Effect of Various Parameters on the Thermal Stability and Corrosion of CO2-Loaded Tertiary Amine Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
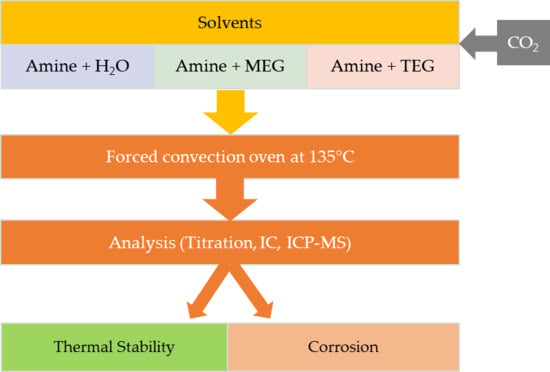
2.2. Methodology
Modeling
3. Results and Discussion
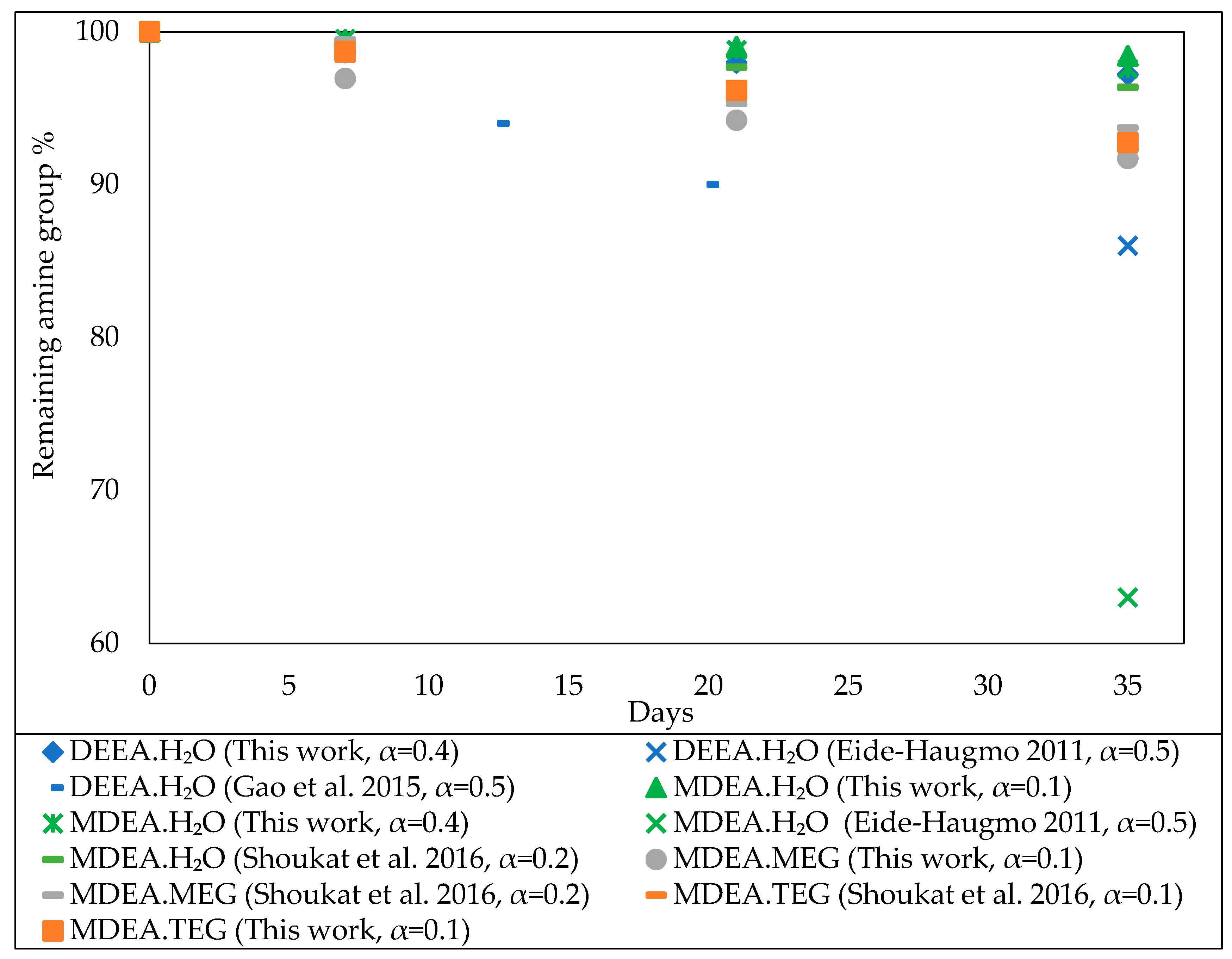
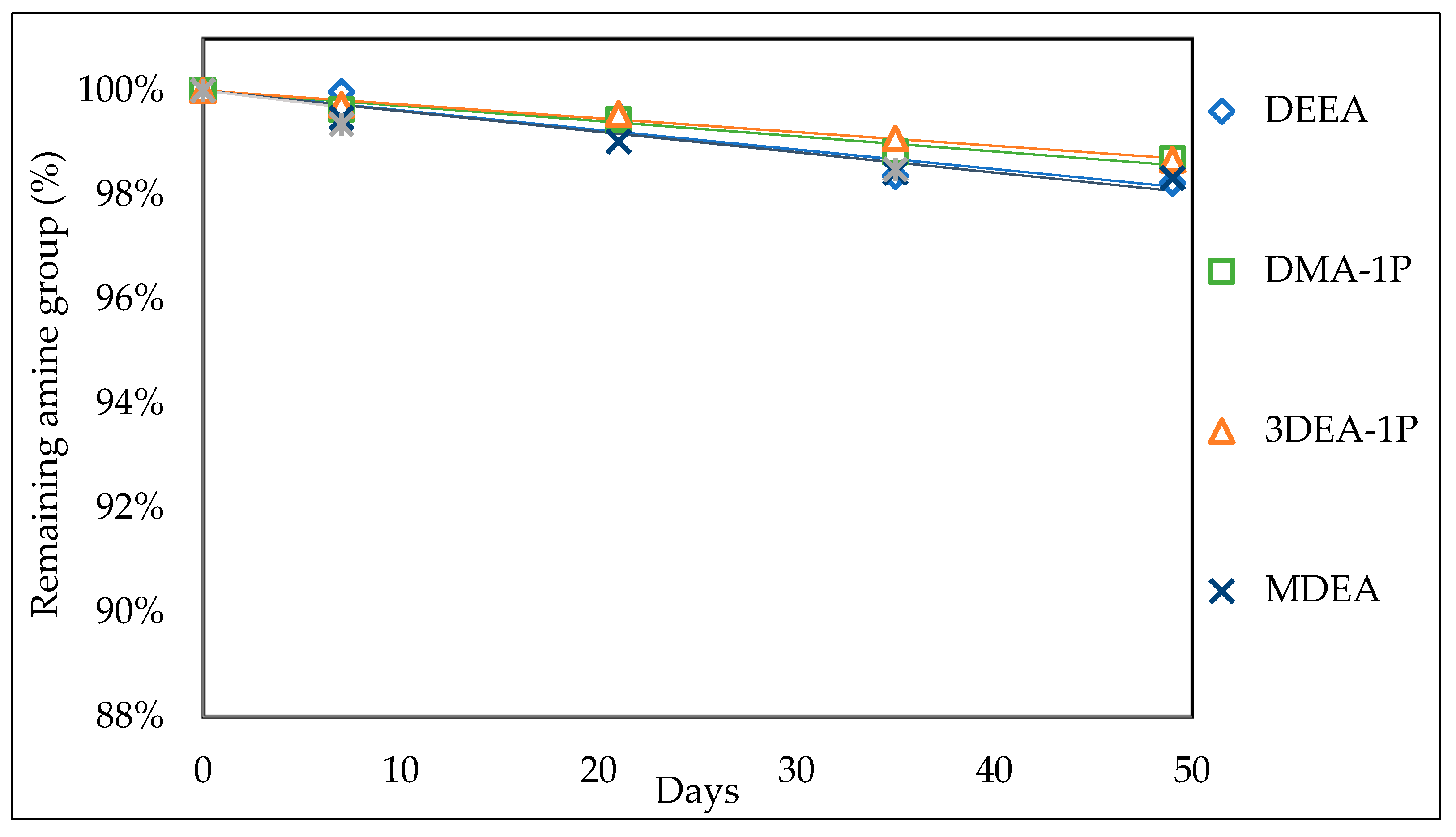
