Pyrolysis Kinetic Parameters of Omari Oil Shale Using Thermogravimetric Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Oil Shale
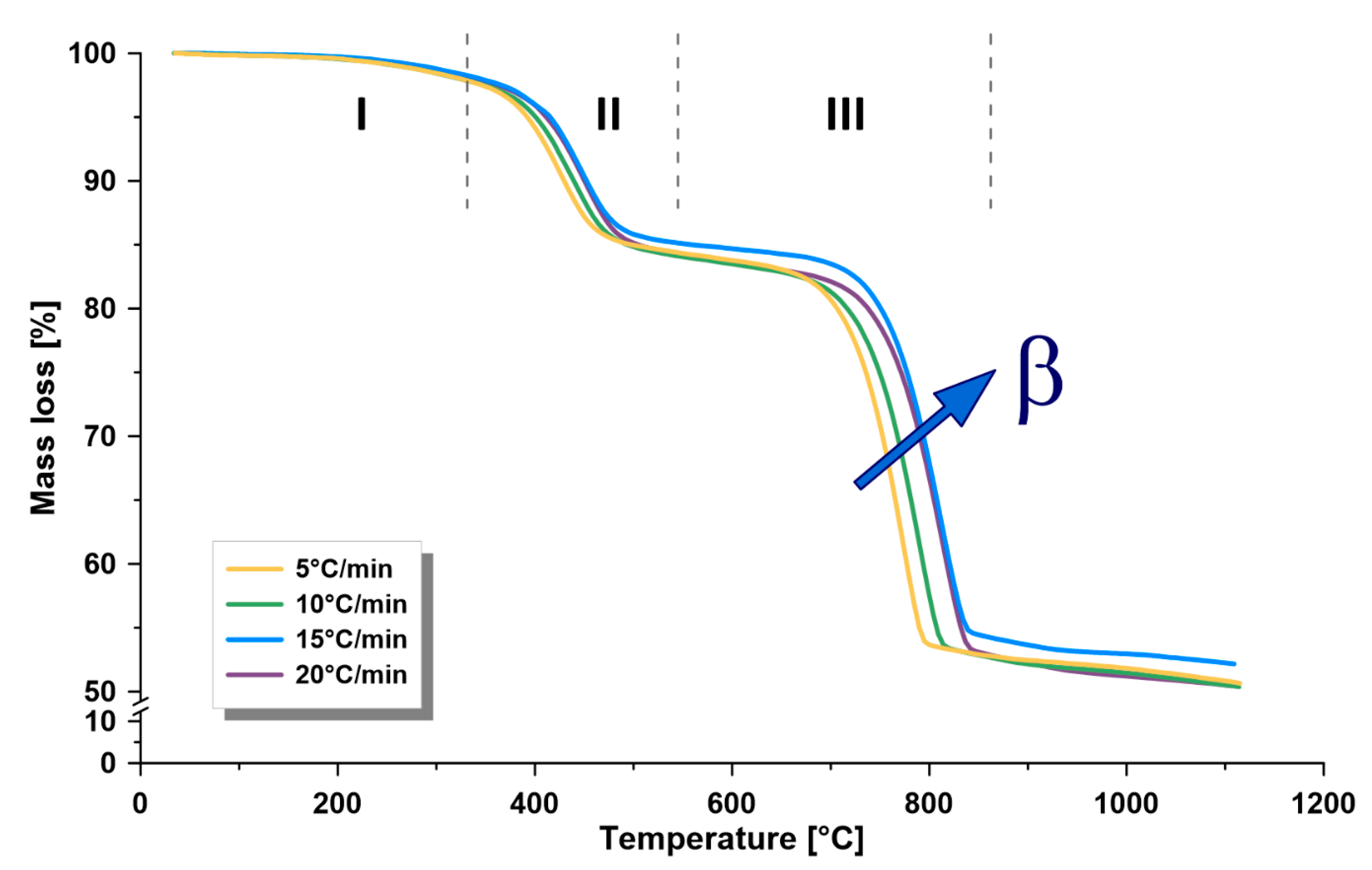
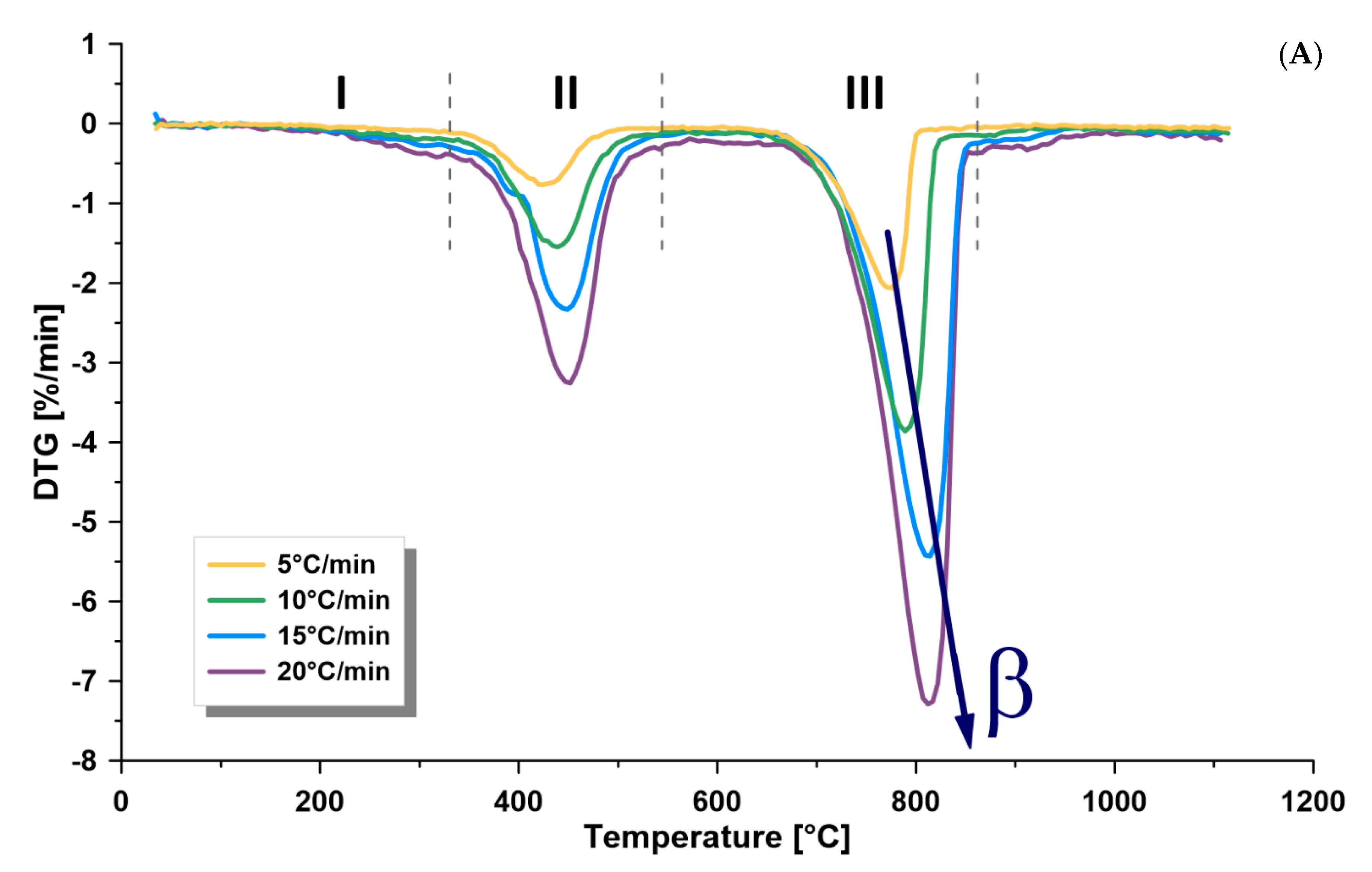
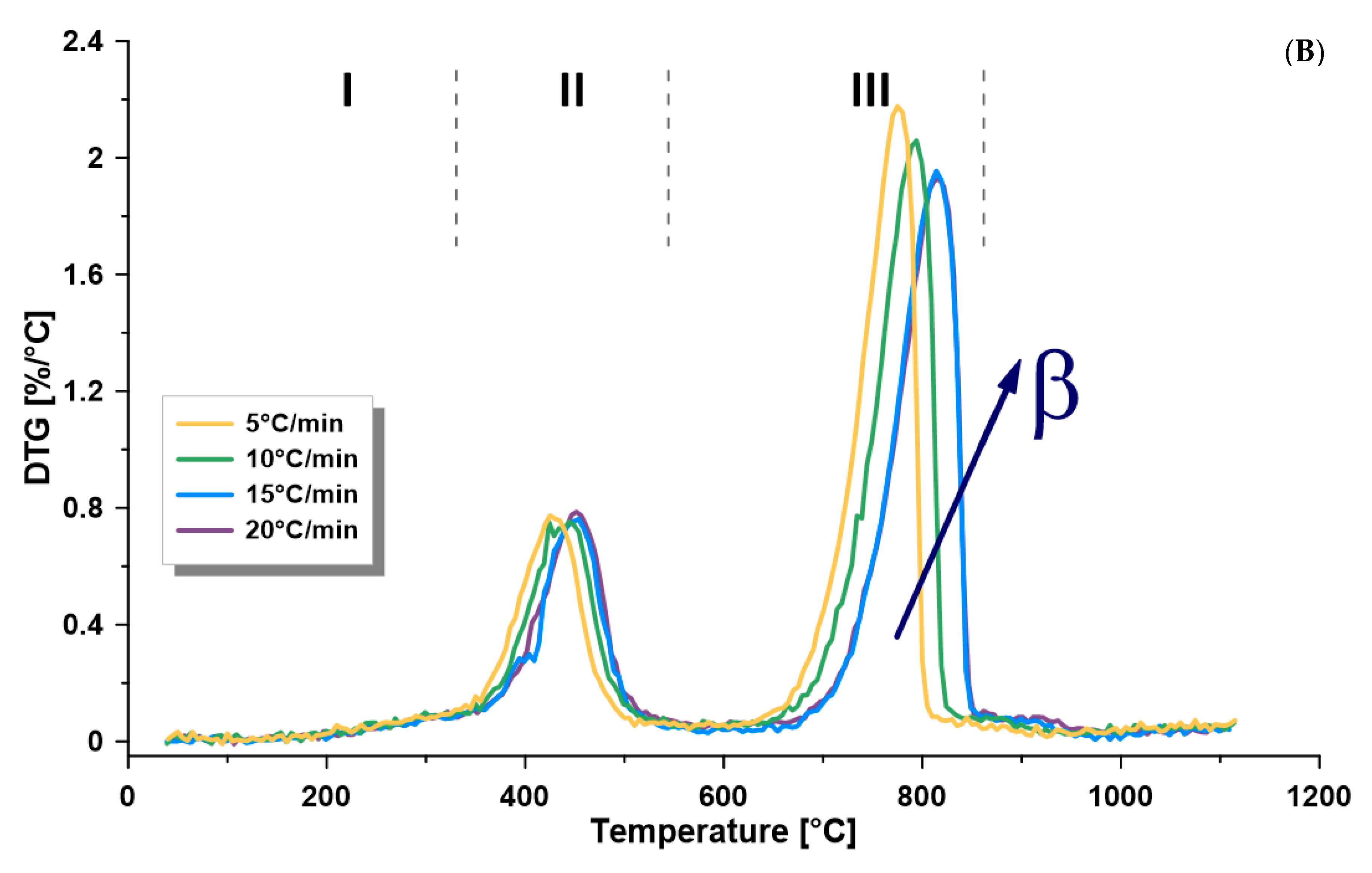
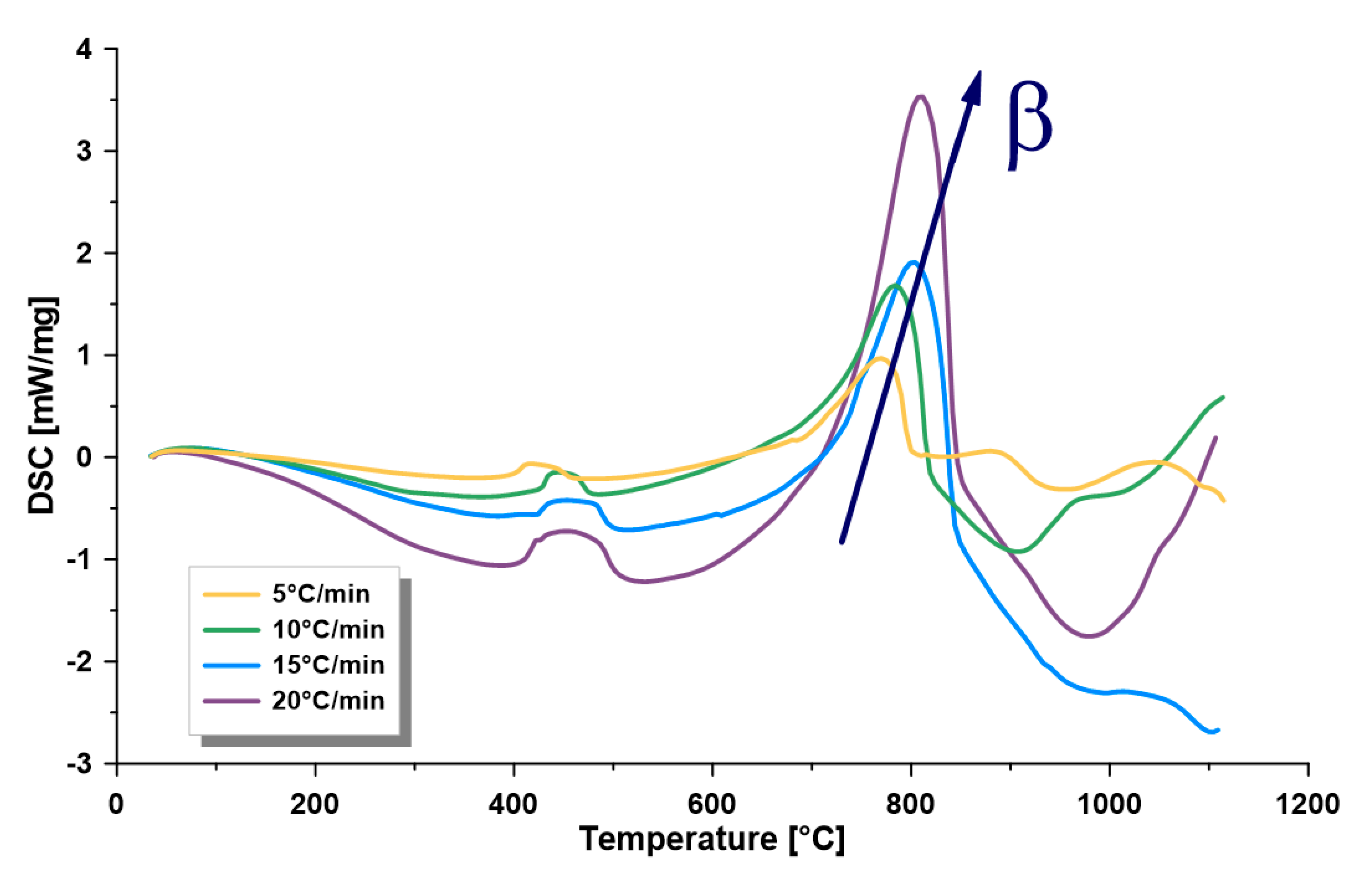
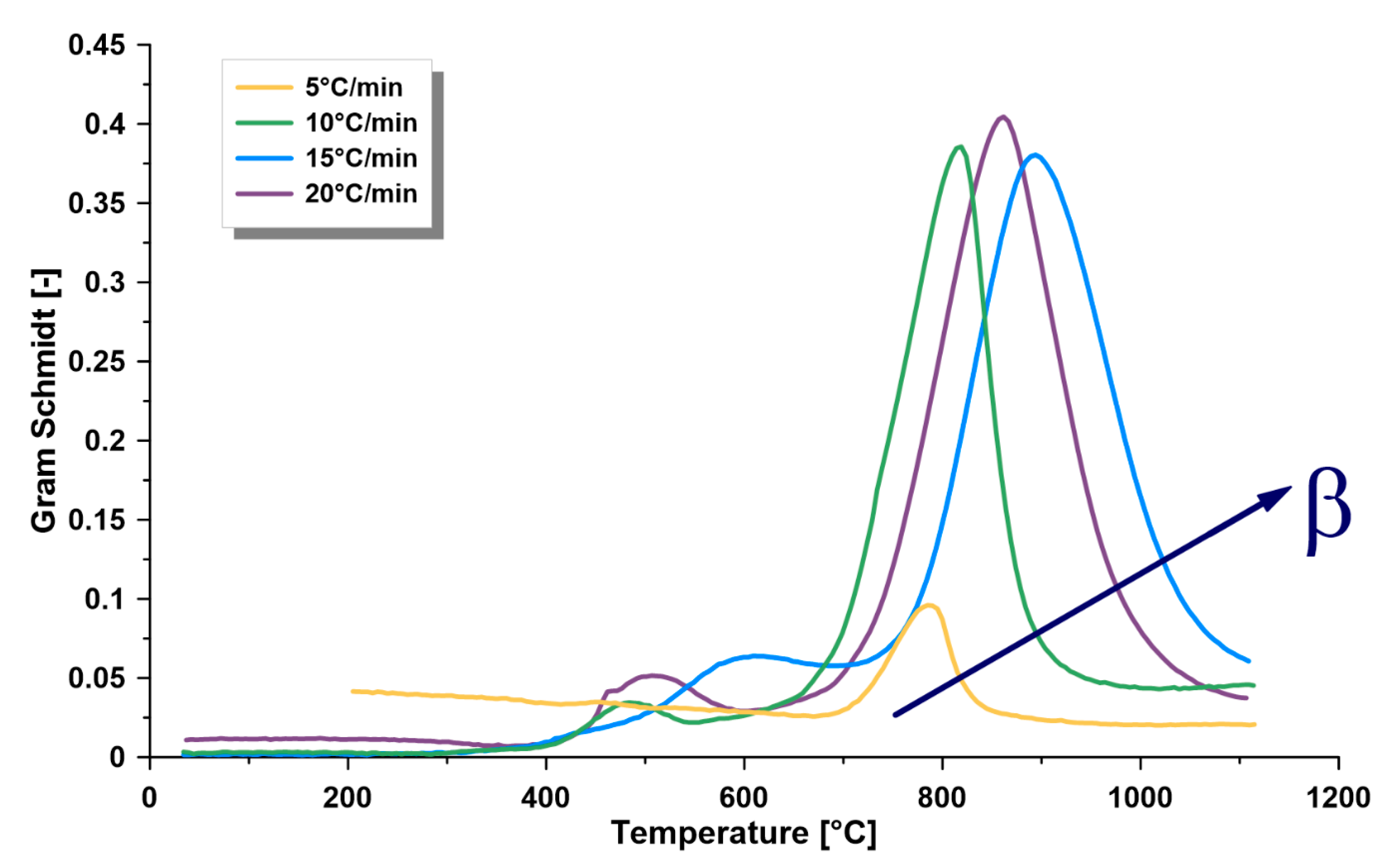
3.2. Thermogravimetric Analysis
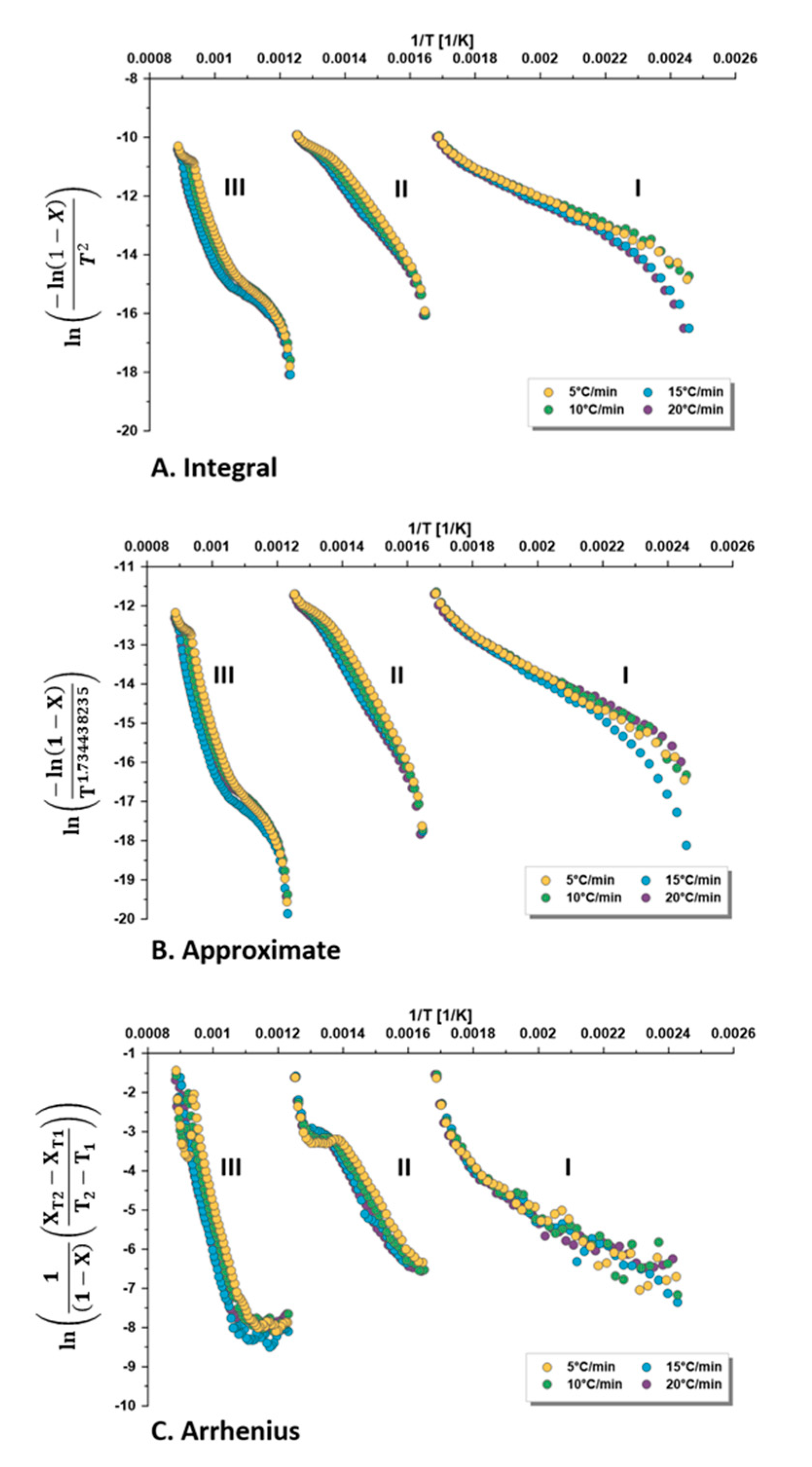
3.3. Selected Kinetic Methods
4. Conclusions
