Abstract
Oil shale is one of the alternative energies and fuel solutions in Jordan because of the scarcity of conventional sources, such as petroleum, coal, and gas. Oil from oil shale reservoirs can be produced commercially by pyrolysis technology. To optimize the process, mechanisms and rates of reactions need to be investigated. Omari oil shale formation in Jordan was selected as a case study, for which no kinetic models are available in the literature. Oil shale was analyzed using the Fischer assay method, proximate analysis (moisture, volatile, and ash), gross calorific value, elemental analysis (CHNS), and X-ray fluorescence (XRF) measurements. Non-isothermal thermogravimetric analysis was applied to study the kinetic parameters (activation energy and frequency factor) at four selected heating rates (5, 10, 15, and 20 °C/min). When oil shale was heated from room temperature to 1100 °C, the weight loss profile exhibited three different zones: drying (devolatilization), pyrolysis, and mineral decomposition. For each zone, the kinetic parameters were calculated using three selected methods: integral, temperature integral approximation, and direct Arrhenius plot. Furthermore, the activation energy in the pyrolysis zone was 112–116 kJ/mol, while the frequency factor was 2.0 × 107 − 1.5 × 109 min−1. Moreover, the heating rate has a directly proportional relationship with the rate constant at each zone. The three different methods gave comparable results for the kinetic parameters with a higher coefficient of determination (R2) for the integral and temperature integral approximation compared with the direct Arrhenius plot. The determined kinetic parameters for Omari formation can be employed in developing pyrolysis reactor models.
1. Introduction
Oil shale can be described as a fossilized organic substance called kerogen, disseminated within the shale mineral structure [1,2]. The quality of extracted oil in commercial reservoirs depends on the expected reserves in the formation, oil yield per ton of shale, calorific value, and concentrations of contaminants (e.g., sulfur) in the collected oil, which requires an upgrading treatment [1]. Oil shale is a promising alternative to petroleum and coal because of its enormous reserves in several countries, among which Jordan is the sixth most prominent [3]. However, this country suffers from challenges in domestic energy supply and fuel, to which oil shale can significantly contribute to its resources. In Jordan, oil shale is distributed over 60% of the area [3], the most important of which is in the northern and central-south regions, with quantities up to 70 billion tons, which is equivalent to 34 billion barrels of oil shale [4].
Commercial oil shale production is usually performed through processes involving pyrolysis retorts. In order to use the most suitable conditions in the retorts to produce oil at the highest productivity and lowest cost, there must be enough knowledge of the specifications of oil shale in the exploited formation, mechanisms, and rates of pyrolysis reactions of kerogen. Oil shale specifications, such as oil content, mineral analysis, and the crystalline structure, helps researchers understand the complex reaction mechanisms of kerogen, which may change as the chemical composition of oil shale varies. On the other hand, the conversion of kerogen to evaporated hydrocarbons may not occur through simple but multiple reactions, depending on the temperature and the chemical composition of kerogen [5,6]. In contrast, the reaction mechanisms and rates depend mainly on the kerogen concentration in the shale, which varies during the pyrolysis process. The oil loss from the shale occurs either through a reaction or evaporation [5]. Therefore, thermogravimetric analysis can be used as a method to measure the concentration or conversion of oil inside the shale as a function of time and temperature. This undoubtedly helps in the formulation of simulation models that study the rates of these reactions and provide performance equations for retorts to improve and optimize their operation [7]. These models are often based on the kinetic rate equation as a function of conversion and reaction order. To solve this equation and find reaction rate kinetic parameters (activation energy and frequency factor), several methods can be used, such as integral, approximate temperature integral, and direct Arrhenius methods, the Coats–Redfern method, the Freeman–Carrol method, the differential method, the Kissinger–Akahira–Sunose method, and the Flynn–Wall–Ozawa method [8,9]. The apparent values of these parameters depend mainly on oil shale properties (such as oil content, elemental analysis, and proximate analysis) and retort operating conditions (such as heating rate and retort temperature) [7].
Isothermal and non-isothermal models have been presented in the literature [10,11]. In the first approach, the rate of the reaction and the kinetic parameters were specified at a fixed pyrolysis temperature. In the second method, time, temperature, and kinetic parameters varied at a constant heating rate. Non-isothermal analyses have some advantages over the isothermal approach, including minimization of errors, absence of thermal induction period, rapid scan for the entire temperature range in the region of interest, and conditions simulation, which are expected to be used in large-scale processes [10,11].
