Performance of Concrete with Low CO2 Emission
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristic of Components and Composition of Tested Cements
2.2. Concrete Compositions and Properties’ Test Methods
3. Results and Discussion
3.1. Properties of Cements’ Constituents and Cements
3.2. Properties of Concretes
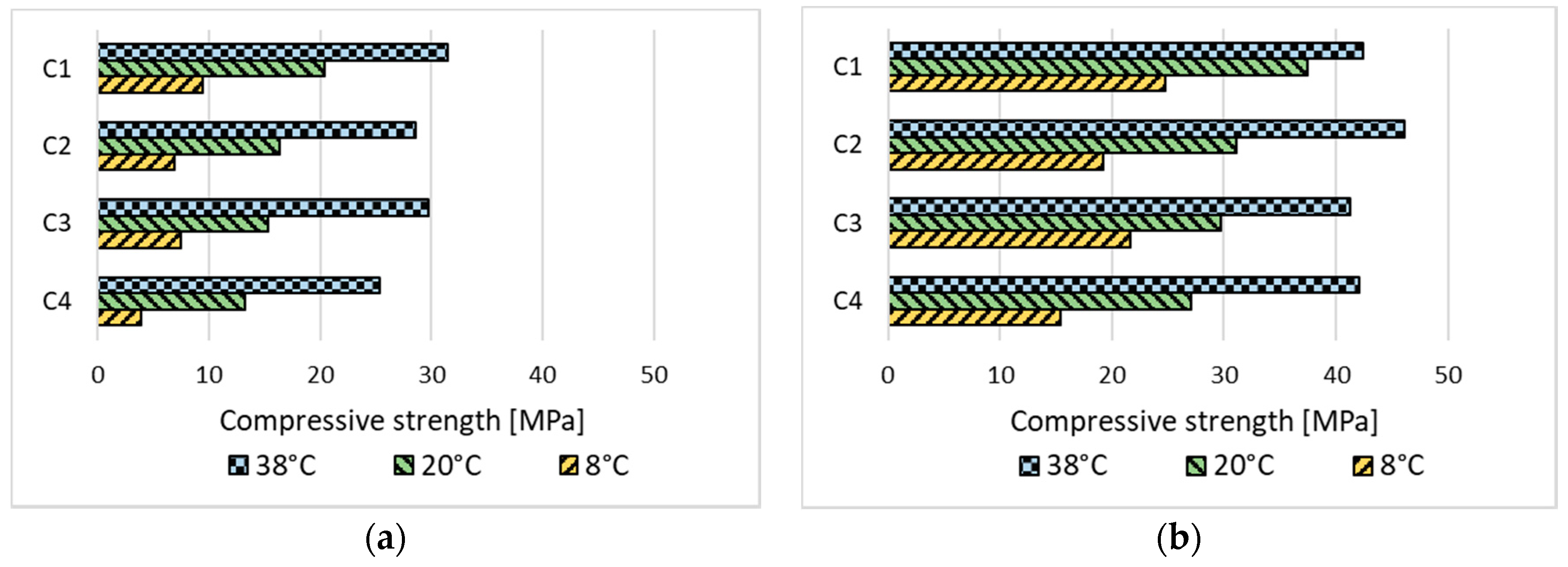
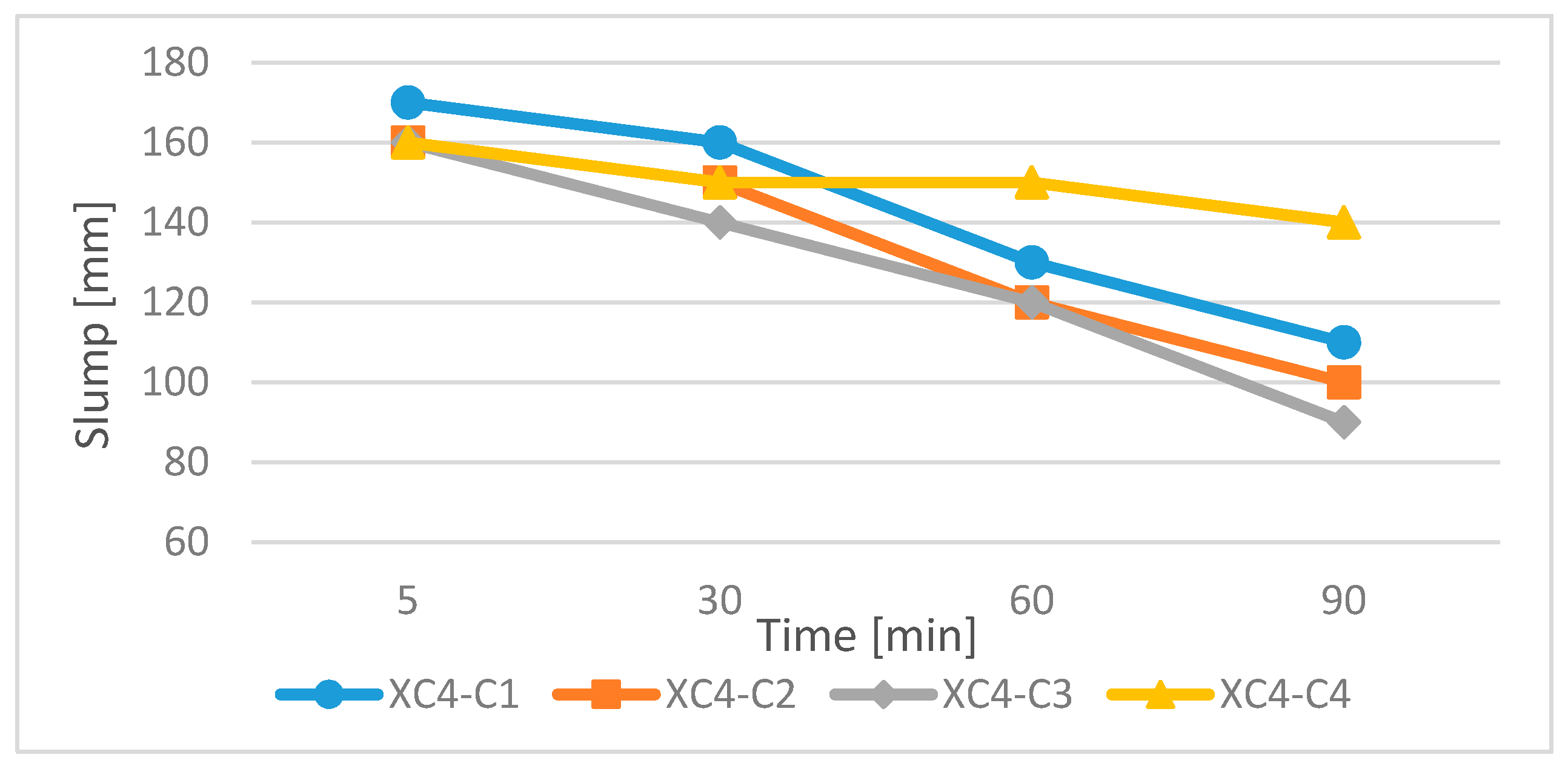
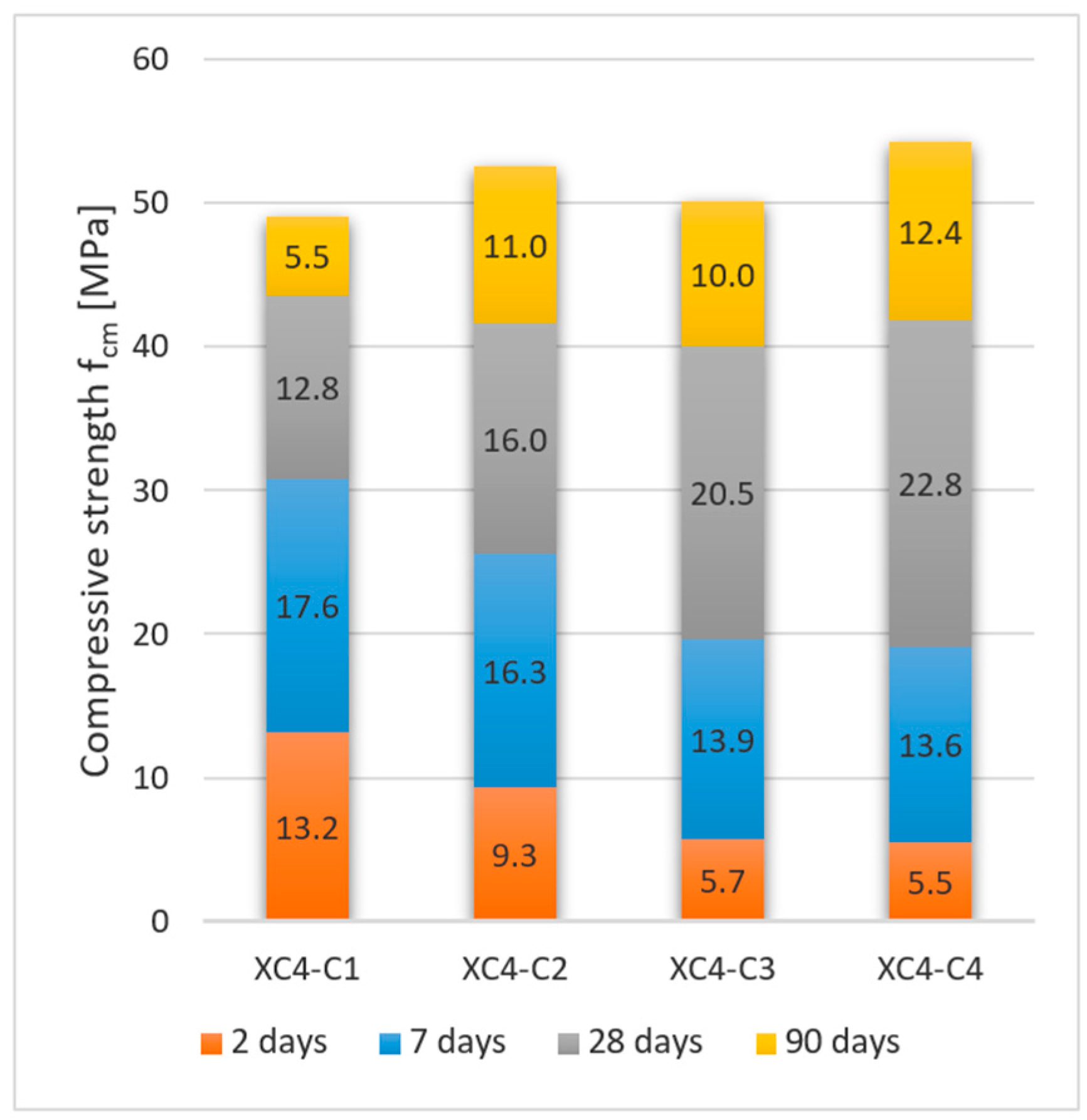
3.2.1. Concrete Exposed to Corrosion Due to Carbonation
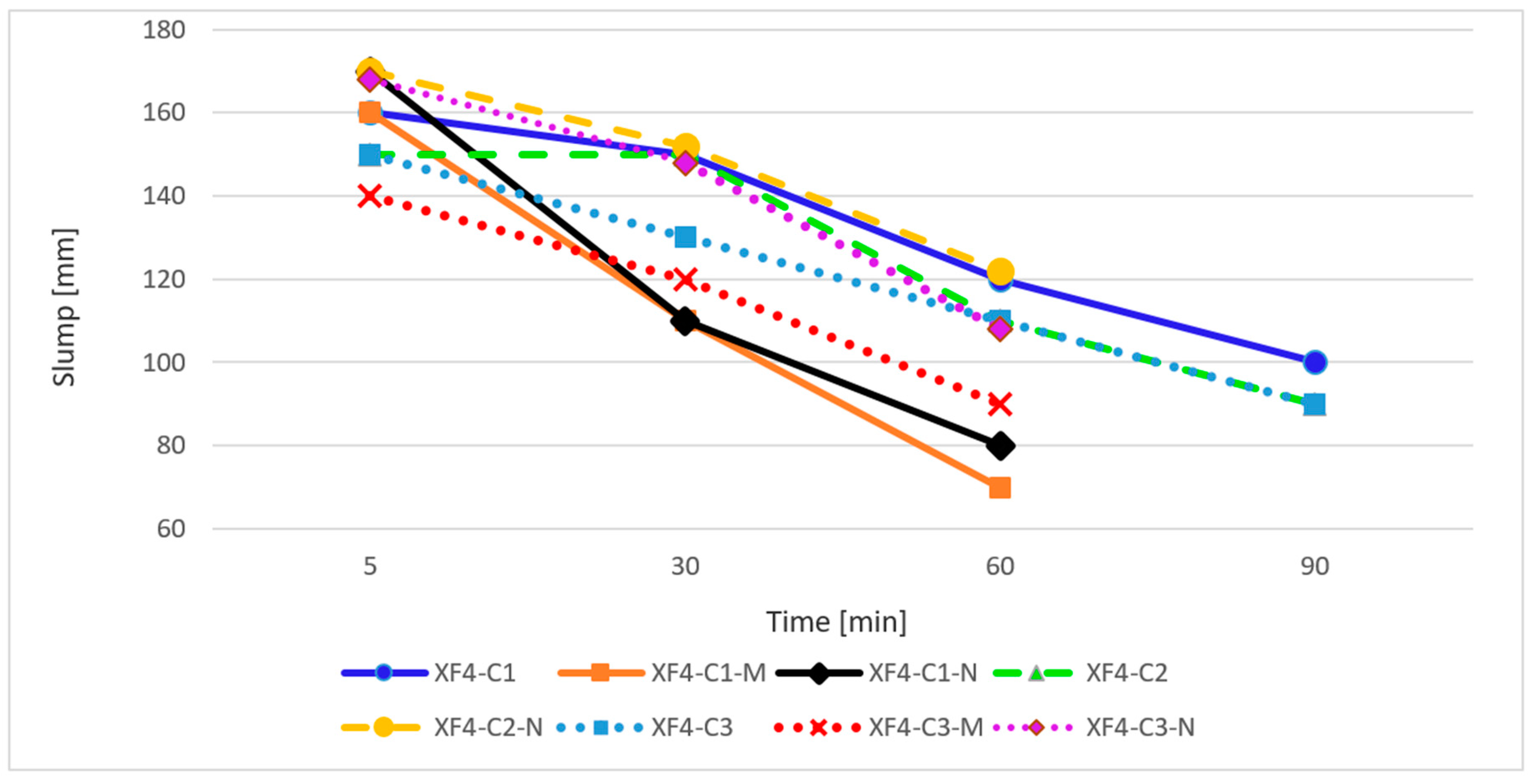
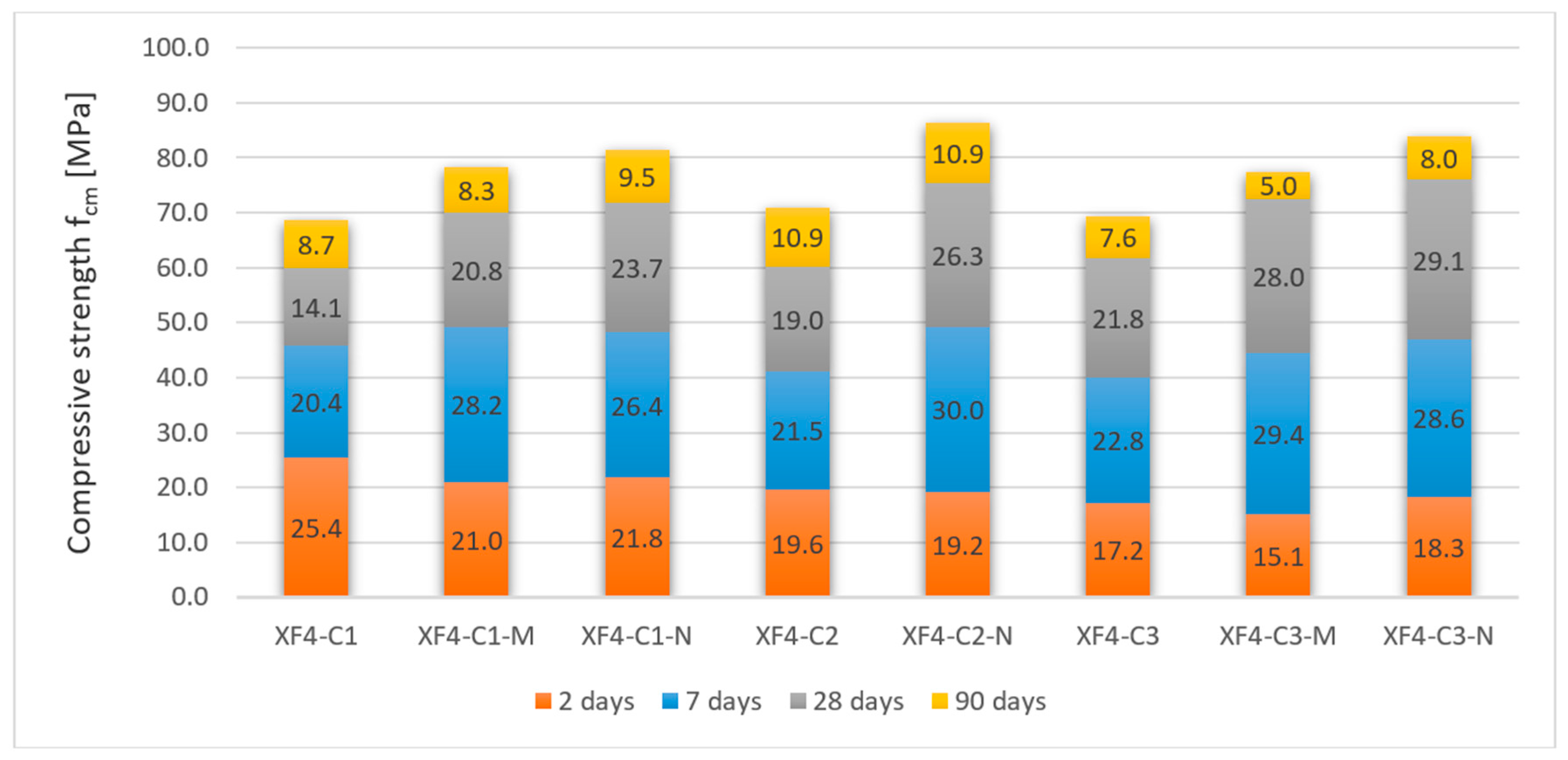
3.2.2. Frost-Resistant Concrete
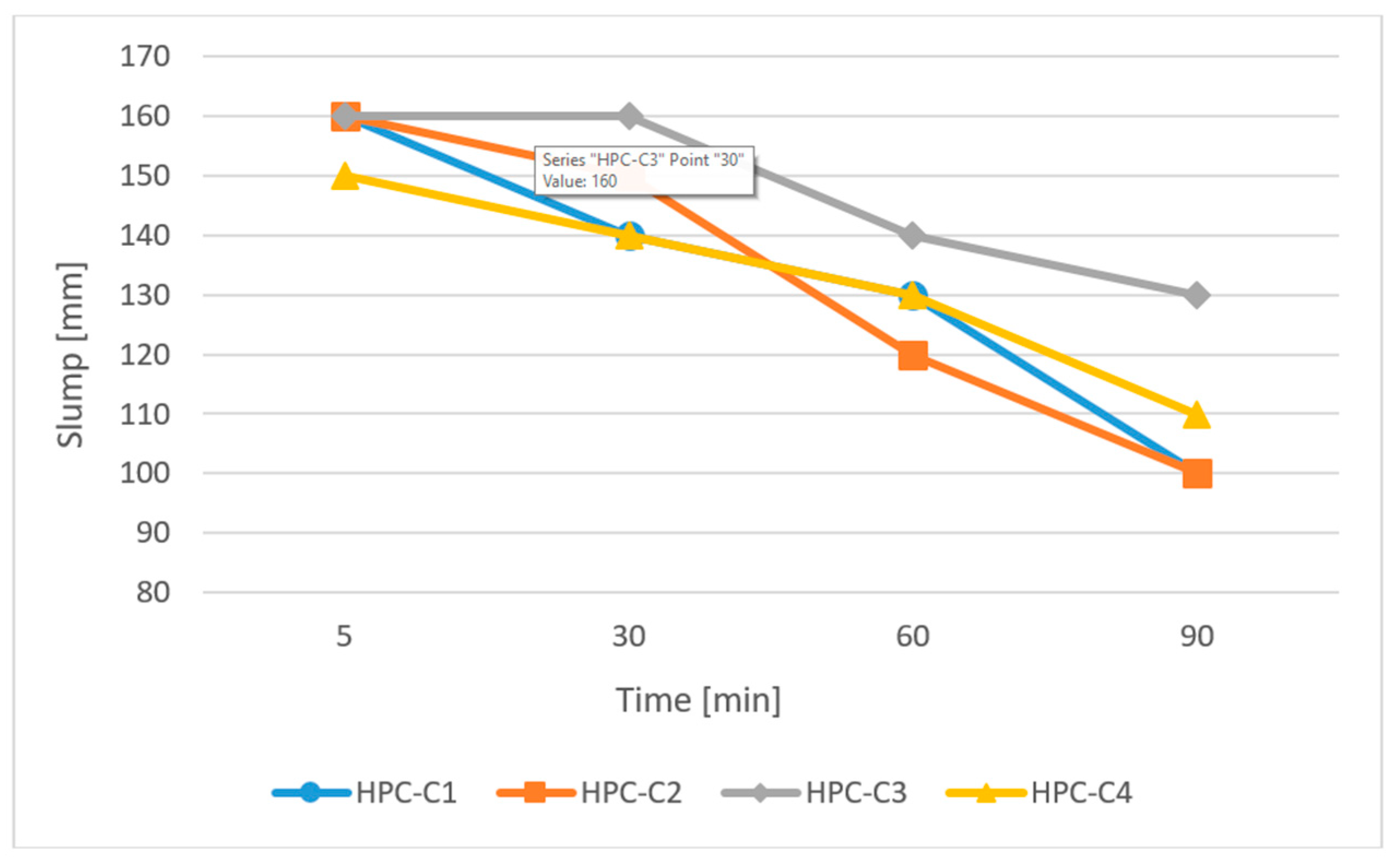
3.2.3. Self-Compacting Concrete (SCC)
3.2.4. High-Performance Concrete (HPC)
4. Summary
5. Conclusions
- Portland multicomponent cements CEM II/C-M (S-LL) and CEM II/C-M (S-V), slag cement CEM III/A, as well as multicomponent cement CEM VI (S-V) perform low emission per Mg of produced cement, ranging from 369.6 (CEM VI) to 470.4 (CEM II/C-M (S-LL) kg CO2.
