Development of Magnetic Multi-Shelled Hollow Catalyst for Biodiesel Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
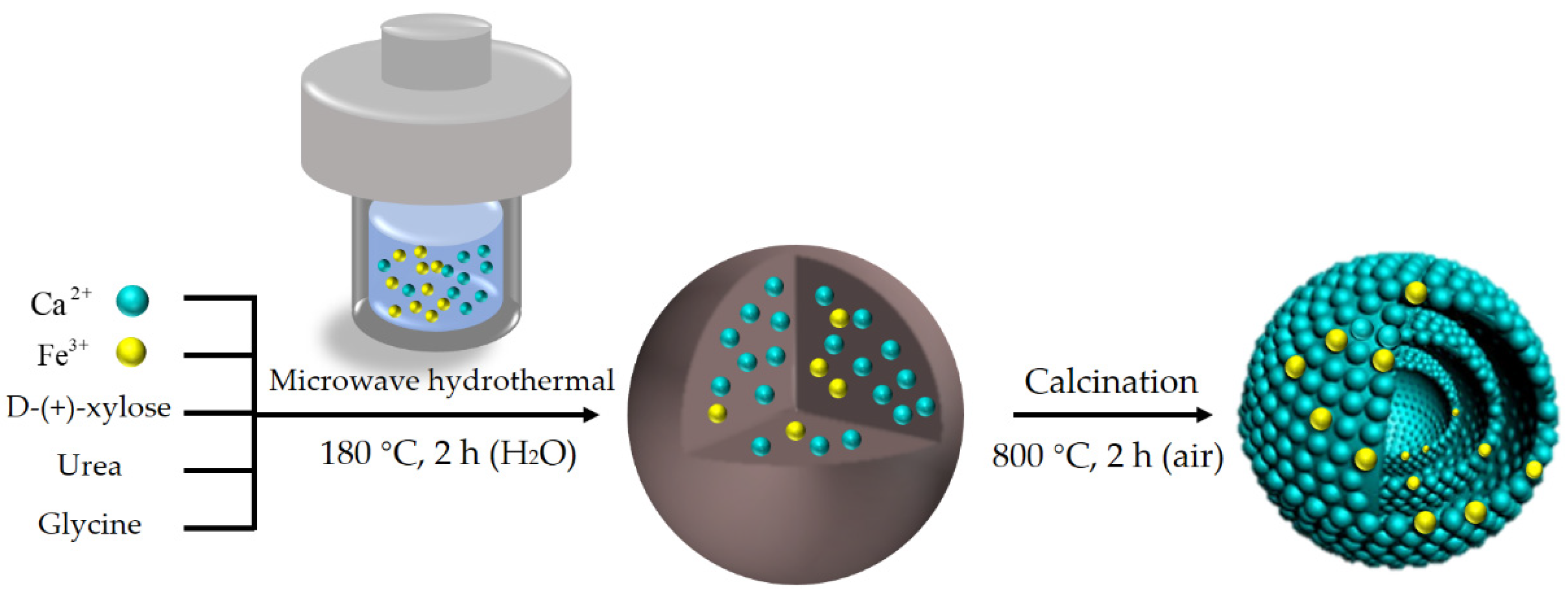
2.2. Catalyst Preparation
2.3. Characterization of Catalyst
2.4. Transesterification Procedure
2.5. Catalyst Reusability
2.6. Response Surface Methodology (RSM) Optimization
3. Results
3.1. Analysis of Catalyst
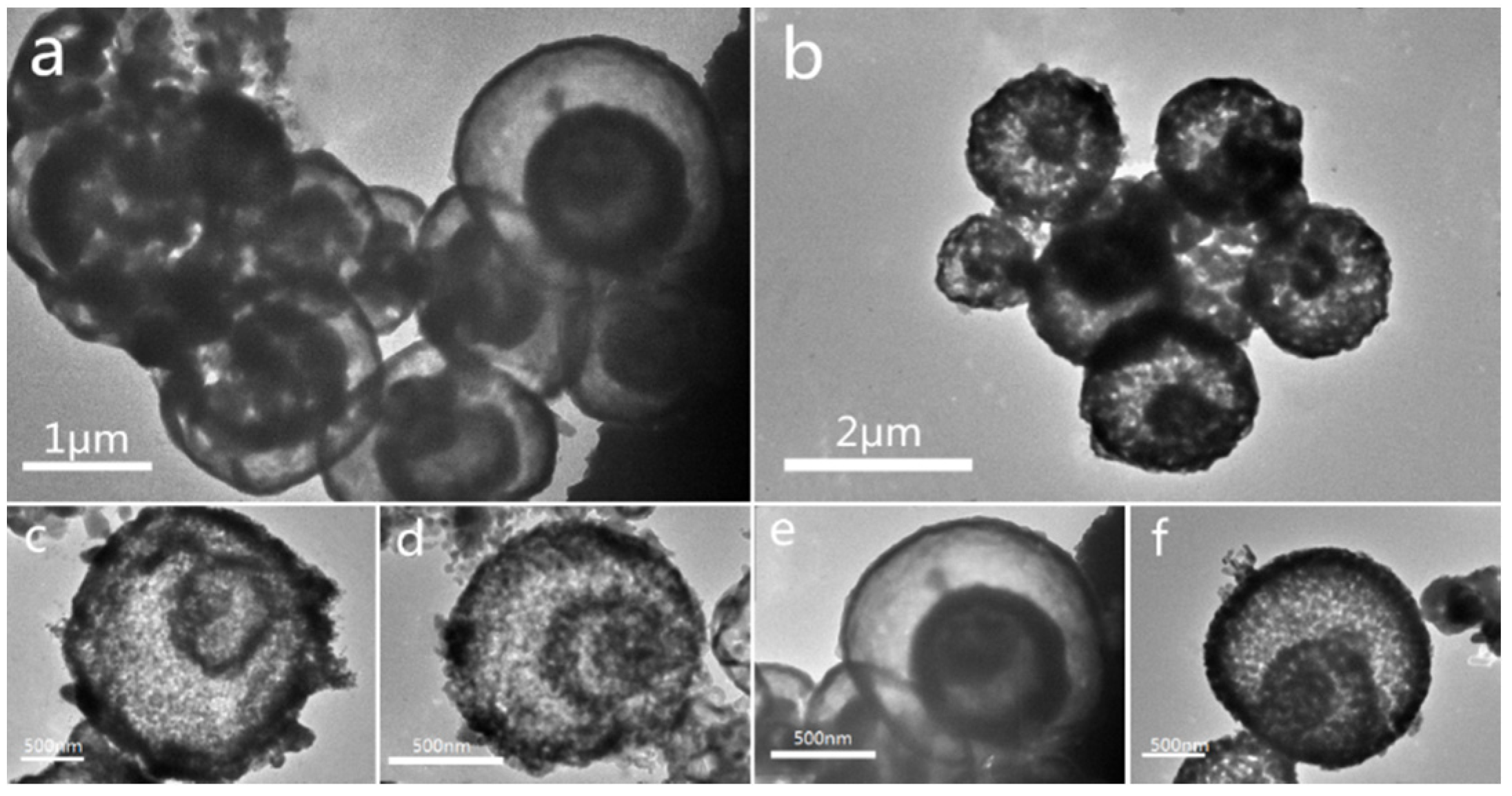
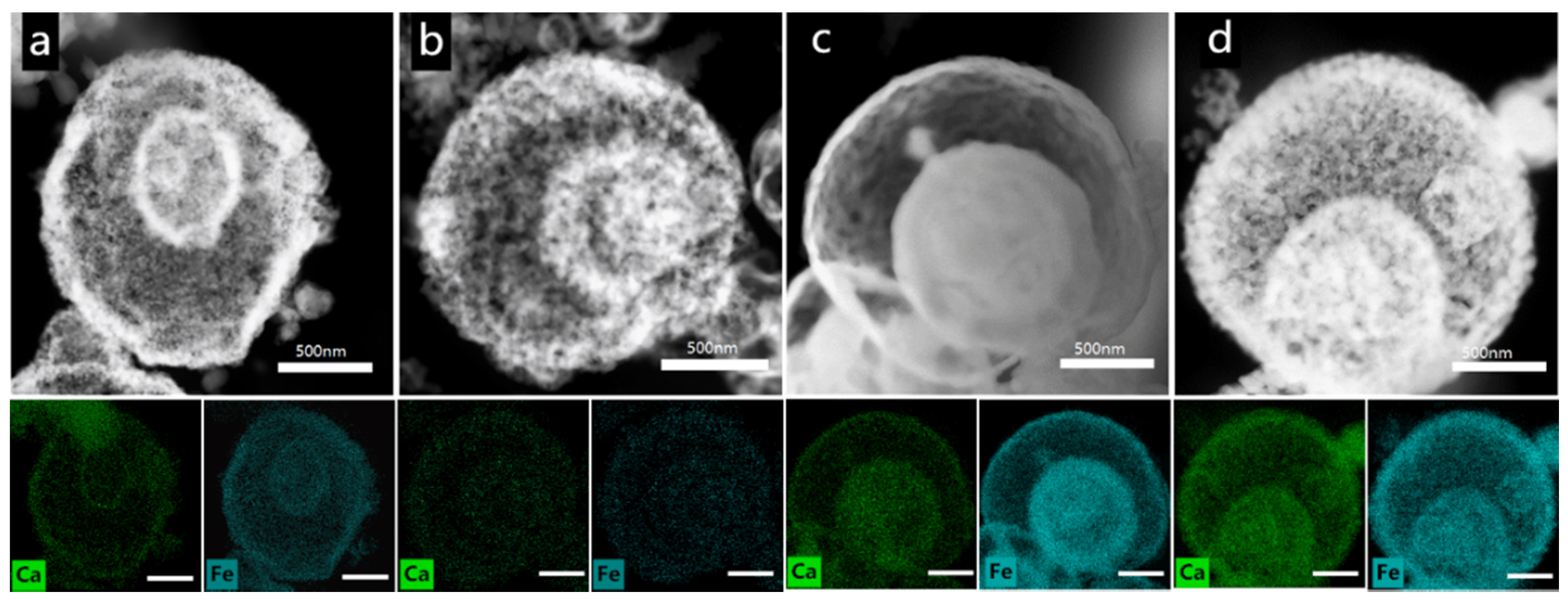
3.1.1. Structure and Composition of the Catalyst
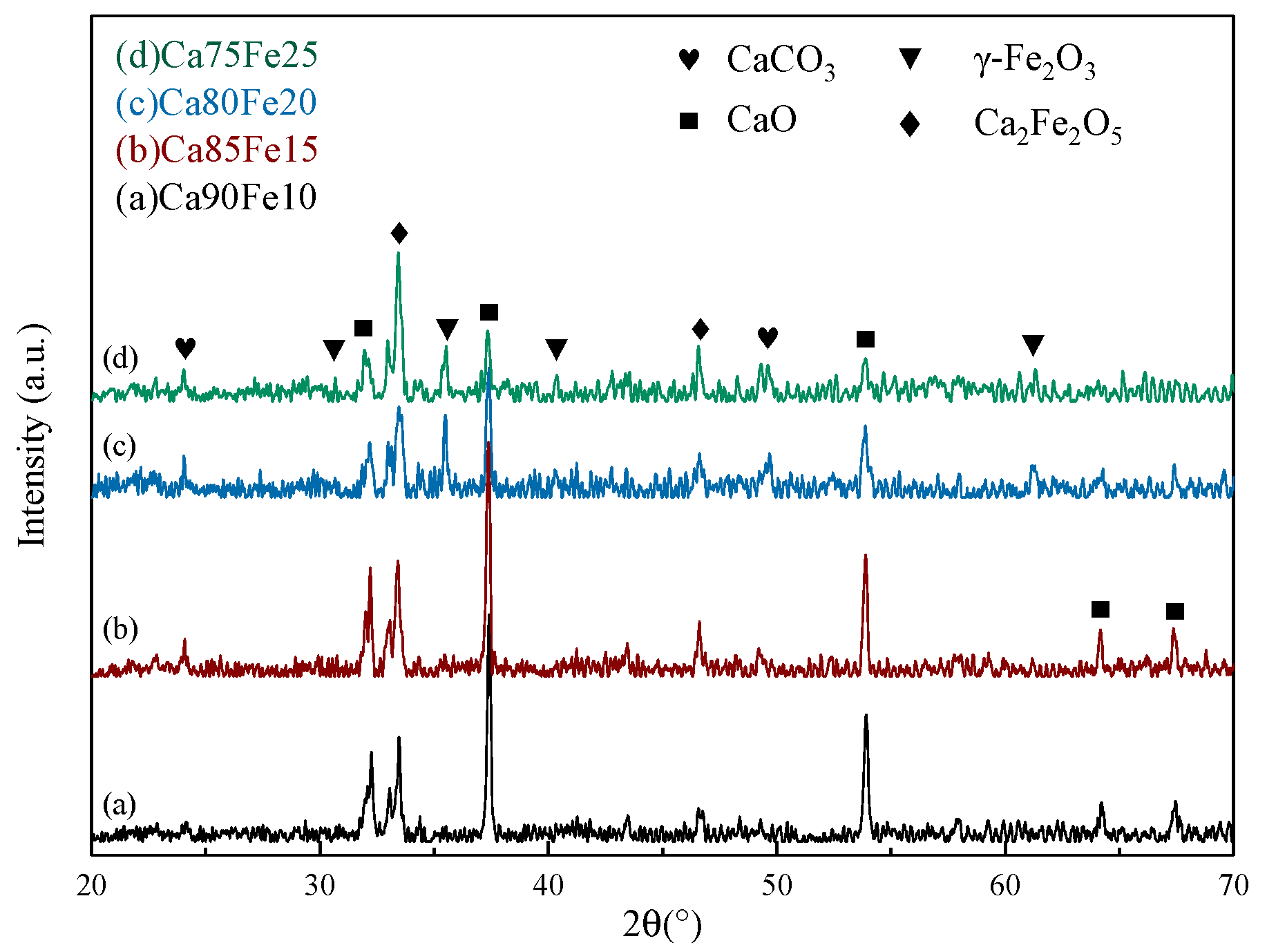
3.1.2. X-ray Diffraction Analysis
3.1.3. BET Analysis
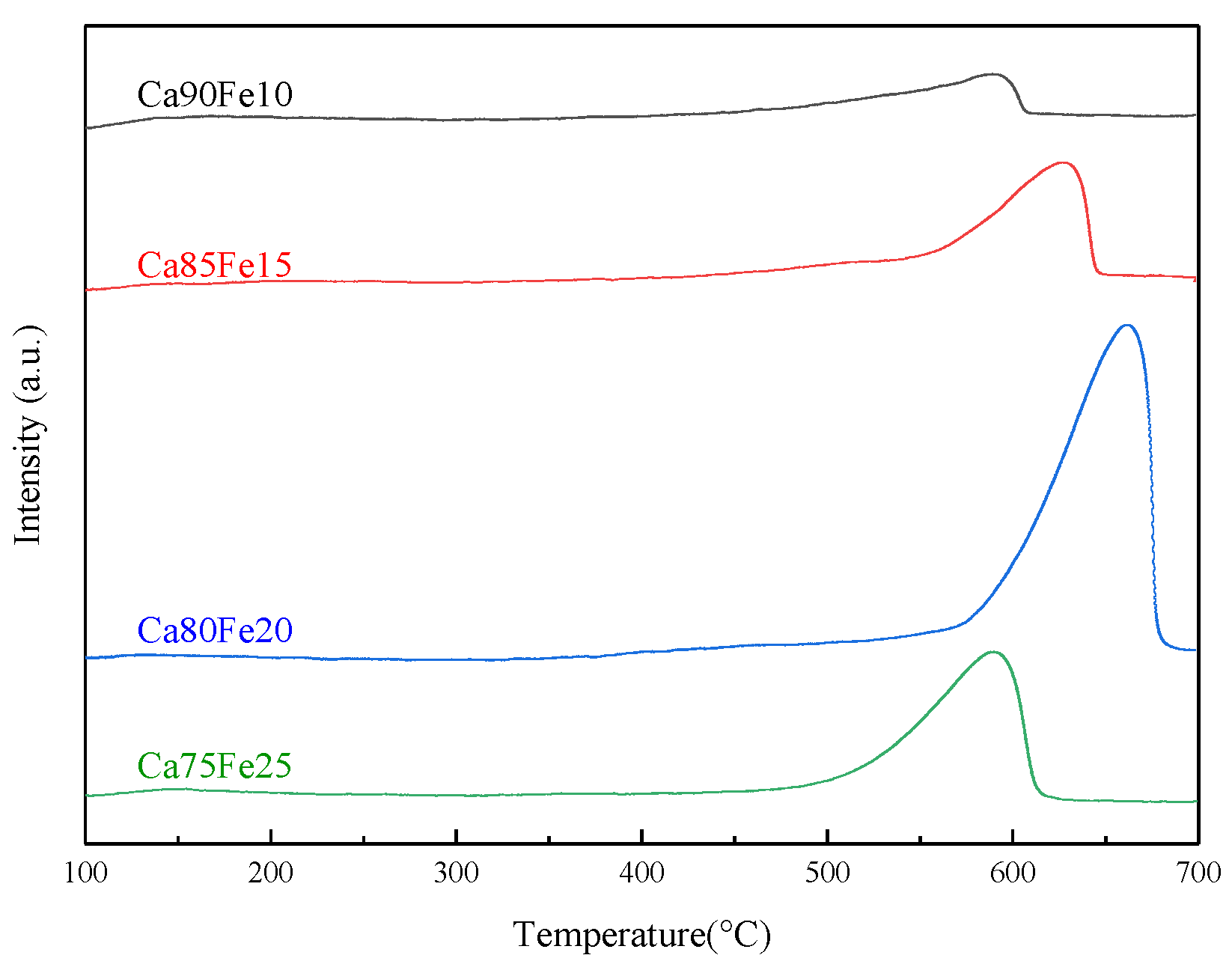
3.1.4. The CO2–TPD Analysis
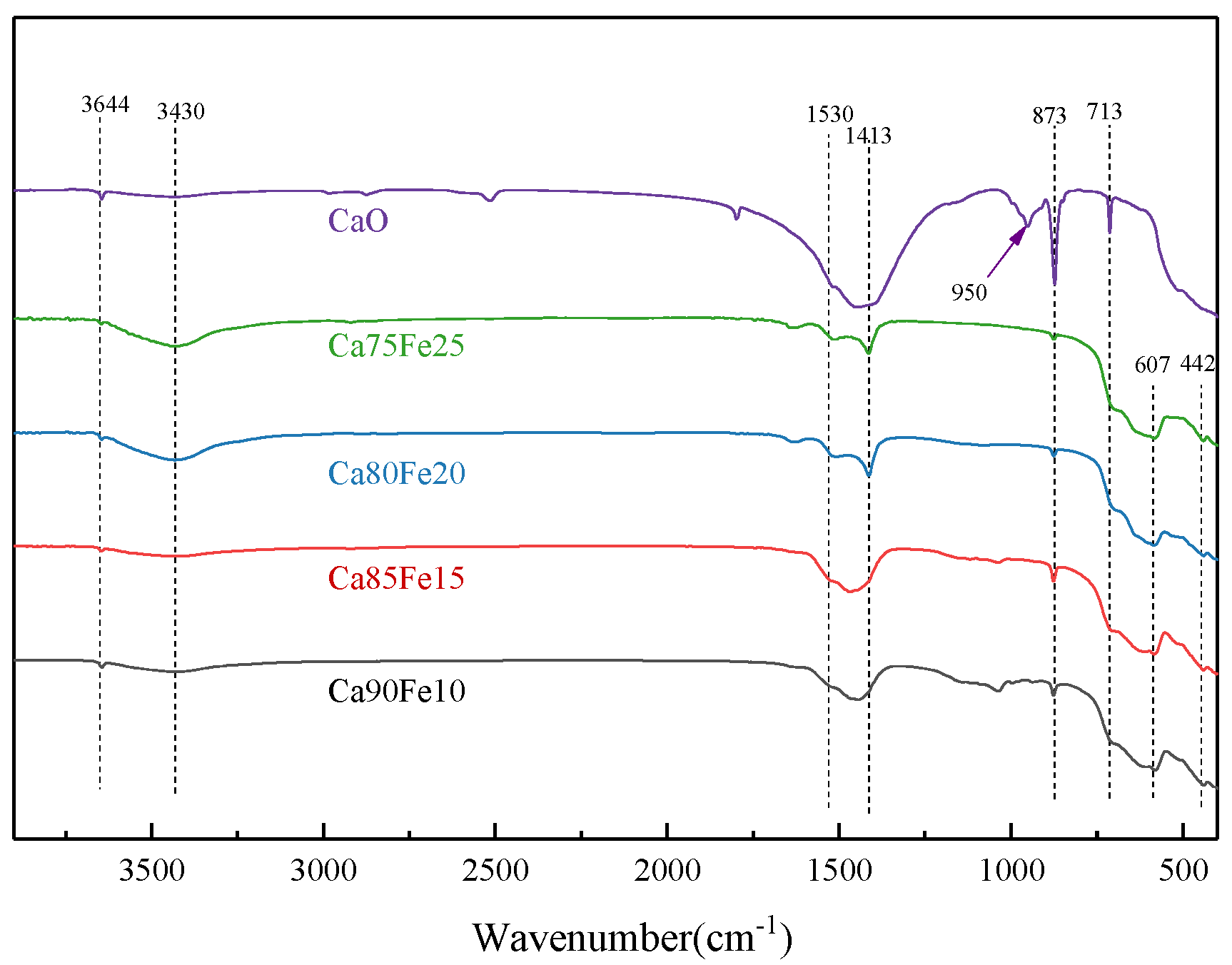
3.1.5. FT–IR Analysis
3.2. Catalyst Performance
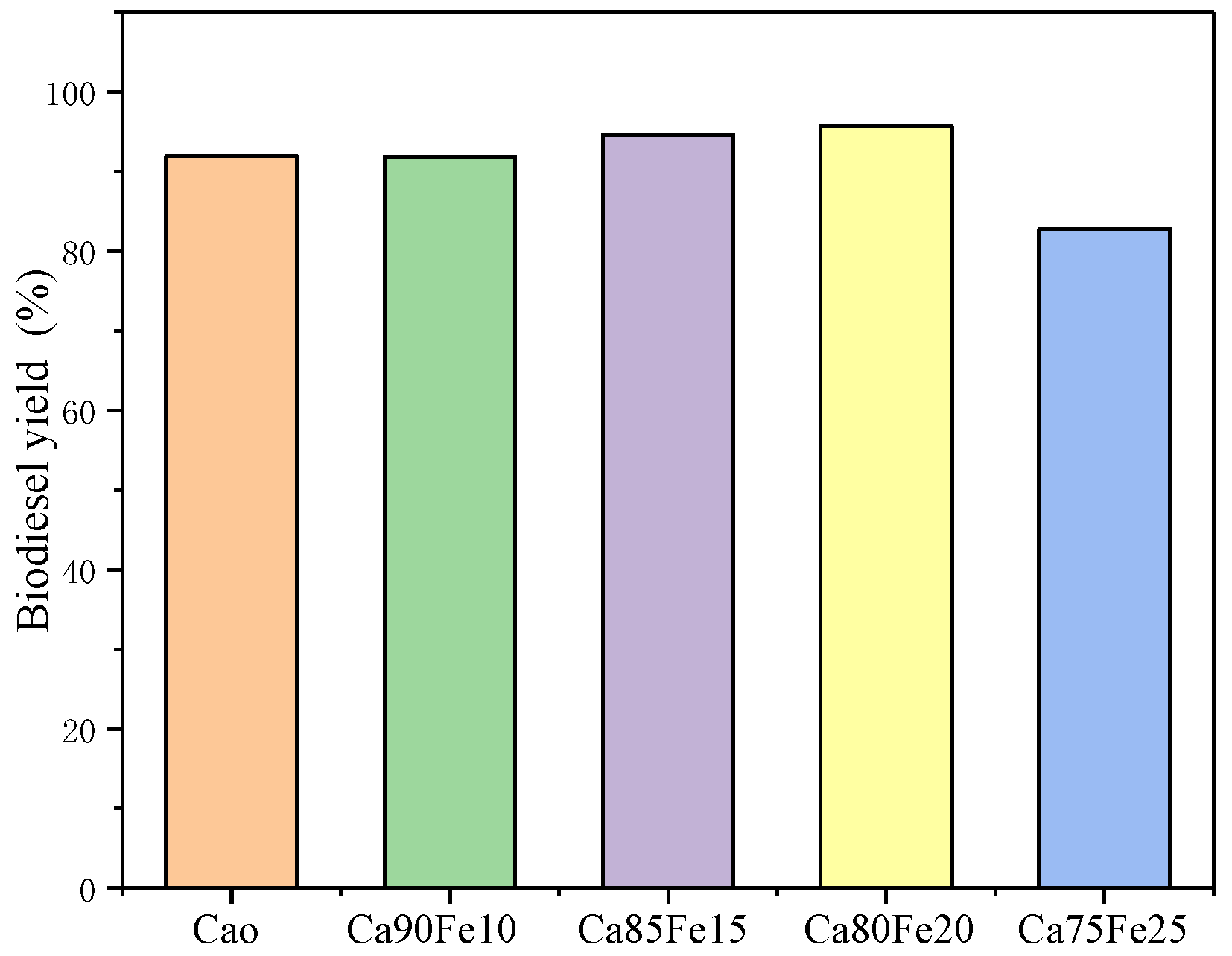
3.2.1. Catalyst Selection
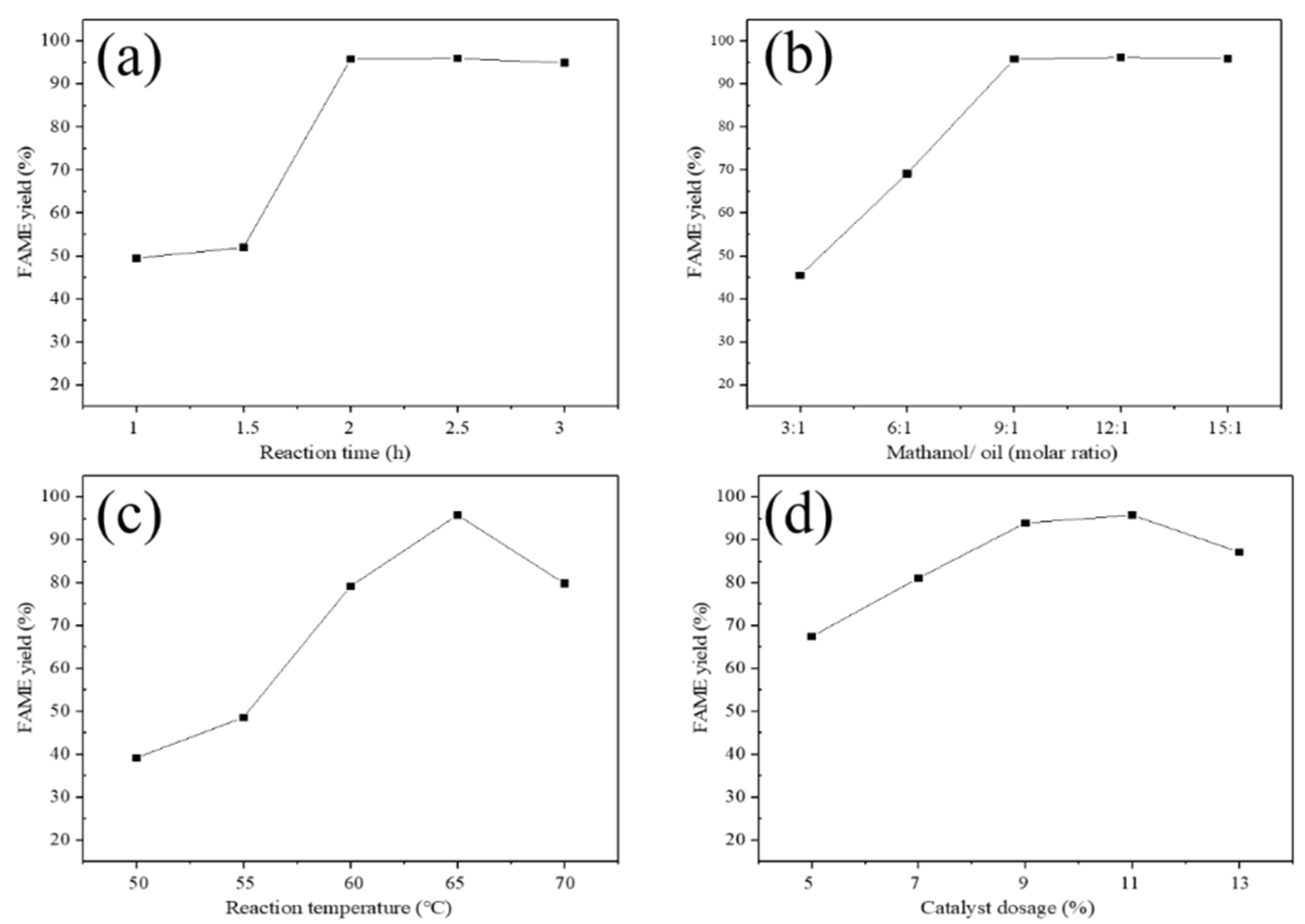
3.2.2. Effect of Reaction Time
3.2.3. Effect of Methanol Molar Ratio
3.2.4. Effect of Reaction Temperature
3.2.5. Effect of Catalyst Dosage
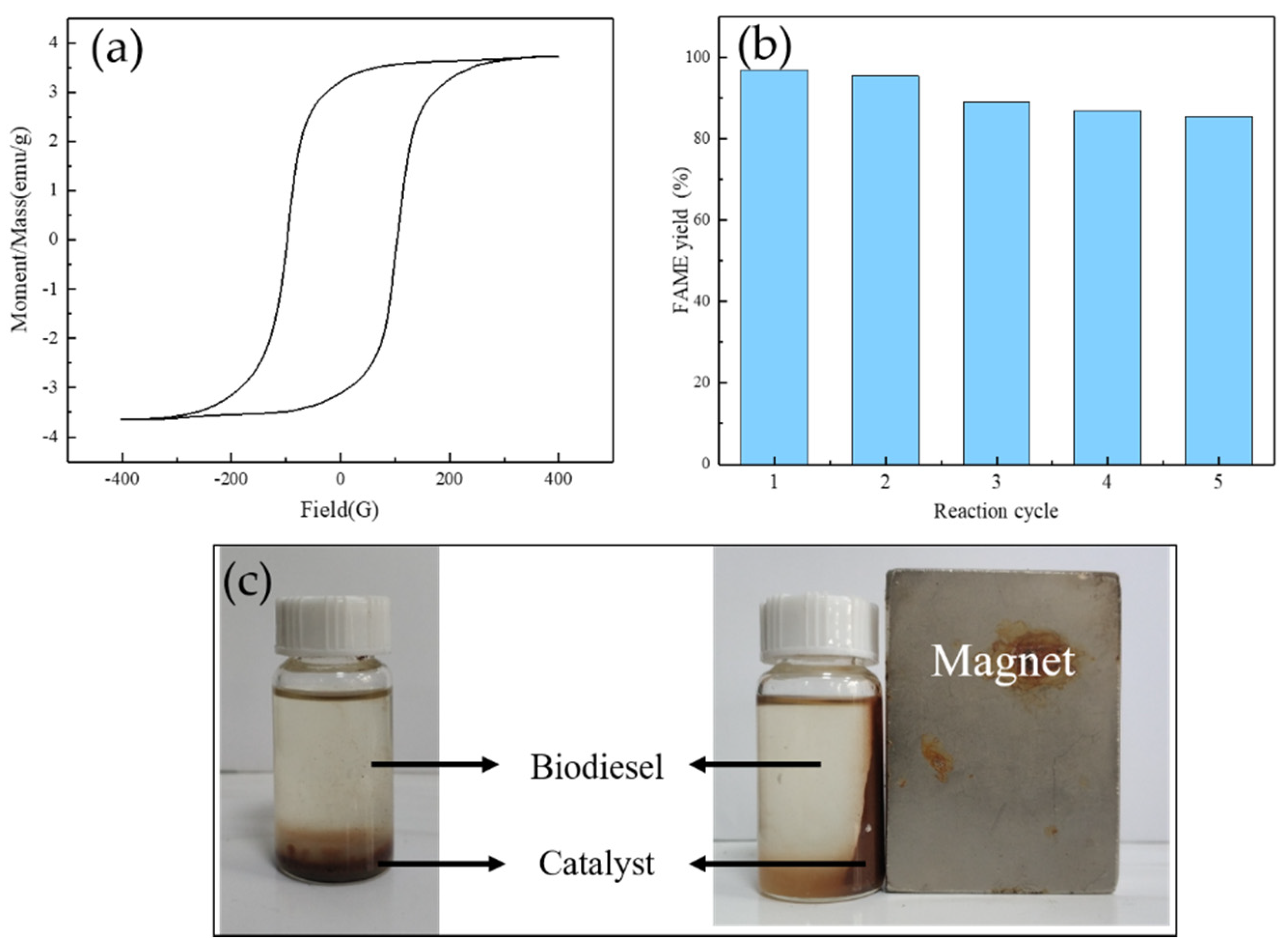
3.2.6. Reusability of Catalyst
3.3. Response Surface Methodology (RSM) Optimization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef] [PubMed]
- Sai, B.A.V.S.L.; Subramaniapillai, N.; Khadhar Mohamed, M.S.B.; Narayanan, A. Optimization of continuous biodiesel production from rubber seed oil (RSO) using calcined eggshells as heterogeneous catalyst. J. Environ. Chem. Eng. 2020, 8, 103603. [Google Scholar]
- Roschat, W.; Phewphong, S.; Thangthong, A.; Moonsin, P.; Yoosuk, B.; Kaewpuang, T.; Promarak, V. Catalytic performance enhancement of CaO by hydration-dehydration process for biodiesel production at room temperature. Energy Convers. Manag. 2018, 165, 1–7. [Google Scholar] [CrossRef]
