Emerging Photovoltaic (PV) Materials for a Low Carbon Economy
Abstract
1. Introduction
2. Methods
2.1. Life Cycle Inventories
2.2. Cost Assessment
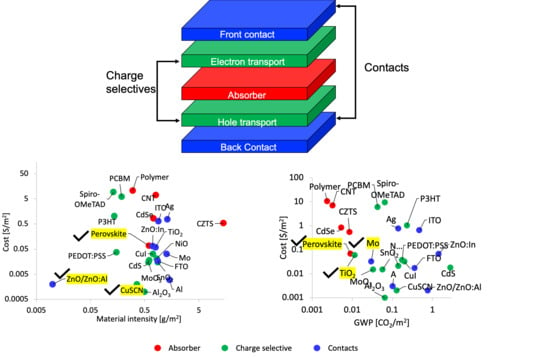
3. Results
3.1. Life Cycle Carbon Emissions
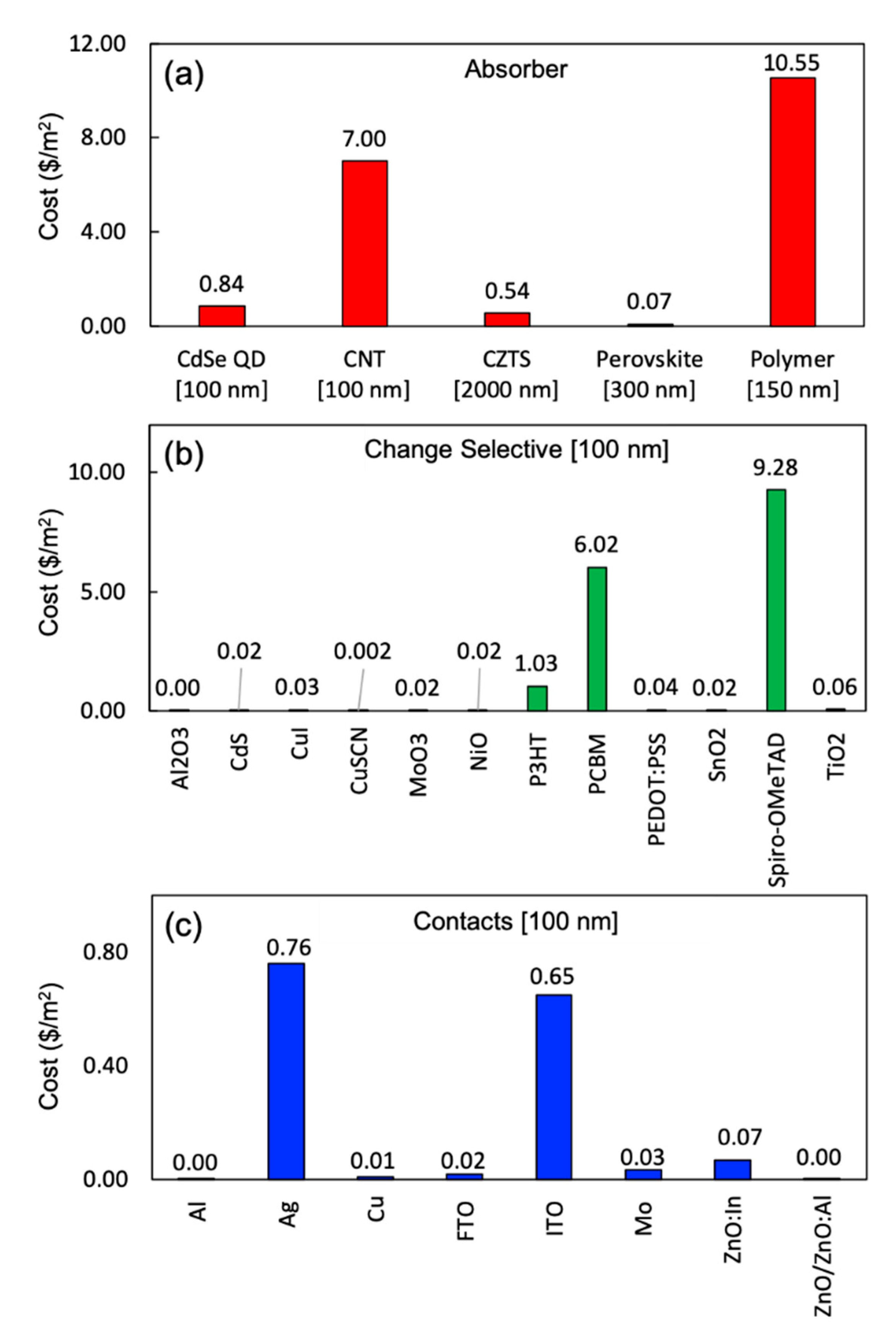
3.2. Life Cycle Costing
3.3. Low-Cost and Eco-Efficient Material Selection for Emerging PVs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Enerdata. Global Energy Statical Yearbook. 2019. Available online: https://www.enerdata.net/about-us/company-news/energy-statistical-yearbook-updated.html (accessed on 8 August 2020).
