Exploring the Biomethane Potential of Different Industrial Hemp (Cannabis sativa L.) Biomass Residues
Abstract
:1. Introduction
2. Materials and Methods
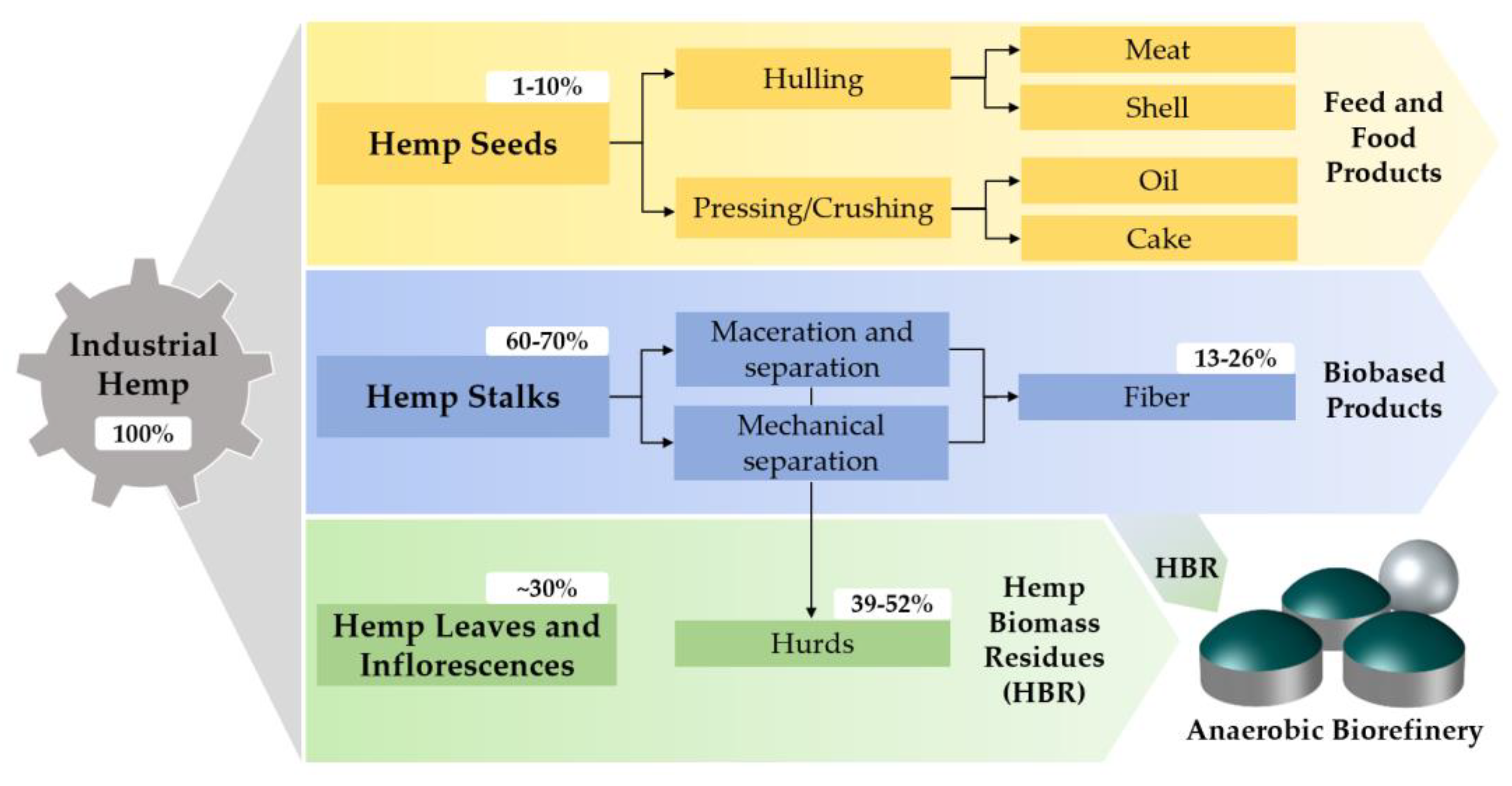
2.1. Raw Hemp Biomass
2.2. Hemp Biomass Residues Processing
2.3. Chemical and Mechanical Pretreatments of Hemp Biomass Residues
2.4. Biochemical Methane Potential Tests
2.5. Analytical Methods and Sampling
2.6. Statistical Analysis
3. Results and Discussion
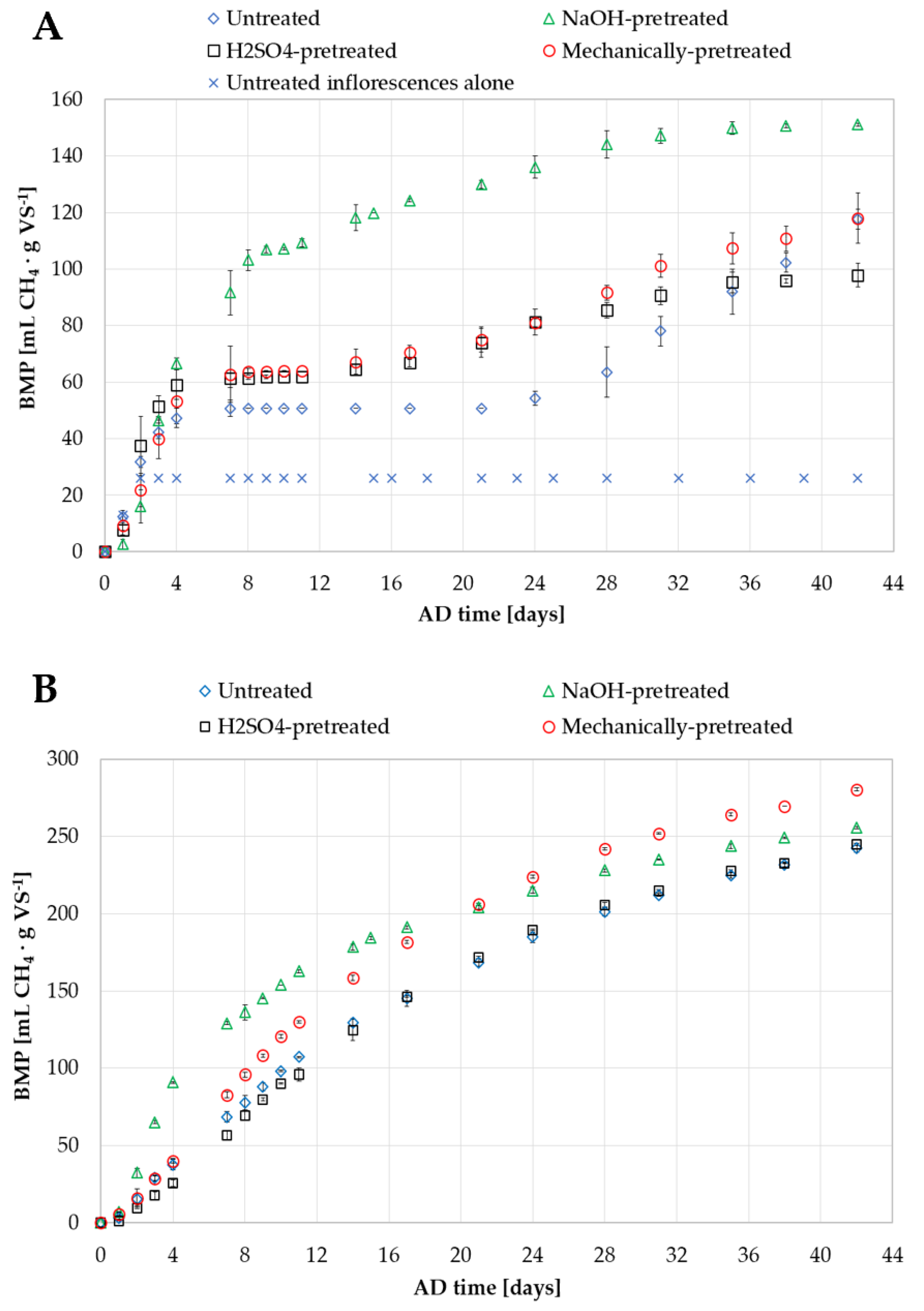
3.1. Biomethane Potential of Untreated Hemp Biomass Residues
3.2. Effect of Alkaline, Acid and Mechanical Pretreatment on the Biomethane Potential of Hemp Biomass Residues
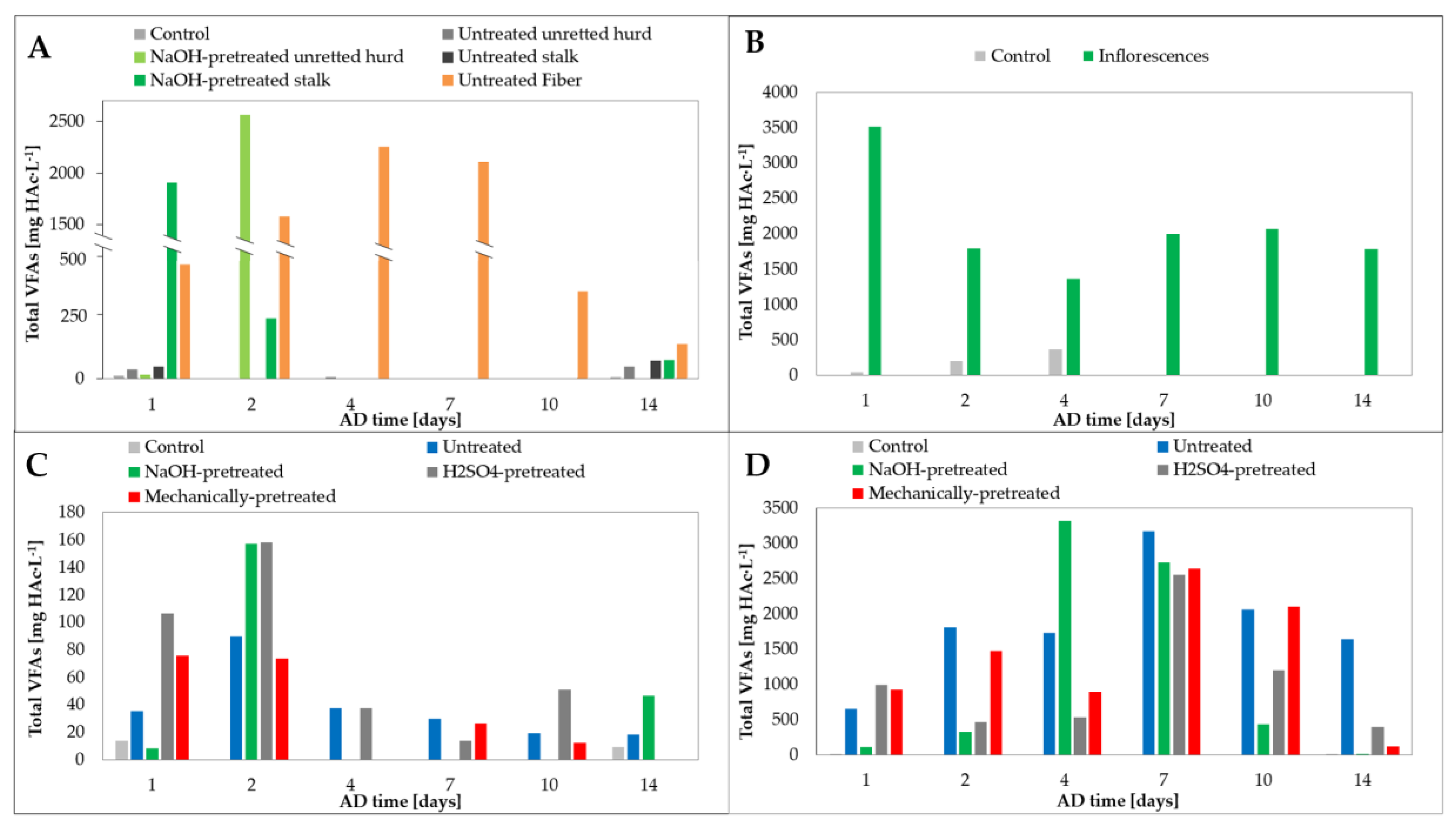
3.3. Evolution of Volatile Fatty Acids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romero-Lankao, P.; McPhearson, T.; Davidson, D.J. The food-energy-water nexus and urban complexity. Nat. Clim. Chang. 