- Mallah, T.A.; Sahito, A.R. Optimization of castor and neem biodiesel blends and development of empirical models to predicts its characteristics. Fuel 2020, 262, 116341. [Google Scholar] [CrossRef]
- Fazal, M.A.; Rubaiee, S.; Al-Zahrani, A. Overview of the interactions between automotive materials and biodiesel obtained from different feedstocks. Fuel Process. Technol. 2019, 196, 106178. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [Green Version]
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of Vegetable Oils with Ethanol and Characterization of the Key Fuel Properties of Ethyl Esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Gad, M.S.; Jayaraj, S. A comparative study on the effect of nano-additives on the performance and emissions of a diesel engine run on Jatropha biodiesel. Fuel 2020, 267, 117168. [Google Scholar] [CrossRef]
- Dahdah, E.; Estephane, J.; Haydar, R.; Youssef, Y.; El Khoury, B.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E.; Aouad, S. Biodiesel production from refined sunflower oil over Ca–Mg–Al catalysts: Effect of the composition and the thermal treatment. Renew. Energy 2020, 146, 1242–1248. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ong, H.C.; Masjuki, H.H.; Amini, Z.; Harrison, M.D.; Wang, C.; Kusumo, F.; Alwi, A. Rice bran oil based biodiesel production using calcium oxide catalyst derived from Chicoreus brunneus shell. Energy 2018, 144, 10–19. [Google Scholar] [CrossRef]
- Sirisomboonchai, S.; Abuduwayiti, M.; Guan, G.; Samart, C.; Abliz, S.; Hao, X.; Kusakabe, K.; Abudula, A. Biodiesel production from waste cooking oil using calcined scallop shell as catalyst. Energy Convers. Manag. 2015, 95, 242–247. [Google Scholar] [CrossRef]