- The characterization tests of the “as received” samples from Omari formation, containing 11.2 wt.% of oil, revealed the following specifications: relatively high sulfur and low nitrogen content, 50.1 wt.% volatile content, 8.18 MJ/kg gross calorific value, 0.114–0.567 kJ/(kg·K) heat capacity of kerogen pyrolysis, and calcite as the main metal oxide (36.9 wt.%).
- The dependence of the kinetics of this formation on temperature, kerogen conversion, and heating rate confirms the complex nature of its kerogen. Furthermore, there is a directly proportional relationship between the heating rate and the kinetic rate at the different zones. Furthermore, the heating rate is directly proportional to the maximum thermal pyrolysis and decomposition temperatures. Therefore, it is recommended to use the heating rate at the maximum allowable operational limits, because some other parameters in commercial retorters may negatively influence, such as heat transfer limitations, if surpassing these limits.
- The three selected methods for calculating the first-order kinetic parameters showed comparable results with higher coefficients of determination (R2) for the integral and approximate methods, compared with the direct Arrhenius method.
- The average activation energy of Omari formation in the pyrolysis zone (330–540 °C) was 112–116 kJ/mol, while the frequency factor was 2.0 × 107 − 1.5 × 109 min−1, depending on the applied heating rate (5, 10, 15, 20 °C/min).
- The activation energy was found to increase when increasing the retorting temperature. Thus, it was the lowest at the drying zone and the maximum at the mineral decomposition zone.
- The kinetic parameters of Omari formation can be employed when developing a pyrolysis reactor model.
- Further kinetic studies need to be conducted on more samples from different locations in the Omari formation.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qian, J.; Yin, L. Oil Shale—Petroleum Alternative; China Petrochemical Press: Beijing, China, 2010. [Google Scholar]
- Wang, Q.; Hou, Y.; Wu, W.; Liu, Q.; Liu, Z. The structural characteristics of kerogens in oil shale with different density grades. Fuel 2018, 219, 151–158. [Google Scholar] [CrossRef]
- Alali, J.; Salah, A.A.; Yasin, S.M.; Omari, W.A. Oil Shale; Ministry of Energy and Mineral Resources: Amman, Jordan, 2015.