Syed et al. [12] evaluated the kinetic parameters at the heating rates (5, 10, 15, and 20 °C/min) for the Lajjun oil shale deposit in Jordan. They compared three methods: integral; direct Arrhenius plot; and temperature integral approximation. They found that the integral method had the highest coefficient of determination and the activation energy was independent of the heating rate. Alayed et al. [13] modeled the kinetics of Lajjun oil shale pyrolysis in a fixed bed retort, applying a second-order rate equation, using the Coats–Redfern differential and integral models. They found an inversely proportional relationship between the heating rate (2 to 10 °C/min) and the activation energy (115 to 71 kJ/mol). Al-Harahsheh et al. [14] studied the effect of the mineral matrix on the activation energy of oil shale pyrolysis at the heating rates (1, 3, 5, 10, 30, and 50 °C/min). They found a directly proportional relationship between the heating rate and pyrolysis activation energy that varied between 70 and 83 kJ/mol for oil shale and 82–112 kJ/mol for isolated kerogen. Yongjiang et al. [10] studied the kinetics of decomposition of Huadian oil shale by non-isothermal and isothermal models at the heating rates (2, 5, 10, 15 and 20 °C/min), up to 600 °C. Both models gave similar kinetic results with activation energy in the range of 131–142 kJ/mol. Foltin et al. [5] studied the kinetics of oil shale pyrolysis from the Irati formation in Brazil over the temperature range 50–900 °C, applying model-free methods validated by isothermal pyrolysis at 400 °C for 3 hours. The apparent activation energy was found in the range 215–255 kJ/mol, which increased as the oil shale conversion increased. Furthermore, the Friedman method gave better results than the Flynn–Wall–Ozawa and Kissinger–Akahira–Sunose methods.
This work aims to develop a kinetic description for the devolatilization of the Omari oil shale formation in Jordan [15] using thermogravimetric analysis (TGA). There have been no published pyrolysis kinetic models applied to this formation. Three methods were selected to find the kinetic parameters: integral, approximate temperature integral, and direct Arrhenius plot. The impact of heating rate on kinetic parameters was also studied. The analysis of the pyrolysis behavior was supported by the characterization of the oil shale using the Fischer assay, proximate and elemental analysis, gross calorific value, and X-ray fluorescence.
2. Materials and Methods
The oil content in oil shale samples was measured by the Fischer Assay method, which is based on the American Society for Testing and Materials (ASTM) D3094-90 standard test method for the determination of oil yield, with the difference in repeatability of less than 4.2 mL/kg (1.0 gal/ton) [16]. Thermal features were determined by a Jupiter STA 449 F5 (Netzsch, Germany) thermogravimetric analyzer combined with a Fourier-transform infrared (FTIR) Vertex 70 V spectrometer (Bruker Optik, Germany). TGA, differential thermal analysis (DTA), and derivative thermogravimetry (DTG) were undertaken in nitrogen between 25 °C and 1100 °C. TGA was done in triplicate using samples of ca. 15 mg, where no influence for the sample weight was noticed. The following heating rates were selected: 5, 10, 15, and 20 °C/min. The thermal resistance and sample stability were defined according to TGA and DTG, while DTA provided data on changes in the energy of materials, such as the enthalpy and specific heat capacity. The application of TGA coupled with FTIR gave the opportunity to analyze gas products online. The average of 16 FTIR scans was collected every 7 s for all heating rates, with a resolution of 4 cm−1. The Gram–Schmidt diagram was used to evaluate the amount of gas production as a function of temperature. The proximate composition of the “as received” oil shale samples were moisture, volatile matter, and ash, which were analyzed in a single analysis, according to ASTM D7582, using a TGA thermostep thermogravimetric analyzer (Eltra, Germany). The gross calorific value of the “as received” oil shale samples were analyzed according to ASTM D5865, using the oxygen bomb calorimeter IKA-C500 (IKA, Germany). The Vario MACRO CHN elemental analyzer (Elementar, Germany) was used for elemental analysis of carbon, hydrogen, and nitrogen. In addition, sulfur was analyzed according to ASTM D5373 by Carbon Sulphur Determinator CS-580 (Eltra, Germany). X-ray fluorescence (XRF), using the sequential wavelength dispersive XRF S4 Pioneer spectrometer (Bruker, Germany), was used for the evaluation of the chemical composition of major oxides and the loss on ignition in the unprocessed oil shale samples, using the automatic fusion technique.
3. Results and Discussion
3.1. Characterization of Oil Shale
The Omari oil shale formation is located east of Jordan near the Iraqi border [3,15]. From this formation, 100 samples with 2.9 to 15 wt.% oil content and an average of 8.44 wt.% were collected from a depth range of 614 to 892 m. These samples were dried at 40 °C, milled into a particle size of 2.36 mm, and homogenized. The samples were characterized by conducting proximate and elemental analysis (Table 1) and XRF analysis (Table 2).