- Veena Singh, L.B.B.S. Biodiesel production using a novel heterogeneous catalyst, magnesium zirconate (Mg2Zr5O12): Process optimization through response surface methodology RSM. Energy Convers. Manag. 2018, 174, 198–207. [Google Scholar] [CrossRef]
- Marinković, D.M.; Stanković, M.V.; Veličković, A.V.; Avramović, J.M.; Miladinović, M.R.; Stamenković, O.O.; Veljković, V.B.; Jovanović, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Banković Ilić, I.B.; Miladinović, M.R.; Stamenković, O.S.; Veljković, V.B. Application of nano CaO–based catalysts in biodiesel synthesis. Renew. Sustain. Energy Rev. 2017, 72, 746–760. [Google Scholar] [CrossRef]
- Risso, R.; Ferraz, P.; Meireles, S.; Fonseca, I.; Vital, J. Highly active Cao catalysts from waste shells of egg, oyster and clam for biodiesel production. Appl. Catal. A Gen. 2018, 567, 56–64. [Google Scholar] [CrossRef]
- Toledo Arana, J.; Torres, J.J.; Acevedo, D.F.; Illanes, C.O.; Ochoa, N.A.; Pagliero, C.L. One-Step Synthesis of CaO-ZnO Efficient Catalyst for Biodiesel Production. Int. J. Chem. Eng. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Yan, B.; Shi, J.; Li, S.; Liu, C. Remarkably enhancing the biodiesel yield from palm oil upon abalone shell-derived CaO catalysts treated by ethanol. Fuel Process. Technol. 2016, 143, 110–117. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Fan, M.; Jiang, P. Biodiesel production from soybean oil catalyzed by magnetic nanoparticle MgFe2O4@CaO. Fuel 2016, 164, 314–321. [Google Scholar] [CrossRef]
