Halide Pb-Free Double–Perovskites: Ternary vs. Quaternary Stoichiometry
Abstract
1. Introduction
2. Ternary Perovskites
2.1. Sn Halide Perovskites
2.2. Ge Halide Perovskites
2.3. Bi and Other Halide Perovskites
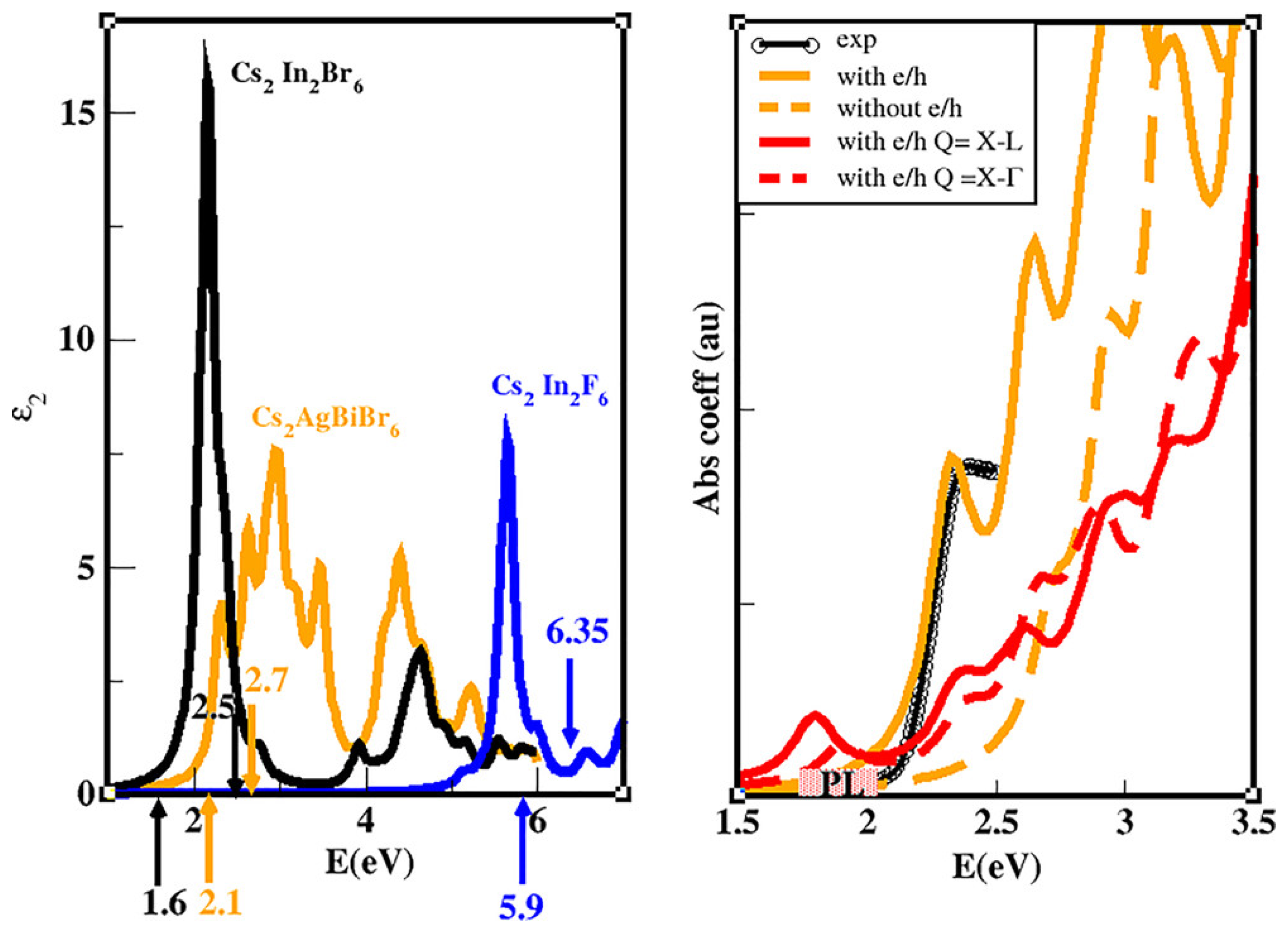
2.4. Ternary Halide Double-Perovskites
3. Quaternary Double-Perovskites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible–Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20190802.pdf (accessed on 10 June 2020).
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Grätzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A 2013, 1, 5628–5641. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.S.; Chang, J.A.; Lee, Y.H.; Kim, H.j.; Sarkar, A.; Nazeeruddin, M.K.; et al. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486. [Google Scholar] [CrossRef]
- Etgar, L.; Gao, P.; Xue, Z.; Peng, Q.; Chandiran, A.K.; Liu, B.; Nazeeruddin, M.K.; Grätzel, M. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J. Am. Chem. Soc. 2012, 134, 17396–17399. [Google Scholar] [CrossRef]
- Laban, W.A.; Etgar, L. Depleted hole conductor-free lead halide iodide heterojunction solar cells. Energy Environ. Sci. 2013, 6, 3249–3253. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef]
- Giorgi, G.; Fujisawa, J.I.; Segawa, H.; Yamashita, K. Small photocarrier effective masses featuring ambipolar transport in methylammonium lead iodide perovskite: A density functional analysis. J. Phys. Chem. Lett. 2013, 4, 4213–4216. [Google Scholar] [CrossRef]
- Giorgi, G.; Yamashita, K. Organic–inorganic halide perovskites: An ambipolar class of materials with enhanced photovoltaic performances. J. Mater. Chem. A 2015, 3, 8981–8991. [Google Scholar] [CrossRef]
- Kawai, H.; Giorgi, G.; Marini, A.; Yamashita, K. The mechanism of slow hot-hole cooling in lead-iodide perovskite: First-principles calculation on carrier lifetime from electron–phonon interaction. Nano Lett. 2015, 15, 3103–3108. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, E.; Amat, A.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. First-principles modeling of mixed halide organometal perovskites for photovoltaic applications. J. Phys. Chem. C 2013, 117, 13902–13913. [Google Scholar] [CrossRef]
- Even, J.; Pedesseau, L.; Jancu, J.M.; Katan, C. Importance of spin–orbit coupling in hybrid organic/inorganic perovskites for photovoltaic applications. J. Phys. Chem. Lett. 2013, 4, 2999–3005. [Google Scholar] [CrossRef]
- Snaith, H.J.; Abate, A.; Ball, J.M.; Eperon, G.E.; Leijtens, T.; Noel, N.K.; Stranks, S.D.; Wang, J.T.W.; Wojciechowski, K.; Zhang, W. Anomalous hysteresis in perovskite solar cells. J. Phys. Chem. Lett. 2014, 5, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, G.; Yamashita, K. Zero-dipole molecular organic cations in mixed organic–inorganic halide perovskites: Possible chemical solution for the reported anomalous hysteresis in the current–voltage curve measurements. Nanotechnology 2015, 26, 442001. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, G.; Fujisawa, J.I.; Segawa, H.; Yamashita, K. Organic–inorganic hybrid lead iodide perovskite featuring zero dipole moment guanidinium cations: A theoretical analysis. J. Phys. Chem. C 2015, 119, 4694–4701. [Google Scholar] [CrossRef]
- Stroppa, A.; Quarti, C.; De Angelis, F.; Picozzi, S. Ferroelectric polarization of CH3NH3PbI3: A detailed study based on density functional theory and symmetry mode analysis. J. Phys. Chem. Lett. 2015, 6, 2223–2231. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Eperon, G.E.; Leijtens, T.C.; McMeekin, D.; Saliba, M.; Zhang, W.; de Bastiani, M.; Petrozza, A.; Herz, L.M.; et al. Charge selective contacts, mobile ions and anomalous hysteresis in organic–inorganic perovskite solar cells. Mater. Horizons 2015, 2, 315–322. [Google Scholar] [CrossRef]
- Tress, W.; Marinova, N.; Moehl, T.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M. Understanding the rate-dependent J–V hysteresis, slow time component, and aging in CH3NH3PbI3 perovskite solar cells: The role of a compensated electric field. Energy Environ. Sci. 2015, 8, 995–1004. [Google Scholar] [CrossRef]
- Li, C.; Tscheuschner, S.; Paulus, F.; Hopkinson, P.E.; Kießling, J.; Köhler, A.; Vaynzof, Y.; Huettner, S. Iodine migration and its effect on hysteresis in perovskite solar cells. Adv. Mater. 2016, 28, 2446–2454. [Google Scholar] [CrossRef] [PubMed]
- Calado, P.; Telford, A.M.; Bryant, D.; Li, X.; Nelson, J.; O’Regan, B.C.; Barnes, P.R. Evidence for ion migration in hybrid perovskite solar cells with minimal hysteresis. Nat. Commun. 2016, 7, 13831. [Google Scholar] [CrossRef] [PubMed]
- Kieslich, G.; Sun, S.; Cheetham, A.K. Solid-state principles applied to organic–inorganic perovskites: New tricks for an old dog. Chem. Sci. 2014, 5, 4712–4715. [Google Scholar] [CrossRef]
- De Marco, N.; Zhou, H.; Chen, Q.; Sun, P.; Liu, Z.; Meng, L.; Yao, E.P.; Liu, Y.; Schiffer, A.; Yang, Y. Guanidinium: A route to enhanced carrier lifetime and open-circuit voltage in hybrid perovskite solar cells. Nano Lett. 2016, 16, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.D.; Zhang, C.; Tiong, V.T.; Zhang, S.; Will, G.; Bou, A.; Bisquert, J.; Shaw, P.E.; Du, A.; Wilson, G.J.; et al. Tailoring crystal structure of FA0.83Cs0.17PbI3 perovskite Through guanidinium doping for Enhanced performance and Tunable hysteresis of planar perovskite solar cells. Adv. Funct. Mater. 2019, 29, 1806479. [Google Scholar] [CrossRef]
- Cheng, N.; Li, W.; Zhang, M.; Wu, H.; Sun, S.; Zhao, Z.; Xiao, Z.; Sun, Z.; Zi, W.; Fang, L. Enhance the performance and stability of methylammonium lead iodide perovskite solar cells with guanidinium thiocyanate additive. Curr. Appl. Phys. 2019, 19, 25–30. [Google Scholar] [CrossRef]
- Gong, J.; Guo, P.; Benjamin, S.E.; Van Patten, P.G.; Schaller, R.D.; Xu, T. Cation engineering on lead iodide perovskites for stable and high-performance photovoltaic applications. J. Energy Chem. 2018, 27, 1017–1039. [Google Scholar] [CrossRef]
- Jodlowski, A.D.; Roldán-Carmona, C.; Grancini, G.; Salado, M.; Ralaiarisoa, M.; Ahmad, S.; Koch, N.; Camacho, L.; De Miguel, G.; Nazeeruddin, M.K. Large guanidinium cation mixed with methylammonium in lead iodide perovskites for 19% efficient solar cells. Nat. Energy 2017, 2, 972–979. [Google Scholar] [CrossRef]
- Kubicki, D.J.; Prochowicz, D.; Hofstetter, A.; Saski, M.; Yadav, P.; Bi, D.; Pellet, N.; Lewinski, J.; Zakeeruddin, S.M.; Grätzel, M.; et al. Formation of Stable mixed guanidinium–methylammonium phases with exceptionally long carrier lifetimes for high-efficiency lead iodide-based perovskite photovoltaics. J. Am. Chem. Soc. 2018, 140, 3345–3351. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.B. Degradation observations of encapsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Mosconi, E.; Azpiroz, J.M.; De Angelis, F. Ab initio molecular dynamics simulations of methylammonium lead iodide perovskite degradation by water. Chem. Mater. 2015, 27, 4885–4892. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden–Popper hybrid lead iodide perovskite 2D homologous semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-efficiency two-dimensional Ruddlesden–Popper perovskite solar cells. Nature 2016, 536, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ke, W.; Pedesseau, L.; Wu, Y.; Katan, C.; Even, J.; Wasielewski, M.R.; Stoumpos, C.C.; Kanatzidis, M.G. Hybrid Dion–Jacobson 2D lead iodide perovskites. J. Am. Chem. Soc. 2018, 140, 3775–3783. [Google Scholar] [CrossRef] [PubMed]
- Mitzi, D.B. Synthesis, structure, and properties of organic-inorganic perovskites and related materials. Prog. Inorg. Chem. 1999, 48, 1–121. [Google Scholar]
- Mitzi, D.B.; Feild, C.; Harrison, W.; Guloy, A. Conducting tin halides with a layered organic-based perovskite structure. Nature 1994, 369, 467–469. [Google Scholar] [CrossRef]
- Mitzi, D.B. Synthesis, crystal structure, and optical and thermal properties of (C4H9NH3) 2MI4 (M= Ge, Sn, Pb). Chem. Mater. 1996, 8, 791–800. [Google Scholar] [CrossRef]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A layered hybrid perovskite solar-cell absorber with enhanced moisture stability. Angew. Chem. Int. Ed. 2014, 53, 11232–11235. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.H.; Stoumpos, C.C.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. 2D homologous perovskites as light-absorbing materials for solar cell applications. J. Am. Chem. Soc. 2015, 137, 7843–7850. [Google Scholar] [CrossRef]
- Giorgi, G.; Yamashita, K.; Palummo, M. Nature of the Electronic and Optical Excitations of Ruddlesden–Popper Hybrid Organic–Inorganic Perovskites: The Role of the Many-Body Interactions. J. Phys. Chem. Lett. 2018, 9, 5891–5896. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Frazer, L.; Clark, D.J.; Kim, Y.S.; Rhim, S.H.; Freeman, A.J.; Ketterson, J.B.; Jang, J.I.; Kanatzidis, M.G. Hybrid germanium iodide perovskite semiconductors: Active lone pairs, structural distortions, direct and indirect energy gaps, and strong nonlinear optical properties. J. Am. Chem. Soc. 2015, 137, 6804–6819. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.P.; Li, Q.S.; Yang, L.N.; Li, Z.S. Theoretical insights into a potential lead-free hybrid perovskite: Substituting Pb2+ with Ge2+. Nanoscale 2016, 8, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Stoumpos, C.C.; Guo, P.; Zhou, N.; Marks, T.J.; Chang, R.P.; Kanatzidis, M.G. Solvent-mediated crystallization of CH3NH3SnI3 films for heterojunction depleted perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 11445–11452. [Google Scholar] [CrossRef] [PubMed]
