Sewage Sludge Valorization via Hydrothermal Carbonization: Optimizing Dewaterability and Phosphorus Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Digested Sewage Sludge
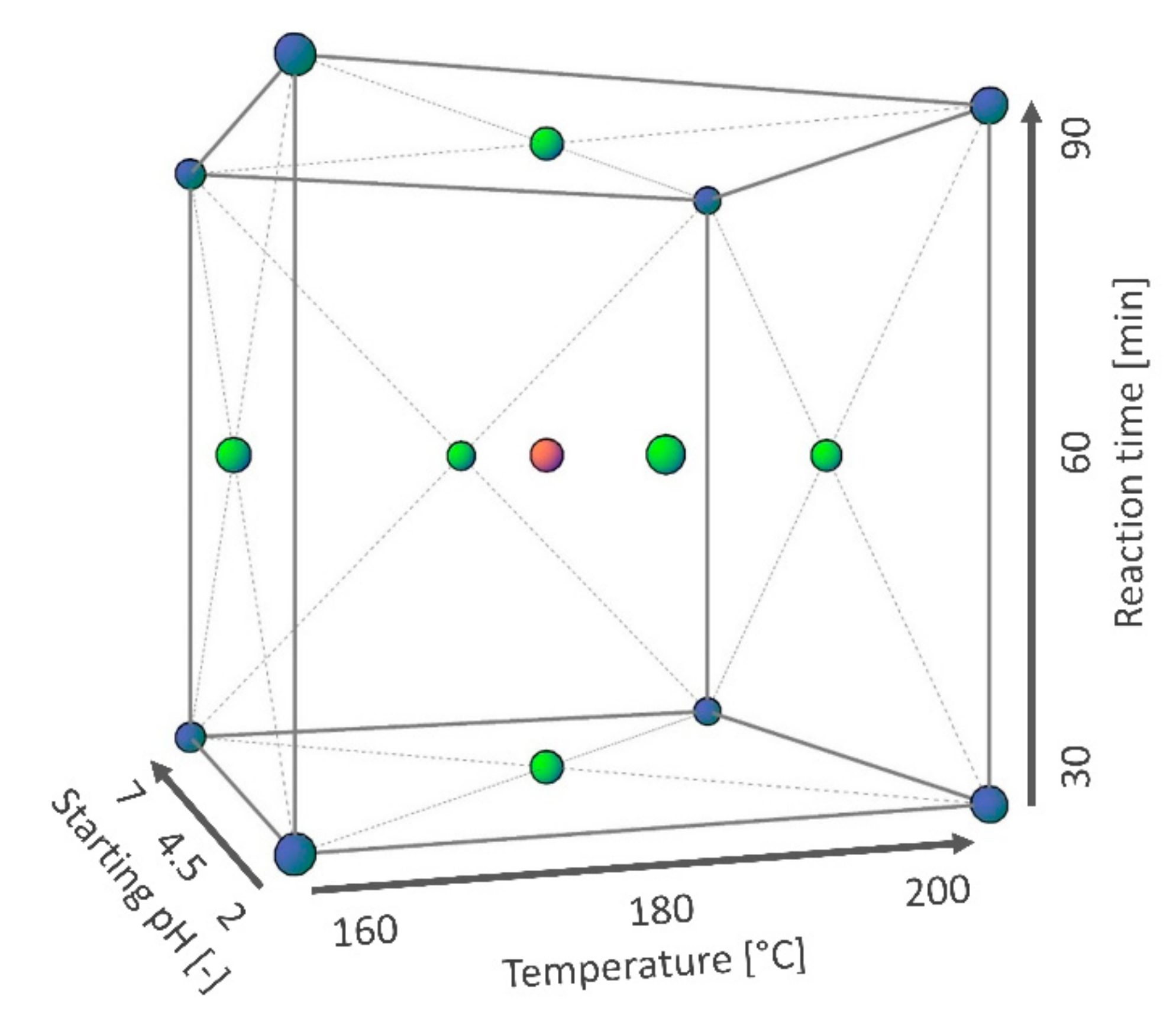
2.2. Experimental Method
2.3. Analytics
2.4. Calculations
2.5. Regression Modeling
3. Results and Discussion
3.1. Regression Modeling
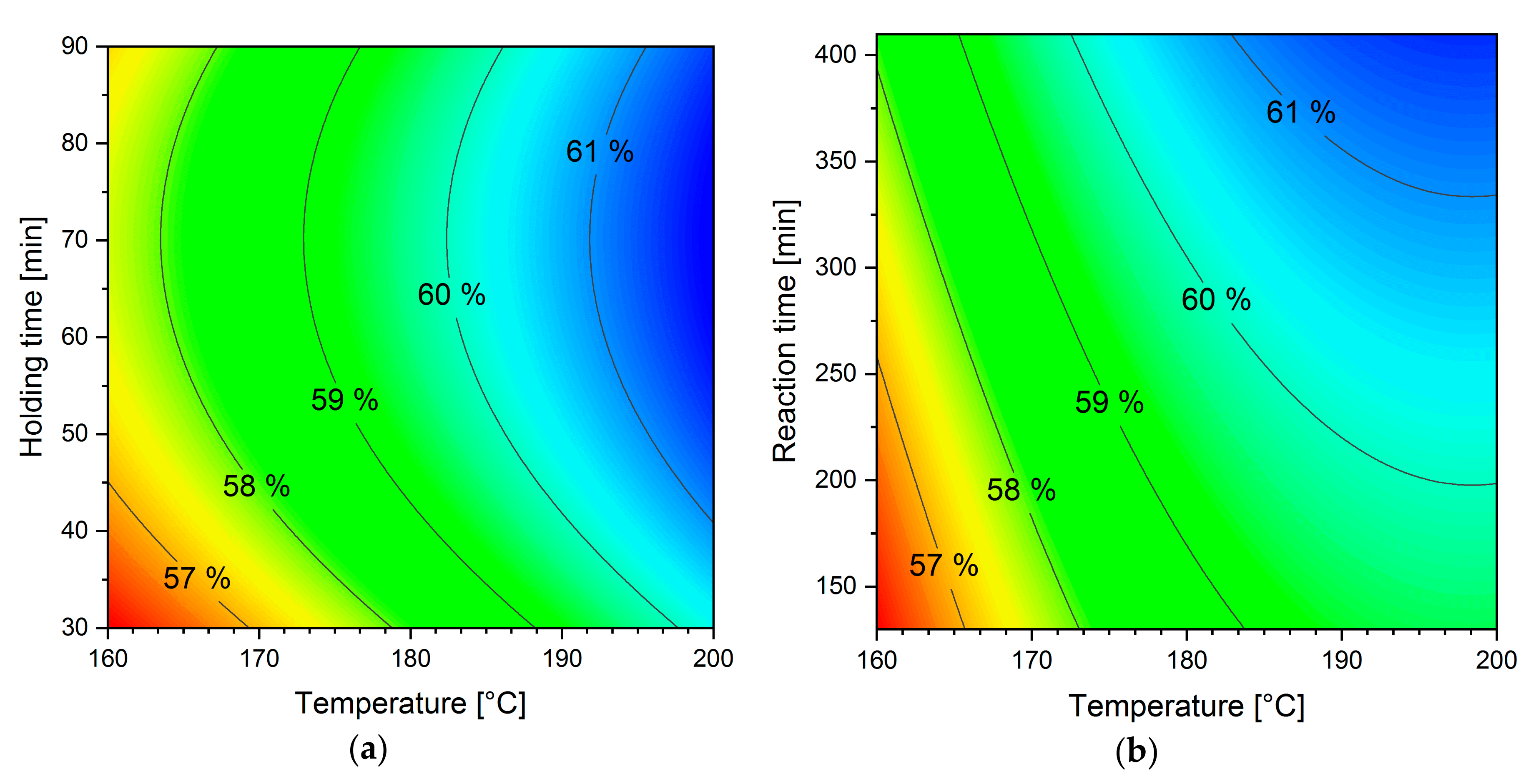
3.2. Holding Time vs. Reaction Time
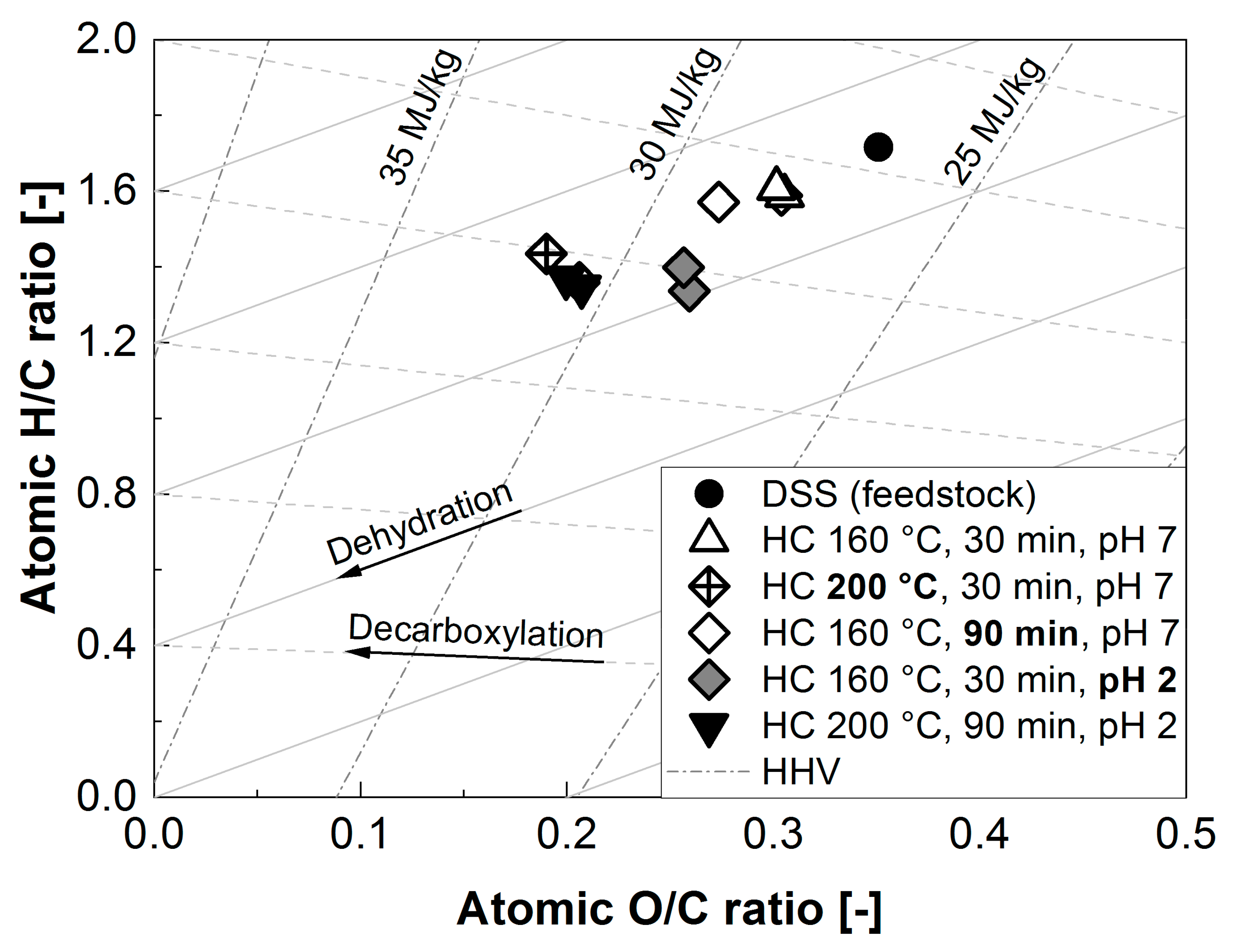
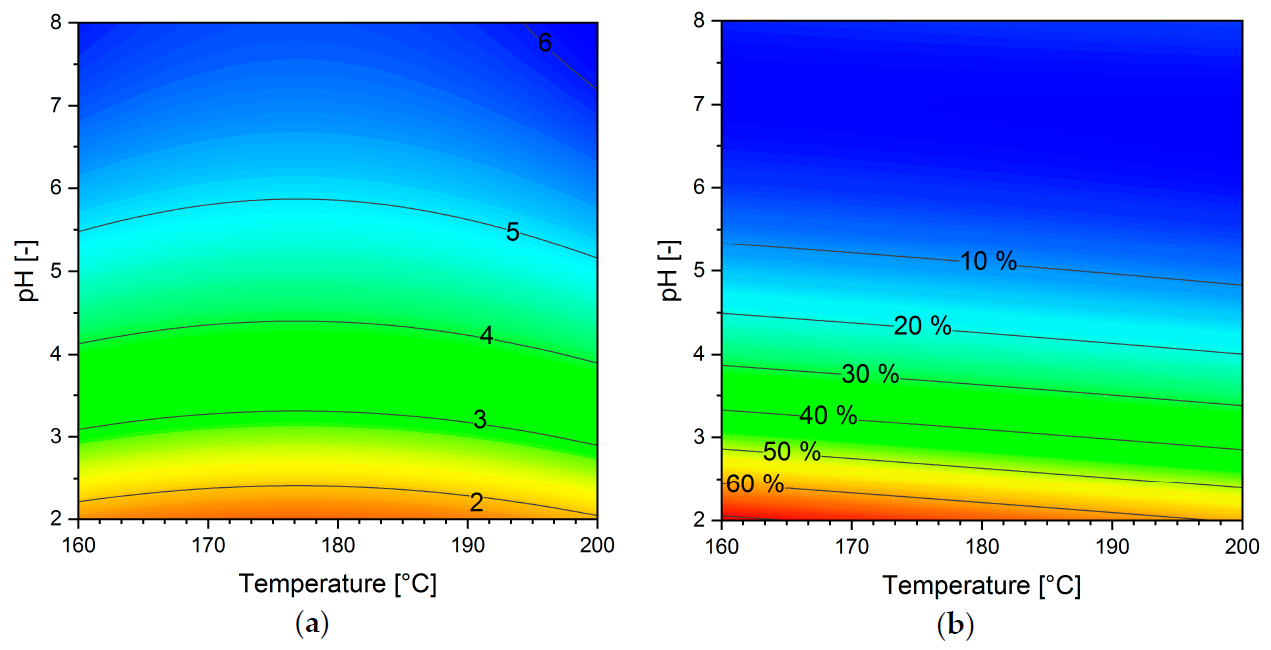
3.3. Hydrochar Properties
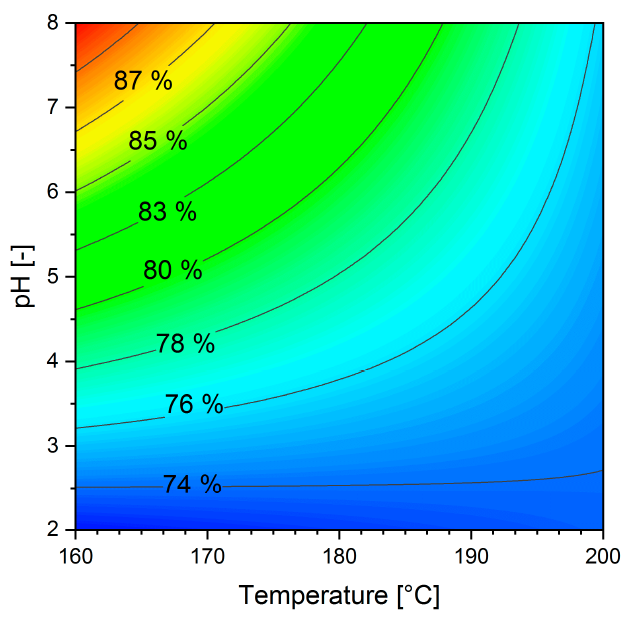
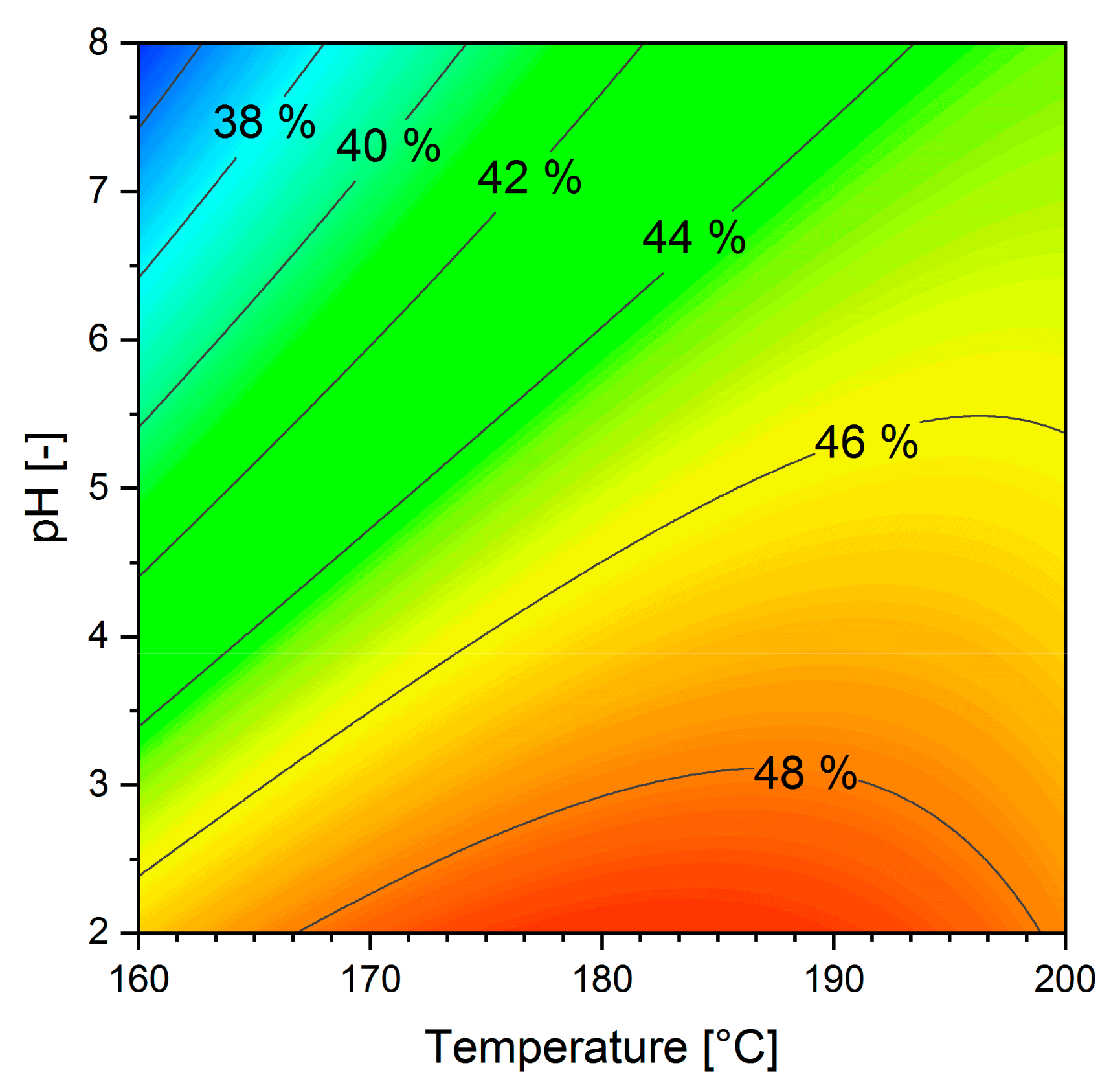
3.4. Phosphorus Release
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Experiment | T (°C) | T (min) | t140 (min) | pH, DoE (-) | pH, Measured (-) |
|---|---|---|---|---|---|
| 1 | 160 | 90 | 275 | 7.0 | 7.8 |
| 2 | 180 | 60 | 251 | 4.5 | 5.6 |
| 3 | 200 | 90 | 411 | 7.0 | 8.1 |
| 4 | 160 | 30 | 130 | 2.0 | 2.1 |
| 5 | 160 | 90 | 274 | 7.0 | 7.7 |
| 6 | 200 | 30 | 247 | 2.0 | 2.3 |
| 7 | 160 | 30 | 144 | 7.0 | 7.7 |
| 8 | 200 | 30 | 207 | 7.0 | 7.8 |
| 9 | 200 | 90 | 365 | 7.0 | 7.1 |
| 10 | 160 | 30 | 147 | 7.0 | 7.4 |
| 11 | 180 | 60 | 221 | 7.0 | 7.3 |
| 12 | 180 | 30 | 178 | 4.5 | 5.3 |
| 13 | 160 | 90 | 274 | 2.0 | 2.2 |
| 14 | 200 | 30 | 236 | 2.0 | 2.2 |
| 15 | 180 | 60 | 264 | 2.0 | 2.1 |
| 16 | 160 | 90 | 280 | 2.0 | 1.9 |
| 17 | 180 | 90 | 352 | 4.5 | 5.5 |
| 18 | 200 | 60 | 324 | 4.5 | 5.5 |