- Hostick, D.; Belzer, D.; Hadley, S.; Markel, T.; Marnay, C. Projecting Electricity Demand in 2050; PNNL: Richland, WA, USA, 2014. [Google Scholar]
- U.S. EIA Annual Energy Outlook 2019 with projections to 2050. Annu. Energy Outlook 2019 Proj. 2050 2019, 44, 1–64.
- Espinosa, N.; Hösel, M.; Angmo, D.; Krebs, F.C. Solar cells with one-day energy payback for the factories of the future. Energy Environ. Sci. 2012, 5, 5117–5132. [Google Scholar] [CrossRef]
- Frei, C.; Whitney, R.; Schiffer, H.-W.; Rose, K.; Rieser, D.A.; Al-Qahtani, A.; Thomas, P.; Turton, H.; Densing, M.; Panos, E.; et al. World Energy Scenarios: Composing Energy Futures to 2050; Conseil Francais de l’energie: Paris, France, 2013. [Google Scholar]
- Anctil, A. Fabrication and Life Cycle Assessment of Organic Photovoltaics; Rochester Institute of Technology: New York, NY, USA, 2011. [Google Scholar]
- Bellini, E. PV Magazine. Available online: https://www.pv-magazine.com/author/emilianobellini/ (accessed on 8 August 2020).
- Markert, E.; Celik, I.; Apul, D. Private and Externality Costs and Benefits of Recycling Crystalline Silicon (c-Si) Photovoltaic Panels. Energies 2020, 13, 3650. [Google Scholar] [CrossRef]
- Maani, T.; Celik, I.; Heben, M.J.; Randall, J. Environmnetal impacts of recycling crystalline silicon (c-Si) and cadmium telluride (CdTe) solar panels. Sci. Total Environ. 2020, 735. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Song, Z.; Cimaroli, A.J.; Yan, Y.; Heben, M.J.; Apul, D. Life Cycle Assessment (LCA) of perovskite PV cells projected from lab to fab. Sol. Energy Mater. Sol. Cells 2016, 156, 157–169. [Google Scholar] [CrossRef]
- Collier, J.; Wu, S.; Apul, D. Life cycle environmental impacts from CZTS (copper zinc tin sulfide) and Zn3P2 (zinc phosphide) thin film PV (photovoltaic) cells. Energy 2014, 74, 314–321. [Google Scholar] [CrossRef]
- Mondal, B.; Kamatham, N.; Samanta, S.R.; Jagadesan, P.; He, J.; Ramamurthy, V. Synthesis, Characterization, Guest Inclusion, and Photophysical Studies of Gold Nanoparticles Stabilized with Carboxylic Acid Groups of Organic Cavitands. Langmuir 2013, 29, 12703–12709. [Google Scholar] [CrossRef]
- Celik, I. Eco-Design of Emerging Photovoltaic (PV) Cells; University of Toledo: Toledo, OH, USA, 2018. [Google Scholar]
- Ahangharnejhad, R.H.; Phillips, A.B.; Ghimire, K.; Koirala, P.; Song, Z.; Barudi, H.M.; Habte, A.; Sengupta, M.; Ellingson, R.J.; Yan, Y.; et al. Irradiance and temperature considerations in the design and deployment of high annual energy yield perovskite/CIGS tandems. Sustain. Energy Fuels 2019, 3, 1841–1851. [Google Scholar] [CrossRef]
- Ahangharnejhad, R.H.; Song, Z.; Phillips, A.B.; Watthage, S.C.; Almutawah, Z.S.; Sapkota, D.R.; Koirala, P.; Collins, R.W.; Yan, Y.; Heben, M.J. Optical design of perovskite solar cells for applications in monolithic tandem configuration with CuInSe2 bottom cells. MRS Adv. 2018, 3, 3111–3119. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, D.; Chen, C.; Ramez H Ahangharnejhad, C.L.; Ghimire, K.; Podraza, N.J.; Heben, M.J.; Zhu, K.; Yan, Y. Monolithic Two-Terminal All-Perovskite Tandem Solar Cells with Power Conversion Efficiency Exceeding 21%. In Proceedings of the 2019 IEEE 46th Photovoltaic Specialists Conference (PVSC), Chicago, IL, USA, 16–21 June 2019; pp. 0743–0746. [Google Scholar]