- Ogomi, Y.; Morita, A.; Tsukamoto, S.; Saitho, T.; Fujikawa, N.; Shen, Q.; Toyoda, T.; Yoshino, K.; Pandey, S.S.; Ma, T.; et al. CH3NH3SnxPb(1−x)I3 Perovskite solar cells covering up to 1060 nm. J. Phys. Chem. Lett. 2014, 5, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, G.; Yamashita, K. Alternative, lead-free, hybrid organic–inorganic perovskites for solar applications: A DFT analysis. Chem. Lett. 2015, 44, 826–828. [Google Scholar] [CrossRef]
- Weber, D. CH3NH3PbX3, ein Pb (II)-system mit kubischer perowskitstruktur/ CH3NH3PbX3, a Pb (II)-system with cubic perovskite structure. Z. Für Naturforschung B 1978, 33, 1443–1445. [Google Scholar] [CrossRef]
- Weber, D. CH3NH3SnBrxI3−x (x = 0–3), ein Sn (II)-System mit kubischer Perowskitstruktur/ CH3NH3SnBrxI3−x (x = 0–3), a Sn (II)-system with cubic perovskite structure. Z. Für Naturforschung B 1978, 33, 862–865. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.; Walsh, A.; Wei, S.H. Crystal and electronic band structure of Cu2ZnSnX4 (X = S and Se) photovoltaic absorbers: First-principles insights. Appl. Phys. Lett. 2009, 94, 041903. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.; Walsh, A.; Wei, S.H. Electronic structure and stability of quaternary chalcogenide semiconductors derived from cation cross-substitution of II-VI and I-III-VI2 compounds. Phys. Rev. B 2009, 79, 165211. [Google Scholar] [CrossRef]
- Zhao, X.G.; Yang, J.H.; Fu, Y.; Yang, D.; Xu, Q.; Yu, L.; Wei, S.H.; Zhang, L. Design of lead-free inorganic halide perovskites for solar cells via cation-transmutation. J. Am. Chem. Soc. 2017, 139, 2630–2638. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, G.; Yamashita, K.; Palummo, M. Two-dimensional optical excitations in the mixed-valence Cs2Au2I6 fully inorganic double perovskite. J. Mater. Chem. C 2018, 6, 10197–10201. [Google Scholar] [CrossRef]
- Palummo, M.; Berrios, E.; Varsano, D.; Giorgi, G. Optical Excitations of Lead-free Double Perovskites by Ab-initio Excited-State Methods. ACS Energy Lett. 2020, 5, 457–463. [Google Scholar] [CrossRef]
- Kieslich, G.; Sun, S.; Cheetham, A.K. An extended tolerance factor approach for organic–inorganic perovskites. Chem. Sci. 2015, 6, 3430–3433. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, V.M. Die gesetze der krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Kubo, A.; Giorgi, G.; Yamashita, K. MgTaO2N photocatalysts: Perovskite versus ilmenite structure. A theoretical investigation. J. Phys. Chem. C 2017, 121, 27813–27821. [Google Scholar] [CrossRef]
- Huang, L.Y.; Lambrecht, W.R. Electronic band structure, phonons, and exciton binding energies of halide perovskites CsSnCl3, CsSnBr3, and CsSnI3. Phys. Rev. B 2013, 88, 165203. [Google Scholar] [CrossRef]
- Huang, L.Y.; Lambrecht, W.R. Vibrational spectra and nonlinear optical coefficients of rhombohedral CsGeX3 halide compounds with X= I, Br, Cl. Phys. Rev. B 2016, 94, 115202. [Google Scholar] [CrossRef]
- Chung, I.; Song, J.H.; Im, J.; Androulakis, J.; Malliakas, C.D.; Li, H.; Freeman, A.J.; Kenney, J.T.; Kanatzidis, M.G. CsSnI3: Semiconductor or metal? High electrical conductivity and strong near-infrared photoluminescence from a single material. High hole mobility and phase-transitions. J. Am. Chem. Soc. 2012, 134, 8579–8587. [Google Scholar] [CrossRef]
- Lee, B.; He, J.; Chang, R.P.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar]
- Qiu, X.; Cao, B.; Yuan, S.; Chen, X.; Qiu, Z.; Jiang, Y.; Ye, Q.; Wang, H.; Zeng, H.; Liu, J.; et al. From unstable CsSnI3 to air-stable Cs2SnI6: A lead-free perovskite solar cell light absorber with bandgap of 1.48 eV and high absorption coefficient. Sol. Energy Mater. Sol. Cells 2017, 159, 227–234. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, S.; Zeng, H.; Song, J. A comprehensive review of doping in perovskite nanocrystals/quantum dots: Evolution of structure, electronics, optics and light-emitting diodes. Mater. Today Nano 2019, 6, 100036. [Google Scholar] [CrossRef]
- Liang, J.; Chen, D.; Yao, X.; Zhang, K.; Qu, F.; Qin, L.; Huang, Y.; Li, J. Recent Progress and Development in Inorganic Halide Perovskite Quantum Dots for Photoelectrochemical Applications. Small 2020, 16, 1903398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tu, J.; Li, T.; Tao, C.; Deng, X.; Li, Z. Convenient preparation of CsSnI3 quantum dots, excellent stability, and the highest performance of lead-free inorganic perovskite solar cells so far. J. Mater. Chem. A 2019, 7, 7683–7690. [Google Scholar] [CrossRef]
- Even, J.; Pedesseau, L.; Jancu, J.M.; Katan, C. DFT and k· p modelling of the phase transitions of lead and tin halide perovskites for photovoltaic cells. Phys. Status Solidi (RRL) Res. Lett. 2014, 8, 31–35. [Google Scholar] [CrossRef]
- Mori, M.; Saito, H. An X-ray study of successive phase transitions in CsSnBr3. J. Phys. C: Solid State Phys. 1986, 19, 2391–2401. [Google Scholar] [CrossRef]
- Kuok, M.H.; Saw, E.L.; Yap, C.T. A Mössbauer Study of the Phase Transitions in CsSnBr3. Phys. Status Solidi (a) 1992, 132, K89–K92. [Google Scholar] [CrossRef]
- Fabini, D.H.; Laurita, G.; Bechtel, J.S.; Stoumpos, C.C.; Evans, H.A.; Kontos, A.G.; Raptis, Y.S.; Falaras, P.; Van der Ven, A.; Kanatzidis, M.G.; et al. Dynamic Stereochemical Activity of the Sn2+ Lone Pair in Perovskite CsSnBr3. J. Am. Chem. Soc. 2016, 138, 11820–11832. [Google Scholar] [CrossRef]
- Sabba, D.; Mulmudi, H.K.; Prabhakar, R.R.; Krishnamoorthy, T.; Baikie, T.; Boix, P.P.; Mhaisalkar, S.; Mathews, N. Impact of Anionic Br– Substitution on Open Circuit Voltage in Lead Free Perovskite (CsSnI3−xBrx) Solar Cells. J. Phys. Chem. C 2015, 119, 1763–1767. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Ding, H.; Yan, C.; Leong, W.L.; Baikie, T.; Zhang, Z.; Sherburne, M.; Li, S.; Asta, M.; Mathews, N.; et al. Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A 2015, 3, 23829–23832. [Google Scholar] [CrossRef]
- Ming, W.; Shi, H.; Du, M.H. Large dielectric constant, high acceptor density, and deep electron traps in perovskite solar cell material CsGeI3. J. Mater. Chem. A 2016, 4, 13852–13858. [Google Scholar] [CrossRef]
- Roknuzzaman, M.; Ostrikov, K.K.; Wang, H.; Du, A.; Tesfamichael, T. Towards lead-free perovskite photovoltaics and optoelectronics by ab-initio simulations. Sci. Rep. 2017, 7, 14025. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Sawada, K. Discovery of Pb-free perovskite solar cells via high-throughput simulation on the K computer. J. Phys. Chem. Lett. 2017, 8, 4826–4831. [Google Scholar] [CrossRef] [PubMed]
- Moussa, J.E.; Schultz, P.A.; Chelikowsky, J.R. Analysis of the Heyd-Scuseria-Ernzerhof density functional parameter space. J. Chem. Phys. 2012, 136, 204117. [Google Scholar] [CrossRef] [PubMed]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Physics Diffraction Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ge, S.; Guan, X.; Wang, Y.; Lin, C.H.; Cui, Y.; Huang, Y.; Zhang, X.; Zhang, R.; Yang, X.; Wu, T. Low-Dimensional Lead-Free Inorganic Perovskites for Resistive Switching with Ultralow Bias. Adv. Funct. Mater. 2020, 30, 2002110. [Google Scholar] [CrossRef]
- Johansson, M.B.; Zhu, H.; Johansson, E.M. Extended photo-conversion spectrum in low-toxic bismuth halide perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3467–3471. [Google Scholar] [CrossRef]
- Park, B.W.; Philippe, B.; Zhang, X.; Rensmo, H.; Boschloo, G.; Johansson, E.M. Bismuth based hybrid perovskites A3Bi2I9 (A: Methylammonium or cesium) for solar cell application. Adv. Mater. 2015, 27, 6806–6813. [Google Scholar] [CrossRef]
- Arakcheeva, A.; Bonin, M.; Chapuis, G.; Zaitsev, A. The phases of Cs3Bi2I9 between RT and 190 K. Z. Fur Kristallographie. 1999, 214, 279–283. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Hong, F.; Mao, X.; Zheng, K.; Yang, S.; Li, Y.; Pullerits, T.; Deng, W.; Han, K. Lead-free, air-stable all-inorganic cesium bismuth halide perovskite nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 12471–12475. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, C.; Lian, L.; Guo, J.; Xia, Y.; Pan, F.; Su, X.; Zhang, J.; Li, H.; Zhang, D. Controlled synthesis and photostability of blue emitting Cs3Bi2Br9 perovskite nanocrystals by employing weak polar solvents at room temperature. J. Mater. Chem. C 2019, 7, 3688–3695. [Google Scholar] [CrossRef]
- Lou, Y.; Fang, M.; Chen, J.; Zhao, Y. Formation of highly luminescent cesium bismuth halide perovskite quantum dots tuned by anion exchange. Chem. Commun. 2018, 54, 3779–3782. [Google Scholar] [CrossRef] [PubMed]
- Maughan, A.E.; Ganose, A.M.; Bordelon, M.M.; Miller, E.M.; Scanlon, D.O.; Neilson, J.R. Defect tolerance to intolerance in the vacancy-ordered double perovskite semiconductors Cs2SnI6 and Cs2TeI6. J. Am. Chem. Soc. 2016, 138, 8453–8464. [Google Scholar] [CrossRef]
- Ju, M.G.; Chen, M.; Zhou, Y.; Garces, H.F.; Dai, J.; Ma, L.; Padture, N.P.; Zeng, X.C. Earth-abundant nontoxic titanium (IV)-based vacancy-ordered double perovskite halides with tunable 1.0 to 1.8 eV bandgaps for photovoltaic applications. ACS Energy Lett. 2018, 3, 297–304. [Google Scholar] [CrossRef]