Table 1.
Proximate and elemental analysis of Omari oil shale samples a.

Table 2.
X-ray fluorescence (XRF) analysis (wt.%) of the tested oil shale sample “as received” from the Omari formation in comparison to samples from Lajjun and Sultani formations.
The proximate and elemental analysis of “as received” samples at room dry conditions are presented in Table 1. The proximate analysis shows the moisture, volatile, and ash content, whereas the fixed carbon is calculated by the difference. The higher volatile content, originated from organic material in kerogen, lead to the generation of more oil via the retorting process. Moreover, the gross calorific value of Omari oil shale was higher than that reported for Jordanian oil shale (5–7 MJ/kg) [17,18,19]. The higher the heating value, the better the quality of the oil shale, especially in power plants that apply direct combustion. Furthermore, the elemental analysis showed relatively high sulfur and low nitrogen content. These values were comparable to other Jordanian oil shales, such as Lajjun and Sultani [18].
The results of XRF analysis in Table 2 reveal that the minerals concentration in the unprocessed samples were in good accordance with the reported data for oil shale deposits in the central part of Jordan formations [3,19]. The main components in the mineral matter of the Omari oil shale were calcite (CaO) and quartz (SiO2), with higher concentrations of calcite and lower content of quartz, compared with those reported for Lajjun and Sultani formations.
3.2. Thermogravimetric Analysis
TGA is a suitable and fast method broadly applied to measure weight loss as a function of temperature. Furthermore, differential scanning calorimetry (DSC) was recorded simultaneously with TGA, which measures the difference of temperature between a reference and the investigated sample, as well as the heat flow. Both techniques can be applied to deliver important data on the behavior of oil shale samples upon heating and assigning the maximum temperature, where kerogen degradation takes place. TGA measurements were applied for Omari oil shale. Samples were heated from 25 °C and 1100 °C at different heating rates (5, 10, 15, and 20 °C/min). Pyrolysis volatiles were subjected to mass flow measurement, as well as FTIR analysis, recorded every 7 s from 400 to 4000 cm−1 during the heating process. The amount of produced gases was verified by the Gram–Schmidt thermogram. The oil shale samples from the Jordanian Omari deposit were tested and characterized by implementing the above-mentioned methods. Figure 1 and Figure 2 show the average TGA and DTG curves of the oil shale sample, respectively. The average total weight loss was 49.0 wt.% up to 1100 °C. Both curves showed three distinct zones, known as: I. drying (up to 320 °C); II. pyrolysis (320–540 °C); and III. mineral decomposition (540–860 °C).
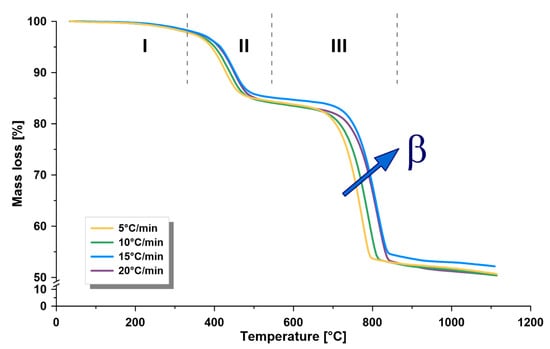
Figure 1.
Thermogravimetric analysis (TGA) curves of the oil shale sample at four heating rates (β) of 5, 10, 15 and 20 °C/min, showing the three decomposition zones of oil shale (I. drying, II. pyrolysis, III. mineral decomposition), using thermogravimetric analysis (under nitrogen).
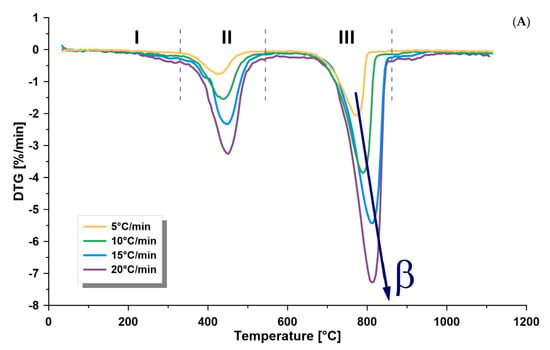
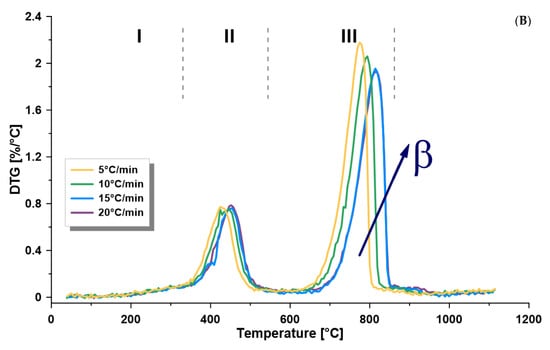
Figure 2.
