Feasibility Study of Co-Firing of Torrefied Empty Fruit Bunch and Coal through Boiler Simulation
Abstract
1. Introduction
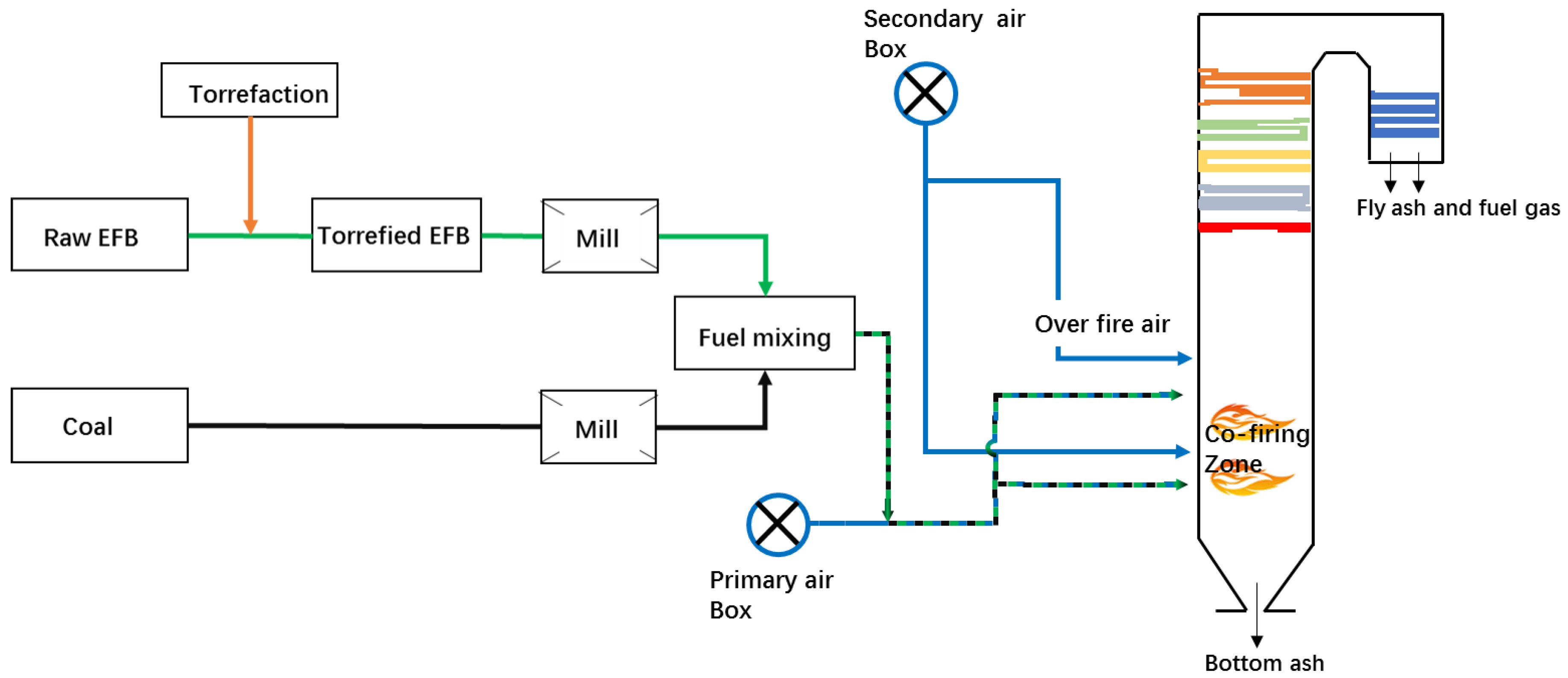
2. Torrefaction-Based Co-Firing System
3. Experimental Section
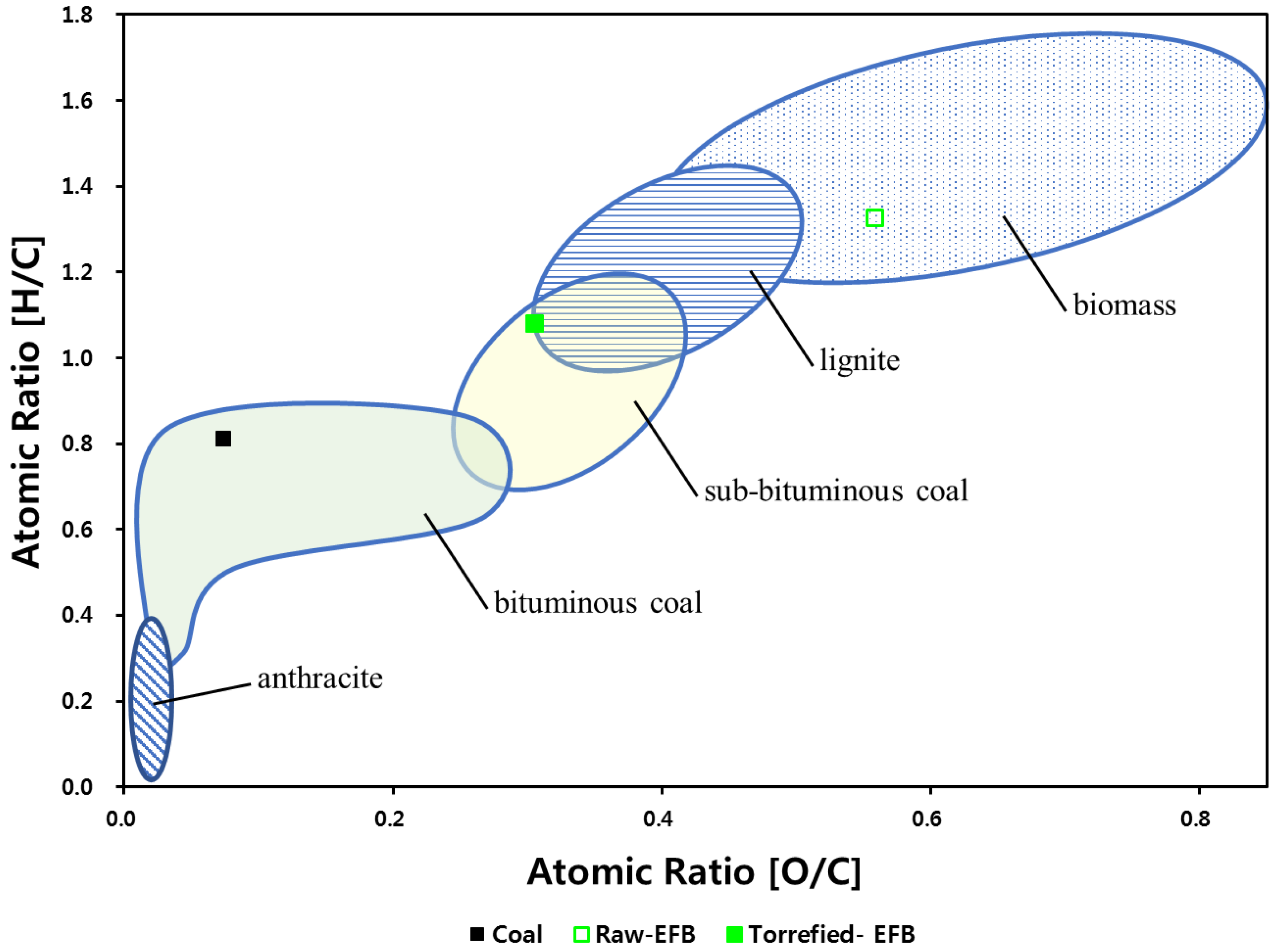
3.1. Material Properties
3.2. Experimental Studies on TG and DTG and Kinetics Tests of T-EFB Biomass
4. Description of Methodology
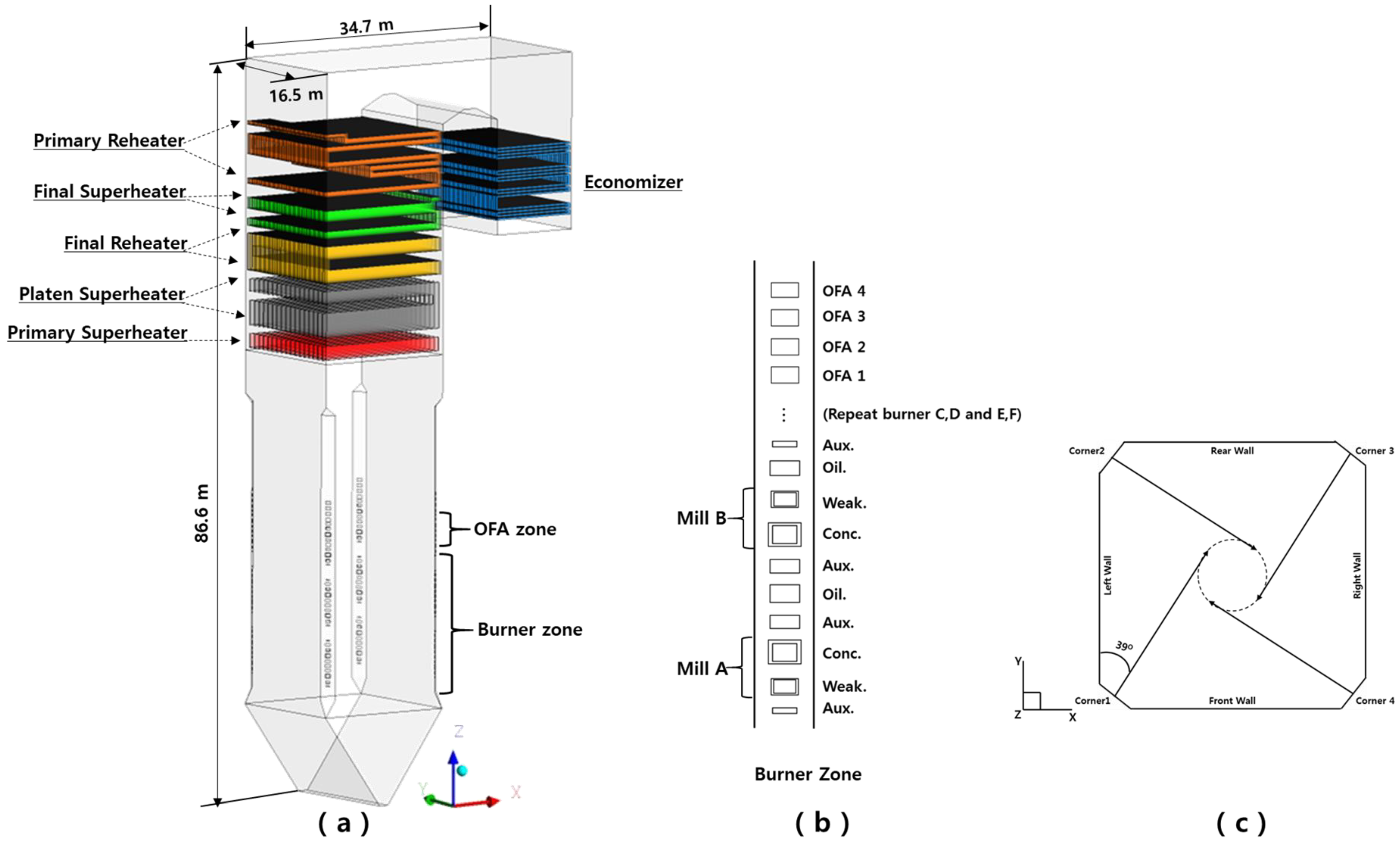
4.1. Boiler Information
4.2. Numerical Simulation
4.3. Boundary Conditions and Simulation Scenarios
5. Results and Discussion
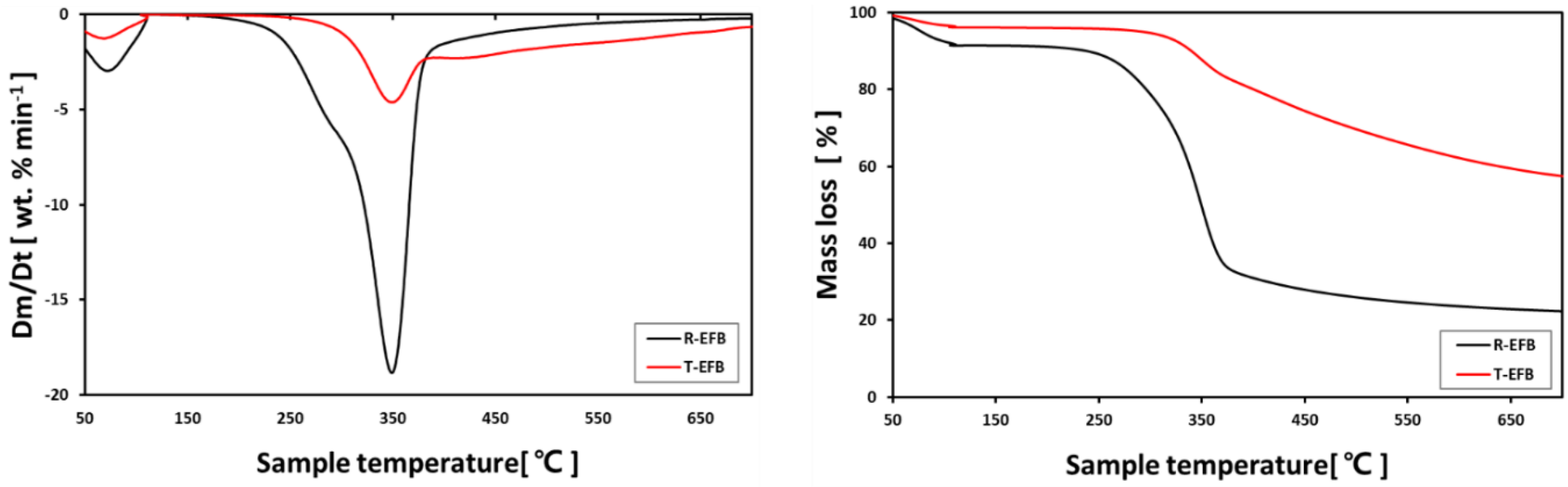
5.1. Comparison of R-EFB and T-EFB Combustion Characteristics with TG/DTG
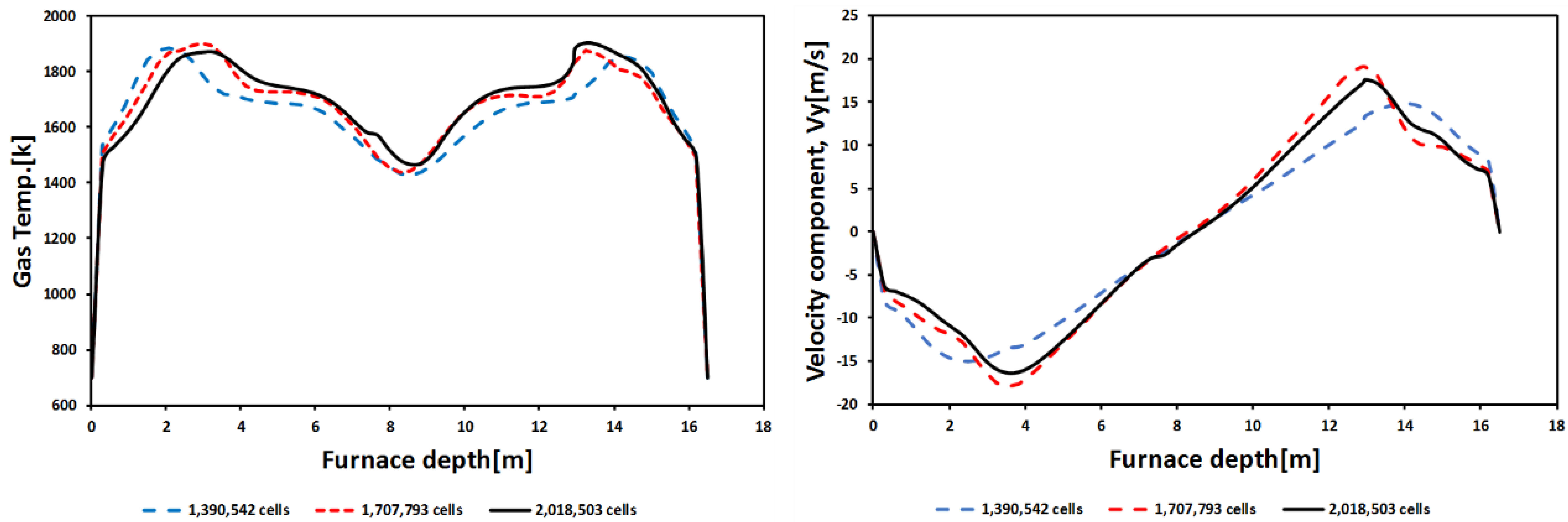
5.2. Grid Independence and Validation of the CFD Simulation
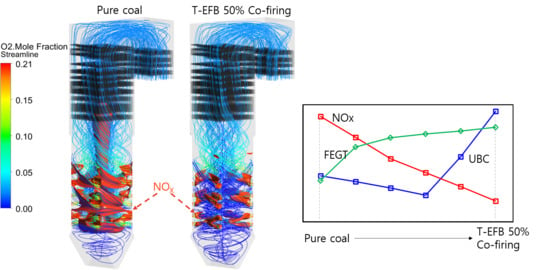
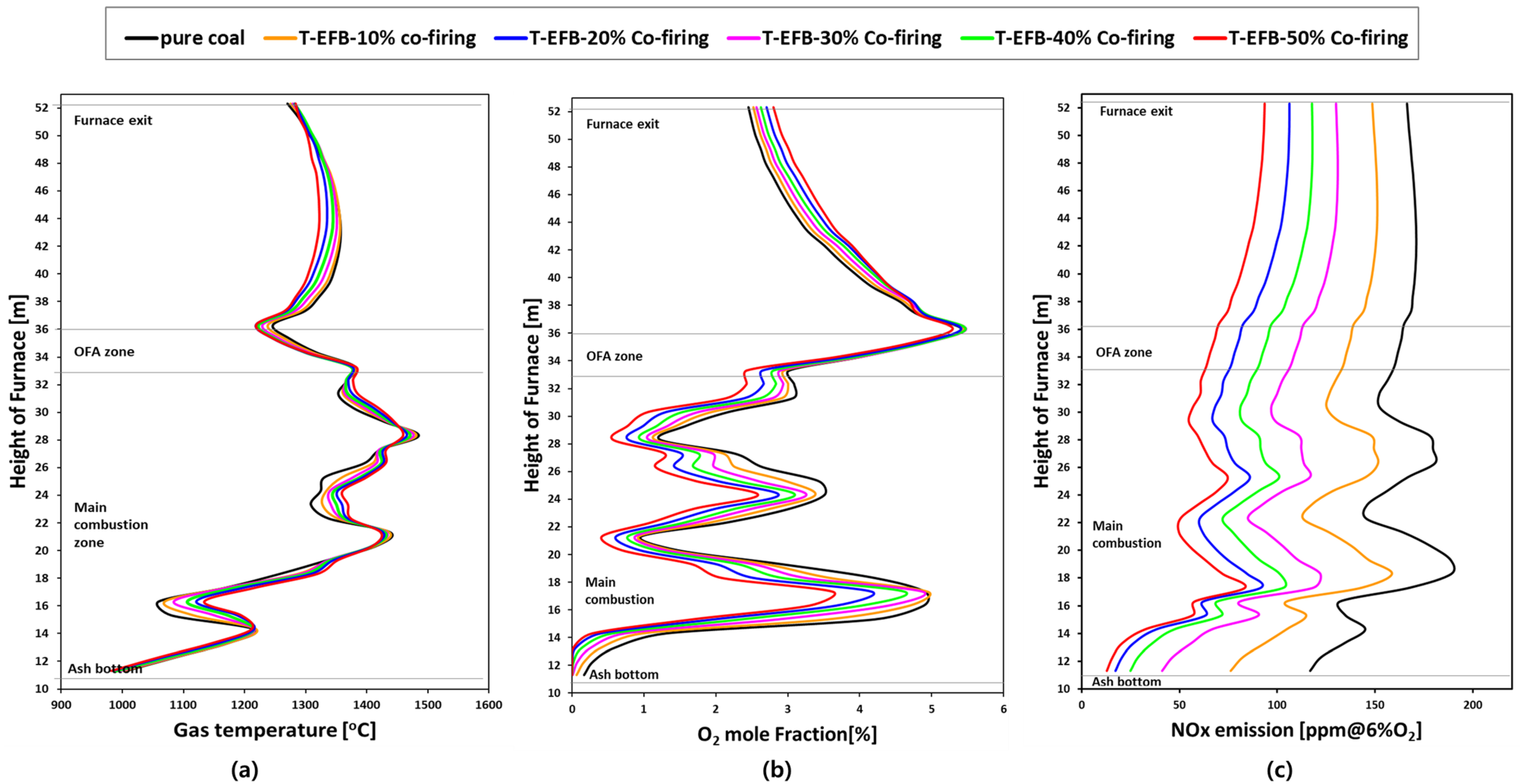
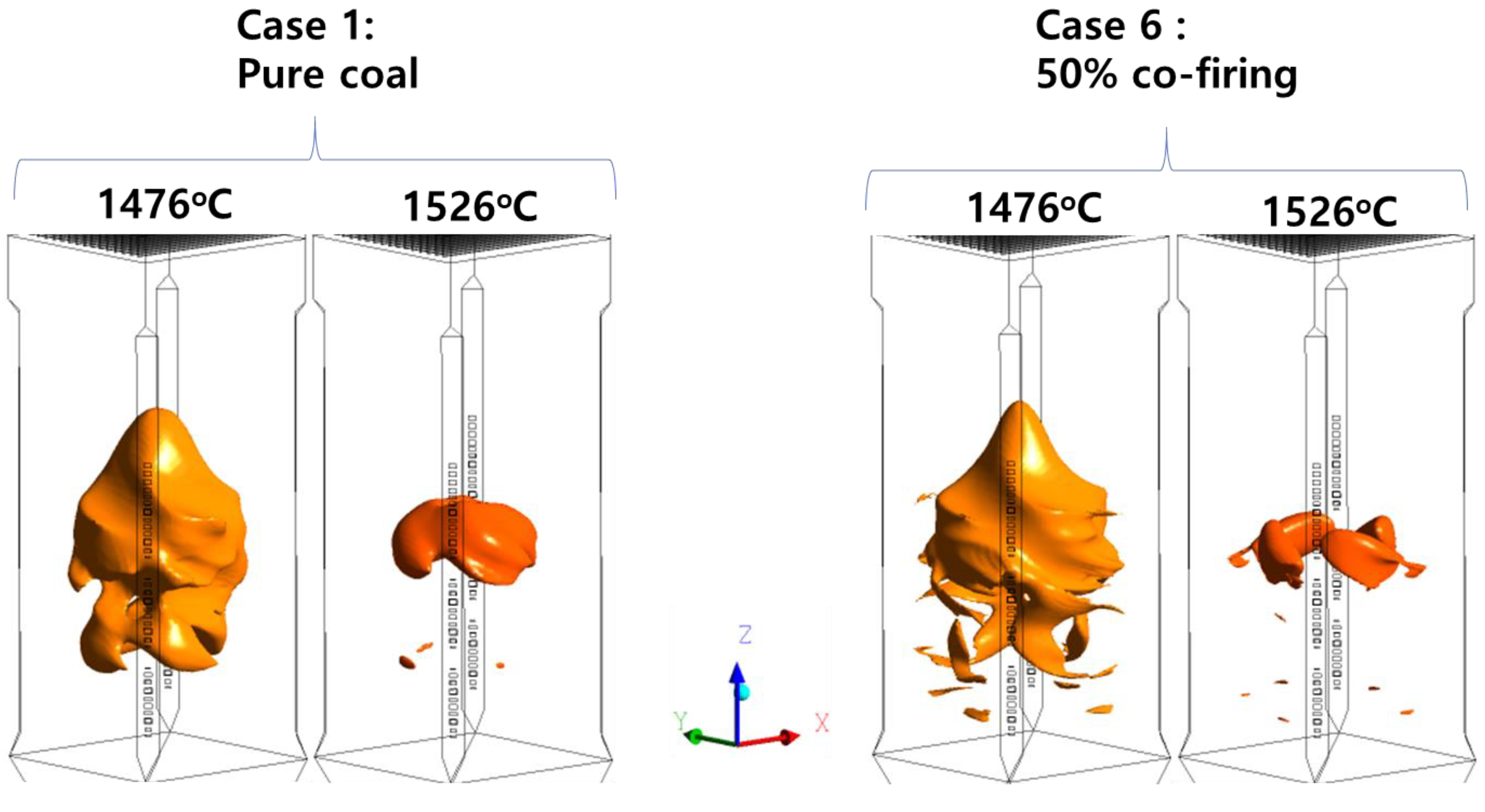