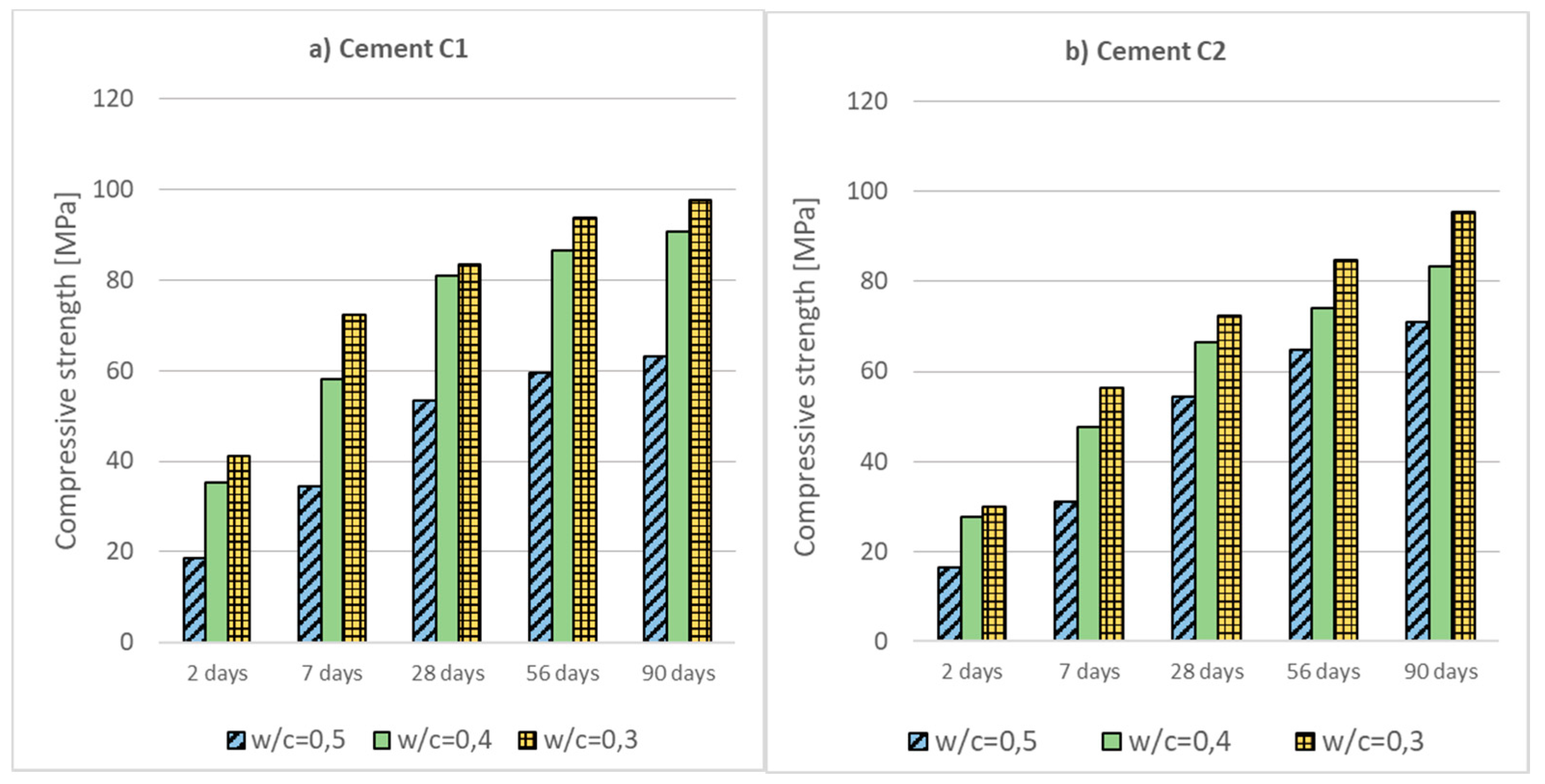
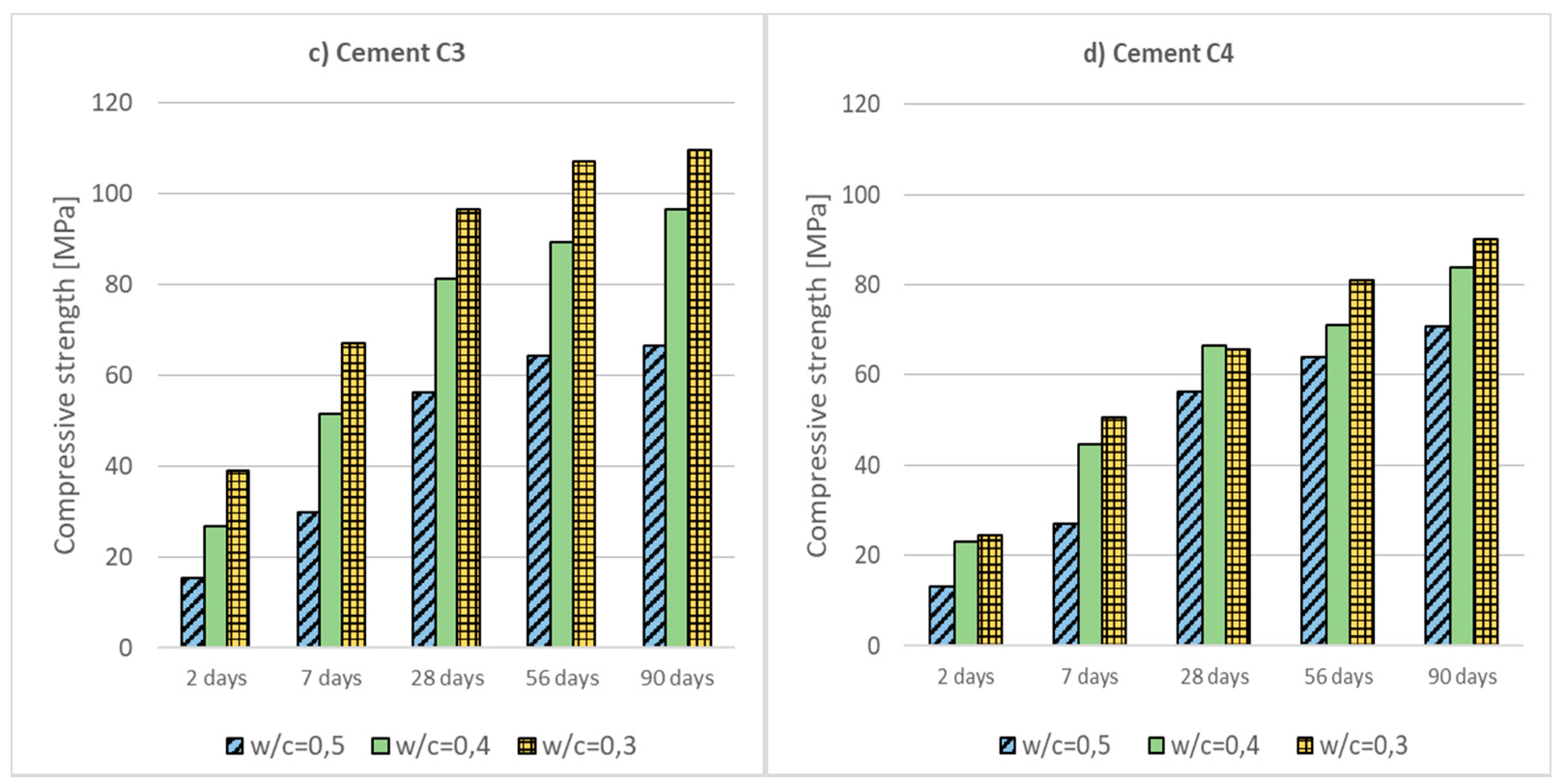
- Low-emission cements CEM II/C-M, CEM III/A and CEM VI with a high content of non-clinker main components (ground granulated blast furnace slag (S), siliceous fly ash (V) and limestone (LL)) are characterized by a moderate increase in compressive strength at the beginning of curing (2 and 7 days) and a significant increase in strength over a longer period of time.
- Reducing the w/c ratio or rising the temperature results in a significant increase in concrete strength, both early and after a longer period of curing. Lowering the temperature has the opposite effect, which can lead to complications with the use of low-emission concretes at low temperatures in the autumn and winter weather.
- Longer curing period and concrete humidity care improve the tightness of cement matrix, which results in an increase in compressive strength and durability. Appropriate selection of concrete mixture composition (w/c = 0.45, adequate aeration of concrete mixture, the use of frost-resistant aggregate, proper care) allows to obtain concrete with a high frost resistance both in the presence of de-icing salts and without them.