First derivative thermogravimetric (TG) curves, with respect to (A) time and (B) temperature (°C), of oil shale sample at four heating rates (β) of 5, 10, 15 and 20 °C/min, showing the three decomposition zones of oil shale (I. drying, II. pyrolysis, III. mineral decomposition), using thermogravimetric analysis (under nitrogen).
The heating rate is an important factor in commercial shale pyrolysis processes. The increase in the heating rate was associated with an increase in the retorting capacity. However, the heating rates in commercial retorts are usually at minimum levels of 1.5–5 °C to reduce the heat transfer limitations exemplified in the temperature difference between the center and internal surface of the retort, which reduces the rates of secondary cracking reactions. Furthermore, the pyrolysis reactions begin before the final temperature is reached, which makes it difficult to control these reactions in commercial retorts. However, new technologies might overcome this limitation and could utilize higher heating rates. It was reported that increasing the heating rate lead to a non-significant increase in the yield of pyrolysis products but changed the quality of the products (higher specific gravity and heavier oil shale fractions) [1].
In the drying zone, there was a relatively low weight loss of less than 1.4 wt.%, which can be related to the devolatilization of surface and adsorbed water, entrapped gases, and light organic materials, as found by the generated FTIR spectra (Figure S1, in Supplementary Materials) [15]. This low water content resulted in less heating requirements and less drying time for evaporation, thereby increasing the thermal efficiency of the retorter. Furthermore, the higher the heating rate, the greater the temperature difference was between the surface and center of the oil shale particle, triggering faster water evaporation, increased expansion in the inner part, and reduced breakup temperature [17].
In the pyrolysis zone, a weight loss of 14 wt.% was recorded. This was due to the thermal decomposition of kerogen to form oil shale and shale gas. In addition, limited decomposition of the mineral material can occur, resulting in carbon dioxide production and the release of combined water. This was supported by the generated FTIR spectra (Figure S1) and our previous work [15], which confirmed the production of CO2, as well as aliphatic and aromatic hydrocarbons.
In the third zone, the mineral decomposition zone, about 33.5 wt.% loss was observed. Here, the different minerals in the shale thermally decomposed to form mainly carbon dioxide, as confirmed by FTIR spectra in Figure S1 and the metal oxide. On the other hand, the maximum thermal decomposition temperature increased from 775 °C to 817 °C.
Two important observations were found, as shown in Figure 1 and Figure 2A, when the heating rate increased: increased temperature lag in the pyrolysis and mineral decomposition zones and increased peak area when measuring DTG (wt.%/min). In the pyrolysis zone, the maximum thermal pyrolysis temperature increased when increasing the heating rate from 430 °C to 451 °C. This shift can be related to heat transfer, a decrease in the heating time when the heating rate is higher, which causes more gases to escape in less time. The effect of time was neutralized, as shown in Figure 2B where DTG (wt.%/°C) is plotted. However, this did not show a perfect overlap, and there was a temperature lag between the curves that increased with decreased heating rates, which can be related to the heat transfer that affected the pyrolysis reaction rates.
The specific heat capacity of oil shale depends on the mineral composition and kerogen content, and principally determines the pyrolysis heat of oil shale [17], which can be determined by the DSC. Accordingly, triplicate DSC analysis was conducted for the samples with no discrepancy found, as demonstrated in Figure 3. The resulted peaks indicate an endothermic behavior in the pyrolysis zone (320–540 °C) with a calculated heat capacity for kerogen decomposition in the range of 0.114–0.567 kJ/(kg·K). Moreover, at elevated temperatures (900–1100 °C), the DSC did not show consistent behavior for the different heating rates, which can be related to the large difference in total exposure time at such elevated temperatures (the duration at a rate of 5 °C/min is four times than that at 20 °C /min). The exposure time difference at such extreme operating temperatures could affect the chemistry of the demineralization zone of the samples. However, temperatures above 900 °C are usually not relevant in the oil shale industry or in pyrolysis research.