2017, 7, 233–235. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Samsatli, S. Biorefineries and the food, energy, water nexus—towards a whole systems approach to design and planning. Curr. Opin. Chem. Eng. 2017, 18, 16–22. [Google Scholar] [CrossRef]
- Pahl-Wostl, C. Governance of the water-energy-food security nexus: A multi-level coordination challenge. Environ. Sci. Policy 2019, 92, 356–367. [Google Scholar] [CrossRef]
- Rulli, M.C.; Bellomi, D.; Cazzoli, A.; De Carolis, G.; D’Odorico, P. The water-land-food nexus of first-generation biofuels. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Moving towards the second generation of lignocellulosic biorefineries in the EU: Drivers, challenges, and opportunities. Renew. Sustain. Energy Rev. 2019, 101, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Ingrao, C.; Lo, A.; Bacenetti, J.; Tricase, C.; Dotelli, G.; Fiala, M.; Siracusa, V.; Mbohwa, C. Energy and environmental assessment of industrial hemp for building applications: A review. Renew. Sustain. Energy Rev. 2015, 51, 29–42. [Google Scholar] [CrossRef]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crop. Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Maalouf, C.; Ingrao, C.; Scrucca, F.; Moussa, T.; Bourdot, A.; Tricase, C.; Presciutti, A.; Asdrubali, F. An energy and carbon footprint assessment upon the usage of hemp-lime concrete and recycled-PET façades for office facilities in France and Italy. J. Clean. Prod. 2018, 170, 1640–1653. [Google Scholar] [CrossRef]
- Devi, V.; Khanam, S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J. Clean. Prod. 2019, 207, 645–657. [Google Scholar] [CrossRef]
- Karus, M.; Vogt, D. European hemp industry: Cultivation, processing and product lines. Euphytica 2004, 140, 7–12. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Amaducci, S. Comparing hemp (Cannabis sativa L.) cultivars for dual-purpose production under contrasting environments. Ind. Crop. Prod. 2016, 87, 33–44. [Google Scholar] [CrossRef]
- Dei Cas, M.; Casagni, E.; Saccardo, A.; Arnoldi, S.; Young, C.; Scotti, S.; Vieira de Manicor, E.; Gambaro, V.; Roda, G. The Italian panorama of cannabis light preparation: Determination of cannabinoids by LC-UV. Forensic Sci. Int. 2020, 307, 110113. [Google Scholar] [CrossRef]
- Markets and Markets. Industrial Hemp Market: Scope, Size, Share, Industry Trends and Market Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/industrial-hemp-market-84188417.html?gclid=Cj0KCQiAtOjyBRC0ARIsAIpJyGO0NASx3M6L47mcp7XhWEWHPF1MhDVeIZ1BU-h_mrHa57HR2BYBGUgaAoLHEALw_wcB (accessed on 29 February 2020).