| 19 | 160 | 60 | 194 | 4.5 | 5.3 |
| 20 | 200 | 90 | 398 | 2.0 | 2.1 |
| 21 | 180 | 60 | 251 | 4.5 | 5.4 |
| 22 | 180 | 60 | 272 | 4.5 | 5.4 |
| 23 | 180 | 60 | 241 | 4.5 | 5.4 |
| 24 | 160 | 30 | 155 | 2.0 | 2.3 |
| 25 | 200 | 30 | 242 | 7.0 | 8.1 |
| 26 | 200 | 90 | 413 | 2.0 | 2.0 |


References
- Sterritt, R.M.; Lester, J.N. The value of sewage sludge to agriculture and effects of the agricultural use of sludges contaminated with toxic elements: A review. Sci. Total Environ. 1980, 16, 55–90. [Google Scholar] [CrossRef]
- Beni, C.; Servadio, P.; Marconi, S.; Neri, U.; Aromolo, R.; Diana, G. Anaerobic Digestate Administration: Effect on Soil Physical and Mechanical Behavior. Commun. Soil Sci. Plant Anal. 2012, 43, 821–834. [Google Scholar] [CrossRef]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- BMU. Verordnung über die Verwertung von Klärschlamm, Klärschlammgemisch und Klärschlammkompost-AbfKlärV. BGBl. I S. 3465. 2017. Available online: https://www.bmu.de/en/law/sewage-sludge-ordinance/ (accessed on 21 August 2020).
- Raheem, A.; Sikarwar, V.S.; He, J.; Dastyar, W.; Dionysiou, D.D.; Wang, W.; Zhao, M. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: A review. Chem. Eng. J. 2018, 337, 616–641. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T. Sewage sludge combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sust. Energ. Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Holdich, R.G.; Wheatley, A.D.; Martin, S.J.; Shama, G. Hydrothermal carbonization of primary sewage sludge and synthetic faeces: Effect of reaction temperature and time on filterability. Environ. Prog. Sustain. Energy 2015, 34, 1279–1290. [Google Scholar] [CrossRef]
- Escala, M.; Zumbühl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal Carbonization as an Energy-Efficient Alternative to Established Drying Technologies for Sewage Sludge: A Feasibility Study on a Laboratory Scale. Energy Fuels 2012, 27, 454–460. [Google Scholar] [CrossRef]
- Meng, D.; Jiang, Z.; Yoshikawa, K.; Mu, H. The effect of operation parameters on the hydrothermal drying treatment. Renew. Energy 2012, 42, 90–94. [Google Scholar] [CrossRef]
- Libra, J.; Ro, K.S.; Kammann, C.I.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Heilmann, S.M.; Molde, J.S.; Timler, J.G.; Wood, B.M.; Mikula, A.L.; Vozhdayev, G.V.; Colosky, E.C.; Spokas, K.A.; Valentas, K.J. Phosphorus reclamation through hydrothermal carbonization of animal manures. Environ. Sci. Technol. 2014, 48, 10323–10329. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Luo, G.; Rao, Y.; Chen, H.; Zhang, S. Hydrothermal conversion of dewatered sewage sludge: Focusing on the transformation mechanism and recovery of phosphorus. Chemosphere 2019, 228, 619–628. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Wang, T.; Xu, B.; Li, C.; Zeng, G. Feedwater pH affects phosphorus transformation during hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2017, 245, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikova, E.; Arauzo, P.J.; Becker, G.C.; Kruse, A. Experimental and thermodynamic studies of phosphate behavior during the hydrothermal carbonization of sewage sludge. Sci. Total Environ. 2019, 692, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of lignocellulosic biomass: Effect of process conditions on hydrochar properties. Appl. Energy 2015, 155, 576–584. [Google Scholar] [CrossRef]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of industrial mixed sludge from a pulp and paper mill. Bioresour. Technol. 2016, 200, 444–450. [Google Scholar] [CrossRef]
- Álvarez-Murillo, A.; Román, S.; Ledesma, B.; Sabio, E. Study of variables in energy densification of olive stone by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 307–314. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.D.; Sabio, E.; Ro, K.S.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review. Energies 2018, 11, 216. [Google Scholar] [CrossRef]
- Kieseler, S.; Neubauer, Y.; Zobel, N. Ultimate and Proximate Correlations for Estimating the Higher Heating Value of Hydrothermal Solids. Energy Fuels 2013, 27, 908–918. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Li, A. Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review. Renew. Sustain. Energ. Rev. 2019, 108, 423–440. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung e. V. Sludge, Treated Biowaste and Soil—Determination of pH (EN 15933:2012); German Version; Beuth Verlag GmbH: Berlin, Germany, 2012; DIN EN 15933:2012-11. [Google Scholar]