- Self-compacting concrete mixtures SCC produced with the use of low-emission cements CEM II/C-M, CEM III/A, CEM VI and the addition of FA fly ash were characterized by adequate flow, stability and viscosity of the concrete mixture in the assumed time of 90 min.
- Low-emission cements can be successfully applied into the production of HPC high-performance concretes (using SF silica fume an additive type II). After 90 days of curing, all analyzed concretes reached compressive strength above 100 MPa.
- Significant increase in strength in the later period of curing (between 28 and 90 days) is a key feature of the tested concretes with low-emission cements, leading to a decrease in the level of emissions associated with obtaining 1 MPa of concretes’ compressive strength.
Author Contributions
Funding
Conflicts of Interest
References
- Neville, A.M. Properties of Concrete; Polish Cement: Cracow, Poland, 2012. (In Polish) [Google Scholar]
- Chatterjee, A.; Sui, T. Alternative fuels—Effects on clinker process and properties. Cem. Conr. Res. 2019, 123, 105777. [Google Scholar] [CrossRef]
- EN 197-1:2011. Cement-Part 1: Composition, Specifications and Conformity Criteria for Common Cements; British Standards Institution: London, UK, 2011. [Google Scholar]
- Prasada Rao, D.V.; Lakshmi Narayana, N. Properties of multi component composite cement concrete. Int. J. Eng. Res. Gen. Sci. 2017, 5, 1. [Google Scholar]
- Yuksel, I. Blast-Furnace Slag. In Waste and Supplementary Cementitious Materials in Concrete; Woodhead Publishing: Cambridge, UK, 2018; pp. 361–415. [Google Scholar]
- Department of Structural Engineering. Properties of Fresh and Hardened Concrete Containing Supplementary Cementitious Materials: State-of-the-Art Report of the RILEM Technical Committee 238-SCM Working Group 4; Soutsos, M., De Belie, N., Gruyaert, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 55–98. [Google Scholar]
- Alberici, S.; de Beer, J.; van der Hoorn, I. Fly Ash and Blast Furnace Slag for Cement Manufacturing, BEIS Research Paper No. 19; Department for Business, Energy & Industrial Strategy: London, UK, 2017; p. 19. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/660888/fly-ash-blast-furnace-slag-cement-manufacturing.pdf (accessed on 7 April 2020).
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- prEN 197-5-Working Document. Available online: https://www.cen.eu/news/brochures/brochures/cen-cenelec_wp_2019.pdf (accessed on 20 April 2020).
- Król, A.; Giergiczny, Z.; Kuterasińska-Warwas, J. Properties of Concrete Made with Low-Emission Cements CEM II/C-M and CEM VI. Materials 2020, 13, 2257. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2014, 55, 374–382. [Google Scholar] [CrossRef]
- Adu-Amankwah, S.; Black, L.; Skocek, J.; Ben Haha, M.; Zajac, M. Effect of sulfate additions on hydration and performance of ternary slag-limestone composite cements. Constr. Build. Mater. 2018, 164, 451–462. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ostnor, T.; Justnes, H.; Ben Haha, M.; Kjellsen, K. Fly-ash limestone synergy in triple cement. In Proceedings of the 1st International Conference on Concrete Sustainability, Tokyo, Japan, 27–29 May 2013; pp. 510–515. [Google Scholar]
- EN 206. Concrete—Specification, Performance, Production and Conformity; CEN: Brussels, Belgium, 2013. [Google Scholar]
- The European Green Deal. Available online: https://eur-lex.europa.eu (accessed on 17 May 2020).
- EN 196-2. Methods of Testing Cement—Part 2: Chemical Analysis of Cement; CEN: Brussels, Belgium, 2013. [Google Scholar]
- EN 196-3. Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness; British Standards Institution: London, UK, 2016. [Google Scholar]
- EN 196-6. Methods of Testing Cement—Part 6: Determination of Fineness; British Standards Institution: London, UK, 2011. [Google Scholar]
- EN 196-9. Methods of Testing Cement—Part 9: Heat of Hydration—Semi-Adiabatic Method; British Standards Institution: London, UK, 2010. [Google Scholar]
- EN 1097-7. Tests for Mechanical and Physical Properties of Aggregates—Part 7: Determination of the Particle Density of Filler-Pyknometer Method; British Standards Institution: London, UK, 2008. [Google Scholar]
- EN 196-1. Methods of Testing Cement—Part 1: Determination of Strength; British Standards Institution: London, UK, 2016. [Google Scholar]
- Sanjuán, M.Á.; Andrade, C.; Mora, P.; Zaragoza, A. Carbon dioxide uptake by cement-based materials. A Spanish case study. Appl. Sci. 2020, 10, 339. [Google Scholar] [CrossRef]
- EN 12350-6. Testing Fresh Concrete-Part 6: Density; CEN: Brussels, Belgium, 2019. [Google Scholar]
- EN 12350-7. Testing Fresh Concrete-Part 7: Air Content. Pressure Methods; CEN: Brussels, Belgium, 2019. [Google Scholar]
- EN 12350-2. Testing Fresh Concrete-Part 2: Slump-Test; CEN: Brussels, Belgium, 2019. [Google Scholar]
- EN 12350-8. Testing Fresh Concrete-Part 8: Self-Compacting Concrete—Slump-Flow Test; CEN: Brussels, Belgium, 2019. [Google Scholar]