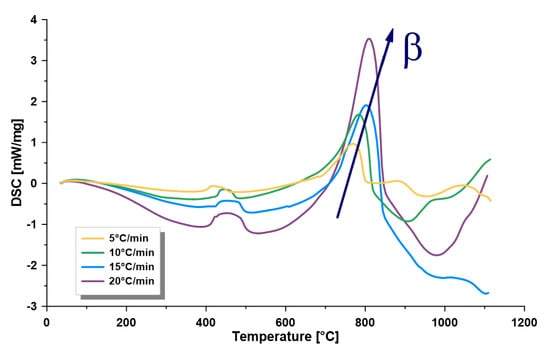
Figure 3.
Representative differential scanning calorimetry (DSC) curve at four heating rates (β) of 5, 10, 15 and 20 °C/min.
The amount of produced gases resulted from the TGA under different heating rates were detected and presented via the Gram–Schmidt thermogram [20] (Figure 4). Similar behavior was recorded for the heating rates 10, 15, and 20 °C/min, with no particular trend. However, the lowest heating rate (5 °C/min) showed a different profile, which might be related to the detection limit of the flow meter.
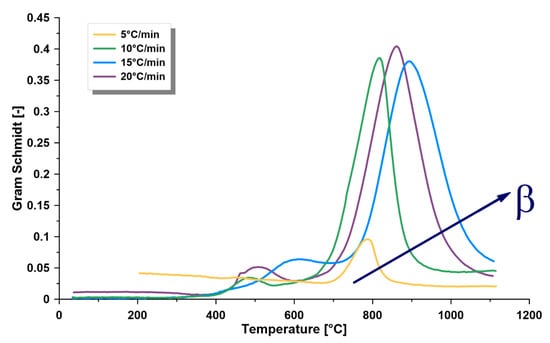
Figure 4.
Gram–Schmidt (GS) signals of the heated oil shale samples at four heating rates (β) of 5, 10, 15, and 20 °C/min and a nitrogen environment showing the gas evolving region in the temperature range of 25–1100 °C, determined as the peak temperatures.
3.3. Selected Kinetic Methods
In order to use optimum conditions for oil shale production in commercial retorts, there must be adequate knowledge on oil shale specifications in the exploited formation, mechanisms of reactions, and rates of kerogen-pyrolysis reactions. The conversion of kerogen to devolatilized hydrocarbons may not occur through simple but multiple reactions, depending on the temperature and the chemical composition of kerogen [5,6,21]. These reactions can be simplified in two-step first-order reactions mechanisms, as presented in Equation (1) [22]. In the first step, the complex kerogen molecules are partially decomposed to bitumen, which is further converted into smaller molecular weight fragments, oil, gas, and char.
where k1 and k2 are the reaction rate constant of kerogen and bitumen decomposition, respectively.
Model-free methods [8,9] can be employed to examine the decay reactions of kerogen without the need for a specific mechanism. Moreover, kinetic models were developed to calculate the apparent kinetic parameters (activation energy and frequency factor) of the three zones, including the pyrolysis region, which is the most important stage in the decomposition of oil shale. The activation energy can be defined as the lowest energy needed to initiate a chemical reaction. However, the calculated activation energies in this study may include heat and mass transfer limitations (which increases with increasing particle size), which affect the values of the kinetic parameters. Therefore, the activation energy is denoted as the apparent activation energy. Moreover, the first stage includes minor mass loss due to the release of moisture and the devolatilization of lighter materials, which has no reactions. However, the kinetic models were applied to the first event to compare the results in this work with the literature.
The chemical conversion of kerogen to oil, gas, and char can be exemplified by the following nth order kinetic equation, as presented in Equation (2):
where X is the conversion, is the reaction time, is the specific rate constant, and is the order of the reaction. Conversion can be represented, as presented in Equation (3):
where X is the conversion, is the initial mass of oil shale at the event of interest, is the mass at time t, and is the final mass of oil shale at the event of interest. In some literature [5,14], was assumed to be the initial mass of oil shale and was the final mass of oil shale after the mineral decomposition ended.
Because we have heterogeneous reactions, a first-order reaction was assumed [1,23]. In addition, the relation between temperature and the rate constant is given by the Arrhenius Equation (4):
where A is the apparent frequency factor , is the apparent activation energy , T is the temperature , and R is the universal gas constant .
The linear relation between heating time and temperature, to account for the heating rate, can be expressed as presented in Equation (5):
Substituting Equations (4) and (5) into (2) then rearranging to give Equation (6):
This equation can be used to calculate the Arrhenius constants (E) and (A). However, there is no definite integral to the right-hand side of the equation. Three methods were selected to solve this integration and find the value of Arrhenius constants by finding a straight-line relation when plotting (1/T) against the variable (Y), explained in Table 3.