- Sakai, N.; Haghighirad, A.A.; Filip, M.R.; Nayak, P.K.; Nayak, S.; Ramadan, A.; Wang, Z.; Giustino, F.; Snaith, H.J. Solution-processed cesium hexabromopalladate (IV), Cs2PdBr6, for optoelectronic applications. J. Am. Chem. Soc. 2017, 139, 6030–6033. [Google Scholar] [CrossRef]
- Ray, D.; Clark, C.; Pham, H.Q.; Borycz, J.; Holmes, R.J.; Aydil, E.S.; Gagliardi, L. Computational study of structural and electronic properties of lead-free CsMI3 Perovskites (M= Ge, Sn, Pb, Mg, Ca, Sr, and Ba). J. Phys. Chem. C 2018, 122, 7838–7848. [Google Scholar] [CrossRef]
- Yang, K.; Zhuravleva, M.; Melcher, C.L. Crystal growth and characterization of CsSr1−xEuxI3 high light yield scintillators. Phys. Status Solidi (RRL) Res. Lett. 2011, 5, 43–45. [Google Scholar] [CrossRef]
- Suta, M.; Wickleder, C. Photoluminescence of CsMI3: Eu2+ (M = Mg, Ca, and Sr)–a spectroscopic probe on structural distortions. J. Mater. Chem. C 2015, 3, 5233–5245. [Google Scholar] [CrossRef]
- Suta, M.; Lavoie-Cardinal, F.; Olchowka, J.; Wickleder, C. Nature of Localized Excitons in CsMgX3 (X = Cl, Br, I) and Their Interactions with Eu2+ Ions. Phys. Rev. Appl. 2018, 9, 064024. [Google Scholar] [CrossRef]
- Suta, M.; Urland, W.; Daul, C.; Wickleder, C. Photoluminescence properties of Yb2+ ions doped in the perovskites CsCaX3 and CsSrX3 (X = Cl, Br, and I)–a comparative study. Phys. Chem. Chem. Phys. 2016, 18, 13196–13208. [Google Scholar] [CrossRef] [PubMed]
- Retuerto, M.; Emge, T.; Hadermann, J.; Stephens, P.; Li, M.; Yin, Z.; Croft, M.; Ignatov, A.; Zhang, S.; Yuan, Z.; et al. Synthesis and properties of charge-ordered thallium halide perovskites, CsTl+0.5 Tl3+0.5X3 (X = F or Cl): Theoretical precursors for superconductivity? Chem. Mater. 2013, 25, 4071–4079. [Google Scholar] [CrossRef]
- Brauer, G.; Sleater, G. Preparation of mixed valent aurate halides. J. Less Common Met. 1970, 21, 283–291. [Google Scholar] [CrossRef]
- Cava, R.J.; Batlogg, B.; Krajewski, J.; Farrow, R.; Rupp, L.; White, A.; Short, K.; Peck, W.; Kometani, T. Superconductivity near 30 K without copper: The Ba0.6K0.4BiO3 perovskite. Nature 1988, 332, 814–816. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Müller, K.A. Possible highT c superconductivity in the Ba- La- Cu- O system. Z. Für Phys. B Condens. Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Kitagawa, H.; Sato, H.; Kojima, N.; Kikegawa, T.; Shimomura, O. Metallization and phase transitions of the three-dimensional halogen-bridge mixed-valence complex Cs2Au2I6 under high pressure. Solid State Commun. 1991, 78, 989–995. [Google Scholar] [CrossRef]
- Kojima, N.; Kitagawa, H.; Ban, T.; Amita, F.; Nakahara, M. Semiconductor-to-metal and metal-to-metal transitions in the three-dimensional mixed-valence compound Cs2Au2I6 under high pressures. Solid State Commun. 1990, 73, 743–745. [Google Scholar] [CrossRef]
- Matsushita, N.; Kitagawa, H.; Kojima, N. A three-dimensional iodo-bridged mixed-valence gold (I, III) compound, Cs2Au(I)Au(III)I6. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 663–666. [Google Scholar] [CrossRef]
- Debbichi, L.; Lee, S.; Cho, H.; Rappe, A.M.; Hong, K.H.; Jang, M.S.; Kim, H. Mixed Valence Perovskite Cs2Au2I6: A Potential Material for Thin-Film Pb-Free Photovoltaic Cells with Ultrahigh Efficiency. Adv. Mater. 2018, 30, 1707001. [Google Scholar] [CrossRef]
- Liu, X.; Matsuda, K.; Moritomo, Y.; Nakamura, A.; Kojima, N. Electronic structure of the gold complexes Cs2Au2X6 (X= I, Br, and Cl). Phys. Rev. B 1999, 59, 7925. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Nardelli, M.B.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Marini, A.; Hogan, C.; Grüning, M.; Varsano, D. Yambo: An ab initio tool for excited state calculations. Comput. Phys. Commun. 2009, 180, 1392–1403. [Google Scholar] [CrossRef]
- Sangalli, D.; Ferretti, A.; Miranda, H.; Attaccalite, C.; Marri, I.; Cannuccia, E.; Melo, P.; Marsili, M.; Paleari, F.; Marrazzo, A.; et al. Many-body perturbation theory calculations using the yambo code. J. Phys. Condens. Matter 2019, 31, 325902. [Google Scholar] [CrossRef]
- Umari, P.; Mosconi, E.; De Angelis, F. Relativistic GW calculations on CH3NH3PbI3 and CH3NH3SnI3 perovskites for solar cell applications. Sci. Rep. 2014, 4, 4467. [Google Scholar] [CrossRef]
- Wierzbowska, M.; Meléndez, J.J.; Varsano, D. Breathing bands due to molecular order in CH3NH3PbI3. Comput. Mater. Sci. 2018, 142, 361–371. [Google Scholar] [CrossRef]
- Baldini, E.; Chiodo, L.; Dominguez, A.; Palummo, M.; Moser, S.; Yazdi-Rizi, M.; Auböck, G.; Mallett, B.P.; Berger, H.; Magrez, A.; et al. Strongly bound excitons in anatase TiO2 single crystals and nanoparticles. Nat. Commun. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Varsano, D.; Giorgi, G.; Yamashita, K.; Palummo, M. Role of Quantum-confinement in Anatase nanosheets. J. Phys. Chem. Lett. 2017, 8, 3867–3873. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.; Bird, S.; Donaldson, J.; Silver, J. The Mössbauer effect in tin (II) compounds. Part XI. The spectra of cubic trihalogenostannates (II). J. Chem. Soc. Inorganic Phys. Theor. 1971, 3105–3108. [Google Scholar] [CrossRef]
- Moghe, D.; Wang, L.; Traverse, C.J.; Redoute, A.; Sponseller, M.; Brown, P.R.; Bulović, V.; Lunt, R.R. All vapor-deposited lead-free doped CsSnBr3 planar solar cells. Nano Energy 2016, 28, 469–474. [Google Scholar] [CrossRef]
- Bose, S.; Satpathy, S.; Jepsen, O. Semiconducting CsSnBr 3. Phys. Rev. B 1993, 47, 4276. [Google Scholar] [CrossRef]
- Gupta, S.; Bendikov, T.; Hodes, G.; Cahen, D. CsSnBr3, a lead-free halide perovskite for long-term solar cell application: Insights on SnF2 addition. ACS Energy Lett. 2016, 1, 1028–1033. [Google Scholar] [CrossRef]
- Yamada, K.; Funabiki, S.; Horimoto, H.; Matsui, T.; Okuda, T.; Ichiba, S. Structural phase transitions of the polymorphs of CsSnI3 by means of rietveld analysis of the X-ray diffraction. Chem. Lett. 1991, 20, 801–804. [Google Scholar] [CrossRef]
- Borriello, I.; Cantele, G.; Ninno, D. Ab initio investigation of hybrid organic-inorganic perovskites based on tin halides. Phys. Rev. B 2008, 77, 235214. [Google Scholar] [CrossRef]
- Thiele, G.; Rotter, H.W.; Schmidt, K.D. Kristallstrukturen und phasentransformationen von caesiumtrihalogenogermanaten (II) CsGeX3 (X = Cl, Br, I). Z. Für Anorg. Und Allg. Chem. 1987, 545, 148–156. [Google Scholar] [CrossRef]
- Chan, M.; Ceder, G. Efficient band gap prediction for solids. Phys. Rev. Lett. 2010, 105, 196403. [Google Scholar] [CrossRef]
- Cai, Y.; Xie, W.; Teng, Y.T.; Harikesh, P.; Ghosh, B.; Huck, P.; Persson, K.A.; Mathews, N.; Mhaisalkar, S.G.; Sherburne, M.; et al. High-throughput Computational Study of Halide Double Perovskite Inorganic Compounds. Chem. Mater. 2019, 31, 5392–5401. [Google Scholar] [CrossRef]
- Kojima, N. Complexes, M2[AuIX2][AuIIIX4](M= Rb, Cs; X = Cl, Br, and I). Gold Valence Transition and Phase Diagram in the Mixed-Valence Bull. Chem. Soc. Jpn. 2000, 73, 1445–1460. [Google Scholar]
- Kangsabanik, J.; Ghorui, S.; Aslam, M.; Alam, A. Optoelectronic Properties and Defect Physics of Lead-free Photovoltaic Absorbers Cs2Au I Au III X6 (X= I, Br). Phys. Rev. Appl. 2020, 13, 014005. [Google Scholar] [CrossRef]
- Fukuhara, F.; Kojima, N.; Matsushita, N. A three-dimensional bromo-bridged mixed-valence gold (I, III) compound, Cs2AuIAuIIIBr6. Acta Crystallogr. Sect. E 2005, 61, i123–i125. [Google Scholar]
- Mortensen, J.J.; Hansen, L.B.; Jacobsen, K.W. Real-space grid implementation of the projector augmented wave method. Phys. Rev. B 2005, 71, 035109. [Google Scholar] [CrossRef]
- Castelli, I.E.; Hüser, F.; Pandey, M.; Li, H.; Thygesen, K.S.; Seger, B.; Jain, A.; Persson, K.A.; Ceder, G.; Jacobsen, K.W. New light-harvesting materials using accurate and efficient bandgap calculations. Adv. Energy Mater. 2015, 5, 1400915. [Google Scholar] [CrossRef]
- Nicholson, K.M.; Kang, S.G.; Sholl, D.S. First principles methods for elpasolite halide crystal structure prediction at finite temperatures. J. Alloys Compd. 2013, 577, 463–468. [Google Scholar] [CrossRef]
- Yang, P.; Doty, F.P.; Rodriguez, M.A.; Sanchez, M.R.; Zhou, X.; Shah, K.S. The synthesis and structures of elpasolite halide scintillators. MRS Online Proc. Libr. Arch. 2009, 1164. [Google Scholar] [CrossRef]
- van Eijk, C.W. Inorganic scintillators for thermal neutron detection. IEEE Trans. Nucl. Sci. 2012, 59, 2242–2247. [Google Scholar] [CrossRef]
- Saeed, Y.; Amin, B.; Khalil, H.; Rehman, F.; Ali, H.; Khan, M.I.; Mahmood, A.; Shafiq, M. Cs2NaGaBr6: A new lead-free and direct band gap halide double perovskite. RSC Adv. 2020, 10, 17444–17451. [Google Scholar] [CrossRef]
- Volonakis, G.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Snaith, H.J.; Giustino, F. Lead-free halide double perovskites via heterovalent substitution of noble metals. J. Phys. Chem. Lett. 2016, 7, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Volonakis, G.; Haghighirad, A.A.; Milot, R.L.; Sio, W.H.; Filip, M.R.; Wenger, B.; Johnston, M.B.; Herz, L.M.; Snaith, H.J.; Giustino, F. Cs2InAgCl6: A new lead-free halide double perovskite with direct band gap. J. Phys. Chem. Lett. 2017, 8, 772–778. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, Z.; Molokeev, M.S.; Zhang, X.; Peng, D.; Liu, Q. Composition design, optical gap and stability investigations of lead-free halide double perovskite Cs2AgInCl6. J. Mater. Chem. A 2017, 5, 15031–15037. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Li, S.; Liu, J.; Guo, Y.; Niu, G.; Yao, L.; Fu, Y.; Gao, L.; Dong, Q.; et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature 2018, 563, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Deng, Z.Y.; He, J.; Wang, M.; Chen, Z.X.; Wei, S.H.; Feng, H.J. Synthesis of Cs2AgSbCl6 and improved optoelectronic properties of Cs2AgSbCl6/TiO2 heterostructure driven by the interface effect for lead-free double perovskites solar cells. Appl. Phys. Lett. 2017, 111, 151602. [Google Scholar] [CrossRef]