- EN 12350-9. Testing Fresh Concrete-Part 9: Self-Compacting Concrete—V-Funnel Test; CEN: Brussels, Belgium, 2012. [Google Scholar]
- EN 12350-10. Testing Fresh Concrete-Part 10: Self-Compacting Concrete—L Box Test; CEN: Brussels, Belgium, 2012. [Google Scholar]
- EN 12390-7. Testing Hardened Concrete-Part 7: Density of Hardened Concrete; CEN: Brussels, Belgium, 2019. [Google Scholar]
- EN 12390-3. Testing Hardened Concrete-Part 3: Compressive Strength of Test Specimens; CEN: Brussels, Belgium, 2019. [Google Scholar]
- EN 12390-8. Testing Hardened Concrete-Part 8: Depth of Penetration of Water Under Pressure; CEN: Brussels, Belgium, 2019. [Google Scholar]
- EN 12390-12. Testing Hardened Concrete-Part 12: Determination of the Carbonation Resistance of Concrete-Accelerated Carbonation Method; CEN: Brussels, Belgium, 2020. [Google Scholar]
- PN-B-06265. Concrete-Specification, Performance, Production and Conformity—National Annex to PN-EN 206+A1:2016-12; PKN: Warsaw, Poland, 2018. [Google Scholar]
- EN 12620. Aggregates for Concrete; British Standards Institution: London, UK, 2002. [Google Scholar]
- EN 450-1. Fly Ash for Concrete—Part 1: Definition, Specifications and, Conformity Criteria; British Standards Institution: London, UK, 2012. [Google Scholar]
- EN 13263-1. Silica Fume for Concrete—Part 1: Definitions, Requirements and Conformity Criteria; CEN: Brussels, Belgium, 2019. [Google Scholar]
- Chi, J.M.; Huang, R.; Yang, C.C. Effects of carbonation on mechanical properties and durability of concrete using accelerated testing method. J. Mar. Sci. Technol. 2002, 10, 14–22. [Google Scholar]
- Balayssac, J.P.; Grandet, J.; Detriche, H. Effects of curing upon carbonation of concrete. Constr. Build. Mater. 1995, 9, 91–95. [Google Scholar] [CrossRef]
- Czarnecki, L.; Woyciechowski, P.; Adamczewski, G. Risk of Concrete Carbonation with Mineral Industrial By-Products. J. Civ. Eng. 2018, 22, 755–764. [Google Scholar] [CrossRef]
- Angst, U.M. Challenges and opportunities in corrosion of steel in concrete. Mater. Struct. 2018, 51, 1–20. [Google Scholar] [CrossRef]
- Poursaee, A. Corrosion of Steel in Concrete Structures; Woodhead Publishing: Sawston, Cambridge, UK, 2016; pp. 19–33. [Google Scholar]
- Jackiewicz-Rek, W.; Wojciechowski, P. Carbonation rate of air-entrained fly ash concretes. Cement Wapno Beton 2011, 5, 249–256. (In Polish) [Google Scholar]
- Boss, P.; Giergiczny, Z. Testing the Frost resistance of concrete with different cement types- experience from laboratory and practice. Archit. Civ. Eng. Environ. 2010, 2, 41–51. [Google Scholar]
- Rusin, Z. Frost Resistant Concrete Technology; Polish Cement: Cracow, Poland, 2002. (In Polish) [Google Scholar]
- Yuan, J.; Lu, H.; Yang, Q.; Ling, J. Mechanisms on the salt-frost scaling of concrete. J. Mater. Civ. Eng. 2015. [Google Scholar] [CrossRef]
- Glinicki, M.A.; Jaskulski, R.; Dąbrowski, M. Design principles and testing of internal frost resistance of concrete for road structures—Critical review. Roads Bridges 2016, 15, 21–43. [Google Scholar]
- Siva, A.; Ravindiran, G. Review on Self Compacting Concrete. Int. J. ChemTech Res. 2018, 10, 62–68. [Google Scholar]
- Khayat, K.H.; De Schutter, G. Mechanical Properties of Self-Compacting Concrete, State-of-the-Art Report of the RILEM Technical Committee 228-MPS; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Zain, M.F.M.; Safiuddin, M.; Mahmud, H. Development of high performance concrete using silica fume at relatively high water–binder ratios. Cem. Concr. Res. 2000, 30, 1501–1505. [Google Scholar] [CrossRef]
- Khaloo, A.; Mobini, M.H.; Hosseini, P. Influence of different types of nano-SiO2 particles on properties of high-performance concrete. Constr. Build. Mater. 2016, 113, 188–201. [Google Scholar] [CrossRef]
- Azmee, N.M.; Shafiq, N. Ultra-high performance concrete: From fundamental to applications. Case Stud. Constr. Mater. 2018, 9, 197. [Google Scholar] [CrossRef]
- Sidodikromo, E.P.; Chen, Z.; Habib, M. Review of the cement-based composite ultra-high-performance concrete (UHPC). Open Civ. Eng. J. 2019, 13, 147–162. [Google Scholar]
- Matsuka, T.; Suzuki, Y.; Sakai, K. Low-Carbon Concrete Using Ground Granulated Blast-Furnace Slag and Fly Ash. Cem. Sci. Concr. Technol. 2010, 64, 295–302. [Google Scholar] [CrossRef][Green Version]
- Adu-Amankwah, S.; Zając, M.; Stabler, C.; Lothenbach, B.; Black, L. Influence of limestone on the hydration of ternary slag cements. Cem. Concr. Res. 2017, 100, 96–109. [Google Scholar] [CrossRef]





| Property | Standard Test Method |
|---|---|
| Chemical composition | EN 196-2:2013-11 [17] |
| Initial setting time | EN 196-3:2016-11 [18] |
| Water demand | EN 196-3:2016-11 [18] |
| Specific surface | EN 196-6:2011 [19] |
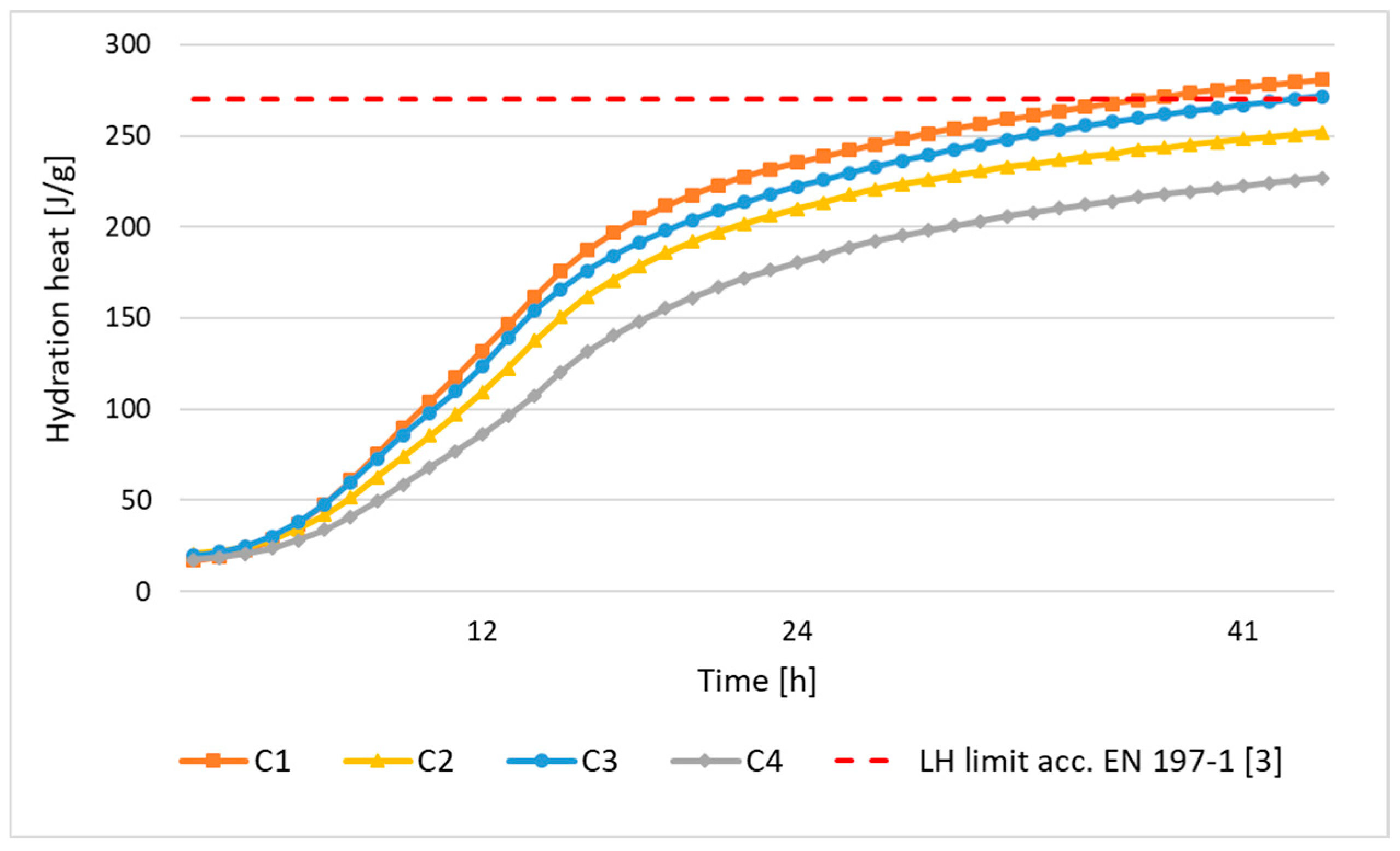
| Cement hydration heat | EN 196-9:2010 [20] |
| Density | EN 1097-7:2008 [21] |
| Compressive strength | EN 196-1:2016-05 [22] |
| Component | Share of Component [%] | Density [g/cm3] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaO | Fe2O3 | SiO2 | Al2O3 | MgO | SO3 | Cl- | Na2O | K2O | ||