- Ezzah-Mahmudah, S.; Lokman, I.M.; Saiman, M.I.; Taufiq-Yap, Y.H. Synthesis and characterization of Fe2O3/CaO derived from Anadara Granosa for methyl ester production. Energy Convers. Manag. 2016, 126, 124–131. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, P.; Fan, M.; Jiang, P.; Dong, Y. Influence of crystal of Fe2O3 in magnetism and activity of nanoparticle CaO@Fe2O3 for biodiesel production. Fuel 2017, 197, 343–347. [Google Scholar] [CrossRef]
- Wan, D.; Yan, C.; Zhang, Q. Facile and Rapid Synthesis of Hollow Magnetic Mesoporous Polydopamine Nanoflowers with Tunable Pore Structures for Lipase Immobilization: Green Production of Biodiesel. Ind. Eng. Chem. Res. 2019, 58, 16358–16369. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Feng, J.; Guo, H.; Wang, S.; Zhao, Y.; Ma, X. Fabrication of multi-shelled hollow Mg-modified CaCO3 microspheres and their improved CO2 adsorption performance. Chem. Eng. J. 2017, 321, 401–411. [Google Scholar] [CrossRef]
- Loy, A.C.M.; Quitain, A.T.; Lam, M.K.; Yusup, S.; Sasaki, M.; Kida, T. Development of high microwave-absorptive bifunctional graphene oxide-based catalyst for biodiesel production. Energy Convers. Manag. 2019, 180, 1013–1025. [Google Scholar] [CrossRef]
- Naeem, M.A.; Armutlulu, A.; Imtiaz, Q.; Donat, F.; Schaublin, R.; Kierzkowska, A.; Muller, C.R. Optimization of the structural characteristics of CaO and its effective stabilization yield high-capacity CO2 sorbents. Nat. Commun. 2018, 9, 2408. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Luo, J.; Zhang, F.; Fang, Z. Biodiesel production from soybean and Jatropha oils by magnetic CaFe2O4–Ca2Fe2O5-based catalyst. Energy 2014, 68, 584–591. [Google Scholar] [CrossRef]