- Prade, T.; Svensson, S.; Andersson, A.; Mattsson, J.E. Biomass and energy yield of industrial hemp grown for biogas and solid fuel. Biomass Bioenergy 2011, 35, 3040–3049. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Kumar, S. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2014, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Solvent Pretreatments of Lignocellulosic Materials to Enhance Biogas Production: A Review. Energy Fuels 2016, 30, 1892–1903. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Effect of N-methylmorpholine-N-oxide pretreatment on biogas production from rice straw, cocoa shell, and hazelnut skin. Environ. Eng. Sci. 2016, 33, 843–850. [Google Scholar] [CrossRef]
- Heiermann, M.; Plöchl, M.; Linke, B.; Schelle, H.; Herrmann, C. Biogas Crops - Part I: Specifications and Suitability of Field Crops for Anaerobic Digestion. Agric. Eng. Int. CIGR Ejournal 2009, XI, 1087. [Google Scholar]
- Kreuger, E.; Prade, T.; Escobar, F.; Svensson, S.; Englund, J.; Bjo, L. Anaerobic digestion of industrial hemp—Effect of harvest time on methane energy yield per hectare. Biomass Bioenergy 2011, 35, 893–900. [Google Scholar] [CrossRef]
- Asquer, C.; Melis, E.; Scano, E.A.; Carboni, G. Opportunities for Green Energy through Emerging Crops: Biogas Valorization of Cannabis sativa L. Residues. Climate 2019, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Kreuger, E.; Sipos, B.; Zacchi, G.; Svensson, S.; Björnsson, L. Bioconversion of industrial hemp to ethanol and methane: The benefits of steam pretreatment and co-production. Bioresour. Technol. 2011, 102, 3457–3465. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Liu, E.; Saeed, A.; Williams, D.W.; Hu, H.; Li, C.; Ray, A.E.; Shi, J. Industrial hemp as a potential bioenergy crop in comparison with kenaf, switchgrass and biomass sorghum. Bioresour. Technol. 2017, 244, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.M.; Dahman, Y. Production and recovery of poly-3-hydroxybutyrate bioplastics using agro-industrial residues of hemp hurd biomass. Bioprocess Biosyst. Eng. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gusovius, H.; Lühr, C.; Musio, S.; Uhrlaub, B. Assessment system to characterise and compare different hemp varieties based on a developed lab-scaled decortication system. Ind. Crop. Prod. 2018, 117, 159–168. [Google Scholar] [CrossRef]
- Idler, C.; Pecenka, R.; Fürll, C.; Gusovius, H. Wet Processing of Hemp: An Overview. J. Nat. fibers 2011, 8, 59–80. [Google Scholar] [CrossRef]
- Papirio, S.; Matassa, S.; Pirozzi, F.; Esposito, G. Anaerobic co-digestion of cheese whey and industrial hemp residues opens new perspectives for the valorization of agri-food waste. Energies 2020, 13, 2820. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Riccardelli, G.; Lens, P.N.L.; Esposito, G. Trace elements dosing and alkaline pretreatment in the anaerobic digestion of rice straw. Bioresour. Technol. 2018, 247, 897–903. [Google Scholar] [CrossRef]
- Papirio, S. Coupling acid pretreatment and dosing of Ni and Se enhances the biomethane potential of hazelnut skin. J. Clean. Prod. 2020, 262, 121407. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [Green Version]
- Bianco, F.; Race, M.; Papirio, S.; Esposito, G. Removal of polycyclic aromatic hydrocarbons during anaerobic biostimulation of marine sediments. Sci. Total Environ. 2020, 709, 136141. [Google Scholar] [CrossRef]
- Adamovičs, A.; Dubrovskis, V.; Platače, R. Productivity of industrial hemp and its utilisation for anaerobic digestion. WIT Trans. Ecol. Environ. 2014, 2, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geffert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties Characterization of Chemically Modified Hemp Hurds. Materials (Basel) 2014, 7, 8131–8150. [Google Scholar] [CrossRef]