- Albrecht, S.; Rech, B. Perovskite solar cells: On top of commercial photovoltaics. Nat. Energy 2017, 2, 16196. [Google Scholar] [CrossRef]
- Jørgensen, M.; Carlé, J.E.; Søndergaard, R.R.; Lauritzen, M.; Dagnæs-Hansen, N.A.; Byskov, S.L.; Andersen, T.R.; Larsen-Olsen, T.T.; Böttiger, A.P.L.; Andreasen, B.; et al. The state of organic solar cells—A meta analysis. Sol. Energy Mater. Sol. Cells 2013, 119, 84–93. [Google Scholar] [CrossRef]
- Katagiri, H.; Jimbo, K.; Maw, W.S.; Oishi, K.; Yamazaki, M.; Akaki, H.; Takeuchi, A. Development of CZTS-based thin film solar cells. Thin Solid Films 2009, 517, 2455–2460. [Google Scholar] [CrossRef]
- Po, R.; Carbonera, C.; Bernardi, A.; Tinti, F.; Camaioni, N. Polymer- and carbon-based electrodes for polymer solar cells: Toward low-cost, continuous fabrication over large area. Sol. Energy Mater. Sol. Cells 2012, 100, 97–114. [Google Scholar] [CrossRef]
- Ellingson, R.J.; Beard, M.C.; Johnson, J.C.; Yu, P.; Micic, O.I.; Nozik, A.J.; Shabaev, A.; Efros, A.L. Highly Efficient Multiple Exciton Generation in Colloidal PbSe and PbS Quantum Dots. Nano Lett. 2005, 5, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Darling, S.B.; You, F. Perovskite photovoltaics: Life-cycle assessment of energy and environmental impacts. Energy Environ. Sci. 2015, 8, 1953–1968. [Google Scholar] [CrossRef]
- Espinosa, N.; Serrano-luján, L.; Urbina, A.; Krebs, F.C. Solution and vapour deposited lead perovskite solar cells: Ecotoxicity from a life cycle assessment perspective. Sol. Energy Mater. Sol. Cells 2015, 137, 303–310. [Google Scholar] [CrossRef]
- Chang, N.L.; Yi Ho-Baillie, A.W.; Basore, P.A.; Young, T.L.; Evans, R.; Egan, R.J. A manufacturing cost estimation method with uncertainty analysis and its application to perovskite on glass photovoltaic modules. Prog. Photovolt. Res. Appl. 2017, 25, 390–405. [Google Scholar] [CrossRef]
- Cai, M.; Wu, Y.; Chen, H.; Yang, X.; Qiang, Y.; Han, L. Cost-Performance Analysis of Perovskite Solar Modules. Adv. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Song, Z.; McElvany, C.L.; Phillips, A.B.; Celik, I.; Krantz, P.W.; Watthage, S.C.; Liyanage, G.K.; Apul, D.; Heben, M.J. A technoeconomic analysis of perovskite solar module manufacturing with low-cost materials and techniques. Energy Environ. Sci. 2017, 10, 1297–1305. [Google Scholar] [CrossRef]
- Şengül, H.; Theis, T.L. An environmental impact assessment of quantum dot photovoltaics (QDPV) from raw material acquisition through use. J. Clean. Prod. 2011, 19, 21–31. [Google Scholar] [CrossRef]
- Tsang, M.P.; Sonnemann, G.W.; Bassani, D.M. Life-cycle assessment of cradle-to-grave opportunities and environmental impacts of organic photovoltaic solar panels compared to conventional technologies. Sol. Energy Mater. Sol. Cells 2016, 156, 37–48. [Google Scholar] [CrossRef]
- Celik, I.; Mason, B.E.; Phillips, A.B.; Heben, M.J.; Apul, D.S. Environmental Impacts from Photovoltaic Solar Cells Made with Single Walled Carbon Nanotubes. Environ. Sci. Technol. 2017, 51, 4722–4732. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Phillips, A.B.; Song, Z.; Yan, Y.; Ellingson, R.J.; Heben, M.J.; Apul, D. Environmental analysis of perovskites and other relevant solar cell technologies in a tandem configuration. Energy Environ. Sci. 2017, 10, 1874–1884. [Google Scholar] [CrossRef]