- Sano, N.; Yamada, K.; Tsunauchi, S.; Tamon, H. A novel solid base catalyst for transesterification of triglycerides toward biodiesel production: Carbon nanohorn dispersed with calcium ferrite. Chem. Eng. J. 2017, 307, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Maneerung, T.; Kawi, S.; Dai, Y.; Wang, C. Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure. Energy Convers. Manag. 2016, 123, 487–497. [Google Scholar] [CrossRef]
- Ramos Guivar, J.A.; Sanches, E.A.; Bruns, F.; Sadrollahi, E.; Morales, M.A.; López, E.O.; Litterst, F.J. Vacancy ordered γ-Fe2O3 nanoparticles functionalized with nanohydroxyapatite: XRD, FTIR, TEM, XPS and Mössbauer studies. Appl. Surf. Sci. 2016, 389, 721–734. [Google Scholar] [CrossRef]
- Khan, I.U.; Yan, Z.; Chen, J. Optimization, Transesterification and Analytical Study of Rhus typhina Non-Edible Seed Oil as Biodiesel Production. Energies 2019, 12, 4290. [Google Scholar] [CrossRef] [Green Version]
- Girdhar Joshi, D.S.R.B.; Kumar, S. Transesterification of Jatropha and Karanja oils by using waste egg shell derived calcium based mixed metal oxides. Energy Convers. Manag. 2015, 96, 258–267. [Google Scholar] [CrossRef]
- Abdollahi Asl, M.; Tahvildari, K.; Bigdeli, T. Eco-friendly synthesis of biodiesel from WCO by using electrolysis technique with graphite electrodes. Fuel 2020, 270, 117582. [Google Scholar] [CrossRef]
- Teo, S.H.; Islam, A.; Chan, E.S.; Thomas Choong, S.Y.; Alharthi, N.H.; Taufiq-Yap, Y.H.; Awual, M.R. Efficient biodiesel production from Jatropha curcus using CaSO4/Fe2O3-SiO2 core-shell magnetic nanoparticles. J. Clean. Prod. 2019, 208, 816–826. [Google Scholar] [CrossRef]
