Hybrid Microencapsulated Phase-Change Material and Carbon Nanotube Suspensions toward Solar Energy Conversion and Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
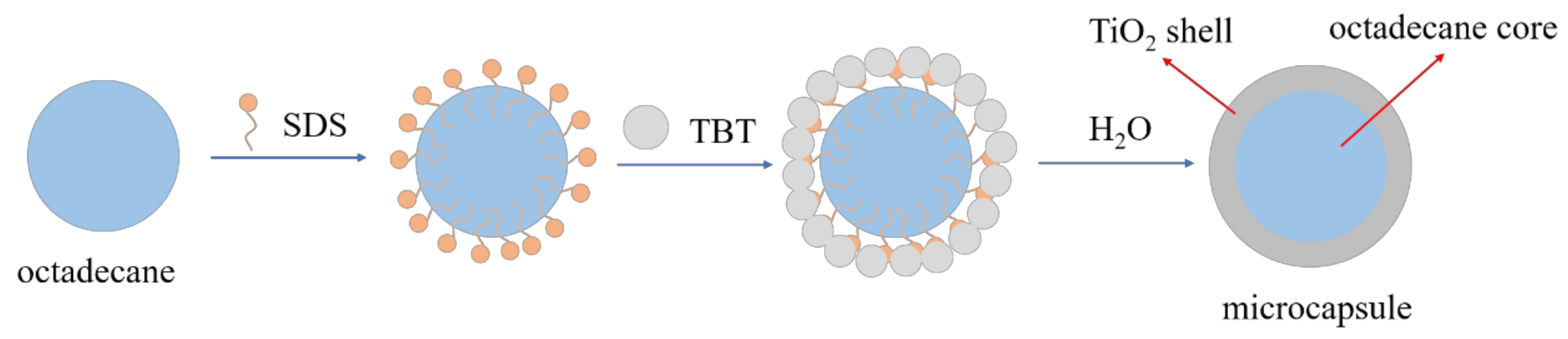
2.2. Synthesis of Octadecane@TiO2 MEPCM
2.3. Characterization and Measurement
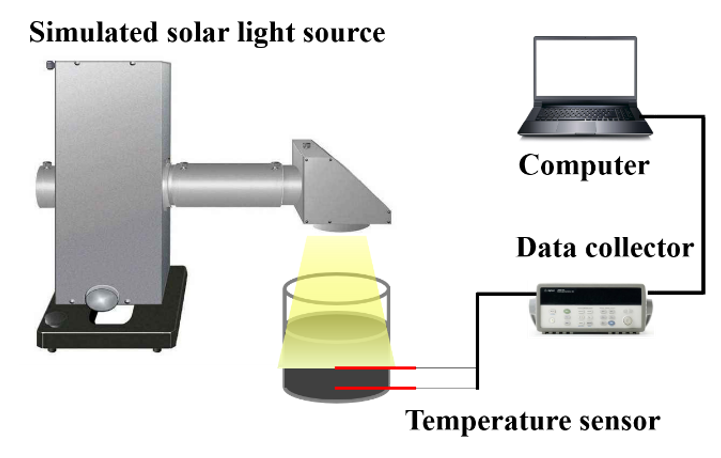
2.4. Photo-Thermal Conversion Performance of MEPCM/CNT Suspension
3. Results and Discussion
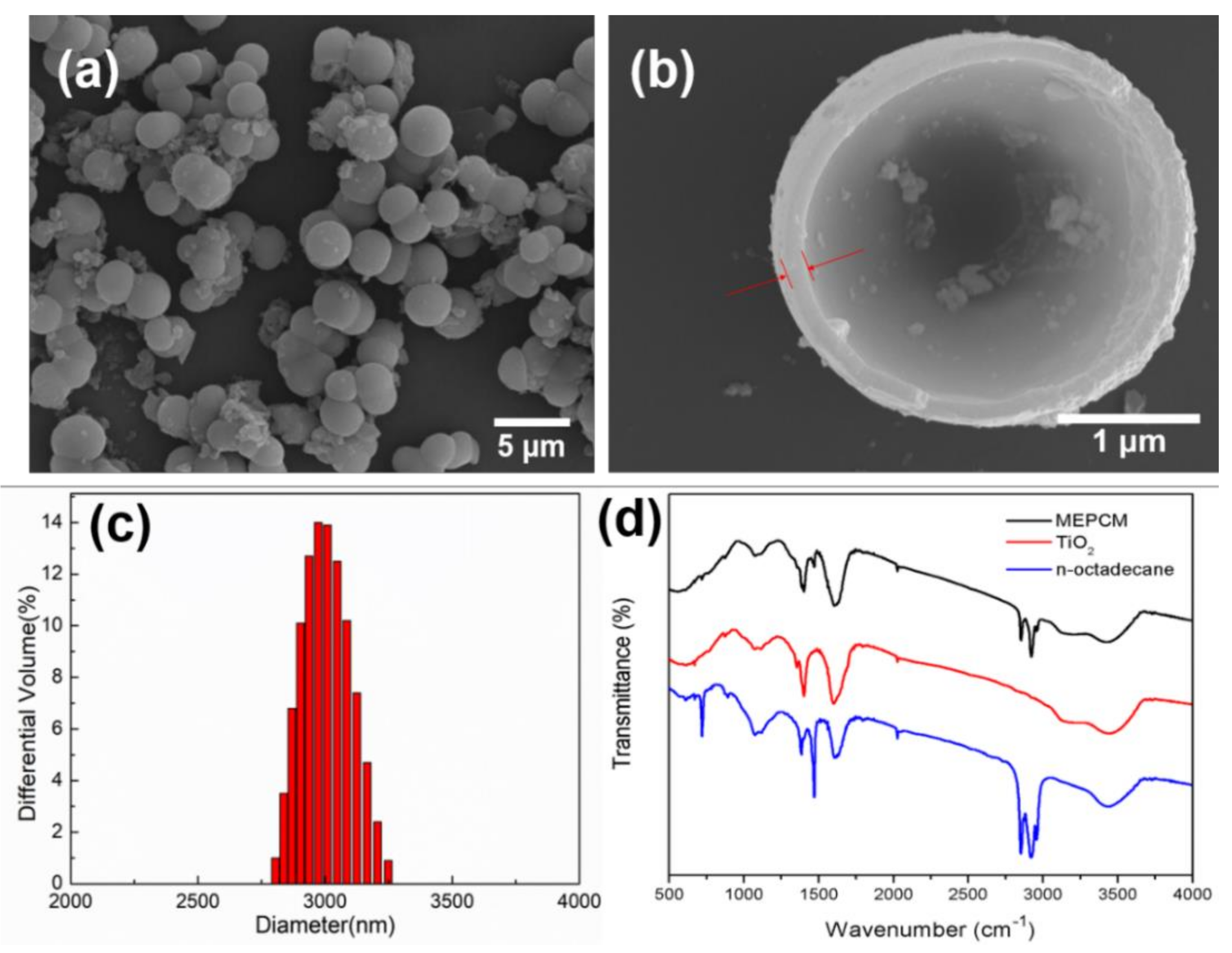
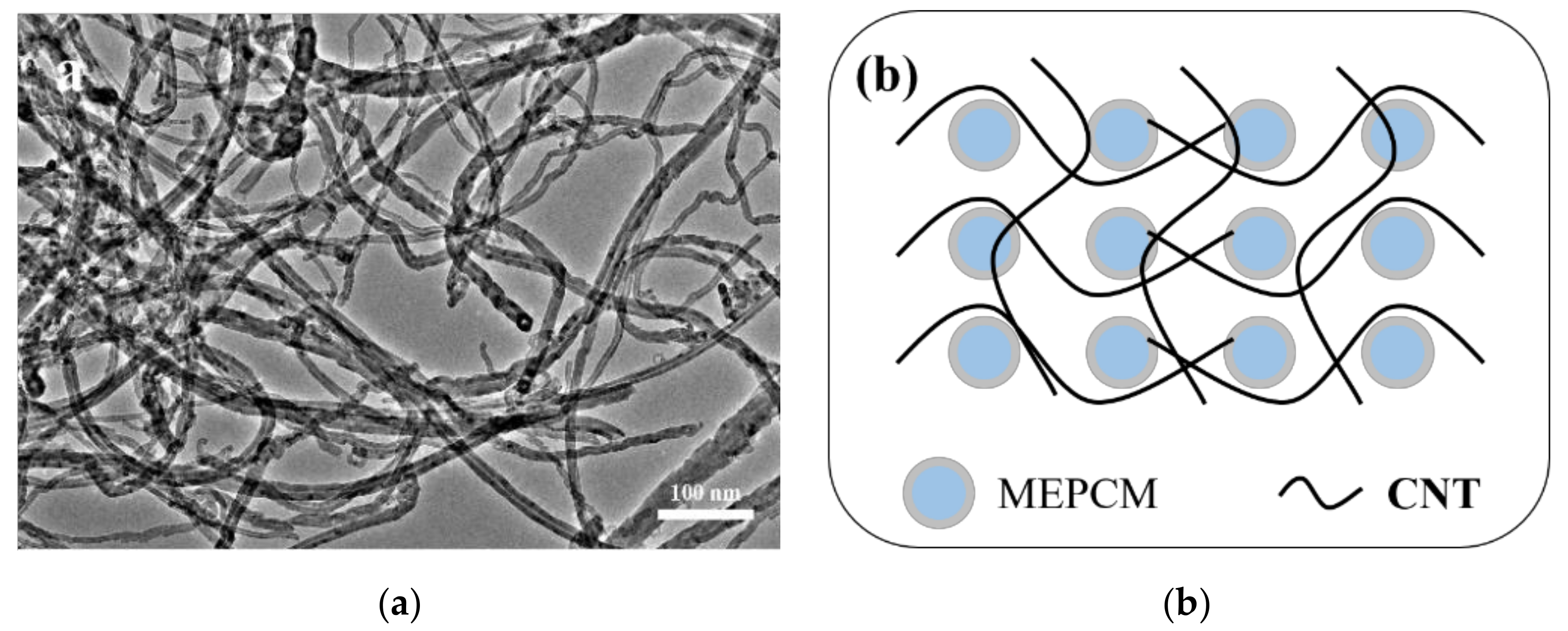
3.1. Morphology and Chemical Structure of MEPCMs
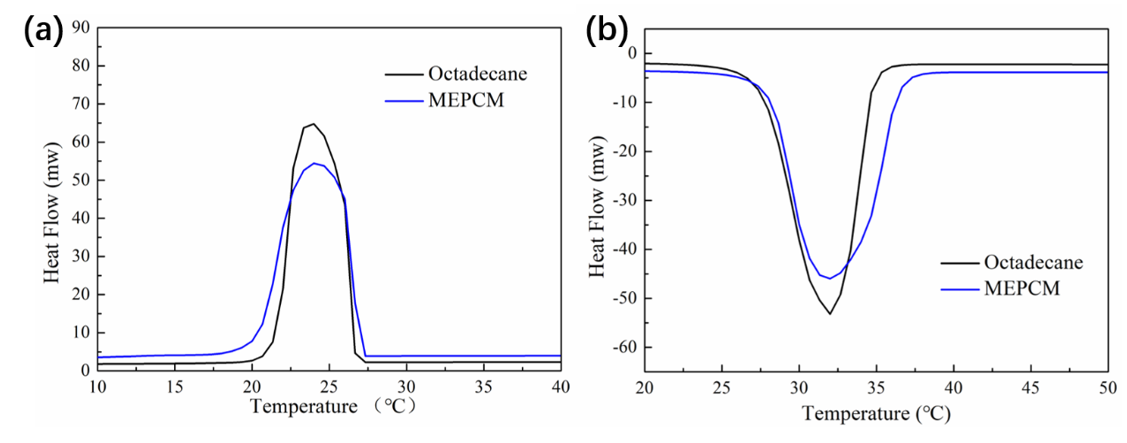
3.2. Thermal Properties of the MEPCMs
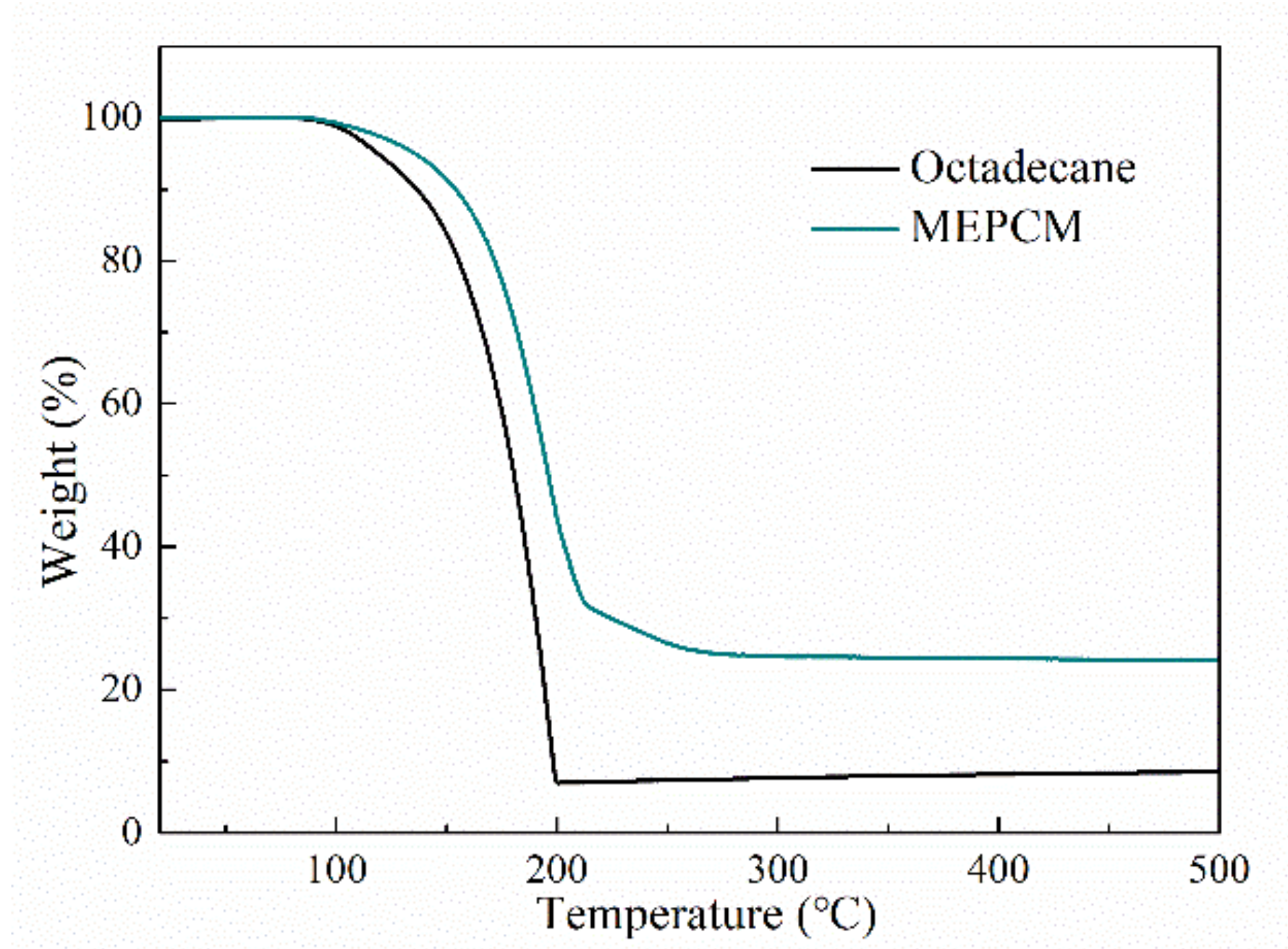
3.3. Thermal Stability of MEPCM
3.4. Dispersion Stability of MEPCM/CNT Suspension
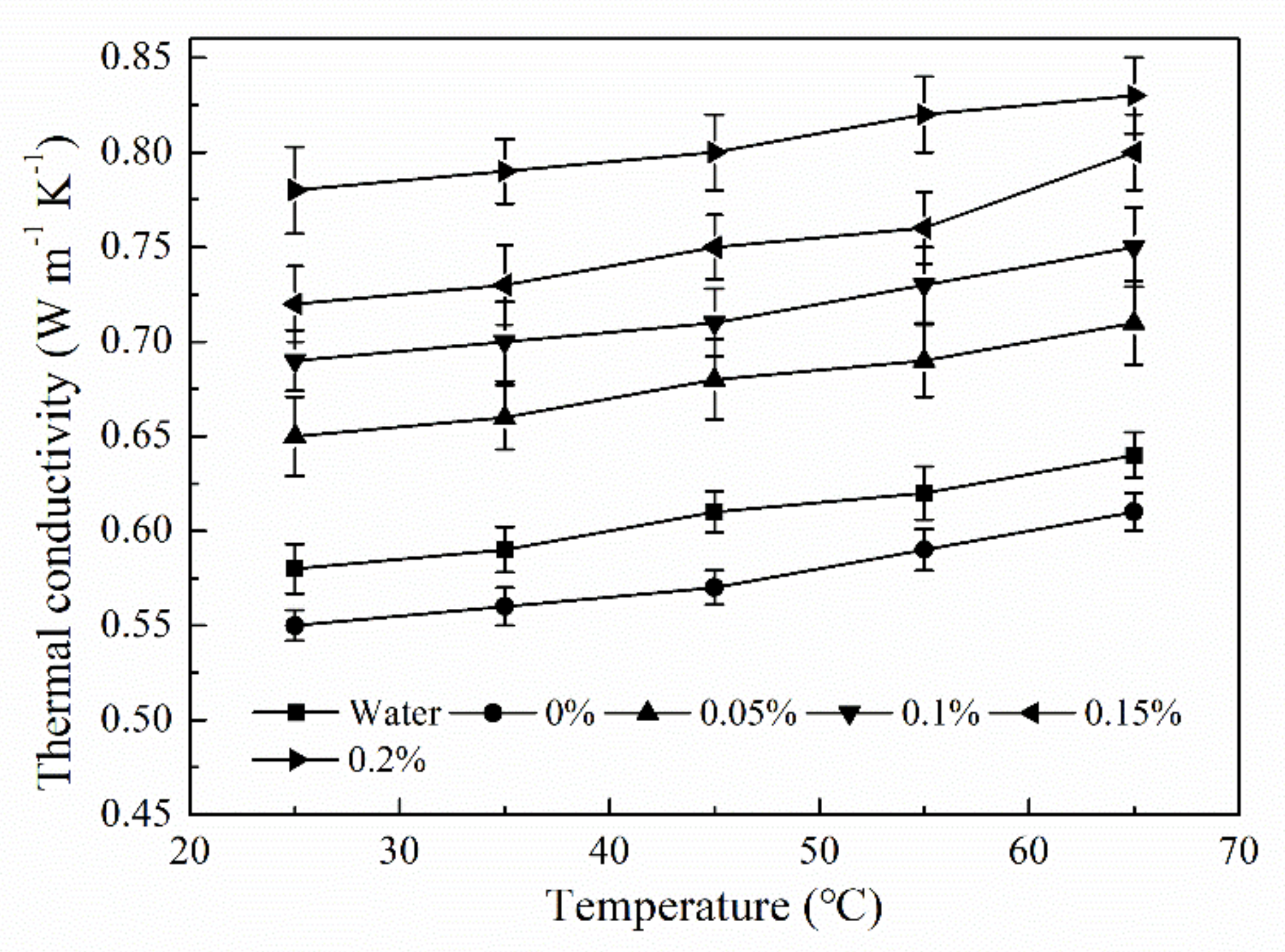
3.5. Thermal-Physical Properties of Hybrid MEPCM/CNT Suspension
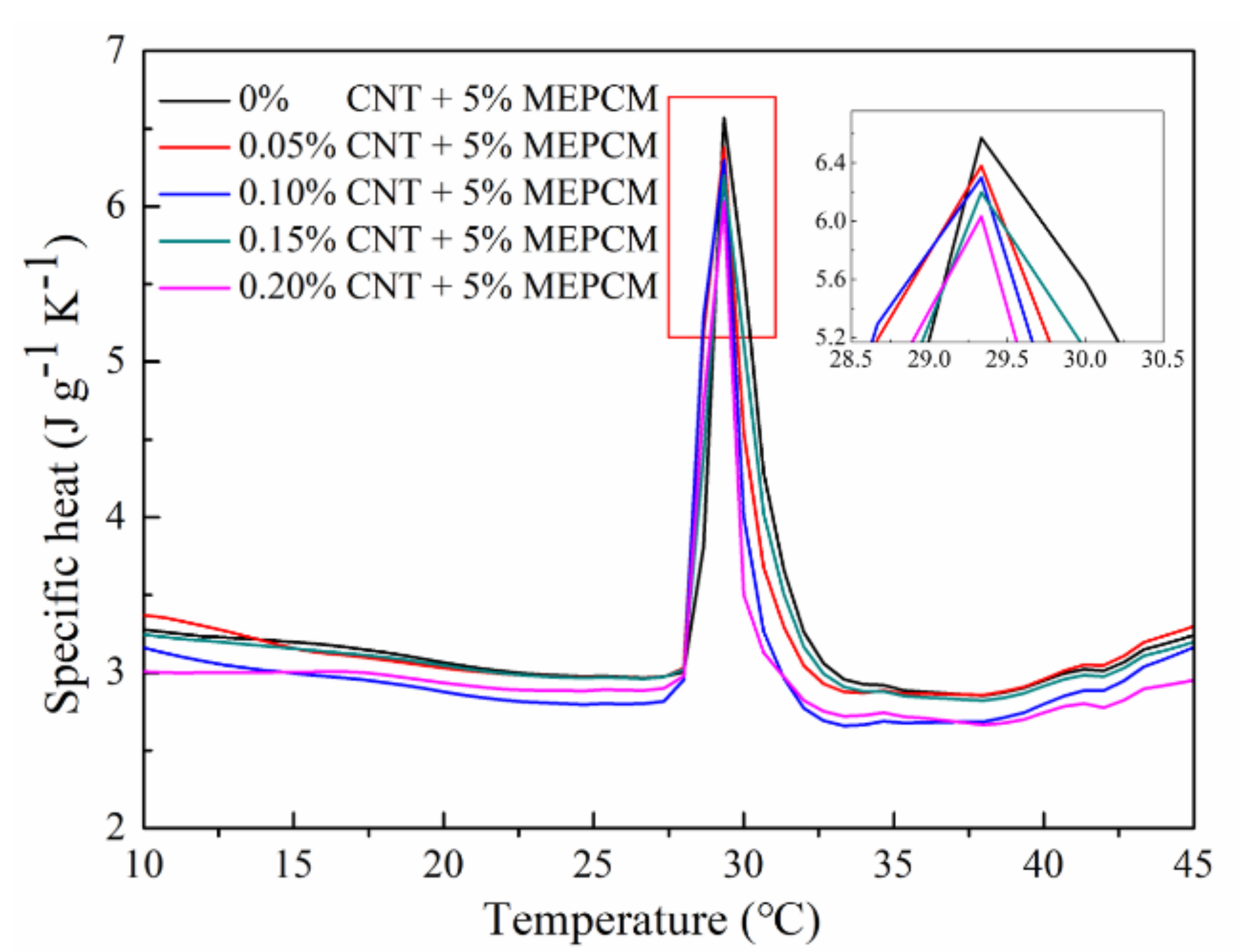
3.6. Specific Heat of Hybrid MEPCM/CNT Suspension
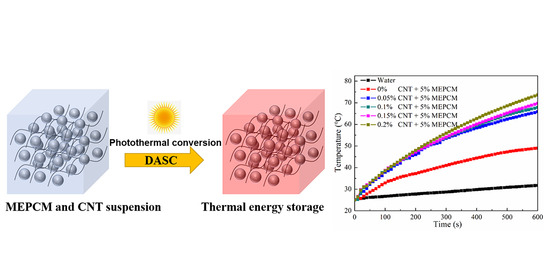
