Modified Activated Graphene-Based Carbon Electrodes from Rice Husk for Supercapacitor Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Rice Husk
2.2. Modification with Ni(OH)2
2.3. Characterizations
2.4. Electrochemical Measurements
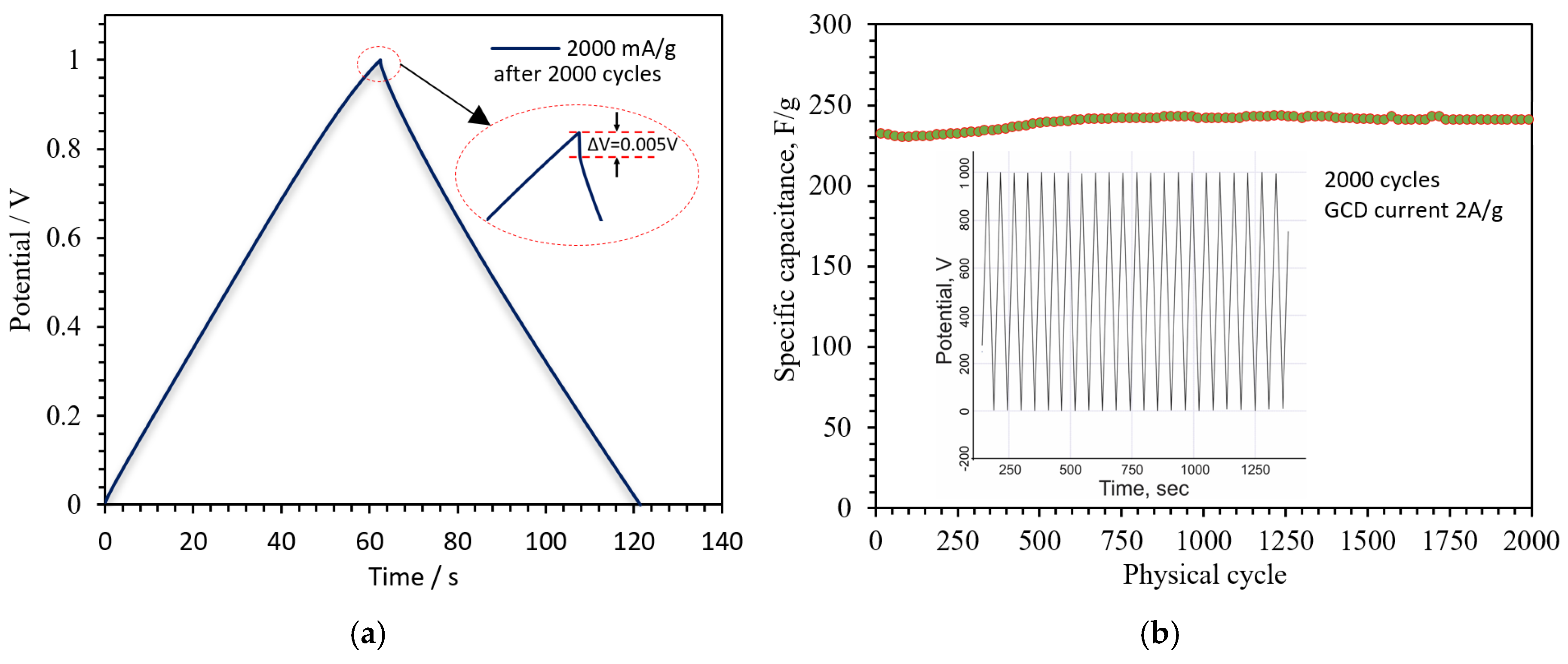
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Quan, H.; Cheng, B.; Xiao, Y.; Lei, S. One-pot synthesis of α-Fe2O3 nanoplates-reduced graphene oxide composites for supercapacitor application. Chem. Eng. J. 2016, 286, 165–173. [Google Scholar] [CrossRef]
- Zou, Z.; Cao, J.; Cao, B.; Chen, W. Evaluation strategy of regenerative braking energy for supercapacitor vehicle. ISA Trans. 2015, 55, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, W.; Zhu, J.; Zhao, W.; Ma, J.; Mhaisalkar, S.; Maria, T.L.; Yang, Y.; Zhang, H.; Hng, H.H.; et al. Synthesis of porous NiO nanocrystals with controllable surface area and their application as supercapacitor electrodes. Nano Res. 2010, 3, 643–652. [Google Scholar] [CrossRef]
- Ansari, S.A.; Fouad, H.; Ansari, S.G.; Sk, M.P.; Cho, M.H. Mechanically exfoliated MoS2 sheet coupled with conductive polyaniline as a superior supercapacitor electrode material. J. Colloid Interface Sci. 2017, 504, 276–282. [Google Scholar] [CrossRef]
- Pavlenko, V.V.; Abbas, Q.; Przygocki, P.; Lesbayev, B.T.; Mansurov, Z.A. Temperature dependent characteristics of activated carbons from walnut shells for improved supercapacitor performance. Eurasian Chem. Technol. J. 2018, 20, 99–105. [Google Scholar] [CrossRef]
- Sultanov, F.; Bakbolat, B.; Mansurov, Z.; Pei, S.-S.; Ebrahim, R.; Daulbayev, C.; Urazgaliyeva, A.; Tulepov, M. Spongy Structures Coated with Carbon Nanomaterialsfor Efficient Oil/Water Separation. Eurasian Chem. Technol. J. 2017, 19, 127. [Google Scholar]
- Sultanov, F.; Bakbolat, B.; Daulbaev, C.; Urazgalieva, A.; Azizov, Z.; Mansurov, Z.; Tulepov, M.; Pei, S.S. Sorptive Activity and Hydrophobic Behavior of Aerogels Based on Reduced Graphene Oxide and Carbon nanotubes. J. Eng. Phys. Thermophys. 2017, 90, 826–830. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef]
- Temirgaliyeva, T.S.; Kuzuhara, S.; Noda, S.; Nazhipkyzy, M.; Kerimkulova, A.R.; Lesbayev, B.T.; Prikhodko, N.G.; Mansurov, Z.A. Self-Supporting Hybrid Supercapacitor Electrodes Based on Carbon Nanotube and Activated Carbons. Eurasian Chem. Technol. J. 2018, 20, 169–175. [Google Scholar] [CrossRef]
- Yuan, C.; Lin, H.; Lu, H.; Xing, E.; Zhang, Y.; Xie, B. Synthesis of hierarchically porous MnO2/rice husks derived carbon composite as high-performance electrode material for supercapacitors. Appl. Energy 2016, 178, 260–268. [Google Scholar] [CrossRef]