- Shi, Y.; Liang, X. Novel carbon microtube based solid acid from pampas grass stick for biodiesel synthesis from waste oils. J Saudi Chem. Soc. 2019, 23, 515–524. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Haapaniemi, E.; Sillanpää, M. Nano-magnetic potassium impregnated ceria as catalyst for the biodiesel production. Renew. Energy 2019, 139, 1428–1436. [Google Scholar] [CrossRef]
- Du, L.; Li, Z.; Ding, S.; Chen, C.; Qu, S.; Yi, W.; Lu, J.; Ding, J. Synthesis and characterization of carbon-based MgO catalysts for biodiesel production from castor oil. Fuel 2019, 258, 116122. [Google Scholar] [CrossRef]
- Li, X.; Lei, T.; Wang, Z.; Li, X.; Wen, M.; Yang, M.; Chen, G.; He, X.; Xu, H.; Guan, Q.; et al. Catalytic pyrolysis of corn straw with magnetic solid acid catalyst to prepare levulinic acid by response surface methodology. Ind. Crop. Prod. 2018, 116, 73–80. [Google Scholar] [CrossRef]
- Da Conceição, L.R.V.; Carneiro, L.M.; Rivaldi, J.D.; de Castro, H.F. Solid acid as catalyst for biodiesel production via simultaneous esterification and transesterification of macaw palm oil. Ind. Crop. Prod. 2016, 89, 416–424. [Google Scholar] [CrossRef]
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Reaction temperature (°C) | 50 | 60 | 70 |
| Reaction time (h) | 1 | 2 | 3 |
| Dosage of catalyst (wt. %) | 5 | 9 | 13 |
| Methanol: oil molar ratio (mol/mol) | 3 | 9 | 15 |
| Sample | Ca: Fe (Molar Ratio) 1 | Crystallite Size (nm) 2 | Surface Area (m2/g) | Average Pore volume (cm3/g) | Average Pore Diameter (Å) |
|---|---|---|---|---|---|
| Ca90Fe10 | 8.8:1.0 | 42.1 | 38.8 | 0.24 | 24.5 |
| Ca85Fe15 | 8.5:1.6 | 48.0 | 43.3 | 0.44 | 75.8 |
| Ca80Fe20 | 8.1:2.0 | 50.8 | 72.2 | 0.25 | 17.3 |
| Ca75Fe25 | 7.2:2.7 | 26.1 | 9.6 | 0.01 | 5.2 |
| Run | A (°C) | B(h) | C (wt. %) | D (mol/mol) | F (%) | G (%) |