- Wei, F.; Deng, Z.; Sun, S.; Hartono, N.T.P.; Seng, H.L.; Buonassisi, T.; Bristowe, P.D.; Cheetham, A.K. Enhanced visible light absorption for lead-free double perovskite Cs2AgSbBr6. Chem. Commun. 2019, 55, 3721–3724. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Hoye, R.L.; Eyre, L.; Wei, F.; Brivio, F.; Sadhanala, A.; Sun, S.; Li, W.; Zhang, K.H.; MacManus-Driscoll, J.L.; Bristowe, P.D.; et al. Fundamental carrier lifetime exceeding 1 μs in Cs2AgBiBr6 double perovskite. Adv. Mater. Interfaces 2018, 5, 1800464. [Google Scholar] [CrossRef]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6 (X = Br, Cl): New Visible Light Absorbing, Lead-Free Halide Perovskite Semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Sui, L.; Yan, J.; Wang, K.; Yuan, K.; Mao, W.L.; Zou, B. Tuning Emission and Electron–Phonon Coupling in Lead-Free Halide Double Perovskite Cs2AgBiCl6 under Pressure. ACS Energy Lett. 2019, 4, 2975–2982. [Google Scholar] [CrossRef]
- Savory, C.N.; Walsh, A.; Scanlon, D.O. Can Pb-free halide double perovskites support high-efficiency solar cells? ACS Energy Lett. 2016, 1, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.R.; Hillman, S.; Haghighirad, A.A.; Snaith, H.J.; Giustino, F. Band gaps of the lead-free halide double perovskites Cs2BiAgCl6 and Cs2BiAgBr6 from theory and experiment. J. Phys. Chem. Lett. 2016, 7, 2579–2585. [Google Scholar] [CrossRef]
- Creutz, S.E.; Crites, E.N.; De Siena, M.C.; Gamelin, D.R. Colloidal nanocrystals of lead-free double-perovskite (elpasolite) semiconductors: Synthesis and anion exchange to access new materials. Nano Lett. 2018, 18, 1118–1123. [Google Scholar] [CrossRef]
- Leppert, L.; Rangel, T.; Neaton, J.B. Towards predictive band gaps for halide perovskites: Lessons from one-shot and eigenvalue self-consistent G W. Phys. Rev. Mater. 2019, 3, 103803. [Google Scholar] [CrossRef]
- Islam, M.T.; Jani, M.R.; Al Amin, S.M.; Sami, M.S.U.; Shorowordi, K.M.; Hossain, M.I.; Devgun, M.; Chowdhury, S.; Banerje, S.; Ahmed, S. Numerical simulation studies of a fully inorganic Cs2AgBiBr6 perovskite solar device. Opt. Mater. 2020, 105, 109957. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Q.; Liu, Y.; Luo, W.; Guo, X.; Huang, Z.; Ting, H.; Sun, W.; Zhong, X.; Wei, S.; et al. The Dawn of Lead-Free Perovskite Solar Cell: Highly Stable Double Perovskite Cs2BiAgBr6 Film. Adv. Sci. 2018, 5, 1700759. [Google Scholar] [CrossRef]
- Gao, W.; Ran, C.; Xi, J.; Jiao, B.; Zhang, W.; Wu, M.; Hou, X.; Wu, Z. High-Quality Cs2AgBiBr6 Double Perovskite Film for Lead-Free Inverted Planar Heterojunction Solar Cells with 2.2% Efficiency. ChemPhysChem 2018, 19, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Greul, E.; Petrus, M.L.; Binek, A.; Docampo, P.; Bein, T. Highly stable, phase pure Cs2AgBiBr6 double perovskite thin films for optoelectronic applications. J. Mater. Chem. A 2017, 5, 19972–19981. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Liu, P.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Simultaneous Power Conversion Efficiency and Stability Enhancement of Cs2AgBiBr6 Lead-Free Inorganic Perovskite Solar Cell through Adopting a Multifunctional Dye Interlayer. Adv. Funct. Mater. 2020, 30, 2001557. [Google Scholar] [CrossRef]
- Xiao, Z.; Du, K.Z.; Meng, W.; Wang, J.; Mitzi, D.B.; Yan, Y. Intrinsic instability of Cs2In (I) M (III) X6 (M= Bi, Sb; X= halogen) double perovskites: A combined density functional theory and experimental study. J. Am. Chem. Soc. 2017, 139, 6054–6057. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Tran, T.T.; Panella, J.R.; Chamorro, J.R.; Morey, J.R.; McQueen, T.M. Designing indirect–direct bandgap transitions in double perovskites. Mater. Horizons 2017, 4, 688–693. [Google Scholar] [CrossRef]
- Bartesaghi, D.; Slavney, A.H.; Gelvez-Rueda, M.C.; Connor, B.A.; Grozema, F.C.; Karunadasa, H.I.; Savenije, T.J. Charge carrier dynamics in Cs2AgBiBr6 double perovskite. J. Phys. Chem. C 2018, 122, 4809–4816. [Google Scholar] [CrossRef] [PubMed]
- Slavney, A.H.; Leppert, L.; Bartesaghi, D.; Gold-Parker, A.; Toney, M.F.; Savenije, T.J.; Neaton, J.B.; Karunadasa, H.I. Defect-induced band-edge reconstruction of a bismuth-halide double perovskite for visible-light absorption. J. Am. Chem. Soc. 2017, 139, 5015–5018. [Google Scholar] [CrossRef]
- Du, K.z.; Meng, W.; Wang, X.; Yan, Y.; Mitzi, D.B. Bandgap Engineering of Lead-Free Double Perovskite Cs 2 AgBiBr 6 through Trivalent Metal Alloying. Angew. Chem. Int. Ed. 2017, 56, 8158–8162. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Pan, W.; Yang, W.; Zou, B.; Tang, J.; Quan, Z. High-Pressure Band-Gap Engineering in Lead-Free Cs2AgBiBr6 Double Perovskite. Angew. Chem. Int. Ed. 2017, 56, 15969–15973. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, J.; Yang, S.; Hong, F.; Sun, L.; Han, P.; Pullerits, T.; Deng, W.; Han, K. Lead-Free Silver-Bismuth Halide Double Perovskite Nanocrystals. Angew. Chem. 2018, 130, 5457–5461. [Google Scholar] [CrossRef]
- Bekenstein, Y.; Dahl, J.C.; Huang, J.; Osowiecki, W.T.; Swabeck, J.K.; Chan, E.M.; Yang, P.; Alivisatos, A.P. The making and breaking of lead-free double perovskite nanocrystals of cesium silver–bismuth halide compositions. Nano Lett. 2018, 18, 3502–3508. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Lin, C.C.; Xie, X.; Meijerink, A. Efficient and stable luminescence from Mn2+ in core and core–isocrystalline shell CsPbCl3 perovskite nanocrystals. Chem. Mater. 2017, 29, 4265–4272. [Google Scholar] [CrossRef]
- Morrs, L.; Robinson, W. Crystal structure of Cs2NaBiCl6. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 653–654. [Google Scholar] [CrossRef]
- Morss, L.R.; Siegal, M.; Stenger, L.; Edelstein, N. Preparation of cubic chloro complex compounds of trivalent metals: Cs2NaMCl6. Inorg. Chem. 1970, 9, 1771–1775. [Google Scholar] [CrossRef]
- Zhao, S.; Yamamoto, K.; Iikubo, S.; Hayase, S.; Ma, T. First-principles study of electronic and optical properties of lead-free double perovskites Cs2NaBX6 (B = Sb, Bi; X = Cl, Br, I). J. Phys. Chem. Solids 2018, 117, 117–121. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, L.; Teo, S.; Guo, Z.; Xu, Z.; Zhao, S.; Ma, T. Design of a novel and highly stable lead-free Cs2NaBiI6 double perovskite for photovoltaic application. Sustain. Energy Fuels 2018, 2, 2419–2428. [Google Scholar] [CrossRef]
- Li, P.; Gao, W.; Ran, C.; Dong, H.; Hou, X.; Wu, Z. Post-Treatment Engineering of Vacuum-Deposited Cs2NaBiI6 Double Perovskite Film for Enhanced Photovoltaic Performance. Phys. Status Solidi (a) 2019, 216, 1900567. [Google Scholar] [CrossRef]
- Steele, J.A.; Pan, W.; Martin, C.; Keshavarz, M.; Debroye, E.; Yuan, H.; Banerjee, S.; Fron, E.; Jonckheere, D.; Kim, C.W.; et al. Photophysical Pathways in Highly Sensitive Cs2AgBiBr6 Double-Perovskite Single-Crystal X-Ray Detectors. Adv. Mater. 2018, 30, 1804450. [Google Scholar] [CrossRef]
- Zelewski, S.; Urban, J.; Surrente, A.; Maude, D.; Kuc, A.; Schade, L.; Johnson, R.; Dollmann, M.; Nayak, P.; Snaith, H.; et al. Revealing the nature of photoluminescence emission in the metal-halide double perovskite Cs2AgBiBr6. J. Mater. Chem. C 2019, 7, 8350–8356. [Google Scholar] [CrossRef]
- Filip, M.R.; Giustino, F. Computational screening of homovalent lead substitution in organic–inorganic halide perovskites. J. Phys. Chem. C 2016, 120, 166–173. [Google Scholar] [CrossRef]
- Kubo, A.; Giorgi, G.; Yamashita, K. Anion Ordering in CaTaO2N: Structural Impact on the Photocatalytic Activity. Insights from First-Principles. Chem. Mater. 2017, 29, 539–545. [Google Scholar] [CrossRef]
- Bernardi, M.; Vigil-Fowler, D.; Lischner, J.; Neaton, J.B.; Louie, S.G. Ab initio study of hot carriers in the first picosecond after sunlight absorption in silicon. Phys. Rev. Lett. 2014, 112, 257402. [Google Scholar] [CrossRef]
- Restrepo, O.; Varga, K.; Pantelides, S. First-principles calculations of electron mobilities in silicon: Phonon and Coulomb scattering. Appl. Phys. Lett. 2009, 94, 212103. [Google Scholar] [CrossRef]
- Onida, G.; Reining, L.; Rubio, A. Electronic excitations: Density-functional versus many-body Green’s-function approaches. Rev. Mod. Phys. 2002, 74, 601. [Google Scholar] [CrossRef]
- Dancoff, S. Non-adiabatic meson theory of nuclear forces. Phys. Rev. 1950, 78, 382. [Google Scholar] [CrossRef]
- Dey, A.; Richter, A.F.; Debnath, T.; Huang, H.; Polavarapu, L.; Feldmann, J. Transfer of Direct to Indirect Bound Excitons by Electron Intervalley Scattering in Cs2AgBiBr6 Double Perovskite Nanocrystals. ACS Nano 2020, 14, 5855–5861. [Google Scholar] [CrossRef]
- A Python3 Implementation of the Spectroscopic Limited Maximum Efficiency (SLME) Analysis of Solar Absorbers. Available online: https://github.com/ldwillia/SL3ME (accessed on 30 August 2019).
