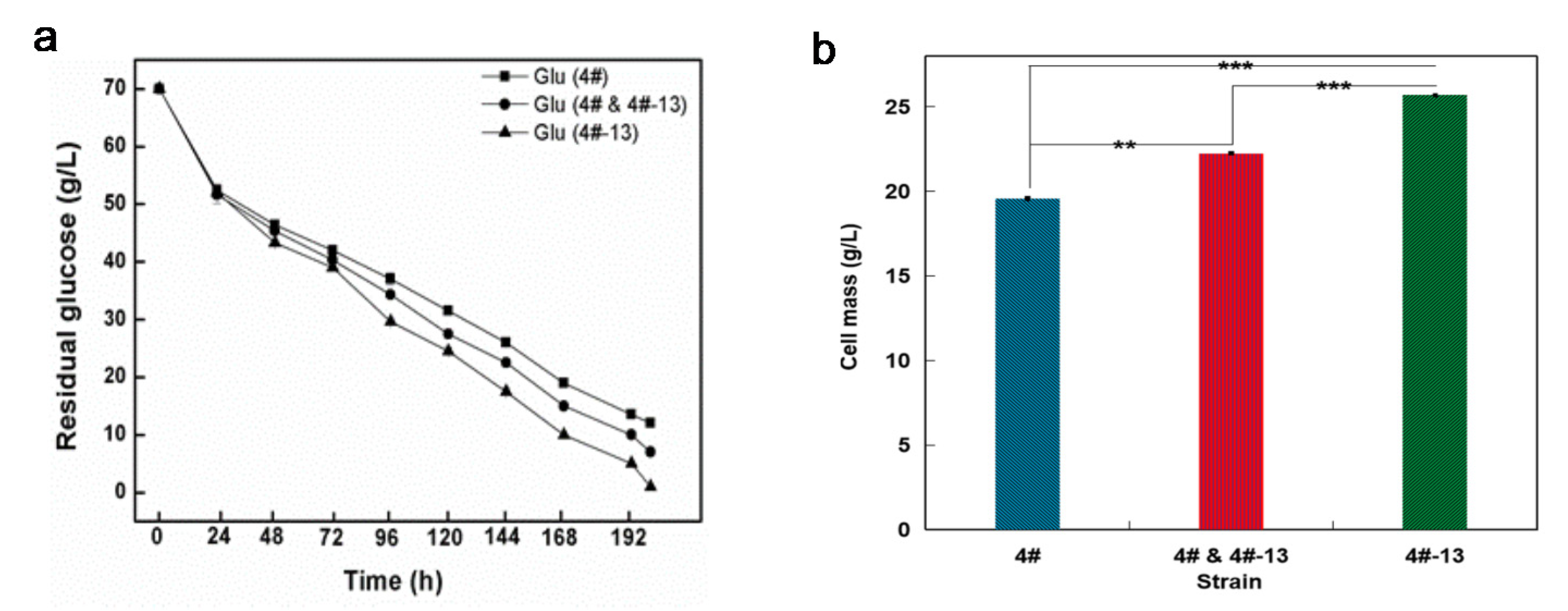
3.4. Lipid Production on Glucose under a Two-Staged Process
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vasconcelos, B.; Teixeira, B.J.; Dragone, G.; Teixeira, J.A. Oleaginous yeasts for sustainable lipid production—From biodiesel to surf boards, a wide range of “green” applications. Appl. Microbiol. Biotechnol. 2019, 103, 3651–3667. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–52. [Google Scholar] [PubMed]
- Koch, B.; Schmidt, C.; Daum, G. Storage lipids of yeasts: A survey of nonpolar lipid metabolism in Saccharomyces cerevisiae, Pichia pastoris, and Yarrowia lipolytica. FEMS Microbiol. Rev. 2014, 38, 892–915. [Google Scholar] [CrossRef] [PubMed]
- Czabany, T.; Athenstaedt, K.; Daum, G. Synthesis, storage and degradation of neutral lipids in yeast. Biochim. Biophys. Acta (BBA)-Mol. Cell Boil. Lipids 2007, 1771, 299–309. [Google Scholar] [CrossRef]
- Bommareddy, R.R.; Sabra, W.; Maheshwari, G.; Zeng, A.P. Metabolic network analysis and experimental study of lipid production in Rhodosporidium toruloides grown on single and mixed substrates. Microb. Cell Fact. 2015, 14, 36. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Z.B.; Bai, F.W. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb. Technol. 2007, 41, 312–317. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Wang, W.; Du, W.; Liu, D. Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem. Eng. J. 2012, 65, 30–36. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass-Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, X.; Zhao, J.; Wu, S.; Zhao, Z.K. Effects of biomass hydrolysis by-products on oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2009, 100, 4843–4847. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, C.M.; Wu, S.G.; Shen, H.W.; Zhao, Z.K. Lipid production by Rhodosporidium toruloides Y4 using different substrate feeding strategies. J. Ind. Microbiol. Biotechnol. 2011, 38, 627–632. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E.; Santos, V.E.; Merchuk, J.C. Oxygen uptake rate in microbial processes: An overview. Biochem. Eng. J. 2010, 49, 289–307. [Google Scholar] [CrossRef]
- Tang, C.C.; Zuo, W.; Tian, Y.; Sun, N.; Wang, Z.W.; Zhang, J. Effect of aeration rate on performance and stability of algal-bacterial symbiosis system to treat domestic wastewater in sequencing batch reactors. Bioresour. Technol. 2016, 222, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Wu, J.Y. Use of n-hexadecane as an oxygen vector to improve Phaffia rhodozyma growth and carotenoid production in shake-flask cultures. J. Appl. Microbiol. 2006, 101, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.G.; Kwak, M.Y.; Rhee, J.S. High density cell culture of Rhodotorula glutinis using oxygen-enriched air. Biotechnol. Lett. 1986, 8, 715–718. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Matsubara, H.; Webster, D.A. Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. Nature 1986, 322, 481–483. [Google Scholar] [CrossRef]
- Orii, Y.; Webster, D.A. Photodissociation of oxygenated cytochromeo(s) (Vitreoscilla) and kinetic studies of reassociation. J. Biol. Chem. 1986, 261, 3544–3547. [Google Scholar]
- Zhang, L.; Li, Y.; Wang, Z.; Xia, Y.; Chen, W.; Tang, K. Recent developments and future prospects of Vitreoscilla hemoglobin application in metabolic engineering. Biotechnol. Adv. 2007, 25, 123–136. [Google Scholar] [CrossRef]