5.3. Effects of T-EFB Co-Firing Substitutions on the Combustion Characteristics
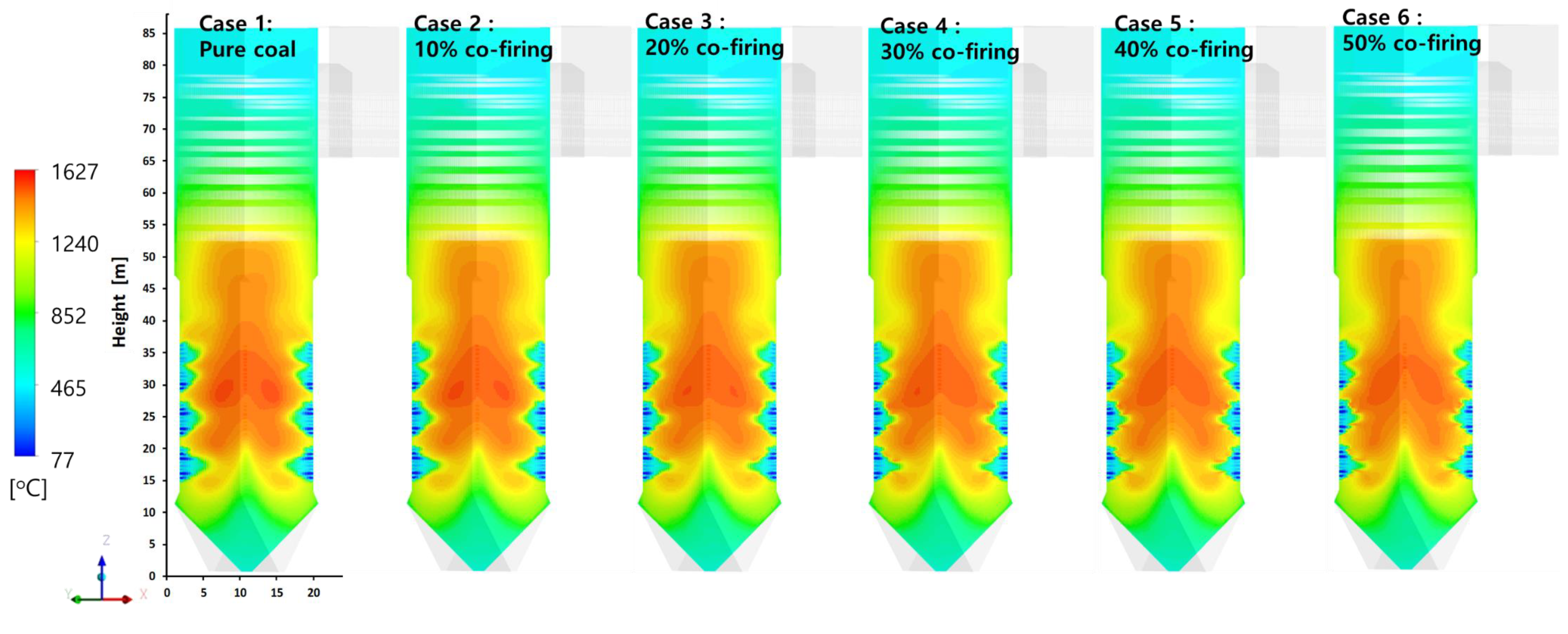
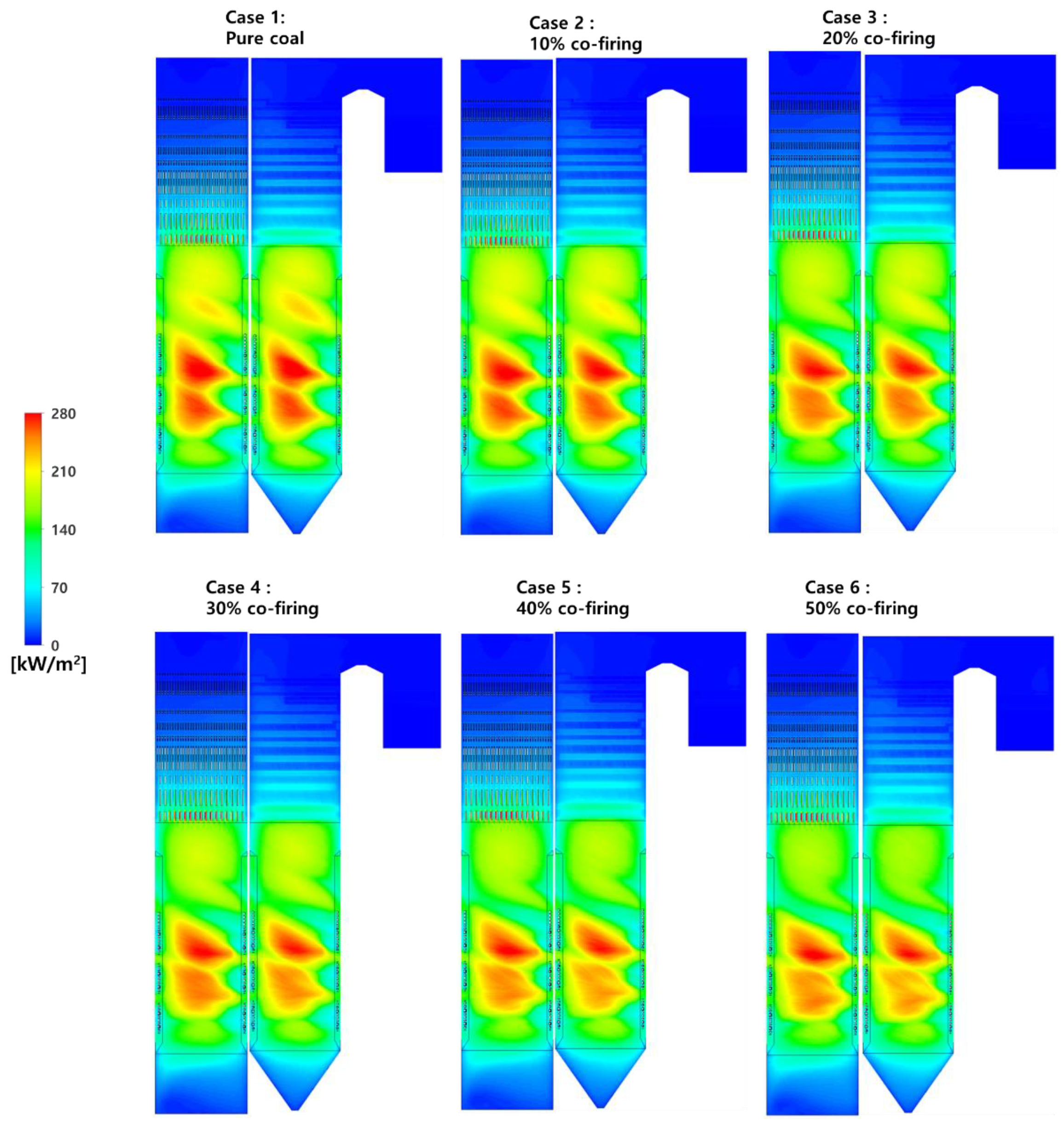
5.4. Effects of T-EFB Co-Firing Substitutions on the Heat Flux Distribution
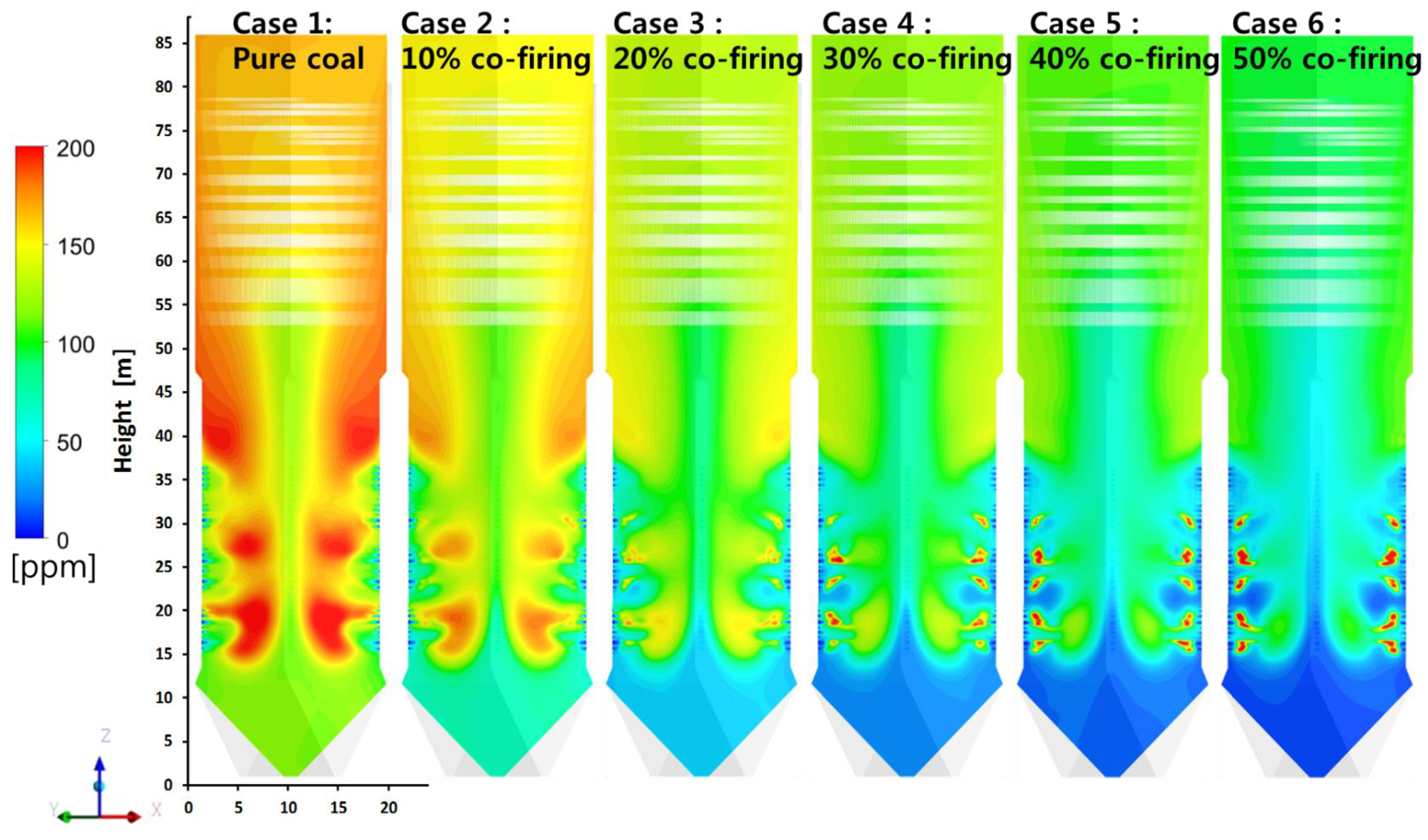
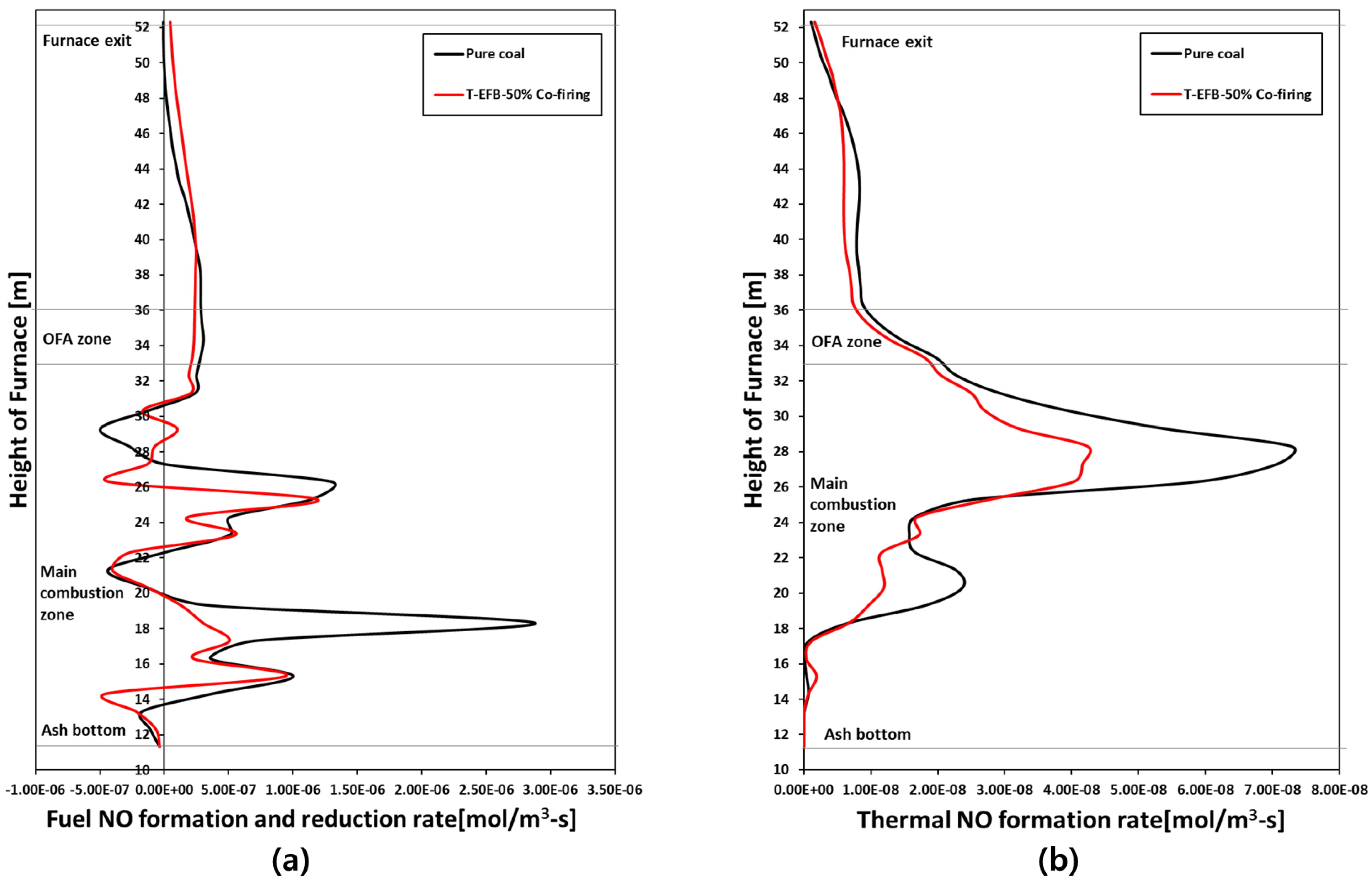
5.5. Effects of T-EFB Co-Firing Substitutions on Gas Emission (NOX, SO2)
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CFD | computational fluid dynamics |
| CO2 | carbon dioxide |
| CO | carbon monoxide |
| DTG | derivative thermogravimetric |
| DTF | drop tube furnace |
| ECO | economizer |
| EFB | empty fruit bunch |
| HHV | higher heating value |
| HT-EFB | hydrothermal empty fruit bunch |
| HT | hydrothermal |
| KIER | Korea institute of energy research |
| OFA | overfire air |
| NOX | oxides of nitrogen |
| O2 | oxygen |
| PKS | palm kernel shells |
| PM | pollution minimum |
| R-EFB | raw empty fruit bunch |