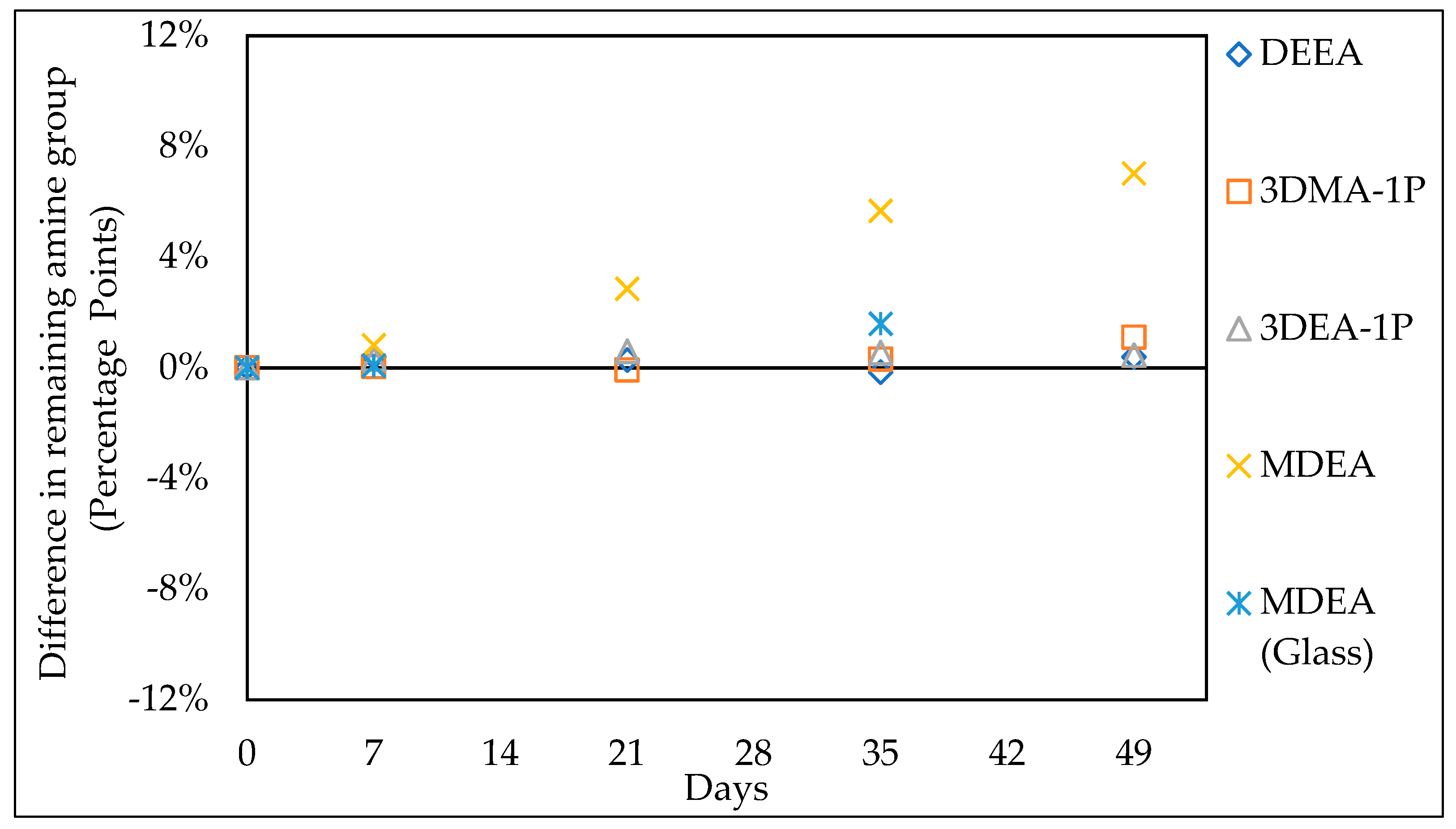
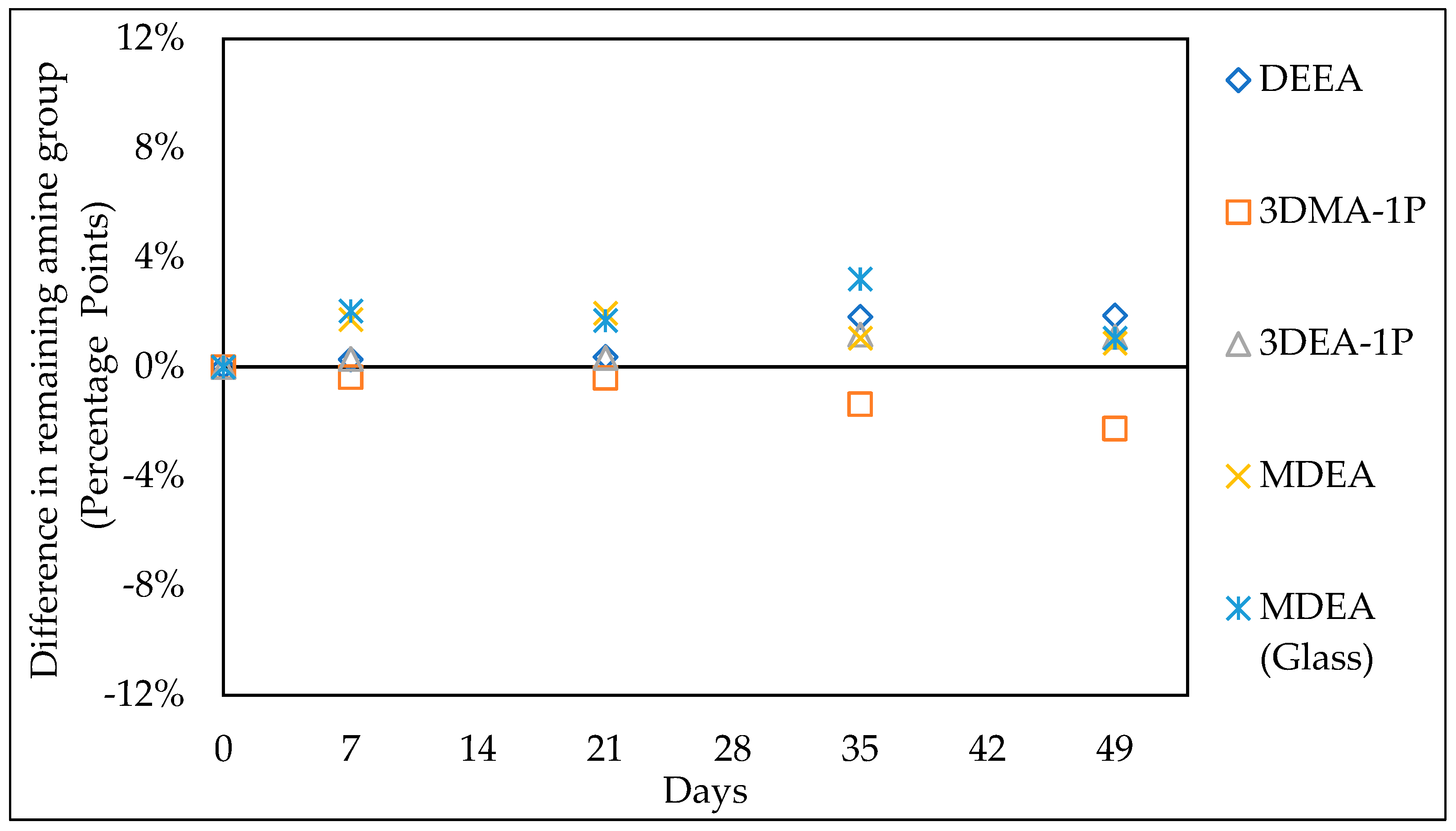
3.1. Comparison with Literature Data