Table 3.
Representative equations of the selected kinetic methods. Adapted from [9,12].
Figure 5 shows the kinetic analysis of the TGA data using the three methods, (A) integral, (B) approximate temperature integral, and (C) direct Arrhenius, at different heating rates of 5, 10, 15, and 20 °C/min for all methods. The expression of Y for each method listed in Table 1 is plotted against 1/T in order to find a linear trend line, from which the kinetic parameters (E) and (A) are found.
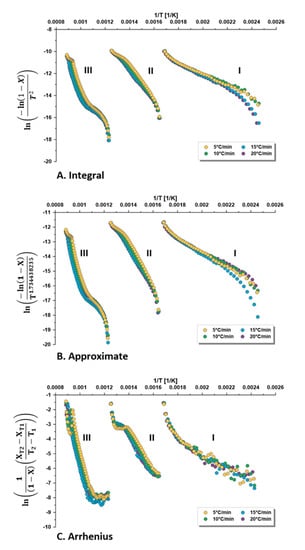
Figure 5.
Kinetic analysis of TGA data of the three decomposition zones of oil shale (I. drying, II. pyrolysis, III. mineral decomposition) using three kinetic methods: (A) integral; (B) approximate temperature integral; and (C) direct Arrhenius, at different heating rates (β) of 5, 10, 15 and 20 °C/min.
The apparent kinetic parameters extracted from the different trend lines in Figure 5 are summarized in Table 4. Thus, for every heating rate, the activation energy and frequency factor were calculated, as well as the arithmetic average of these parameters. Results were also compared with the found kinetic parameters, which resulted from one trend line representing the data of all heating rates. Furthermore, the value of the reaction kinetic constant is a function of the temperature and the kinetic parameters, as explained in Equation (4). Therefore, we can calculate and compare the rate constant at the average zone temperature as a suggested temperature of comparison.

Table 4.
Effect of heating rate on the apparent kinetic parameters and the rate constant at the average zone temperatures for the three kinetic methods.
The coefficient of determination (R2) indicates that the integral and approximate methods have a better correlation than the direct Arrhenius method. Therefore, these two methods were considered in the further discussion below. Furthermore, the coefficient of determination of all data (All) was not equivalent to the arithmetic average (Average) of the data and its trend line showed weaker regression. Syed et al. [12] found the highest regression for the integral method.
The activation energy increased when increasing the temperature or conversion. Therefore, the activation energy increased when moving up from the first to the last zone. This can be related to the fact that less activation energy was required for kerogen decomposition compared to demineralization.
In the first zone (drying), there was a directly proportional effect for the heating rate on the kinetic parameters and the rate constant. The arithmetic average kinetic parameters were 45 kJ/mol for the activation energy, and the frequency factor values were 1.2 × 104 and 1.5 × 104 min−1 for the integral and approximate methods, respectively. The activation energy of this zone was the lowest and represented the loss in oil shale weight attributed to the release of moisture and devolatilization of lighter organic materials, which requires the least amount of energy compared to the other zones. Furthermore, the average activation energy was relatively high compared with the average drying activation energy for the Lajjun formation found by Syed et al., (10 kJ/mol). This may indicate that more moisture in the Omari formation is trapped in the pores, which makes it more difficult to be released.
In the second zone (pyrolysis), there was a directly proportional relationship between the heating rate and both of the kinetic parameters, as well as the kinetic constant, which agrees with the results found by Al-Harahsheh et al. [14]. The activation energy of the pyrolysis stage is the most important parameter in the performance equation of the oil shale retorts. This zone has intermediate activation energy compared to the other two zones, where the loss in oil shale weight was mainly related to the loss of hydrocarbon materials. The average values of the activation energy were 112 and 117 kJ/mol, whereas the frequency factor had the values of 2.0 × 107 and 1.5 × 109 min−1 for the integral and approximate methods, respectively. These values of the activation energy were relatively higher than those reported for Lajjun (80 kJ/mol) [1].
In the third zone (mineral decomposition), there was an inversely proportional relationship between the heating rate and the kinetic parameters. The values for this zone ranged from 134 to 167 kJ/mol for the activation energy, while the frequency factor was 7.2 × 105 and 2.0 × 107 min−1 for the integral and approximate methods, respectively. This zone had the highest activation energy, which was related to the decomposition of carbonate minerals to produce carbon dioxide. This carbon dioxide reacted later with the residue of oil shale to increase the loss in the oil shale weight. The zone had a lower coefficient of determination than the pyrolysis zone, which might be related to the selected temperature range, especially 540 to 700 °C. This part of the zone might be an extension to the pyrolysis zone, where both kerogen pyrolysis and catalytic cracking by mineral matter may occur, as found by Foltin et al. [5]. In our case, the mineral matter in Omari samples was mainly CaCO3 and SiO2, where the calcium carbonate decomposition becomes effective at temperatures higher than 700 °C [24].