| RH | reheaters |
| SO2 | sulfur dioxide |
| SH | superheaters |
| T-EFB | torrefied empty fruit bunch |
| TGA | thermogravimetric analysis |
| UBC | unburned carbon |
| TG | weight loss curves |
| FEGT | furnace exit gas temperature |
| PA | primary air |
| SA | secondary air |
References
- IPCC. Climate Change 2014: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Li, J.; Brzdekiewicz, A.; Yang, W.H.; Tognotti, L.; Blasiak, W. Co-firing based on biomass torrefaction in a pulverized coal boiler with aim of 100% fuel switching. Appl. Energy 2012, 99, 344–354. [Google Scholar] [CrossRef]
- Tarigan, S.D.; Widyaliza, S. Expansion of oil palm plantations and forest cover changes in Bungo and Merangin districts, Jambi Province, Indonesia. Proc. Environ. Sci. 2015, 24, 199–205. [Google Scholar] [CrossRef][Green Version]
- Aziz, M.; Prawisudha, P.; Prabowo, B.; Budiman, B.A. Integration of energy-efficient empty fruit bunch drying with gasification/combined cycle systems. Appl. Energy 2015, 139, 188–195. [Google Scholar] [CrossRef]
- Puspasari, I.; Meor Talib, M.Z.; Wan Daud, W.R.; Tasirin, S.M. Drying kinetics of oil palm frond particles in an agitated fluidized bed dryer. Dry Technol. 2012, 30, 619–630. [Google Scholar] [CrossRef]
- Tabet, F.; Gökalp, I. Review on CFD based models for co-firing coal and biomass. Renew. Sustain. Energy Rev. 2015, 51, 1101–1114. [Google Scholar] [CrossRef]
- Darmawan, A.; Budianto, D.; Aziz, M.; Tokimatsu, K. Retrofitting existing coal power plants through cofiring with hydrothermally treated empty fruit bunch and a novel integrated system. Appl. Energy 2017, 204, 1138–1147. [Google Scholar] [CrossRef]
- Paepatung, N.; Nopharatana, A.; Songkasiri, W. Bio-methane potential of biological solid materials and agricultural wastes. Asian J. Energy Environ. 2009, 10, 19–27. [Google Scholar]