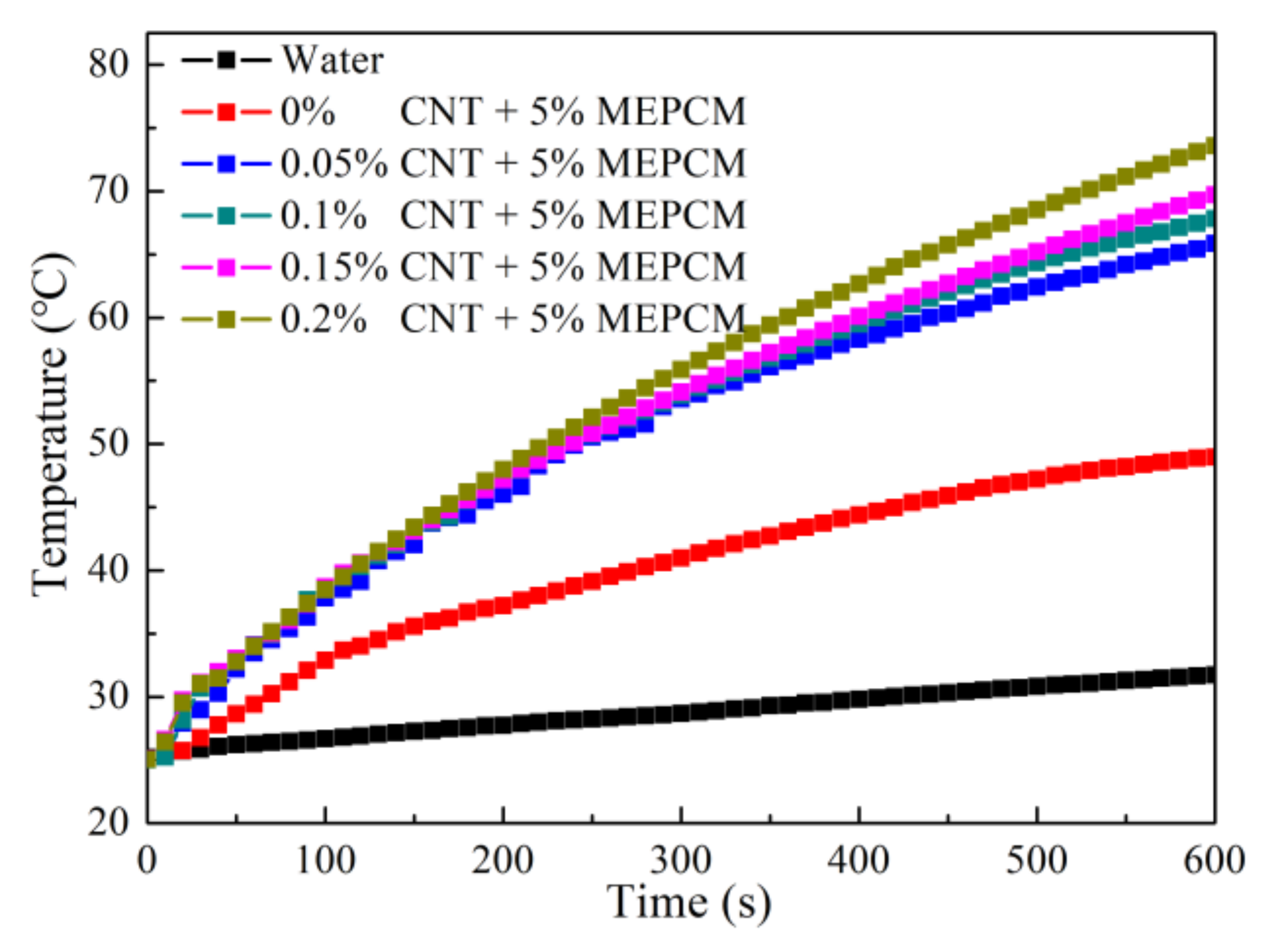
3.7. Photo-Thermal Performance of Hybrid MEPCM/CNT Suspension
4. Conclusions
- The n-octadecane@TiO2 composite microcapsules were successfully synthesized through an in situ hydrolysis and polycondensation reaction in a nonaqueous emulsion. The morphology and thermal properties of the MEPCMs were characterized. The SEM results showed that the prepared microcapsules displayed a uniform spherical shape and a well-defined core-shell structure with a smooth surface, the thickness of the shell was about 100 nm. The average diameter of the MEPCMs was about 2.9 μm and had a narrow distribution, which was confirmed by the DLS test. The FT-IR results confirmed the successful encapsulation of n-octadecane into TiO2 shell. The DSC measurements indicated that the MEPCMs had a large latent heat of 154 J/g and the encapsulated ratio was about 65.84%. The TGA results showed that the TiO2 shell of the MEPCMs improved thermal stability.