4. Conclusions
In this work, non-isothermal thermogravimetric analysis was used to study the kinetic parameters (activation energy and frequency factor) of oil shale devolatilization at different heating rates (5, 10, 15, 20 °C/min). These parameters were calculated using three different methods: the integral method, temperature integral approximation method, and direct Arrhenius plot method. The study was applied to samples obtained from Omari oil shale formation in Jordan, which were analyzed using the Fischer assay method, proximate and elemental analysis, gross calorific value, and X-ray fluorescence. Under the test conditions, the following conclusions were drawn:
- The characterization tests of the “as received” samples from Omari formation, containing 11.2 wt.% of oil, revealed the following specifications: relatively high sulfur and low nitrogen content, 50.1 wt.% volatile content, 8.18 MJ/kg gross calorific value, 0.114–0.567 kJ/(kg·K) heat capacity of kerogen pyrolysis, and calcite as the main metal oxide (36.9 wt.%).
- The dependence of the kinetics of this formation on temperature, kerogen conversion, and heating rate confirms the complex nature of its kerogen. Furthermore, there is a directly proportional relationship between the heating rate and the kinetic rate at the different zones. Furthermore, the heating rate is directly proportional to the maximum thermal pyrolysis and decomposition temperatures. Therefore, it is recommended to use the heating rate at the maximum allowable operational limits, because some other parameters in commercial retorters may negatively influence, such as heat transfer limitations, if surpassing these limits.
- The three selected methods for calculating the first-order kinetic parameters showed comparable results with higher coefficients of determination (R2) for the integral and approximate methods, compared with the direct Arrhenius method.
- The average activation energy of Omari formation in the pyrolysis zone (330–540 °C) was 112–116 kJ/mol, while the frequency factor was 2.0 × 107 − 1.5 × 109 min−1, depending on the applied heating rate (5, 10, 15, 20 °C/min).
- The activation energy was found to increase when increasing the retorting temperature. Thus, it was the lowest at the drying zone and the maximum at the mineral decomposition zone.
- The kinetic parameters of Omari formation can be employed when developing a pyrolysis reactor model.
- Further kinetic studies need to be conducted on more samples from different locations in the Omari formation.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1073/13/16/4060/s1, Figure S1: FTIR spectra of the three decomposition zones of oil shale samples at the four heating rates (β) of (A) 5 °C/min, (B) 10 °C/min, (C) 15 °C/min, (D) 20 °C/min.
Author Contributions
Conceptualization, Z.A.E.-R.; data curation, J.K., S.A.-G., and Z.A.E.-R.; formal analysis, J.K. and S.A.-G.; funding acquisition, Z.A.E.-R.; investigation Z.A.E.-R., J.K., and S.A.-G.; methodology Z.A.E.-R.; project administration, Z.A.E.-R.; resources, Z.A.E.-R., S.A.-G., and J.K.; supervision, Z.A.E.-R.; validation, Z.A.E.-R., S.A.-G., and J.K.; visualization, J.K. and Z.A.E.-R.; writing—original draft, Z.A.E.-R.; writing—review and editing, Z.A.E.-R., J.K., and S.A.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Jordanian University, project number SAMS 33/2016.
Acknowledgments
The authors would like to thank Jordan Oil Shale Company (JOSCO) for their support in providing the oil shale samples and their Fischer assay analysis and Manaseer Industrial Complex for their support in providing proximate and ultimate analysis of oil shale samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qian, J.; Yin, L. Oil Shale—Petroleum Alternative; China Petrochemical Press: Beijing, China, 2010. [Google Scholar]
- Wang, Q.; Hou, Y.; Wu, W.; Liu, Q.; Liu, Z. The structural characteristics of kerogens in oil shale with different density grades. Fuel 2018, 219, 151–158. [Google Scholar] [CrossRef]
- Alali, J.; Salah, A.A.; Yasin, S.M.; Omari, W.A. Oil Shale; Ministry of Energy and Mineral Resources: Amman, Jordan, 2015.