- Shahidi, F. Bailey’s Industrial Oil and Fat Products, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Damartzis, T.; Michailos, S.; Zabaniotou, A. Energetic assessment of a combined heat and power integrated biomass gasification-internal combustion engine system by using Aspen Plus (R). Fuel Process. Technol. 2012, 95, 37–44. [Google Scholar] [CrossRef]
- Aziz, M.; Oda, T.; Kashiwagi, T. Innovative steam drying of empty fruit bunch with high energy efficiency. Dry Technol. 2015, 33, 395–405. [Google Scholar] [CrossRef]
- Zaini, I.N.; Novianti, S.; Nurdiawati, A.; Irhamna, A.R.; Aziz, M.; Yoshikawa, K. Investigation of the physical characteristics of washed hydrochar pellets made from empty fruit bunch. Fuel Process. Technol. 2017, 160, 109–120. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. Review: A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef]
- Luk, H.T.; Lam, T.Y.G.; Oyedun, A.O.; Gebreegziabher, T.; Hui, C.W. Drying of biomass for power generation: A case study on power generation from empty fruit bunch. Energy 2013, 63, 205–215. [Google Scholar] [CrossRef]
- Ninduangdee, P.; Kuprianov, V.I. A study on combustion of oil palm empty fruit bunch in a fluidized bed using alternative bed materials: Performance, emissions, and time-domain changes in the bed condition. Appl. Energy 2016, 176, 34–48. [Google Scholar] [CrossRef]
- Aziz, M.; Kurniawan, T.; Oda, T.; Kashiwagi, T. Advanced power generation using biomass wastes from palm oil mills. Appl. Thermal Eng. 2017, 114, 1378–1386. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Boersma, A.R.; Zwart, R.W.H.; Kiel, J.H.A. Development of Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations. ECN Report ECN-C 2005-05-013. 2005. Available online: https://publicaties.ecn.nl/PdfFetch.aspx?nr=ECN-C--05-013 (accessed on 11 June 2020).