|---|---|---|---|---|---|---|
| 1 | 60.00 | 1.00 | 9.00 | 3.00 | 36.80 | 36.04 |
| 2 | 70.00 | 1.00 | 9.00 | 9.00 | 67.92 | 66.15 |
| 3 | 60.00 | 1.00 | 9.00 | 15.00 | 51.80 | 52.08 |
| 4 | 60.00 | 3.00 | 5.00 | 9.00 | 65.75 | 59.35 |
| 5 | 60.00 | 2.00 | 13.00 | 15.00 | 78.88 | 78.61 |
| 6 | 60.00 | 3.00 | 9.00 | 3.00 | 35.00 | 32.63 |
| 7 | 60.00 | 3.00 | 13.00 | 9.00 | 72.89 | 69.98 |
| 8 | 50.00 | 2.00 | 9.00 | 3.00 | 23.71 | 23.53 |
| 9 | 70.00 | 3.00 | 9.00 | 9.00 | 62.76 | 70.81 |
| 10 | 60.00 | 3.00 | 9.00 | 15.00 | 85.74 | 84.41 |
| 11 | 60.00 | 1.00 | 13.00 | 9.00 | 52.86 | 58.16 |
| 12 | 50.00 | 3.00 | 9.00 | 9.00 | 46.01 | 50.96 |
| 13 | 50.00 | 2.00 | 13.00 | 9.00 | 41.79 | 46.60 |
| 14 | 60.00 | 2.00 | 9.00 | 9.00 | 72.45 | 71.34 |
| 15 | 60.00 | 2.00 | 13.00 | 3.00 | 32.23 | 27.93 |
| 16 | 60.00 | 2.00 | 9.00 | 9.00 | 70.14 | 71.34 |
| 17 | 50.00 | 2.00 | 9.00 | 15.00 | 37.80 | 32.54 |
| 18 | 60.00 | 2.00 | 9.00 | 9.00 | 75.45 | 71.34 |
| 19 | 60.00 | 2.00 | 9.00 | 9.00 | 70.21 | 71.34 |
| 20 | 50.00 | 2.00 | 5.00 | 9.00 | 21.22 | 21.77 |
| 21 | 60.00 | 2.00 | 9.00 | 9.00 | 68.45 | 71.34 |
| 22 | 70.00 | 2.00 | 13.00 | 9.00 | 67.32 | 64.68 |
| 23 | 70.00 | 2.00 | 9.00 | 15.00 | 87.99 | 87.08 |
| 24 | 60.00 | 2.00 | 5.00 | 3.00 | 27.98 | 31.44 |
| 25 | 50.00 | 1.00 | 9.00 | 9.00 | 31.57 | 26.70 |
| 26 | 60.00 | 1.00 | 5.00 | 9.00 | 40.44 | 42.25 |
| 27 | 70.00 | 2.00 | 5.00 | 9.00 | 69.88 | 62.97 |
| 28 | 60.00 | 2.00 | 5.00 | 15.00 | 41.08 | 48.57 |
| 29 | 70.00 | 2.00 | 9.00 | 3.00 | 24.12 | 28.28 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 11,091.53 | 14 | 792.25 | 24.95 | <0.0001 | significant |