- Deutsches Institut für Normung e. V. Solid Biofuels—Sample Preparation (ISO 14780:2017); German Version; Beuth Verlag GmbH: Berlin, Germany, 2017; DIN EN ISO 14780:2017-08. [Google Scholar]
- Deutsches Institut für Normung e. V. Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 3: Moisture in General Analysis Sample (ISO 18134-3:2015); German Version; Beuth Verlag GmbH: Berlin, Germany, 2015; DIN EN ISO 18134-3:2015-12. [Google Scholar]
- Deutsches Institut für Normung e. V. Solid biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen—Instrumental Methods (EN 15104:2011); German Version; Beuth Verlag GmbH: Berlin, Germany, 2011; DIN EN 15104:2011-04. [Google Scholar]
- Deutsches Institut für Normung e. V. Solid Biofuels—Determination of Ash Content (EN 14775:2009); German Version; Verlag GmbH: Berlin, Germany, 2012; DIN EN 14775:2012-11. [Google Scholar]
- Deutsches Institut für Normung e. V. Solid Biofuels—Determination of Calorific Value (ISO 18125:2017); German Version; Verlag GmbH: Berlin, Germany, 2017; DIN EN ISO 18125:2017-08. [Google Scholar]
- Deutsches Institut für Normung e. V. Water quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) (ISO 11885:2007); German Version; Beuth Verlag GmbH: Berlin, Germany, 2009; DIN EN ISO 11885:2009-09. [Google Scholar]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons Inc.: Singapore, 2013; ISBN 978-1-118-09793-9. [Google Scholar]