- Yu, L.; Zunger, A. Identification of potential photovoltaic absorbers based on first-principles spectroscopic screening of materials. Phys. Rev. Lett. 2012, 108, 068701. [Google Scholar] [CrossRef] [PubMed]
- Pantaler, M.; Cho, K.T.; Queloz, V.I.; Garcia Benito, I.; Fettkenhauer, C.; Anusca, I.; Nazeeruddin, M.K.; Lupascu, D.C.; Grancini, G. Hysteresis-free lead-free double-perovskite solar cells by interface engineering. ACS Energy Lett. 2018, 3, 1781–1786. [Google Scholar] [CrossRef]
- Manzhos, S.; Pal, A.; Chen, Y.; Giorgi, G. Effect of organic cation states on electronic properties of mixed organic–inorganic halide perovskite clusters. Phys. Chem. Chem. Phys. 2019, 21, 8161–8169. [Google Scholar] [CrossRef]
- Giorgi, G.; Yoshihara, T.; Yamashita, K. Structural and electronic features of small hybrid organic–inorganic halide perovskite clusters: A theoretical analysis. Phys. Chem. Chem. Phys. 2016, 18, 27124–27132. [Google Scholar] [CrossRef]
- Giorgi, G.; Yamashita, K. Zero-dimensional hybrid organic–inorganic halide perovskite modeling: Insights from first principles. J. Phys. Chem. Lett. 2016, 7, 888–899. [Google Scholar] [CrossRef]
- Mailoa, J.P.; Bailie, C.D.; Johlin, E.C.; Hoke, E.T.; Akey, A.J.; Nguyen, W.H.; McGehee, M.D.; Buonassisi, T. A 2-terminal perovskite/silicon multijunction solar cell enabled by a silicon tunnel junction. Appl. Phys. Lett. 2015, 106, 121105. [Google Scholar] [CrossRef]
- Sahli, F.; Werner, J.; Kamino, B.A.; Bräuninger, M.; Monnard, R.; Paviet-Salomon, B.; Barraud, L.; Ding, L.; Leon, J.J.D.; Sacchetto, D.; et al. Fully textured monolithic perovskite/silicon tandem solar cells with 25.2% power conversion efficiency. Nat. Mater. 2018, 17, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, L.; Wienands, K.; Kim, T.W.; Uchida, S.; Bett, A.J.; Rafizadeh, S.; Goldschmidt, J.C.; Glunz, S.W. Detailed investigation of evaporated perovskite absorbers with high crystal quality on different substrates. ACS Appl. Mater. Interfaces 2018, 10, 26293–26302. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.A.; Palmstrom, A.F.; Zhengshan, J.Y.; Boccard, M.; Cheacharoen, R.; Mailoa, J.P.; McMeekin, D.P.; Hoye, R.L.; Bailie, C.D.; Leijtens, T.; et al. 23.6%-efficient monolithic perovskite/silicon tandem solar cells with improved stability. Nat. Energy 2017, 2, 17009. [Google Scholar] [CrossRef]
- Duong, T.; Pham, H.; Kho, T.C.; Phang, P.; Fong, K.C.; Yan, D.; Yin, Y.; Peng, J.; Mahmud, M.A.; Gharibzadeh, S.; et al. High Efficiency Perovskite-Silicon Tandem Solar Cells: Effect of Surface Coating versus Bulk Incorporation of 2D Perovskite. Adv. Energy Mater. 2020, 10, 1903553. [Google Scholar] [CrossRef]