- He, X.; Ling, P.; Yu, M.; Wang, X.; Zhang, X.; Zheng, M. Rice husk-derived porous carbons with high capacitance by ZnCl2 activation for supercapacitors. Electrochim. Acta 2013, 105, 635–641. [Google Scholar] [CrossRef]
- Seitzhanova, M.A.; Chenchik, D.I.; Yeleuov, M.A.; Mansurov, Z.A.; Di Capua, R.; Elibaeva, N.S. Synthesis and characterization of graphene layers from rice husks. Chem. Bull. Kazakh Natl. Univ. 2018, 12–18. [Google Scholar] [CrossRef]
- Seitzhanova, M.A.; Mansurov, Z.A.; Yeleuov, M.; Roviello, V.; Di Capua, R. The Characteristics of Graphene Obtained from Rice Husk and Graphite. Eurasian Chem. Technol. J. 2019, 21, 149–156. [Google Scholar] [CrossRef]
- Muramatsu, H.; Kim, Y.A.; Yang, K.S.; Cruz-Silva, R.; Toda, I.; Yamada, T.; Terrones, M.; Endo, M.; Hayashi, T.; Saitoh, H. Rice husk-derived graphene with nano-sized domains and clean edges. Small 2014, 10, 2766–2770. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chan-Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 320–3213. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.C.; Zhou, J.; Guan, M.; Lin, M.C.; Pennycook, S.J. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Zhang, L.; Jiang, J.; Yan, J.; Huang, Y.; Lin, J.; Fan, H.J.; Shen, Z.X. A flexible alkaline rechargeable Ni/Fe battery based on graphene foam/carbon nanotubes hybrid film. Nano Lett. 2014, 14, 7180–7187. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, C.H.; Gong, H. A high energy density asymmetric supercapacitor from nano-architectured Ni(OH)2/carbon nanotube electrodes. Adv. Funct. Mater. 2012, 22, 1272–1278. [Google Scholar] [CrossRef]
- Yang, G.W.; Xu, C.L.; Li, H.L. Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance. Chem. Commun. 2008, 48, 6537–6539. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, C.; Gong, H. Capacitance decay of nanoporous nickel hydroxide. J. Power Sources 2010, 195, 6977–6981. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Yuen, M.F.; Zhang, J. Hierarchical Composite Electrodes of Nickel Oxide Nanoflake 3D Graphene for Hig-Performance Pseudocapacitors. Adv. Funct. Mater. 2014, 24, 6372–6380. [Google Scholar] [CrossRef]
- Cheah, W.K.; Ooi, C.H.; Yeoh, F.Y. Rice husk and rice husk ash reutilization into nanoporous materials for adsorptive biomedical applications: A review. Open Mater. Sci. 2016, 3, 27–38. [Google Scholar] [CrossRef]