| A | 2636.96 | 1 | 2636.96 | 83.05 | <0.0001 | |
| B | 627.04 | 1 | 627.04 | 19.75 | 0.0006 | |
| C | 528.09 | 1 | 528.09 | 16.63 | 0.0011 | |
| D | 3449.52 | 1 | 3449.52 | 108.64 | <0.0001 | |
| AB | 96.07 | 1 | 96.07 | 3.03 | 0.1039 | |
| AC | 133.70 | 1 | 133.70 | 4.21 | 0.0594 | |
| AD | 619.53 | 1 | 619.53 | 19.51 | 0.0006 | |
| BC | 6.97 | 1 | 6.97 | 0.22 | 0.6467 | |
| BD | 319.27 | 1 | 319.27 | 10.05 | 0.0068 | |
| CD | 281.50 | 1 | 281.50 | 8.87 | 0.0100 | |
| A2 | 1105.79 | 1 | 1105.79 | 34.83 | <0.0001 | |
| B2 | 138.86 | 1 | 138.86 | 4.37 | 0.0552 | |
| C2 | 558.62 | 1 | 558.62 | 17.59 | 0.0009 | |
| D2 | 1543.61 | 1 | 1543.61 | 48.61 | <0.0001 | |
| Residual | 444.54 | 14 | 31.75 | |||
| Lack of Fit | 415.38 | 10 | 41.54 | 5.70 | 0.0541 | not significant |
| Pure Error | 29.16 | 4 | 7.29 | |||
| Cor Total | 11,536.06 | 28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Yao, J.; Ren, Z.; Yu, Z.; Cai, H. Development of Magnetic Multi-Shelled Hollow Catalyst for Biodiesel Production. Energies 2020, 13, 2754. https://doi.org/10.3390/en13112754
Zhou L, Yao J, Ren Z, Yu Z, Cai H. Development of Magnetic Multi-Shelled Hollow Catalyst for Biodiesel Production. Energies. 2020; 13(11):2754. https://doi.org/10.3390/en13112754
Chicago/Turabian StyleZhou, Liang, Jingang Yao, Zhaoxia Ren, Zhenqiang Yu, and Hongzhen Cai. 2020. "Development of Magnetic Multi-Shelled Hollow Catalyst for Biodiesel Production" Energies 13, no. 11: 2754. https://doi.org/10.3390/en13112754
APA StyleZhou, L., Yao, J., Ren, Z., Yu, Z., & Cai, H. (2020). Development of Magnetic Multi-Shelled Hollow Catalyst for Biodiesel Production. Energies, 13(11), 2754. https://doi.org/10.3390/en13112754