- Weisberg, S. Applied Linear Regression, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005; ISBN 9780471663799. [Google Scholar]
- Wang, L.; Li, A. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: The dewatering performance and the characteristics of products. Water Res. 2015, 68, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal carbonisation of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Gao, N.; Li, Z.; Quan, C.; Miscolczi, N.; Egedy, A. A new method combining hydrothermal carbonization and mechanical compression in-situ for sewage sludge dewatering: Bench-scale verification. J. Anal. Appl. Pyrolysis 2019, 139, 187–195. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, A. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: Influence of operating conditions and the process energetics. Water Res. 2014, 65, 85–97. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Michael, J.; Antal, J.R.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
| C ‡ | H ‡ | N ‡ | S ‡ | O ‡ | Ash † | HHVmeas † | HHVcalc |
|---|---|---|---|---|---|---|---|
| (%db) | (MJ kgdb−1) | ||||||
| 32.5 | 4.7 | 5 | 1.7 | 15.2 | 40.9 | 14.3 | 13.4 |
| (±0.24) | (±0.05) | (±0.04) | (±0.01) | (±0.33) | (±0.11) | (±0.10) | |
| Target Value | Coded Regression Model Coefficients | Metrics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| xT | xt | xpH | xT × xpH | xT² | xt² | xpH² | R2adj | R2pred | |
| Hydrochar Characteristics | |||||||||
| Dry matter (%) | 2.70 | −3.90 | 2.22 | −1.87 | 0.89 | 0.84 | |||
| Carbon content (%daf) | 2.12 | 0.61 | 2.52 | 1.31 | −0.90 | 0.92 | 0.90 | ||
| HHV (MJ kgdb−1) | −0.23 | −0.16 | 0.31 | 0.63 | 0.53 | ||||
| Solid yield (%db) | −3.36 | 5.19 | −4.09 | 0.72 | 0.69 | ||||
| Carbon yield (%db) | −4.39 | 4.62 | −3.40 | 0.72 | 0.69 | ||||
| Energy yield (%db) | −4.95 | 4.04 | −4.01 | 0.78 | 0.74 | ||||
| Process Water Properties | |||||||||
| P content (mg L−1) | −159.55 | −2260.21 | 163.81 | 1720.72 | 0.99 | 0.99 | |||
| P share (%) | −2.56 | −31.46 | 3.64 | 24.97 | 0.99 | 0.98 | |||
| Final pH (-) | 0.10 | 0.11 | 2.17 | 0.32 | −0.87 | 0.99 | 0.99 | ||
| Target Value | Coded Regression Model Coefficients | Metrics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| xT | xt,140 | xpH | xT*xpH | xt140*xpH | xT2 | xpH2 | R2adj | R2pred | |
| Hydrochar characteristics | |||||||||
| Carbon content (%daf) | 1.72 | 1.04 | 2.56 | 1.34 | −0.93 | 0.91 | 0.89 | ||
| HHV (MJ kgdb−1) | −0.17 | −0.15 | −0.17 | 0.16 | 0.35 | 0.73 | 0.61 | ||
| Process water properties | |||||||||
| Final pH (-) | 0.24 | 2.17 | −0.59 | 0.99 | 0.99 | ||||
| T (°C) | t (min) | pH (-) | C Content (%daf) | N Content (%daf) | HHV (MJ kgdb−1) | Solid Yield (%db) | C Yield (%db) | Energy Yield (%db) |
|---|---|---|---|---|---|---|---|---|