- Due to the net structure of CNT in the water, the dispersion stability of the MEPCM/CNT suspension was higher than that of MEPCM suspension. The thermal conductivity of the MEPCM/CNT suspension can reach 0.83 W/(m·K), which was about 29.7% higher than that of the pure water. The specific heats of the MEPCM/CNT suspension were higher than water due to the phase change of the MEPCM. The photo-thermal conversion efficiency of MEPCM/CNT suspension reaches 86.0% because the hybrid suspension exhibited higher thermal conductivity and optical absorption property than the pure water. It can be concluded that the hybrid MEPCM/CNT suspension with enhanced thermophysical and photo-thermal conversion performance paves the way for the application of the binary system of MEPCM and nanoparticles towards improving the effective receiver efficiency of DASC.
Author Contributions
Funding
Conflicts of Interest
References
- Xu, B.; Xu, J.; Chen, Z. Heat transfer study in solar collector with energy storage. Int. J. Heat Mass Transf. 2020, 156, 119778. [Google Scholar] [CrossRef]
- Timilsina, G.R.; Kurdgelashvili, L.; Narbel, P.A. A review of solar energy: Markets, economics and policies. Policy Res. Work. Pap. 2011, 16, 1. [Google Scholar]
- Huang, Z.F.; Pan, L.; Zou, J.J.; Zhang, X.; Wang, L. Nanostructured bismuth vanadate-based materials for solar-energy-driven water oxidation: A review on recent progress. Nanoscale 2014, 6, 14044–14063. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, R.; Wang, Z.; Wang, Q. Photo-thermal conversion properties of hybrid CuO-MWCNT/H2O nanofluids for direct solar thermal energy harvest. Appl. Therm. Eng. 2019, 147, 390–398. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Zeng, S.; Zheng, Z. Experimental investigation on photothermal properties of nanofluids for direct absorption solar thermal energy systems. Energy Convers. Manag. 2013, 73, 150–157. [Google Scholar] [CrossRef]