- Prikhod’ko, N.A.; Mansurov, Z.A.; Auelkhankyzy, M.; Lesbaev, B.T.; Nazhipkyzy, M.; Smagulova, G.T. Flame Synthesis of Graphene Layers at Low Presssure. Russ. J. Phys. Chem. B 2015, 9, 743–747. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2017, 143, 47–57. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, X.; Li, J.; Dai, C.; Gamboa, S.; Sebastian, P.J. Nickel hydroxide/activated carbon composite electrodes for electrochemical capacitors. J. Power Sources 2007, 164, 425–429. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Khomenko, V.; Frackowiak, E.; Beguin, F. Determination of the specific capacitance of conducting polymer/nanotubes composite electrodes using different cell configurations. Electrochim. Acta 2005, 50, 2499–2506. [Google Scholar] [CrossRef]
- Dubal, D.P.; Kim, J.G.; Kim, Y.; Holze, R.; Lokhande Ch, D.; Kim, W.B. Supercapacitors Based on Flexible Substrates: An Overview. Energy Technol. 2014, 2, 325–341. [Google Scholar] [CrossRef]




| Scan Rate/mV s−1 | Ni(OH)2 Content/wt.% | ||||
|---|---|---|---|---|---|
| 0 | 4.5 | 9 | 13.5 | 18 | |
| 1 | 212 | n.d. | 258 | n.d. | n.d. |
| 5 | 202 | 213 | 236 | 182 | 172 |
| 10 | 187 | 206 | 230 | 177 | 163 |
| 20 | 178 | 200 | 225 | 173 | 153 |
| 40 | 176 | 194 | 218 | 168 | 142 |
| Current Density/mA g−1 | Specific Capacitance/F g−1 | |
|---|---|---|
| ARH | 9 wt.% Ni(OH)2 | |
| 50 | 236 | 300 |
| 100 | 232 | 290 |
| 250 | 209 | 277 |
| 500 | 203 | 267 |
| 1000 | 197 | 256 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeleuov, M.; Seidl, C.; Temirgaliyeva, T.; Taurbekov, A.; Prikhodko, N.; Lesbayev, B.; Sultanov, F.; Daulbayev, C.; Kumekov, S. Modified Activated Graphene-Based Carbon Electrodes from Rice Husk for Supercapacitor Applications. Energies 2020, 13, 4943. https://doi.org/10.3390/en13184943
Yeleuov M, Seidl C, Temirgaliyeva T, Taurbekov A, Prikhodko N, Lesbayev B, Sultanov F, Daulbayev C, Kumekov S. Modified Activated Graphene-Based Carbon Electrodes from Rice Husk for Supercapacitor Applications. Energies. 2020; 13(18):4943. https://doi.org/10.3390/en13184943
Chicago/Turabian StyleYeleuov, Mukhtar, Christopher Seidl, Tolganay Temirgaliyeva, Azamat Taurbekov, Nicholay Prikhodko, Bakytzhan Lesbayev, Fail Sultanov, Chingis Daulbayev, and Serik Kumekov. 2020. "Modified Activated Graphene-Based Carbon Electrodes from Rice Husk for Supercapacitor Applications" Energies 13, no. 18: 4943. https://doi.org/10.3390/en13184943
APA StyleYeleuov, M., Seidl, C., Temirgaliyeva, T., Taurbekov, A., Prikhodko, N., Lesbayev, B., Sultanov, F., Daulbayev, C., & Kumekov, S. (2020). Modified Activated Graphene-Based Carbon Electrodes from Rice Husk for Supercapacitor Applications. Energies, 13(18), 4943. https://doi.org/10.3390/en13184943