- Moscicki, K.J.; Niedzwiecki, L.; Owczarek, P.; Wnukowski, M. Commoditization of biomass: Dry torrefaction and pelletization-a review. J. Power Technol. 2014, 94, 233–249. [Google Scholar]
- Vander Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Minaret, J. Review on comparative study of dry and wet torrefaction. Sustain. Energy Technol. Assess 2015, 12, 26–37. [Google Scholar] [CrossRef]
- Uslu, A.; Faaij, A.P.; Bergman, P.C. Pre-treatment technologies, and their effect on international bioenergy supply chain logistics. Techno-economic evaluation of torrefaction, fast pyrolysis and pelletisation. Energy 2008, 33, 1206–1223. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, K.Y. Thermal and chemical characteristics of torrefied biomass derived from a generated volatile atmosphere. Energy 2018, 165, 235–245. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Szufa, S.; Dzikuć, M.; Adrian, Ł.; Piersa, P.; Romanowska-Duda, Z.; Lewandowska, W.; Marcza, M.; Błaszczuk, A.; Piwowar, A. Torrefaction of oat straw to use as solid biofuel, an additive to organic fertilizers for agriculture purposes and activated carbon—TGA analysis, kinetics. E3S Web Conf. 2020, 154, 02004. [Google Scholar] [CrossRef]
- Miedema, J.H.; Benders, R.M.J.; Moll, H.C.; Pierie, F. Renew, reduce or become more efficient? The climate contribution of biomass co-combustion in a coal-fired power plant. Appl. Energy 2017, 187, 873–885. [Google Scholar] [CrossRef]
- Falah, A.; Jan, P.B.; Alexander, S.; Jochen, S.; Bernd, E. Experimental measurements for torrefied biomass Co-combustion in a 1 M Wth pulverized coal-fired furnace. J. Energy Inst. 2020, 93, 833–846. [Google Scholar]
- Alexander, S.; Falah, A.; Jan-Peter, B.; Jochen, S.; Bernd, E. 3-D numerical simulation for co-firing of torrefied biomass in a pulverized-fired 1 MWth combustion chamber. Energy 2015, 85, 105–116. [Google Scholar]
- Emad, R.; Ren, X.H.; Panahi, A.; Yiannis, A.L. Emissions of SO2, NOx, C O2, and HCl from Co-firing of coals with raw and torrefied biomass fuels. Fuel 2018, 211, 363–374. [Google Scholar]
- Krevelen, V.W.D. Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284. [Google Scholar]
- Ansys Fluent 17.1-Theory Guide; Fluent Inc.: New York, NY, USA, 2016.
- Ma, L.; Fang, Q.; Yin, C.; Wang, H.; Zhang, C.; Chen, G. A novel corner-fired boiler system of improved efficiency and coal flexibility and reduced NOx emissions. Appl. Energy 2019, 238, 453–465. [Google Scholar] [CrossRef]
- Zhao, S.N.; Fang, Q.Y.; Yin, C.G.; Wei, T.S.; Wang, H.J.; Zhang, C.; Chen, G. New Fuel Air Control Strategy for Reducing NOx Emissions from Corner-Fired Utility Boilers at Medium−Low Loads. Energy Fuels 2017, 31, 6689–6699. [Google Scholar] [CrossRef]
- Haider, A.; Levenspiel, O. Drag coefficient and terminal velocity of spherical and nonspherical particles. Powder Technol. 1989, 58, 63–70. [Google Scholar] [CrossRef]
- Hwang, M.Y.; Kim, S.M.; Kim, G.B.; Lee, B.H.; Song, J.H.; Park, M.S.; Jeon, C.H. Simulation studies on direct ash recycling and reburning technology in a tangentially fired 500 MW pulverized coal boiler. Fuel 2013, 114, 78–87. [Google Scholar] [CrossRef]
- Badzioch, S.; Hawksley, P.G.W. Kinetics of Thermal Decomposition of Pulverized Coal Particles. Ind. Eng. Chem. Process Des. Dev. 1970, 9, 521–530. [Google Scholar] [CrossRef]
- Sheng, C.D.; Moghtaderi, B.; Gupta, R.; Wall, T.F. A computational fluid dynamics based study of the combustion characteristics of coal blends in pulverised coal-fired furnace. Fuel 2001, 83, 1543–1552. [Google Scholar] [CrossRef]
- Wang, J.; Lou, H.H.; Yang, F.; Cheng, F. Numerical simulation of a decoupling and Re-burning combinative Low-NOx coal grate boiler. J. Clean. Prod. 2018, 188, 977–988. [Google Scholar] [CrossRef]
- Vodicka, M.; Haugen, N.E.; Gruber, A.; Hrdlicka, J. NOX formation in oxy-fuel combustion of lignite in a bubbling fluidized bed - modelling and experimental verification. Int. J. Greenh. Gas Control 2018, 76, 208–214. [Google Scholar] [CrossRef]
- Hanson, R.K.; Salimian, S. Survey of Rate Constants in the N/H/O System. In Combustion Chemistry; Springer: New York, NY, USA, 1984; pp. 361–421. [Google Scholar]
- De Soete, G.G. Overall reaction rates of NO and N2 formation from fuel nitrogen. Symp. Int. Combust. 1975, 15, 1093–1102. [Google Scholar] [CrossRef]
- Winter, F.; Wartha, C.; Loffler, G.; Hofbauer, H. The NO and formation Mechanism During Devolatilization and Char Combustion Under Fluidized Bed Conditions. In Proceedings of the 26th Symposium (Int’l) on Combustion, The Combustion Institute, Pittsburgh, PA, USA, 28 July–2 August 1996; pp. 3325–3334. [Google Scholar]
- Li, J.; Biagini, E.; Yang, W.H.; Tognotti, L.; Blasiak, W. Flame characteristics of pulverized torrefied-biomass combusted with high-temperature air. Combust. Flame 2014, 160, 2585–2594. [Google Scholar] [CrossRef]
- Choi, C.R.; Kim, C.N. Numerical investigation on the flow, combustion and NOx emission characteristics in a 500 MWe tangentially fired pulverized-coal boiler. Fuel 2009, 88, 1720–1731. [Google Scholar] [CrossRef]