| Portland clinker with set controlling agent | 62.86 | 2.47 | 19.70 | 5.25 | 1.44 | 3.79 | 0.097 | 0.121 | 0.809 | 3.13 |
| Granulated blast furnace slag | 43.85 | 0.88 | 38.58 | 7.11 | 6.4 | 1.31 | 0.011 | 0.44 | 0.32 | 2.94 |
| Siliceous fly ash (V) (*) | 2.6 | 5.5 | 51.58 | 27.17 | 2.29 | 0.06 | 0.013 | 0.87 | 3.22 | 2.20 |
| Limestone (**) | 53.1 | 0.2 | 2.8 | 0.45 | 0.36 | 0.1 | 0.020 | - | - | 2.75 |
| Cement Designation | Cement Type | Component Content [%] | CO2 Emission Level from Mg of Cement [kg] | |||
|---|---|---|---|---|---|---|
| Clinker | Granulated Slag | Siliceous Fly Ash | Limestone | |||
| K (C *) | S | V | LL | |||
| C1 | CEM II/C-M (S-LL) [10] | 56 (60) | 30 | - | 10 | 470.4 |
| C2 | CEM II/C-M (S-V) [10] | 50 (53) | 47 | 420.0 | ||
| C3 | CEM III/A [3] | 47 (50) | 50 | - | 394.8 | |
| C4 | CEM VI (S-V) [10] | 44 (47) | 53 | - | 369.6 | |
| Tested Material | Property | Standard Test Method (Procedures) |
|---|---|---|
| Concrete mixture | Density | EN 12350-6:2019-08 [24] |
| Air content | EN 12350-7:2019-08 [25] | |
| Consistency | EN 12350-2:2019-07 [26] | |
| Consistency—SCC concretes | EN 12350-8:2019-08 [27] | |
| V-funnel | EN 12350-9:2012 [28] | |
| L-box | EN 12350-10:2012 [29] | |
| Hardened concrete | Density | EN 12390-7:2019-08 [30] |
| Compressive strength | EN 12390-3:2019-07 [31] | |
| Depth of water penetration under pressure | EN 12390-8:2019-08 [32] | |
| Depth of carbonation | EN 12390-12:2020-06 [33] | |
| Frost resistance | PN-B-06265:2018-10 [34] | |
| De-icing salts frost resistance | PN-B-06265:2018-10 [34] |
| Content of Components [kg/m3] in Concrete Composition | ||||||
|---|---|---|---|---|---|---|
| Component | XC4 b | XF4 | XF4-M | XF4-N | SCC | HPC |
| Fine aggregate | 722 | 665 | 684 | 694 | 765 | 623 |
| Coarse gravel aggregate (Dmax = 16 mm, F1 a) | 1116 | - | - | - | 864 | - |
| Crashed coarse aggregate (Dmax = 16 mm, F1 a, FNaCl1 a) | - | 1323 | 1359 | 1380 | - | 1212 |
| Cement (C1–C4) | 300 | 350 | 350 | 350 | 380 | 480 |
| Water | 165 | 155 | 155 | 155 | 175 | 166 |
| Siliceous Fly Ash FA | - | - | - | - | 120 | - |
| Silica Fume SF | - | - | - | - | - | 30 |
| Polymer microspheres | - | - | 2.1 | - | - | - |
| Plasticizer | - | - | - | - | 1.52 | 1.44 |
| Superplasticizer | 1.65 | 1.90 | 2.80 | 2.80 | 4.00 | 3.50 |
| Air entraining agent | - | 0.40 | - | - | - | - |
| w/c ratio | 0.55 | 0.45 | 0.45 | 0.45 | 0.46 | 0.36 |
| w/s ratio (c + 0,4 × FA); (c + SF) | 0.55 | 0.45 | 0.45 | 0.45 | 0.41 | 0.33 |
| Cement Designation | Density [g/cm3] | Specific Surface Area [cm2/g] | Water Demand [%] | Initial Setting Time [min] | Compressive Strength [MPa] | |||
|---|---|---|---|---|---|---|---|---|
| 2 Days | 7 Days | 28 Days | 90 Days | |||||
| C1 | 3.06 | 4380 | 27.6 | 200 | 18.5 | 34.4 | 53.3 | 63.2 |
| C2 | 2.88 | 3648 | 27.8 | 215 | 16.3 | 31.1 | 54.5 | 71.1 |
| C3 | 3.02 | 3940 | 32.5 | 240 | 15.3 | 29.7 | 56.3 | 66.6 |
| C4 | 2.90 | 3769 | 27.8 | 225 | 13.2 | 27.1 | 56.4 | 70.7 |
| Concrete | Clinker Content per m3 of Concrete [kg] | Concrete Density [kg/m3] | Curing Time [days] | Depth of Water Penetration under Pressure [mm] | Compressive Strength fcm [MPa] | CO2 Emission per 1 MPa [kg] | CO2 Emission per 1 m3 of Concrete [kg] | CO2 Emission per 1 Mg of Concrete [kg] |
|---|---|---|---|---|---|---|---|---|
| XC4-C1 | 168 | 2344 | 28 | 25 | 43.6 | 3.24 | 141.12 | 60.20 |
| 90 | 19 | 49.1 | 2.87 | |||||
| XC4-C2 | 150 | 2302 | 28 | 24 | 41.6 | 3.03 | 126.00 | 54.74 |
| 90 | 18 | 52.6 | 2.40 | |||||
| XC4-C3 | 141 | 2265 | 28 | 21 | 40.1 | 2.95 | 118.44 | 52.29 |
| 90 | 10 | 50.1 | 2.36 | |||||
| XC4-C4 | 132 | 2299 | 28 | 19 | 41.9 | 2.65 | 110.88 | 48.23 |
| 90 | 14 | 54.3 | 2.04 |
| Property | XF4-C1 | XF4-C1-M | XF4-C1-N | XF4-C2 | XF4-C2-N | XF4-C3 | XF4-C3-M | XF4-C3-N |
|---|---|---|---|---|---|---|---|---|
| Air Content [% vol.] | 5.0 | 2.1 | 2.0 | 5.9 | 2.2 | 5.1 | 1.5 | 2.3 |
| Density [kg/m3] | 2481 | 2551 | 2580 | 2446 | 2571 | 2471 | 2540 | 2565 |
| Concrete | Clinker Content per m3 of Concrete [kg] | Concrete Density [kg/m3] | Curing Time [days] | Depth of Water Penetration under Pressure [mm] | Compressive Strength fcm [MPa] | CO2 Emission per 1 MPa [kg] | CO2 Emission per 1 m3 of Concrete [kg] | CO2 Emission per 1 Mg of Concrete [kg] |
|---|---|---|---|---|---|---|---|---|
| XF4-C1 | 196.0 | 2487 | 28 | 16 | 59.9 | 2.75 | 164.64 | 66.44 |
| 90 | 12 | 68.6 | 2.40 | |||||
| XF4-C1-M | 196.0 | 2571 | 28 | 17 | 70.0 | 2.35 | 164.64 | 63.94 |
| 90 | 14 | 78.3 | 2.10 | |||||
| XF4-C1-N | 196.0 | 2583 | 28 | 17 | 71.9 | 2.29 | 164.64 | 63.74 |
| 90 | 14 | 81.4 | 2.02 | |||||
| XF4-C2 | 175.0 | 2465 | 28 | 28 | 60.1 | 2.45 | 147.00 | 59.15 |
| 90 | 18 | 71.0 | 2.07 | |||||
| XF4-C2-N | 175.0 | 2577 | 28 | 18 | 75.5 | 1.95 | 147.00 | 57.04 |
| 90 | 15 | 86.4 | 1.60 | |||||
| XF4-C3 | 164.5 | 2487 | 28 | 17 | 61.8 | 2.24 | 138.18 | 55.70 |
| 90 | 11 | 69.4 | 1.99 | |||||