- Jami, T.; Karade, S.R.; Singh, L.P. A review of the properties of hemp concrete for green building applications. J. Clean. Prod. 2019, 239, 117852. [Google Scholar] [CrossRef]
- Sierra-Alvarez, R.; Lettinga, G. The methanogenic toxicity of wood resin constituents. Biol. Wastes 1990, 33, 211–226. [Google Scholar] [CrossRef]
- Wikandari, R.; Gudipudi, S.; Pandiyan, I.; Millati, R.; Taherzadeh, M.J. Inhibitory effects of fruit flavors on methane production during anaerobic digestion. Bioresour. Technol. 2013, 145, 188–192. [Google Scholar] [CrossRef]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa – From plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Alvira, P.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Xiao, K.K.; Guo, C.H.; Zhou, Y.; Maspolim, Y.; Wang, J.Y.; Ng, W.J. Acetic acid inhibition on methanogens in a two-phase anaerobic process. Biochem. Eng. J. 2013, 75, 1–7. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]

| Substrate | Parameter | Condition | |||
|---|---|---|---|---|---|
| Untreated | Alkaline Pretreated (NaOH) a | Acid Pretreated (H2SO4) b | Mechanically Pretreated (Grinding) c | ||
| Inoculum | TS (%) | 6.65–7.27 | n.a. | n.a. | n.a. |
| VS (%) | 4.51–5.20 | ||||
| N–NH4+ (g N–NH4+/L) | 0.86–1.12 | ||||
| pH | 7.6–7.8 | ||||
| Fibers | TS (%) | 89.33 ± 0.18 | n.a. | n.a. | n.a. |
| VS (%) | 87.43 ± 1.90 | ||||
| Inflorescences | TS (%) | 87.19 ± 0.55 | n.a. | n.a. | n.a. |
| VS (%) | 79.44 ± 0.55 | ||||
| Stalks | TS (%) | 89.93 ± 0.44 | 93.14 ± 0.64 | n.a. | n.a. |
| VS (%) | 85.77 ± 0.50 | 89.54 ± 0.17 | |||
| Unretted hurds | TS (%) | 89.88 ± 0.04 | 94.25 ±1.76 | n.a. | n.a |
| VS (%) | 88.38 ± 0.07 | 90.57 ±1.71 | |||
| Retted hurds | TS (%) | 89.66 ± 0.19 * | 92.03 ±1.16 | 96.66 ± 0.12 | 89.66 ± 0.19 |
| VS (%) | 85.77 ± 0.50 * | 88.68 ± 0.35 | 95.95 ± 0.06 | 85.77 ± 0.50 | |
| Mix of leaves and inflorescences | TS (%) | 89.07 ± 0.18 | 92.03 ± 0.54 | 92.39 ± 0.54 | 89.07 ± 0.18 |
| VS (%) | 71.61 ± 0.88 | 72.14 ± 0.48 | 79.80 ± 0.48 | 71.61 ± 0.88 | |
| HBRs | Condition | |||
|---|---|---|---|---|
| Untreated | Alkaline (NaOH) a | Acid (H2SO4) b | Mechanical (Grinding) c | |
| Fibers | 422 ± 20 | n.a. | n.a. | n.a. |
| Inflorescences alone | 26 ± 5 | n.a. | n.a. | n.a. |
| Stalks | 275 ± 7 | 249 ± 33 ns | n.a. | n.a. |
| (−9.5%) | ||||
| Unretted hurds | 239 ± 10 | 277 ± 13 * | n.a. | n.a. |
| (+15.9%) | ||||
| Retted hurds | 242 ± 13 d | 255 ± 1 ns | 245 ± 13 ns | 280 ± 4 * |
| (+5.7%) | (+1.3%) | (+15.9%) | ||
| Mix of leaves and inflorescences | 118 ± 8 | 151 ± 8 * | 98 ± 10 ns | 118 ± 17 ns |
| (+28.5%) | (−16.6%) | (+0.3%) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matassa, S.; Esposito, G.; Pirozzi, F.; Papirio, S. Exploring the Biomethane Potential of Different Industrial Hemp (Cannabis sativa L.) Biomass Residues. Energies 2020, 13, 3361. https://doi.org/10.3390/en13133361
Matassa S, Esposito G, Pirozzi F, Papirio S. Exploring the Biomethane Potential of Different Industrial Hemp (Cannabis sativa L.) Biomass Residues. Energies. 2020; 13(13):3361. https://doi.org/10.3390/en13133361
Chicago/Turabian StyleMatassa, Silvio, Giovanni Esposito, Francesco Pirozzi, and Stefano Papirio. 2020. "Exploring the Biomethane Potential of Different Industrial Hemp (Cannabis sativa L.) Biomass Residues" Energies 13, no. 13: 3361. https://doi.org/10.3390/en13133361
APA StyleMatassa, S., Esposito, G., Pirozzi, F., & Papirio, S. (2020). Exploring the Biomethane Potential of Different Industrial Hemp (Cannabis sativa L.) Biomass Residues. Energies, 13(13), 3361. https://doi.org/10.3390/en13133361