- El-Hasan, T.; Szczerba, W.; Buzanich, G.; Radtke, M.; Riesemeier, H.; Kersten, M. Cr(VIi)/Cr(III) and As(V)/As(III) ratio assessments in Jordanian spent oil shale produced by aerobic combustion and anaerobic pyrolysis. Environ. Sci. Technol. 2011, 45, 9799–9805. [Google Scholar] [CrossRef] [PubMed]
- Foltin, J.P.; Lisboa, A.C.L.; de Klerk, A. Oil shale pyrolysis: Conversion dependence of kinetic parameters. Energy Fuels 2017, 31, 6766–6776. [Google Scholar] [CrossRef]
- Pan, L.; Dai, F.; Li, G.; Liu, S. A TGA/DTA-MS investigation to the influence of process conditions on the pyrolysis of Jimsar oil shale. Energy 2015, 86, 749–757. [Google Scholar] [CrossRef]
- Strizhakova, Y.A.; Usova, T.V. Current trends in the pyrolysis of oil shale: A review. Solid Fuel Chem. 2008, 42, 197–201. [Google Scholar] [CrossRef]
- Abdul Jameel, A.G.; Han, Y.; Brignoli, O.; Telalović, S.; Elbaz, A.M.; Im, H.G.; Roberts, W.L.; Sarathy, S.M. Heavy fuel oil pyrolysis and combustion: Kinetics and evolved gases investigated by TGA-FTIR. J. Anal. Appl. Pyrolysis 2017, 127, 183–195. [Google Scholar] [CrossRef]
- Foltin, J.P.; Prado, G.N.; Lisbôa, A.C.L. Analysis of kinetics parameters of oil shale pyrolysis. Chem. Eng. Trans. 2017, 61, 439–444. [Google Scholar] [CrossRef]
- Chaohe, F.; Zhiping, L.; Hongyan, W.; Huaqing, X.; Yongjiang, X. Kinetics of isothermal and non-isothermal pyrolysis of oil shale. Oil Shale 2011, 28. [Google Scholar] [CrossRef]
- Torrente, M.C.; Galan, M.A. Kinetics of the thermal decomposition of oil shale from Puertollano (Spain). Fuel 2001, 80, 327–334. [Google Scholar] [CrossRef]
- Syed, S.; Qudaih, R.; Talab, I.; Janajreh, I. Kinetics of pyrolysis and combustion of oil shale sample from thermogravimetric data. Fuel 2011, 90, 1631–1637. [Google Scholar] [CrossRef]
- Al-Ayed, O.S.; Suliman, M.R.; Rahman, N.A. Kinetic modeling of liquid generation from oil shale in fixed bed retort. Appl. Energy 2010, 87, 2273–2277. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Al-Ayed, O.; Robinson, J.; Kingman, S.; Al-Harahsheh, A.; Tarawneh, K.; Saeid, A.; Barranco, R. Effect of demineralization and heating rate on the pyrolysis kinetics of Jordanian oil shales. Fuel Process. Technol. 2011, 92, 1805–1811. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Kujawa, J.; Albarahmieh, E.A.; Al-Rifai, N.; Qaimari, F.; Al-Gharabli, S. High throughput screening and characterization methods of Jordanian oil shale as a case study. Energies 2019, 12. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Oil from Oil Shale (Resource Evaluation by the Fischer Assay Procedure); ASTM: D3904; American Society for Testing and Materials: West Conshohocken, PA, USA, 1990. [Google Scholar]
- Speight, J. Shale Oil Production Processes; Gulf Professional Publishing: Oxford, UK, 2012. [Google Scholar]
- Jaber, J.O.; Probert, S.D. Pyrolysis and gasification kinetics of Jordanian oil-shales. Appl. Energy 1999, 63, 269–286. [Google Scholar] [CrossRef]
- Jaber, J.O.; Probert, S.D. Exploitation of Jordanian oil-shales. Appl. Energy 1997, 58, 161–175. [Google Scholar] [CrossRef]
- Mingshu, C.; Xiyan, L.; Hongpeng, L.; Yang, X.; Qing, W. Gaseous emission and thermal analysis during co-combustion of oil shale semi-coke and sawdust using TG-FTIR. Oil Shale 2015, 32. [Google Scholar] [CrossRef]
- Khraisha, Y.A. Kinetics of isothermal pyrolysis of Jordan oil shales. Energy Convers. Manag. 1998, 39, 157–165. [Google Scholar] [CrossRef]