| XF4-C3-M | 164.5 | 2558 | 28 | 14 | 72.5 | 1.91 | 138.18 | 54.79 |
| 90 | 10 | 77.5 | 1.78 | |||||
| XF4-C3-N | 164.5 | 2569 | 28 | 11 | 76.0 | 1.82 | 138.18 | 53.79 |
| 90 | 7 | 84.0 | 1.65 |
| Concrete | Test | Ordinary Frost Resistance F150 | ||
|---|---|---|---|---|
| Curing Time | Average Mass Loss ∆mF | Average Decrease in Strength ∆fF | Assessment Criteria | |
| [Days] | [%] | |||
| XF4-C1 | 28 | 0.04 | 1.92 | Samples prove no cracking ∆mF ≤ 5 ∆fF ≤ 20 |
| 90 | 0.12 | 1.06 | ||
| XF4-C1-M | 28 | 0.68 | 17.21 | |
| 90 | 0.90 | 19.11 | ||
| XF4-C1-N | 28 | 0.58 | 38.79 | |
| 90 | 0.52 | 31.88 | ||
| XF4-C2 | 28 | 0.25 | 2.41 | |
| 90 | 0.04 | 1.27 | ||
| XF4-C2-N | 28 | 1.41 | 43.62 | |
| 90 | 0.98 | 34.87 | ||
| XF4-C3 | 28 | 0.39 | 4.17 | |
| 90 | 0.11 | 1.49 | ||
| XF4-C3-M | 28 | 0.39 | 11.02 | |
| 90 | 0.72 | 12.39 | ||
| XF4-C3-N | 28 | 1.78 | 52.16 | |
| 90 | 1.16 | 39.16 | ||
| Test | Results after 90 min | Consistency Class | |||
|---|---|---|---|---|---|
| SCC-C1 | SCC-C2 | SCC-C3 | SCC-C4 | ||
| Slump [mm] | 650 | 690 | 680 | 670 | SF2 |
| V-funnel [s] | 4.3 | 4.9 | 6.5 | 7.1 | VF1 |
| L-box [-] | 0.85 | 0.98 | 0.92 | 0.89 | PL2 |
| Concrete | Clinker Content per m3 of Concrete [kg] | Concrete Density [kg/m3] | Curing Time [days] | Depth of Water Penetration under Pressure [mm] | Compressive Strength fcm [MPa] | CO2 Emission per 1 MPa [kg] | CO2 Emission per 1 m3 of Concrete [kg] | CO2 Emission per Mg of Concrete [kg] |
|---|---|---|---|---|---|---|---|---|
| SCC-C1 | 212.8 | 2281 | 28 | 18 | 70.6 | 2.53 | 178.75 | 78.37 |
| 90 | 13 | 78.4 | 2.04 | |||||
| SCC-C2 | 190.0 | 2254 | 28 | 23 | 65.1 | 2.45 | 159.60 | 70.81 |
| 90 | 15 | 79.5 | 2.01 | |||||
| SCC-C3 | 178.6 | 2279 | 28 | 18 | 63.4 | 2.37 | 150.02 | 65.83 |
| 90 | 15 | 72.3 | 2.08 | |||||
| SCC-C4 | 167.2 | 2283 | 28 | 23 | 62.2 | 2.26 | 140.45 | 61.52 |
| 90 | 16 | 81.3 | 1.73 |
| Concrete | Clinker Content per m3 of Concrete [kg] | Concrete Density [kg/m3] | Curing Time [days] | Depth of Water Penetration under Pressure [mm] | Compressive Strength fcm [MPa] | CO2 Emission per 1 MPa [kg] | CO2 Emission per 1 m3 of Concrete [kg] | CO2 Emission per Mg of Concrete [kg] |
|---|---|---|---|---|---|---|---|---|
| HPC-C1 | 268.8 | 2531 | 28 | 14 | 108.9 | 2.07 | 225.79 | 89.21 |
| 90 | 11 | 117.9 | 1.71 | |||||
| HPC-C2 | 240.0 | 2472 | 28 | 17 | 96.1 | 2.10 | 201.60 | 81.55 |
| 90 | 7 | 108.4 | 1.86 | |||||
| HPC-C3 | 225.6 | 2491 | 28 | 15 | 101.4 | 1.87 | 189.50 | 76.08 |
| 90 | 10 | 106.8 | 1.77 | |||||
| HPC-C4 | 211.2 | 2484 | 28 | 21 | 101.6 | 1.75 | 177.41 | 71.42 |
| 90 | 9 | 115.1 | 1.54 |
| Concrete | Frost Resistance F150 | |||||
|---|---|---|---|---|---|---|
| Curing Time | Average Mass Loss ∆mF | Average Strength of Test Specimens fF1 | Average Strength of Frozen Samples fF2 | Average Strength Loss ∆fF | Assessment Criteria | |
| [days] | [%] | [MPa] | [%] | |||
| HPC-C1 | 28 | 0.15 | 103.0 | 101.8 | 1.2 | Samples prove no cracking ∆mF ≤ 5 ∆fF ≤ 20 |
| 90 | 0.10 | 104.3 | 103.8 | 0.5 | ||
| HPC-C2 | 28 | 0.22 | 94.4 | 92.2 | 2.3 | |
| 90 | 0.10 | 113.7 | 108.0 | 3.0 | ||
| HPC-C3 | 28 | 0.18 | 91.6 | 89.6 | 2.2 | |
| 90 | 0.13 | 98.1 | 96.5 | 1.6 | ||
| HPC-C4 | 28 | 0.37 | 98.9 | 95.4 | 3.5 | |
| 90 | 0.14 | 109.1 | 106.7 | 2.2 | ||
| Concrete Designation | Cement Content [kg/m3] | Clinker Content [kg/m3] | w/c Ratio | CO2 Emission Level per 1 MPa | |
|---|---|---|---|---|---|
| 28 Days of Curing | 90 Days of Curing | ||||
| XC4-C1 | 300 | 168.0 | 0.55 | 3.24 | 2.87 |
| XC4-C2 | 150.0 | 3.03 | 2.40 | ||
| XC4-C3 | 141.0 | 2.95 | 2.36 | ||
| XC4-C4 | 132.0 | 2.65 | 2.04 | ||
| XF4-C1 | 350 | 196.0 | 0.45 | 2.75 | 2.40 |
| XF4-C1-M | 2.35 | 2.10 | |||
| XF4-C1-N | 2.29 | 2.02 | |||
| XF4-C2 | 175.0 | 2.45 | 2.07 | ||
| XF4-C2-N | 1.95 | 1.60 | |||
| XF4-C3 | 164.5 | 2.24 | 1.99 | ||
| XF4-C3-M | 1.91 | 1.78 | |||
| XF4-C3-N | 1.82 | 1.65 | |||
| SCC-C1 | 380 (+120 FA) | 212.8 | 0.46 (0.41) * | 2.53 | 2.04 |
| SCC-C2 | 190.0 | 2.45 | 2.01 | ||
| SCC-C3 | 178.6 | 2.37 | 2.08 | ||
| SCC-C4 | 167.2 | 2.26 | 1.73 | ||
| HPC-C1 | 480 (+30 SF) | 268.8 | 0.36 (0.33) ** | 2.07 | 1.71 |
| HPC-C2 | 240.0 | 2.10 | 1.86 | ||
| HPC-C3 | 225.6 | 1.87 | 1.77 | ||
| HPC-C4 | 211.2 | 1.75 | 1.54 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giergiczny, Z.; Król, A.; Tałaj, M.; Wandoch, K. Performance of Concrete with Low CO2 Emission. Energies 2020, 13, 4328. https://doi.org/10.3390/en13174328
Giergiczny Z, Król A, Tałaj M, Wandoch K. Performance of Concrete with Low CO2 Emission. Energies. 2020; 13(17):4328. https://doi.org/10.3390/en13174328
Chicago/Turabian StyleGiergiczny, Zbigniew, Anna Król, Michał Tałaj, and Karol Wandoch. 2020. "Performance of Concrete with Low CO2 Emission" Energies 13, no. 17: 4328. https://doi.org/10.3390/en13174328
APA StyleGiergiczny, Z., Król, A., Tałaj, M., & Wandoch, K. (2020). Performance of Concrete with Low CO2 Emission. Energies, 13(17), 4328. https://doi.org/10.3390/en13174328