- Giorgi, G. Structural and electronic features of Si/CH3NH3PbI3 interfaces with optoelectronic applicability: Insights from first-principles. Nano Energy 2020, 67, 104166. [Google Scholar] [CrossRef]
- Mariotti, S.; Al Turkestani, M.; Hutter, O.S.; Papageorgiou, G.; Major, J.D.; Swallow, J.; Nayak, P.K.; Snaith, H.J.; Dhanak, V.R.; Durose, K. Direct Silicon Heterostructures With Methylammonium Lead Iodide Perovskite for Photovoltaic Applications. IEEE J. Photovoltaics 2020, 10, 945–951. [Google Scholar] [CrossRef]
- Liu, J.Q.; Gao, Y.; Wu, G.A.; Tong, X.W.; Xie, C.; Luo, L.B.; Liang, L.; Wu, Y.C. Silicon/Perovskite Core–Shell Heterojunctions with Light-Trapping Effect for Sensitive Self-Driven Near-Infrared Photodetectors. ACS Appl. Mater. Interfaces 2018, 10, 27850–27857. [Google Scholar] [CrossRef]
- Pan, W.; Wu, H.; Luo, J.; Deng, Z.; Ge, C.; Chen, C.; Jiang, X.; Yin, W.J.; Niu, G.; Zhu, L.; et al. Cs2AgBiBr6 single-crystal X-ray detectors with a low detection limit. Nat. Photonics 2017, 11, 726–732. [Google Scholar] [CrossRef]
- Ghosh, J.; Ghosh, R.; Giri, P. Mesoporous Si nanowire templated controlled fabrication of organometal halide perovskite nanoparticles with high photoluminescence quantum yield for light-emitting applications. ACS Appl. Nano Mater. 2018, 1, 1551–1562. [Google Scholar] [CrossRef]
- Wu, Y.; Lazic, P.; Hautier, G.; Persson, K.; Ceder, G. First principles high throughput screening of oxynitrides for water-splitting photocatalysts. Energy Environ. Sci. 2013, 6, 157–168. [Google Scholar] [CrossRef]
- Castelli, I.E.; Thygesen, K.S.; Jacobsen, K.W. Computational High-throughput Screening for Solar Energy Materials. In Theoretical Modeling of Organohalide Perovskites for Photovoltaic Applications; Giorgi, G., Yamashita, K., Eds.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2017. [Google Scholar]
- Castelli, I.E.; García-Lastra, J.M.; Thygesen, K.S.; Jacobsen, K.W. Bandgap calculations and trends of organometal halide perovskites. APL Mater. 2014, 2, 081514. [Google Scholar] [CrossRef]
- Chakraborty, S.; Xie, W.; Mathews, N.; Sherburne, M.; Ahuja, R.; Asta, M.; Mhaisalkar, S.G. Rational design: A high-throughput computational screening and experimental validation methodology for lead-free and emergent hybrid perovskites. ACS Energy Lett. 2017, 2, 837–845. [Google Scholar] [CrossRef]
- Körbel, S.; Marques, M.A.; Botti, S. Stability and electronic properties of new inorganic perovskites from high-throughput ab initio calculations. J. Mater. Chem. C 2016, 4, 3157–3167. [Google Scholar] [CrossRef]