| 160 | 30 | 7.5 ± 0.23 | 57.3 ± 0.04 | 4.6 ± 0.16 | 13.7 ± 0.27 | 90.0 ± 0.88 | 86.4 ± 2.02 | 92.1 ± 2.71 |
| 200 | 30 | 7.9 ± 0.23 | 63.8 ± 0.63 | 3.7 ± 0.19 | 13.0 ± 0.52 | 76.9 ± 0.69 | 71.9 ± 2.32 | 74.9 ± 3.68 |
| 160 | 90 | 7.7 ± 0.07 | 58.3 ± 1.02 | 4.4 ± 0.04 | 13.6 ± 0.00 | 85.8 ± 7.44 | 82.2 ± 6.94 | 87.5 ± 7.58 |
| 160 | 30 | 2.2 ± 0.14 | 55.5 ± 0.13 | 3.6 ± 0.09 | 14.5 ± 0.62 | 62.7 ± 18.60 | 62.4 ± 15.93 | 67.4 ± 17.24 |
| 200 | 90 | 2.1 ± 0.05 | 57.9 ± 0.45 | 3.1 ± 0.08 | 13.3 ± 0.07 | 74.2 ± 1.77 | 68.1 ± 1.46 | 73.5 ± 1.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lühmann, T.; Wirth, B. Sewage Sludge Valorization via Hydrothermal Carbonization: Optimizing Dewaterability and Phosphorus Release. Energies 2020, 13, 4417. https://doi.org/10.3390/en13174417
Lühmann T, Wirth B. Sewage Sludge Valorization via Hydrothermal Carbonization: Optimizing Dewaterability and Phosphorus Release. Energies. 2020; 13(17):4417. https://doi.org/10.3390/en13174417
Chicago/Turabian StyleLühmann, Taina, and Benjamin Wirth. 2020. "Sewage Sludge Valorization via Hydrothermal Carbonization: Optimizing Dewaterability and Phosphorus Release" Energies 13, no. 17: 4417. https://doi.org/10.3390/en13174417
APA StyleLühmann, T., & Wirth, B. (2020). Sewage Sludge Valorization via Hydrothermal Carbonization: Optimizing Dewaterability and Phosphorus Release. Energies, 13(17), 4417. https://doi.org/10.3390/en13174417