- Burnham, A.K. Chemistry and kinetics of oil shale retorting. In Oil Shale: A Solution to the Liquid Fuel Dilemma; American Chemical Society: Washington, DC, USA, 2010; pp. 115–134. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Lee, S. Oil Shale Technology; CRC Press Inc.: Boca Raton, FL, USA, 1991. [Google Scholar]
| Proximate Analysis (wt.%) | Elemental Analysis (wt.%) | ||
|---|---|---|---|
| Moisture | 0.5 | C | 23.2 |
| Volatile | 50.1 | H | 1.7 |
| Fixed carbon b | 4.6 | N | 0.4 |
| Ash | 44.8 | S | 1.1 |
| Total | 100 | O c | 28.1 |
| Gross calorific value (MJ/kg) | 8.18 | - | - |
| Formation | FA a (wt.%) | CaO | SiO2 | SO3 | Al2O3 | P2O5 | Fe2O3 | Na2O | MgO | K2O | TiO2 | MnO | LOI b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Omari | 11.2 | 36.9 | 7.76 | 4.57 | 1.21 | 0.83 | 0.73 | 0.51 | 0.39 | 0.09 | 0.05 | - | 46.96 |
| Lajjun [3] | 10.5 | 30.43 | 16.13 | 4.83 | 3.77 | 3.30 | 1.55 | 0.10 | 0.65 | 0.00 | 0.16 | 0.01 | 38.13 |
| Sultani [3] | 7.5 | 26.3 | 26.26 | 4.38 | 2.87 | 3.48 | 1.12 | 0.27 | 0.95 | 1.37 | 0.13 | - | 33.0 |
| Method | Equation | Y |
|---|---|---|
| Integral | ||
| Approximate temperature integral | ||
| Direct Arrhenius plot |
| Zone | Heating Rate (°C/min) | Kinetic Parameters E (kJ/mol), A (min−1), k (min−1) c | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integral Method | Approximate Method | Arrhenius Method | |||||||||||
| E | A | k | R2 | E | A | k | R2 | E | A | k | R2 | ||
| I. Drying (100–330 °C) | 5 | 44 | 0.02 | 0.99 | 44 | 0.03 | 0.99 | 48 | 0.03 | 0.91 | |||
| 10 | 43 | 0.05 | 0.98 | 43 | 0.06 | 0.98 | 47 | 0.06 | 0.98 | ||||
| 15 | 54 | 0.06 | 0.96 | 55 | 0.07 | 0.97 | 50 | 0.08 | 0.92 | ||||
| 20 | 38 | 0.09 | 0.98 | 39 | 0.11 | 0.98 | 45 | 0.12 | 0.86 | ||||
| Average a | 45 | 0.19 | 0.98 | 45 | 0.22 | 0.98 | 47 | 0.08 | 0.92 | ||||
| All b | 45 | 0.06 | 0.95 | 45 | 0.07 | 0.95 | 47 | 0.08 | 0.89 | ||||
| II. Pyrolysis (330–540 °C) | 5 | 112 | 0.10 | 0.96 | 112 | 0.12 | 0.96 | 83 | 0.10 | 0.93 | |||
| 10 | 116 | 0.18 | 0.98 | 116 | 0.20 | 0.98 | 95 | 0.18 | 0.97 | ||||
| 15 | 115 | 0.23 | 0.99 | 115 | 0.26 | 0.99 | 101 | 0.25 | 0.97 | ||||
| 20 | 117 | 0.29 | 0.99 | 117 | 0.32 | 0.99 | 100 | 0.32 | 0.98 | ||||
| Average a | 115 | 0.22 | 0.98 | 115 | 0.25 | 0.98 | 95 | 0.42 | 0.96 | ||||
| All b | 116 | 0.22 | 0.97 | 112 | 0.30 | 0.96 | 83 | 0.26 | 0.93 | ||||
| III. Mineral Decomposition (540–860 °C) | 5 | 166 | 0.02 | 0.96 | 166 | 0.03 | 0.96 | 166 | 0.03 | 0.90 | |||
| 10 | 151 | 0.04 | 0.94 | 151 | 0.04 | 0.94 | 164 | 0.04 | 0.87 | ||||
| 15 | 142 | 0.03 | 0.91 | 142 | 0.04 | 0.91 | 167 | 0.04 | 0.82 | ||||
| 20 | 134 | 0.05 | 0.91 | 134 | 0.05 | 0.91 | 156 | 0.06 | 0.82 | ||||
| Average a | 148 | 0.07 | 0.93 | 148 | 0.08 | 0.93 | 163 | 0.04 | 0.85 | ||||
| All b | 148 | 0.04 | 0.93 | 148 | 0.04 | 0.91 | 162 | 0.04 | 0.84 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu El-Rub, Z.; Kujawa, J.; Al-Gharabli, S. Pyrolysis Kinetic Parameters of Omari Oil Shale Using Thermogravimetric Analysis. Energies 2020, 13, 4060. https://doi.org/10.3390/en13164060
Abu El-Rub Z, Kujawa J, Al-Gharabli S. Pyrolysis Kinetic Parameters of Omari Oil Shale Using Thermogravimetric Analysis. Energies. 2020; 13(16):4060. https://doi.org/10.3390/en13164060
Chicago/Turabian StyleAbu El-Rub, Ziad, Joanna Kujawa, and Samer Al-Gharabli. 2020. "Pyrolysis Kinetic Parameters of Omari Oil Shale Using Thermogravimetric Analysis" Energies 13, no. 16: 4060. https://doi.org/10.3390/en13164060
APA StyleAbu El-Rub, Z., Kujawa, J., & Al-Gharabli, S. (2020). Pyrolysis Kinetic Parameters of Omari Oil Shale Using Thermogravimetric Analysis. Energies, 13(16), 4060. https://doi.org/10.3390/en13164060