- Sani, E.; Mercatelli, L.; Barison, S.; Pagura, C.; Agresti, F.; Colla, L.; Sansoni, P. Potential of carbon nanohorn-based suspensions for solar thermal collectors. Sol. Energy Mater. Sol. Cells 2011, 95, 2994–3000. [Google Scholar] [CrossRef]
- Zeiny, A.; Jin, H.; Bai, L.; Lin, G.; Wen, D. A comparative study of direct absorption nano fluids for solar thermal applications. Sol. Energy 2018, 161, 74–82. [Google Scholar] [CrossRef]
- Gorji, T.B.; Ranjbar, A.A.; Mirzababaei, S.N. Optical properties of carboxyl functionalized carbon nanotube aqueous nanofluids as direct solar thermal energy absorbers. Sol. Energy 2015, 119, 332–342. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Wen, X.; Yin, H.; Liu, J. A novel CNT encapsulated phase change material with enhanced thermal conductivity and photo-thermal conversion performance. Sol. Energy Mater. Sol. Cells 2018, 184, 82–90. [Google Scholar] [CrossRef]
- Gao, F.; Wang, X.; Wu, D. Design and fabrication of bifunctional microcapsules for solar thermal energy storage and solar photocatalysis by encapsulating para ffi n phase change material into cuprous oxide. Sol. Energy Mater. Sol. Cells 2017, 168, 146–164. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhang, G.H. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhao, C.Y. Thermal property investigation of aqueous suspensions of microencapsulated phase change material and carbon nanotubes as a novel heat transfer fluid. Renew. Energy 2013, 60, 433–438. [Google Scholar] [CrossRef]
- Ho, C.J.; Huang, J.B.; Tsai, P.S.; Yang, Y.M. Preparation and properties of hybrid water-based suspension of Al2O3 nanoparticles and MEPCM particles as functional forced convection fluid. Int. Commun. Heat Mass Transf. 2010, 37, 490–494. [Google Scholar] [CrossRef]
- Jia, L.; Li, Y.; Chen, Y.; Wang, J.; Mo, S.; Li, J.; Liu, G. New hybrid suspension of MEPCM/GO particles with enhanced dispersion stability and thermo-physical properties. Appl. Energy 2019, 255, 113827. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Li, H.; Fang, G.; Liu, X. Synthesis of shape-stabilized paraffin/silicon dioxide composites as phase change material for thermal energy storage. J. Mater. Sci. 2010, 45, 1672–1676. [Google Scholar] [CrossRef]
- Connor, P.A.; Dobson, K.D.; James McQuillan, A. Infrared spectroscopy of the TiO2/aqueous solution interface. Langmuir 1999, 15, 2402–2408. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Buccheri, M.A.; Sanz, R.; Piluso, N.; Reitano, R.; La Via, F.; Grimaldi, M.G.; Privitera, V. Photocatalytical activity of amorphous hydrogenated TiO2 obtained by pulsed laser ablation in liquid. Mater. Sci. Semicond. Process. 2016, 42, 28–31. [Google Scholar] [CrossRef]

| Sample | Melting | Solidifying | Thermal Conductivity (W m−1 K−1) | ||
|---|---|---|---|---|---|
| Peak Temperature (°C) | Latent Heat (J/g) | Peak Temperature (°C) | Latent Heat (J/g) | ||
| N-octadecane | 32.71 | 234.26 ± 0.94 | 23.67 | 229.18 ± 1.02 | 0.16 ± 0.03 |