- El Mellouhi, F.; Alharbi, F.H.; Motta, C.; Rashkeev, S.; Sanvito, S.; Kais, S. Alloys and Environmental Related Issues: Toward the Computational Design of Pb-Free and Stable Hybrid Materials for Solar Cells. In Theoretical Modeling of Organohalide Perovskites for Photovoltaic Applications; Giorgi, G., Yamashita, K., Eds.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2017; pp. 135–164. [Google Scholar]
- Saidi, W.A.; Shadid, W.; Castelli, I.E. Machine-learning structural and electronic properties of metal halide perovskites using a hierarchical convolutional neural network. NPJ Comput. Mater. 2020, 6, 36. [Google Scholar] [CrossRef]
- Li, Y.; Yang, K. High-throughput computational design of organic–inorganic hybrid halide semiconductors beyond perovskites for optoelectronics. Energy Environ. Sci. 2019, 12, 2233–2243. [Google Scholar] [CrossRef]
- Li, Y.; Maldonado-Lopez, D.; Ríos Vargas, V.; Zhang, J.; Yang, K. Stability diagrams, defect tolerance, and absorption coefficients of hybrid halide semiconductors: High-throughput first-principles characterization. J. Chem. Phys. 2020, 152, 084106. [Google Scholar] [CrossRef] [PubMed]
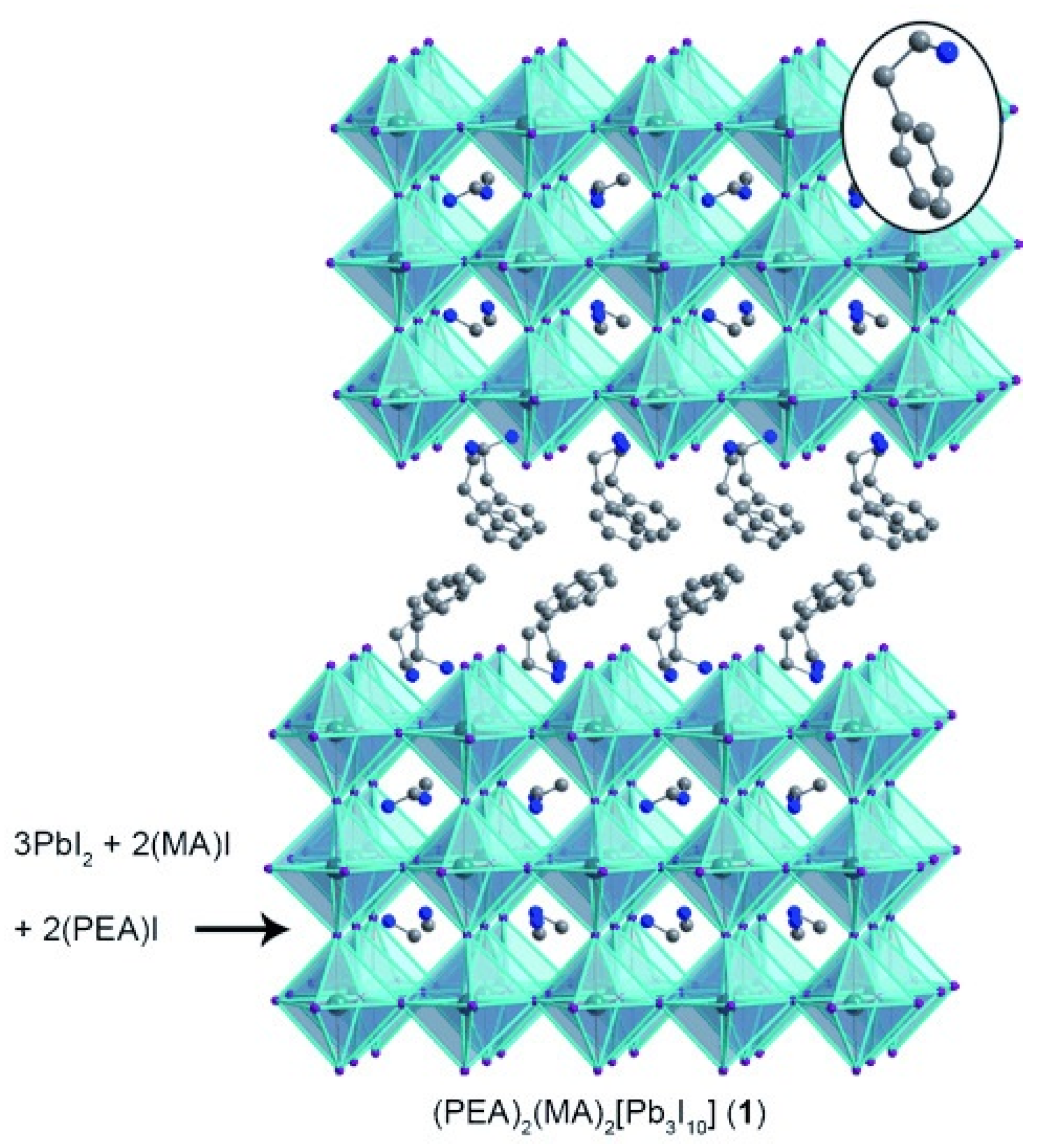
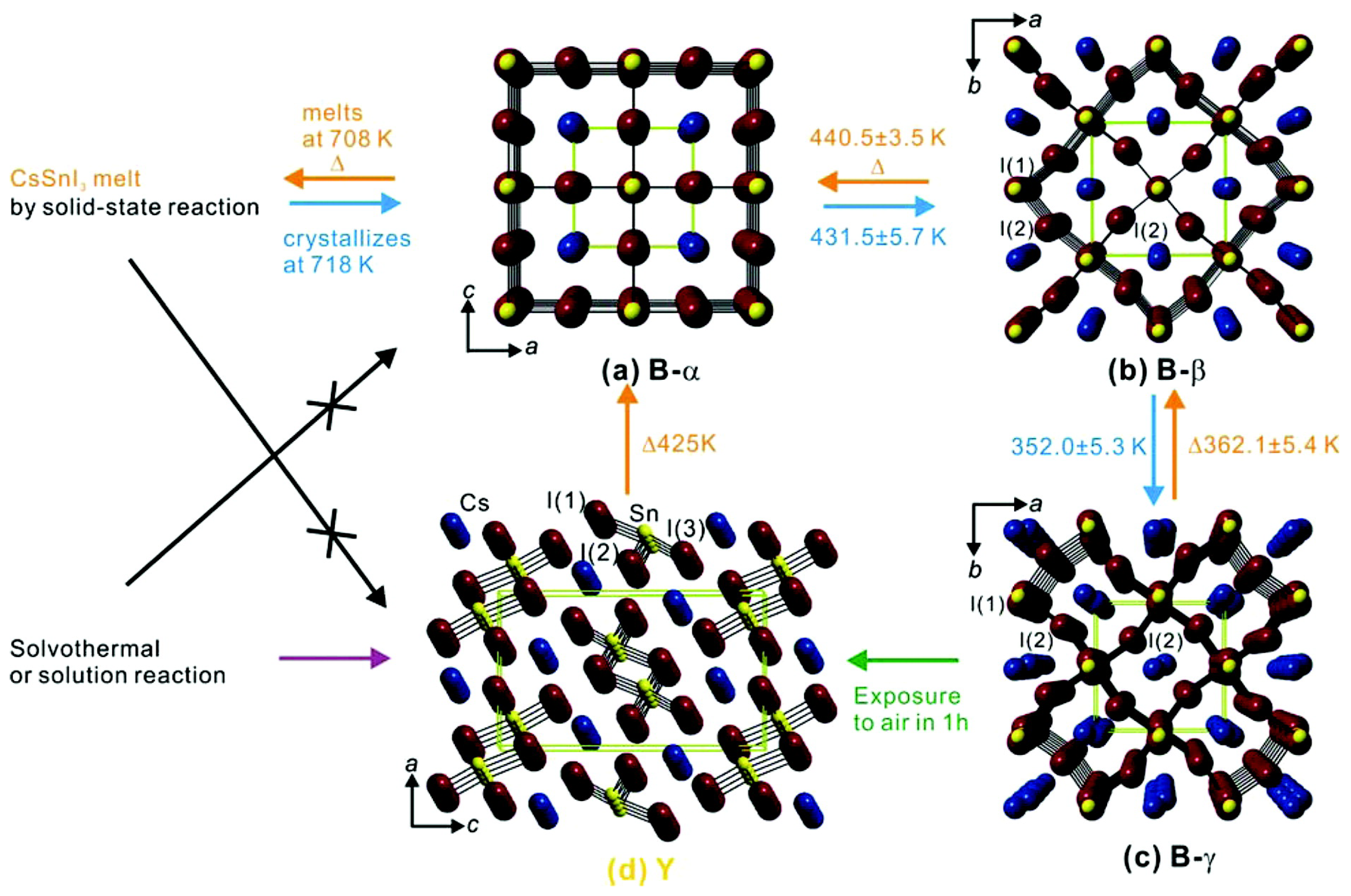
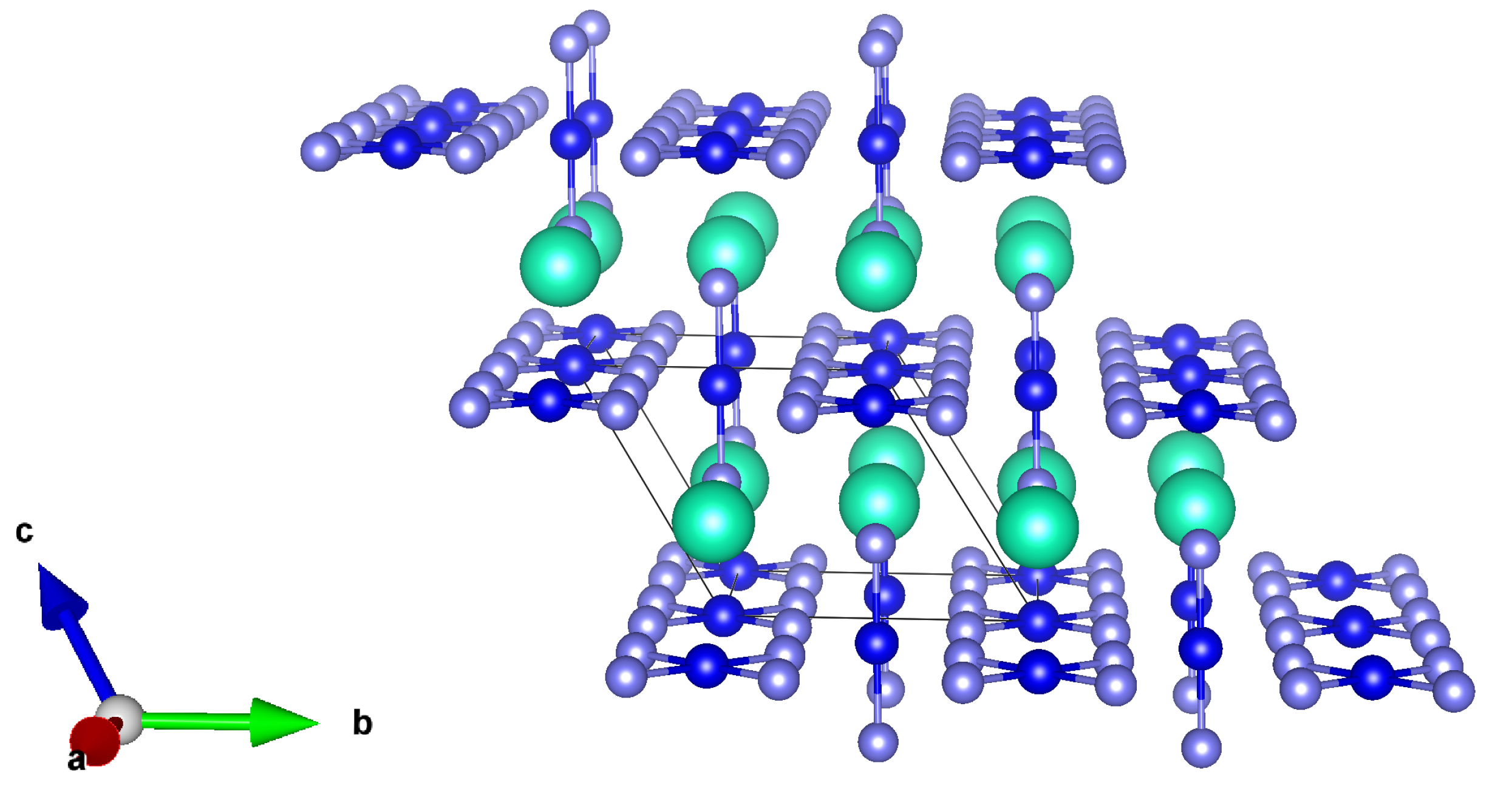
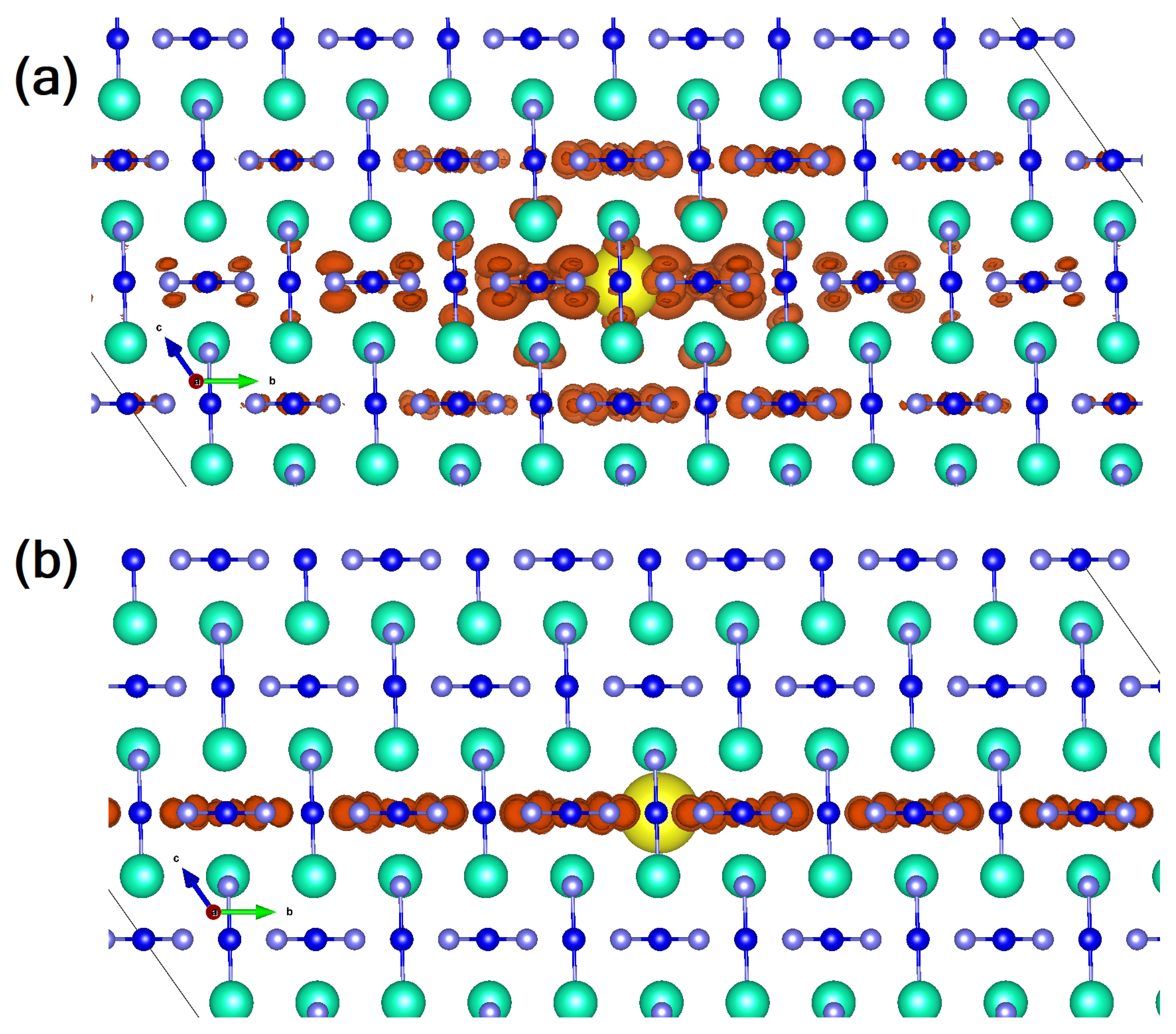
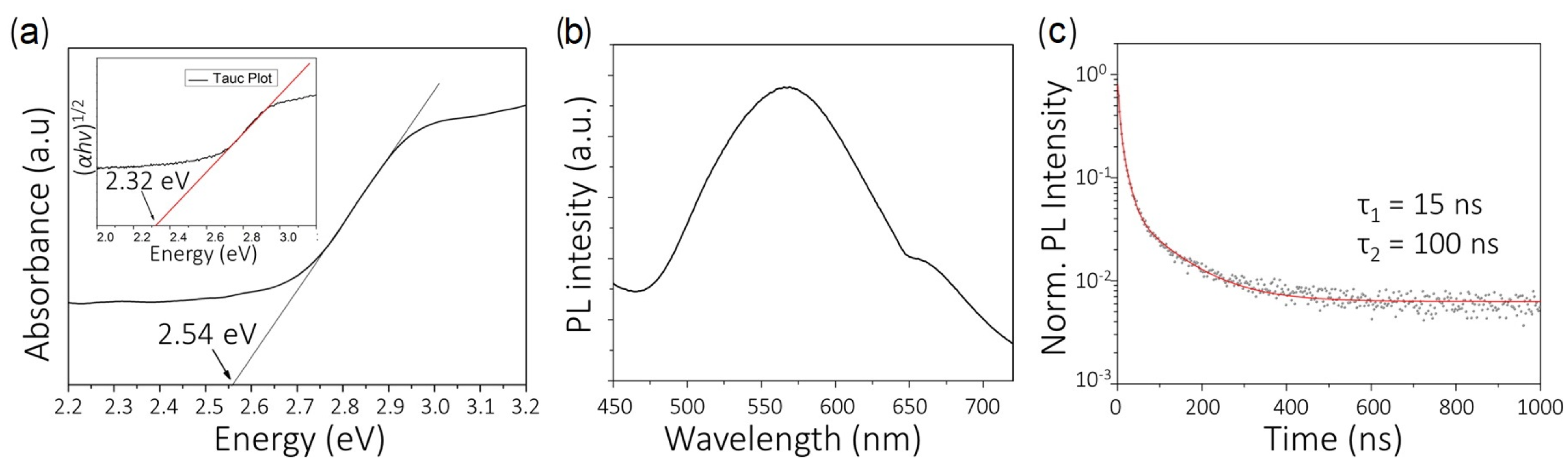
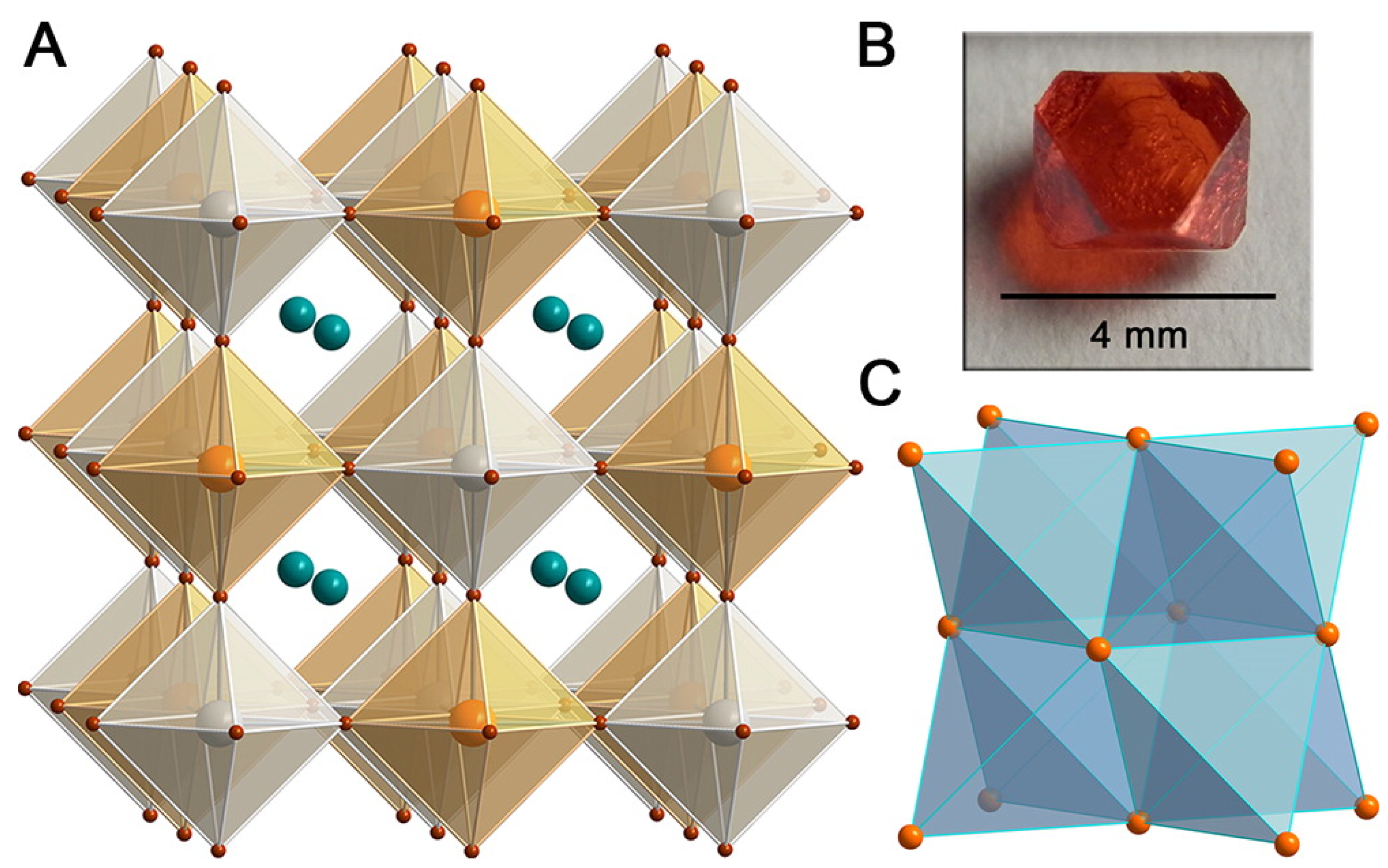
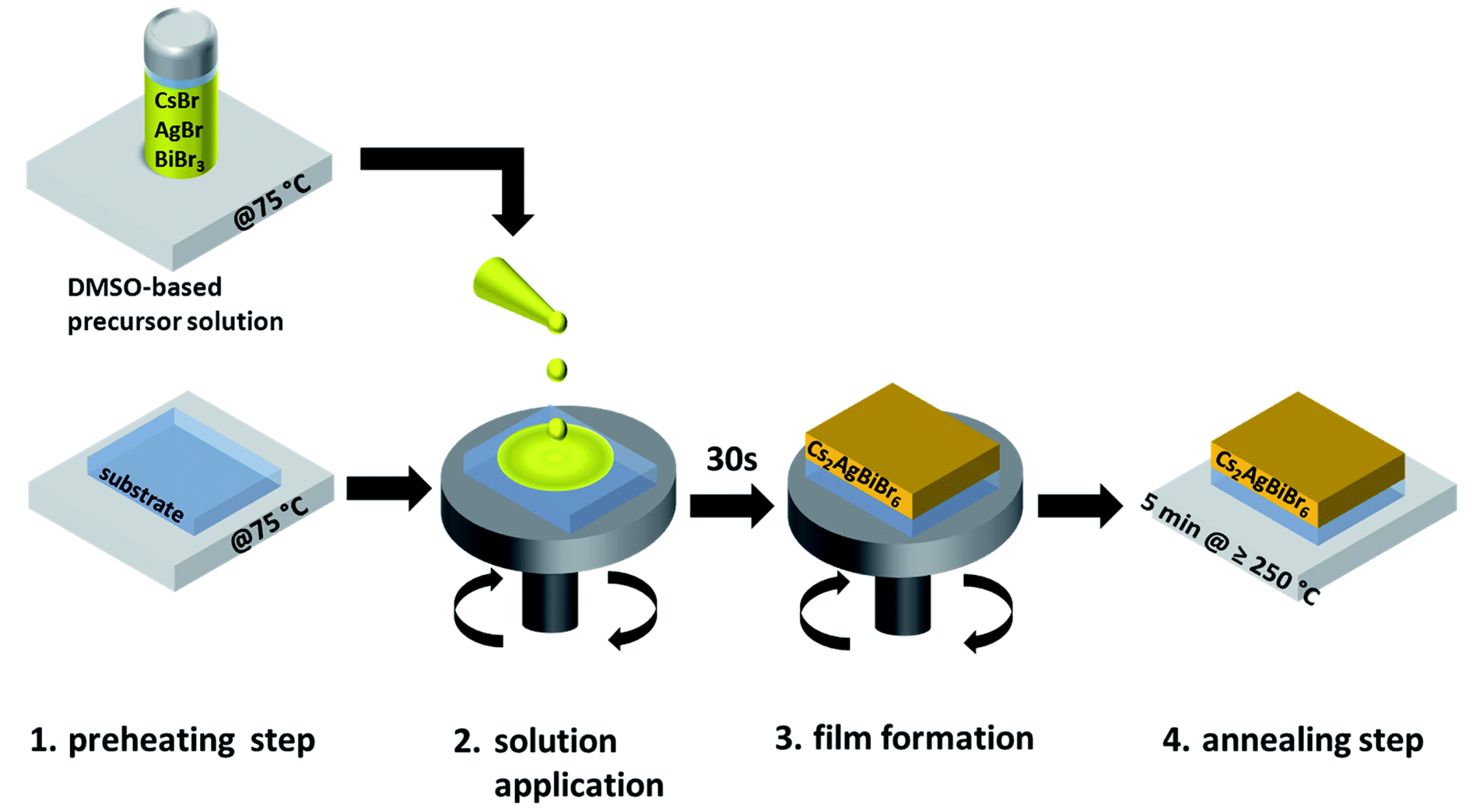

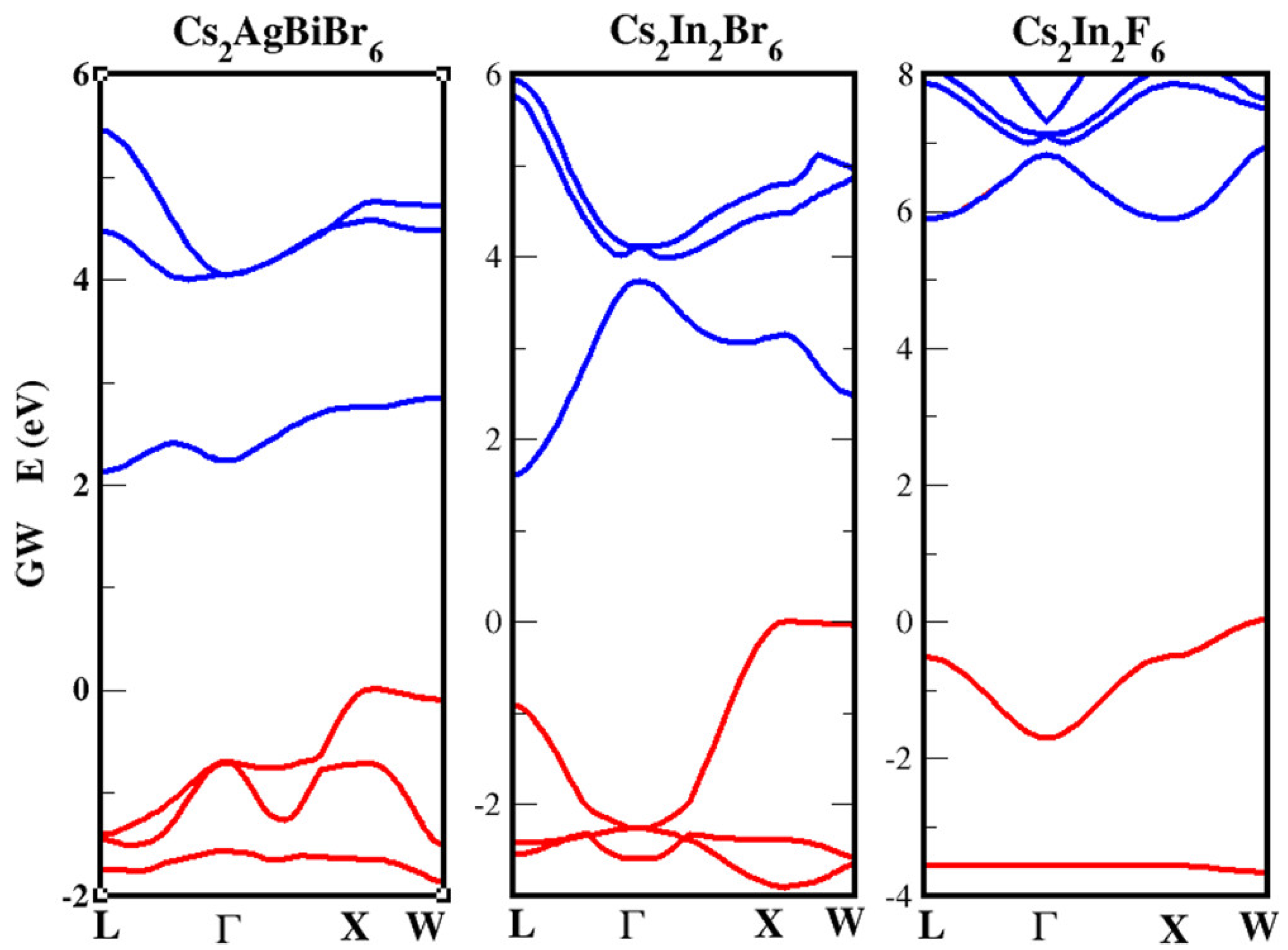
| Lattice | E | J(mA cm); V (V); | |
|---|---|---|---|
| Parameters (Å) | (eV) | FF (%); (%) | |
| single-perovskite | |||
| -CsSnBr (P) | a = 5.804 ; 5.797 | 1.8 (Abs) ; 1.75 (Abs) ; 0.351 (LDA, FP-LMTO), 1.690 (QSGW), 1.382 (QSGW+ SOC) , 0.58 (LMTO-ASA) e | 1.57; 0.19; 0.34; 0.10 with SnF: 3.99; 0.41; 0.58; 0.95 ; 0.4; 0.1; 33; 0.01 w SnF: 9.1; 0.42; 57; 2.17 (rev) |
| CsSnIBr | a = b = c = 5.916 | 1.65 (Abs) | w SnF 11.57; 0.311; 0.43; 1.56 |
| CsSnIBr | a = 8.610, b = 8.580, c = 12.393 | 1.37 (Abs) | w SnF 15.06; 0.289; 0.38; 1.67 |
| -CsSnI (Pnam/Pnma) | a = 8.6885, b = 12.3775, c = 8.6384 (Pnam) ; a = 8.688, b = 8.643, c = 12.378 (Pnma) ; a = 8.711, b = 8.640, c = 12.398 (Pnma) | 1.27 (Abs) ; 1.3 (Abs) , 0.503 (LDA, FP- LMTO) , 0.561 (GGA) | 0.22; 0.0023; 0.57; 3.0×10 ; w SnF: 27.67; 0.201; 0.29; 1.66 |
| L-CsGeI (R) | a = 5.983, = 88.61 ; a = 5.98, = 88.6 | 1.63 (Abs) | 5.7; 0.074; 0.27; 0.11 |
| H-CsGeI (P) | a = 6.05 ; a = 5.99 | 0.62 (GGA) , 1.93( ) | |
| double-perovskite | |||
| CsInF (F) | a = 9.461 (PBE) | 5.50 (d)(BSE + SOC) | SLME: 0.1 (BSE + SOC) |
| CsInBr (F) | a = 11.4771 (PBE) | 2.15 (d) (BSE + SOC) | SLME:11.5 (BSE + SOC) ; SLME: 11.2 (HSE06 + SOC) |
| CsAuCl (I) | a = 7.495, c = 10.880 o | 2.04 (Reflec) ; 2.08 (HSE06 + GW) | 12.20; 1.72; 0.92; SLME: 19.40 (HSE06 + GW) |