- Novianti, S.; Zaini, I.N.; Nurdiawati, A.; Yoshikawa, K. Low potassium Content Pellet Production by Hydrothermal-W\shing Co-treatment. Int. J. Chem. Chem. Eng. Syst. 2016, 1, 28–38. [Google Scholar]
- Balasundram, V.; Alias, N.; Ibrahim, N.; Kasmani, R.M.; Isha, R.; Hamid, M.K.A.; Hasbullah, H. Thermal Characterization of Malaysian Biomass via Thermogravimetric Analysis. J. Energy Saf. Technol. 2018, 1, 31–38. [Google Scholar] [CrossRef]
- Becher, V.; Jan-Peter, B.; Goanta, A.; Hartmut, S. A combustion concept for oxyfuel processes with low recirculation rate experimental validation. Combust. Flame 2011, 158, 1542–1552. [Google Scholar] [CrossRef]

| Fuel Samples | Coal | R-EFB | T-EFB |
|---|---|---|---|
| Proximate analysis (as-received basis, wt%) | |||
| Moisture | 14.20 | 60.00 | 2.99 |
| Volatile matter | 39.70 | 34.84 | 65.19 |
| Fixed Carbon | 40.90 | 3.71 | 19.00 |
| Ash | 5.20 | 1.46 | 12.82 |
| Ultimate analysis (dry ash-free basis, wt%) | |||
| Carbon (C) | 81.80 | 46.62 | 59.45 |
| Hydrogen (H) | 5.40 | 6.45 | 5.36 |
| Nitrogen (N) | 2.20 | 1.21 | 1.06 |
| Sulfur (S) | 0.40 | 0.035 | 0.15 |
| Oxygen (O) | 10.20 | 45.66 | 24.16 |
| Calorific value (as-received basis, MJ/Kg) | |||
| HHV | 30.18 | 17.02 | 20.9 |
| Parameter | Coal a | T-EFB b |
|---|---|---|
| Devolatilization kinetics | ||
| A (s−1) | 3.12 × 105 | 1.1 × 104 |
| E (kJ/mol) | 7.4 × 104 | 8.87 × 104 |
| Char oxidation kinetics | ||
| A (s−1) | 0.0043 | 6.4 |
| E (kJ/mol) | 83.7 | 110 |
| Item | Operation Boiler | Simulation Cases | ||||
|---|---|---|---|---|---|---|
| Case | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
| Combustion type | Pure coal | Co-firing | ||||
| T-EFB bleeding ratio (%, thermal basis) | 0 | 10 | 20 | 30 | 40 | 50 |
| Fuel mills in service (burn zone) | A B C D E | |||||
| Coal feed rates (kg/s) | 51.3 | 46.2 | 41.1 | 35.9 | 30.8 | 25.7 |
| Biomass feed rates (kg/s) | - | 6.2 | 12.5 | 18.7 | 24.9 | 31.1 |
| PA a flow rate (kg/s) | 109.8 | 110.7 | 111.6 | 112.4 | 113.2 | 113.8 |
| SA a flow rate (kg/s) | 276.9 | 279.3 | 281.4 | 283.5 | 285.4 | 287.1 |
| OFA a flow rate (kg/s) | 90.7 | 91.5 | 92.2 | 92.9 | 93.5 | 94.0 |
| Temperature of PA (°C) | 58.5 | |||||
| Temperature of SA (°C) | 303.5 | |||||
| Temperature of OFA (°C) | 303.5 | |||||
| Burner zone stoichiometry ratio | 0.91 | |||||
| Case Type | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|---|
| Pure Coal | T-EFB 10% Co-Firing | T-EFB 20% Co-Firing | T-EFB 30% Co-Firing | T-EFB 40% Co-Firing | T-EFB 50% Co-Firing | ||
| Method | Experimental | Simulation | Simulation | Simulation | Simulation | Simulation | Simulation |
| Furnace peak temperature (°C) | - | 1484.5 | 1480.2 | 1476.5 | 1470.3 | 1465.1 | 1459.4 |
| Furnace exit gas temperature (°C) | 1257 | 1258.8 | 1266 | 1268 | 1268.8 | 1269.4 | 1270.2 |
| Gas temperature at boiler exit (°C) | 333.5 | 353.5 | 360.4 | 361.2 | 362.1 | 362.9 | 363.6 |
| O2 content at boiler exit (vol%) | 2.2 | 2.2 | 2.3 | 2.36 | 2.41 | 2.46 | 2.54 |
| CO content at boiler exit (vol%) | - | 8.8 × 10−11 | 4.9 × 10−11 | 6.64 × 10−9 | 8.02 × 10−9 | 9.9 × 10−9 | 1.29 × 10−8 |
| CO2 content at boiler exit (vol%) | - | 14.60 | 14.84 | 14.65 | 14.65 | 14.65 | 14.62 |
| SO2 content at boiler exit (ppm, at 6% O2) | 474.2 | 476.1 | 441.4 | 402.4 | 363.1 | 331.7 | 292.5 |
| Discrepancies a (%) | - | 0.4 | 7.3 | 15.5 | 23.7 | 30.3 | 38.6 |
| NOX emission at boiler exit (ppm, at 6% O2) | 159.2 | 170 | 152.2 | 133.9 | 122 | 110.1 | 97.8 |
| Discrepancies a (%) | - | 6.8 | 10.5 | 21.2 | 28.2 | 35.2 | 42.5 |
| Average residence time of fuel particles (s) | - | 20.24 | 20.19 | 20.13 | 19.85 | 19.6 | 19.5 |
| Unburned carbon content (wt%) | 3.48 | 3.21 | 3.12 | 3.02 | 2.91 | 3.5 | 4.2 |
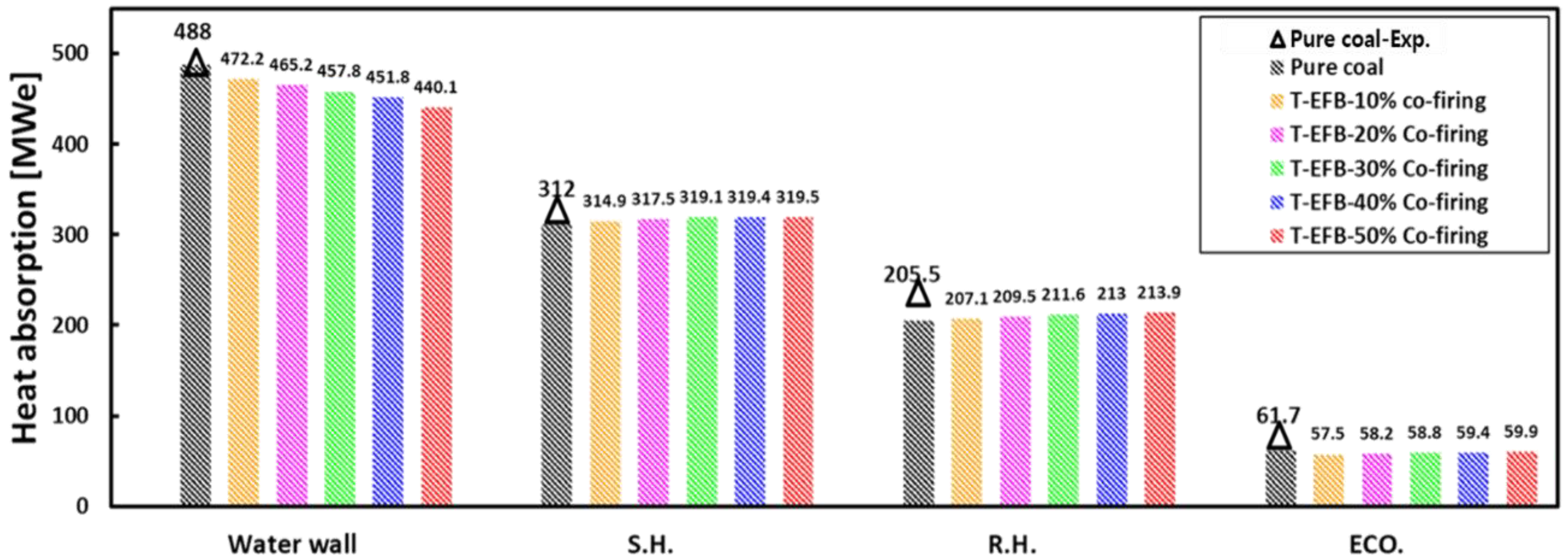
| Economizer heat absorption (MW) | 64.3 | 61.7 | 57.5 | 58.2 | 58.8 | 59.4 | 59.9 |
| Reheater heat absorption (MW) | 224 | 205.5 | 207.1 | 209.5 | 211.6 | 213 | 213.9 |
| Superheater heat absorption (MW) | 317 | 312 | 314.9 | 317.5 | 319.1 | 319.4 | 319.5 |
| Water wall heat absorption (MW) | 482.1 | 488 | 472.2 | 465.2 | 457.8 | 451.8 | 440.1 |
| Total heat absorption (MW) | 1087 | 1067.2 | 1051.7 | 1050.4 | 1047.3 | 1043.6 | 1033.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Park, K.-H.; Jeon, C.-H. Feasibility Study of Co-Firing of Torrefied Empty Fruit Bunch and Coal through Boiler Simulation. Energies 2020, 13, 3051. https://doi.org/10.3390/en13123051
Jiang Y, Park K-H, Jeon C-H. Feasibility Study of Co-Firing of Torrefied Empty Fruit Bunch and Coal through Boiler Simulation. Energies. 2020; 13(12):3051. https://doi.org/10.3390/en13123051
Chicago/Turabian StyleJiang, Yu, Kyeong-Hoon Park, and Chung-Hwan Jeon. 2020. "Feasibility Study of Co-Firing of Torrefied Empty Fruit Bunch and Coal through Boiler Simulation" Energies 13, no. 12: 3051. https://doi.org/10.3390/en13123051
APA StyleJiang, Y., Park, K.-H., & Jeon, C.-H. (2020). Feasibility Study of Co-Firing of Torrefied Empty Fruit Bunch and Coal through Boiler Simulation. Energies, 13(12), 3051. https://doi.org/10.3390/en13123051