| MEPCM | 33.41 | 154.24 ± 1.09 | 24.82 | 154.26 ± 1.11 | 0.55 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jia, L.; Li, L.; Huang, Z.; Chen, Y. Hybrid Microencapsulated Phase-Change Material and Carbon Nanotube Suspensions toward Solar Energy Conversion and Storage. Energies 2020, 13, 4401. https://doi.org/10.3390/en13174401
Li J, Jia L, Li L, Huang Z, Chen Y. Hybrid Microencapsulated Phase-Change Material and Carbon Nanotube Suspensions toward Solar Energy Conversion and Storage. Energies. 2020; 13(17):4401. https://doi.org/10.3390/en13174401
Chicago/Turabian StyleLi, Jun, Lisi Jia, Longjian Li, Zehang Huang, and Ying Chen. 2020. "Hybrid Microencapsulated Phase-Change Material and Carbon Nanotube Suspensions toward Solar Energy Conversion and Storage" Energies 13, no. 17: 4401. https://doi.org/10.3390/en13174401
APA StyleLi, J., Jia, L., Li, L., Huang, Z., & Chen, Y. (2020). Hybrid Microencapsulated Phase-Change Material and Carbon Nanotube Suspensions toward Solar Energy Conversion and Storage. Energies, 13(17), 4401. https://doi.org/10.3390/en13174401