| CsAuBr (I) | a = 7.7592, c = 11.3079 ; a = 7.759, c = 11.308 o | 1.60 (Reflec) ; 1.61 (HSE06 + GW) | 22.90; 1.31; 0.91; SLME: 27.19 (HSE06 + GW) |
| CsAuI (I) | a = 8.284, c = 12.092 ; a = 8.284, c = 12.092 o | 1.3 (Reflec) ; 0.79 (PBE) ; 1.21 (HSE06) ; 1.35 (BSE + SOC) 1.45 (HSE06 + GW) ; 1.34 (GLLB-SC ) | 33.02; 1.04; 0.89; SLME: 30.41 (HSE06 + GW) ; SLME: 30 (BSE + SOC) |
| Lattice Parameter (Å) | E (eV) | (%) | |
|---|---|---|---|
| CsAgBiCl (F) | a = 10.785 ; a = 10.777 ; a = 10.7774 ; a = 10.6959 (PBESol) | 2.84 (Abs) ; 2.2 (PL) ; 2.15 (PL) e; 2.77 (Abs) ; 2.89 (Abs,i) ; 2.62 (HSE06 + SOC) ; 2.35(i)–2.87(d) (HSE06 + SOC) | 3.90 (HSE06 + SOC) |
| CsAgBiBr (F) | a = 11.25 ; a = 11.2711 ; a = 11.2011 (PBESol) | 1.95 (i), 2.21 (d) (Abs) ; 2.19 (Abs) ; 2.33 (Abs,i) ; 1.87–2.01 (PBE-GW) ; 1.79(i)–2.45(d) (HSE06 + SOC); 1.8(i)–2.36 (d) (BSE + SOC) | 7.25 (simul.) ; 1.44 ; 2.23 ; 2.43 ; 2.84 (+N719 interlayer) o; 7.92 (HSE06 + SOC) ; 10.5 (BSE + SOC) |
| CsAgBiI (F) | a = 11.931 (PBESol) | 1.89 (Abs,i) ; 1.08(i)–1.79(d) (HSE06 + SOC) | 12.37 (HSE06 + SOC) |
| CsAgInCl (F) | 10.48059 ; a = 10.467, a = 10.20 (LDA) ; 10.60 (HSE06) | 3.23 (PL) ; 3.3 (Abs), 2.1–2.6 (HSE06), 2.9–3.3 (PBE0) | |
| CsAgInBr (F) | a = 10.74 (LDA) | 1.49 (HSE06 + SOC) | 22.5 (HSE06 + SOC) |
| CsInBiCl (F) | a = 11.48(PBE). 11.42 (HSE06) | 0.28 (HSE06 + SOC) ; 0.88 (HSE06 + SOC) | 10.25 (HSE06 + SOC) ; 30 (HSE06 + SOC) ; 24.9 (HSE06 + SOC) |
| CsInBiBr (F) | a = 11.95 (GGA) ; a = 11.93 (PBE) | 0.36 (HSE06 + SOC) ; 0.33 (HSE06 + SOC) ; 0.29 (HSE06 + SOC) | 10.43 (HSE06 + SOC) ; 31.9 (HSE06 + SOC) |
| CsInBiI (F) | 12.69 (PBE) | 0.0 (HSE06 + SOC) ; 0.21 (HSE06 + SOC) | |
| CsAgSbCl (F) | 10.664 ; 10.84 (PBE) | 2.54 (Abs) ; 2.40 (HSE06 + SOC) | |
| CsAgSbBr (F) | a = 11.1583 , 11.1602 (optB86b-vdW) | 1.89 (Abs, i), 1.64 (Reflec); 1.46 (HSE06 + SOC) | 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palummo, M.; Varsano, D.; Berríos, E.; Yamashita, K.; Giorgi, G. Halide Pb-Free Double–Perovskites: Ternary vs. Quaternary Stoichiometry. Energies 2020, 13, 3516. https://doi.org/10.3390/en13143516
Palummo M, Varsano D, Berríos E, Yamashita K, Giorgi G. Halide Pb-Free Double–Perovskites: Ternary vs. Quaternary Stoichiometry. Energies. 2020; 13(14):3516. https://doi.org/10.3390/en13143516
Chicago/Turabian StylePalummo, Maurizia, Daniele Varsano, Eduardo Berríos, Koichi Yamashita, and Giacomo Giorgi. 2020. "Halide Pb-Free Double–Perovskites: Ternary vs. Quaternary Stoichiometry" Energies 13, no. 14: 3516. https://doi.org/10.3390/en13143516
APA StylePalummo, M., Varsano, D., Berríos, E., Yamashita, K., & Giorgi, G. (2020). Halide Pb-Free Double–Perovskites: Ternary vs. Quaternary Stoichiometry. Energies, 13(14), 3516. https://doi.org/10.3390/en13143516