- Ouyang, P.; Wang, H.; Hajnal, I.; Wu, Q.; Guo, Y.; Chen, G.Q. Increasing oxygen availability for improving poly (3-hydroxybutyrate) production by Halomonas. Metab. Eng. 2018, 45, 20–31. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Xie, Y.; Jia, S.; Hou, Y.; Zou, Y.; Zhong, C. Enhanced bacterial cellulose production by Gluconacetobacter xylinus via expression of Vitreoscilla hemoglobin and oxygen tension regulation. Appl. Microbiol. Biotechnol. 2017, 102, 1155–1165. [Google Scholar] [CrossRef]
- Vélez-Lee, A.E.; Cordova-Lozano, F.; Bandala, E.R.; Sanchez-Salas, J.L. Cloning and expression of vgb gene in Bacillus cereus, improve phenol and p-nitrophenol biodegradation. Phys. Chem. Earth Parts A/B/C 2016, 91, 38–45. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, X.; Song, B.; Xue, W.; Su, X.; Chen, X.; Dong, Z. Improving cellulase production in submerged fermentation by the expression of a Vitreoscilla hemoglobin in Trichoderma reesei. AMB Express 2017, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jiao, X.; Liu, X.J.; Zhang, Y.; Zhang, S.; Zhao, Z.K. Analysis of codon usage in Rhodosporidium toruloides. Mycosystema 2018, 37, 1454–1465. [Google Scholar]
- Lin, X.; Wang, Y.; Zhang, S.; Zhu, Z.; Zhou, Y.J.; Yang, F.; Sun, W.Y.; Wang, X.Y.; Zhao, Z.K. Functional integration of multiple genes into the genome of the oleaginous yeast Rhodosporidium toruloides. FEMS Yeast Res. 2014, 14, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Van den Ent, F.; Löwe, J. RF cloning: A restriction-free method for inserting target genes into plasmids. J. Biochem. Biophys. Methods 2006, 67, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, S.; Zhang, S. Enhanced production of total flavones and exopolysaccharides via Vitreoscilla hemoglobin biosynthesis in Phellinus igniarius. Bioresour. Technol. 2011, 102, 1747–1751. [Google Scholar] [CrossRef]
- Yang, X.; Jin, G.; Gong, Z.; Shen, H.; Bai, F.; Zhao, Z.K. Recycling biodiesel-derived glycerol by the oleaginous yeast Rhodosporidium toruloides Y4 through the two-stage lipid production process. Biochem. Eng. J. 2014, 91, 86–91. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Y.; Cui, Q.; Song, X. Expression of Vitreoscilla hemoglobin enhances production of arachidonic acid and lipids in Mortierella alpina. BMC Biotechnol. 2017, 17, 68. [Google Scholar] [CrossRef]
- Shen, H.; Jin, G.; Hu, C.; Gong, Z.; Bai, F.; Zhao, Z.K. Effects of dilution rate and carbon-to-nitrogen ratio on lipid accumulation by Rhodosporidium toruloides under chemostat conditions. Chin. J. Biotechnol. 2012, 28, 57–65. [Google Scholar]
- Zhang, H.; Kang, X.; Xiao, N.; Gao, M.; Zhao, Y.; Zhang, B.; Song, Y. Intracellular expression of Vitreoscilla hemoglobin improves lipid production in Yarrowia lipolytica. Lett. Appl. Microbiol. 2019, 68, 248–257. [Google Scholar] [CrossRef]
- Xue, S.J.; Jiang, H.; Chen, L.; Ge, N.; Liu, G.L.; Hu, Z.; Chi, Z.M.; Chi, Z. Over-expression of Vitreoscilla hemoglobin (VHb) and flavohemoglobin (FHb) genes greatly enhances pullulan production. Int. J. Biol. Macromol. 2019, 132, 701–709. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Zhu, Z.; Shen, H.; Lin, X.; Jin, X.; Jiao, X.; Zhao, Z.K. Systems analysis of phosphate-limitation-induced lipid accumulation by the oleaginous yeast Rhodosporidium toruloides. Biotechnol. Biofuels 2018, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Zhang, Y.; Shen, H.; Yang, X.; Xie, H.; Zhao, Z.K. Fatty acid ethyl esters production in aqueous phase by the oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2013, 150, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.R.; Neto, C.J.D.; Soccol, V.T.; Sydney, E.B.; da Costa, E.S.F.; Medeiros, A.B.P.; Vandenberghe, L.P.S. Pilot scale biodiesel production from microbial oil of Rhodosporidium toruloides DEBB 5533 using sugarcane juice: Performance in diesel engine and preliminary economic study. Bioresour. Technol. 2017, 223, 259–268. [Google Scholar] [CrossRef]
- Park, Y.K.; Nicaud, J.M.; Ledesma-Amaro, R. The engineering potential of Rhodosporidium toruloides as a workhorse for biotechnological applications. Trends Biotechnol. 2018, 36, 304–317. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, S.; Odoh, C.K.; Jin, M.; Zhao, Z.K. Rhodosporidium toruloides—A potential red yeast chassis for lipids and beyond. FEMS Yeast Res. 2020, 20, foaa038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Pötter, M.; Sun, W.; Li, L.; Yang, X.; Jiao, X.; Zhao, Z.K. Overexpression of Δ12-fatty acid desaturase in the oleaginous yeast Rhodosporidium toruloides for production of linoleic acid-rich lipids. Appl. Biochem. Biotechnol. 2016, 180, 1497–1507. [Google Scholar] [CrossRef]
- Pimienta, J.A.P.; Papa, G.; Rodrigue, A.; Carolina, A.; Barcelos, C.A.; Liang, L.; Stavila, V.; Sanchez, A.; Gladden, J.M.; Simmons, B.A. Pilot-scale hydrothermal pretreatment and optimized saccharification enables bisabolene production from multiple feedstock. Green Chem. 2019, 21, 3152–3164. [Google Scholar] [CrossRef]
- Geiselman, G.M.; Zhuang, X.; Kirby, J.; Tran-Gyamfi, M.B.; Prahl, J.-P.; Sundstrom, E.R.; Gao, Y.; Munoz, N.M.; Nicora, C.D.; Clay, D.M.; et al. Production of ent-kaurene from lignocellulosic hydrolysate in Rhodosporidium toruloides. Microb. Cell Fact. 2020, 19, 24. [Google Scholar] [CrossRef]
- Ratledge, C.; Hall, M.J. Oxygen demand by lipid-accumulating yeasts in continuous culture. Appl. Environ. Microbiol. 1977, 34, 230–231. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Hegde, K.; Brar, S.K.; Kermanshahipour, A.; Avalos-Ramírez, A. Challenges in lipid production from lignocellulosic biomass using Rhodosporidium sp.; A look at the role of lignocellulosic inhibitors. Biofuels Bioprod. Bioref. 2018, 13, 740–759. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Kamal, R.; Zhang, Y.; Zhou, R.; Lv, L.; Huang, Q.; Qian, S.; Zhang, S.; Zhao, Z.K. Expression of VHb Improved Lipid Production in Rhodosporidium toruloides. Energies 2020, 13, 4446. https://doi.org/10.3390/en13174446
Wang S, Kamal R, Zhang Y, Zhou R, Lv L, Huang Q, Qian S, Zhang S, Zhao ZK. Expression of VHb Improved Lipid Production in Rhodosporidium toruloides. Energies. 2020; 13(17):4446. https://doi.org/10.3390/en13174446
Chicago/Turabian StyleWang, Shuang, Rasool Kamal, Yue Zhang, Renhui Zhou, Liting Lv, Qitian Huang, Siriguleng Qian, Sufang Zhang, and Zongbao Kent Zhao. 2020. "Expression of VHb Improved Lipid Production in Rhodosporidium toruloides" Energies 13, no. 17: 4446. https://doi.org/10.3390/en13174446
APA StyleWang, S., Kamal, R., Zhang, Y., Zhou, R., Lv, L., Huang, Q., Qian, S., Zhang, S., & Zhao, Z. K. (2020). Expression of VHb Improved Lipid Production in Rhodosporidium toruloides. Energies, 13(17), 4446. https://doi.org/10.3390/en13174446