- Celik, I.; Song, Z.; Phillips, A.B.; Heben, M.J.; Apul, D. Life cycle analysis of metals in emerging photovoltaic (PV) technologies: A modeling approach to estimate use phase leaching. J. Clean. Prod. 2018, 186, 632–639. [Google Scholar] [CrossRef]
- Song, Z.; Adam, B.; Phillips, A.B.; Celik, I.; Watthage, S.C.; Zhao, D.; Apul, D.; Yan, Y.; Heben., M.J. Manufacturing Cost Analysis of Perovskite Solar Modules in Single-Junction and All-Perovskite Tandem Configurations. In Proceedings of the IEEE 7th World Conference on Photovoltaic Energy Conversion (WCPEC) (A Joint Conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC), Waikoloa Village, HI, USA, 10–15 June 2018; pp. 1134–1138. [Google Scholar]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent databae version 3 (part 1): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Espinosa, N.; García-Valverde, R.; Urbina, A.; Krebs, F.C. A life cycle analysis of polymer solar cell modules prepared using roll-to-roll methods under ambient conditions. Sol. Energy Mater. Sol. Cells 2011, 95, 1293–1302. [Google Scholar] [CrossRef]
- Chatzisideris, M.D.; Espinosa, N.; Laurent, A.; Krebs, F.C. Ecodesign perspectives of thin-film photovoltaic technologies: A review of life cycle assessment studies. Sol. Energy Mater. Sol. Cells 2016, 156, 2–10. [Google Scholar] [CrossRef]
- Cha, K.; Lim, S.; Hur, T. Eco-efficiency approach for global warming in the context of Kyoto Mechanism. Ecol. Econ. 2007, 7, 4–10. [Google Scholar] [CrossRef]



| Deposition Methods | Layers | Material [g/m2] | Electricity [kWh/m2] |
|---|---|---|---|
| Chemical bath deposition + Stirring + Heating | CdS | 0.60 | 0.10 |
| Doctor blading + heating | CNT | 0.20 | 0.03 |
| Printing + heating | CuSCN, CdSe, CuI, Perovskite, NiO | CuSCN = 0.36 | 0.03 |
| CdSe = 0.73 | 0.12 | ||
| CuI = 0.71 | 0.03 | ||
| Perovskite = 0.13 | 0.05 | ||
| NiO = 0.83 | 0.15 | ||
| Spinning + heating | CZTS, PCBM, PEDOT:PSS, Spiro-OMeTAD, TiO2 | CZTS = 0.71 | 0.22 |
| PCBM = 0.19 | 0.03 | ||
| PEDOT:PSS = 0.15 | 0.03 | ||
| Spiro-OMeTAD = 0.13 | 0.03 | ||
| TiO2 = 0.53 | 0.21 | ||
| Sputtering | Al2O3, Cu, ITO, Mo, ZnO, SnO2 | Al2O3 = 0.49 | 3.47 |
| Cu = 1.12 | 2.89 | ||
| ITO = 0.90 | 2.89 | ||
| Mo = 1.28 | 2.89 | ||
| SnO2 = 0.87 | 0.11 | ||
| ZnO = 0.70 | 2.89 | ||
| Thermal evaporation | Al, Ag, MoO3 | Al = 1.44 | 0.15 |
| Ag = 1.31 | 0.21 | ||
| MoO3 = 0.59 | 0.11 |
| c-Si PV | CdTe PV | CIGS PV | |
|---|---|---|---|
| kg CO2/ m2 | 130.2 | 56.3 | 51.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celik, I.; Hosseinian Ahangharnejhad, R.; Song, Z.; Heben, M.; Apul, D. Emerging Photovoltaic (PV) Materials for a Low Carbon Economy. Energies 2020, 13, 4131. https://doi.org/10.3390/en13164131
Celik I, Hosseinian Ahangharnejhad R, Song Z, Heben M, Apul D. Emerging Photovoltaic (PV) Materials for a Low Carbon Economy. Energies. 2020; 13(16):4131. https://doi.org/10.3390/en13164131
Chicago/Turabian StyleCelik, Ilke, Ramez Hosseinian Ahangharnejhad, Zhaoning Song, Michael Heben, and Defne Apul. 2020. "Emerging Photovoltaic (PV) Materials for a Low Carbon Economy" Energies 13, no. 16: 4131. https://doi.org/10.3390/en13164131
APA StyleCelik, I., Hosseinian Ahangharnejhad, R., Song, Z., Heben, M., & Apul, D. (2020). Emerging Photovoltaic (PV) Materials for a Low Carbon Economy. Energies, 13(16), 4131. https://doi.org/10.3390/en13164131