- El-Hasan, T.; Szczerba, W.; Buzanich, G.; Radtke, M.; Riesemeier, H.; Kersten, M. Cr(VIi)/Cr(III) and As(V)/As(III) ratio assessments in Jordanian spent oil shale produced by aerobic combustion and anaerobic pyrolysis. Environ. Sci. Technol. 2011, 45, 9799–9805. [Google Scholar] [CrossRef] [PubMed]
- Foltin, J.P.; Lisboa, A.C.L.; de Klerk, A. Oil shale pyrolysis: Conversion dependence of kinetic parameters. Energy Fuels 2017, 31, 6766–6776. [Google Scholar] [CrossRef]
- Pan, L.; Dai, F.; Li, G.; Liu, S. A TGA/DTA-MS investigation to the influence of process conditions on the pyrolysis of Jimsar oil shale. Energy 2015, 86, 749–757. [Google Scholar] [CrossRef]
- Strizhakova, Y.A.; Usova, T.V. Current trends in the pyrolysis of oil shale: A review. Solid Fuel Chem. 2008, 42, 197–201. [Google Scholar] [CrossRef]
- Abdul Jameel, A.G.; Han, Y.; Brignoli, O.; Telalović, S.; Elbaz, A.M.; Im, H.G.; Roberts, W.L.; Sarathy, S.M. Heavy fuel oil pyrolysis and combustion: Kinetics and evolved gases investigated by TGA-FTIR. J. Anal. Appl. Pyrolysis 2017, 127, 183–195. [Google Scholar] [CrossRef]
- Foltin, J.P.; Prado, G.N.; Lisbôa, A.C.L. Analysis of kinetics parameters of oil shale pyrolysis. Chem. Eng. Trans. 2017, 61, 439–444. [Google Scholar] [CrossRef]
- Chaohe, F.; Zhiping, L.; Hongyan, W.; Huaqing, X.; Yongjiang, X. Kinetics of isothermal and non-isothermal pyrolysis of oil shale. Oil Shale 2011, 28. [Google Scholar] [CrossRef]
- Torrente, M.C.; Galan, M.A. Kinetics of the thermal decomposition of oil shale from Puertollano (Spain). Fuel 2001, 80, 327–334. [Google Scholar] [CrossRef]
- Syed, S.; Qudaih, R.; Talab, I.; Janajreh, I. Kinetics of pyrolysis and combustion of oil shale sample from thermogravimetric data. Fuel 2011, 90, 1631–1637. [Google Scholar] [CrossRef]
- Al-Ayed, O.S.; Suliman, M.R.; Rahman, N.A. Kinetic modeling of liquid generation from oil shale in fixed bed retort. Appl. Energy 2010, 87, 2273–2277. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Al-Ayed, O.; Robinson, J.; Kingman, S.; Al-Harahsheh, A.; Tarawneh, K.; Saeid, A.; Barranco, R. Effect of demineralization and heating rate on the pyrolysis kinetics of Jordanian oil shales. Fuel Process. Technol. 2011, 92, 1805–1811. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Kujawa, J.; Albarahmieh, E.A.; Al-Rifai, N.; Qaimari, F.; Al-Gharabli, S. High throughput screening and characterization methods of Jordanian oil shale as a case study. Energies 2019, 12. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Oil from Oil Shale (Resource Evaluation by the Fischer Assay Procedure); ASTM: D3904; American Society for Testing and Materials: West Conshohocken, PA, USA, 1990. [Google Scholar]
- Speight, J. Shale Oil Production Processes; Gulf Professional Publishing: Oxford, UK, 2012. [Google Scholar]
- Jaber, J.O.; Probert, S.D. Pyrolysis and gasification kinetics of Jordanian oil-shales. Appl. Energy 1999, 63, 269–286. [Google Scholar] [CrossRef]
- Jaber, J.O.; Probert, S.D. Exploitation of Jordanian oil-shales. Appl. Energy 1997, 58, 161–175. [Google Scholar] [CrossRef]
- Mingshu, C.; Xiyan, L.; Hongpeng, L.; Yang, X.; Qing, W. Gaseous emission and thermal analysis during co-combustion of oil shale semi-coke and sawdust using TG-FTIR. Oil Shale 2015, 32. [Google Scholar] [CrossRef]
- Khraisha, Y.A. Kinetics of isothermal pyrolysis of Jordan oil shales. Energy Convers. Manag. 1998, 39, 157–165. [Google Scholar] [CrossRef]
- Burnham, A.K. Chemistry and kinetics of oil shale retorting. In Oil Shale: A Solution to the Liquid Fuel Dilemma; American Chemical Society: Washington, DC, USA, 2010; pp. 115–134. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Lee, S. Oil Shale Technology; CRC Press Inc.: Boca Raton, FL, USA, 1991. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).