3.1.1. Thermal Stability
3.1.2. Corrosion
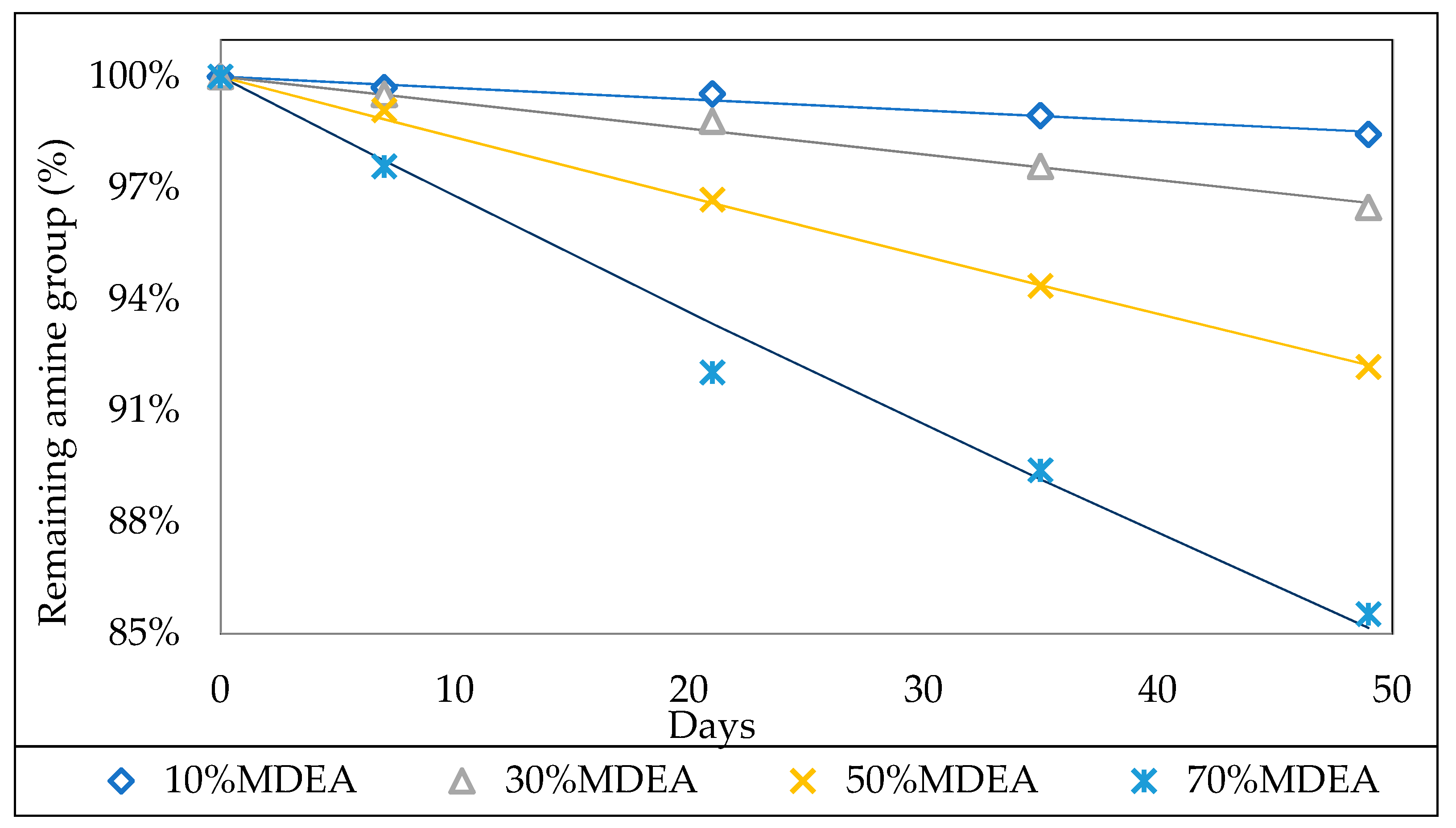
3.2. Thermal Stability
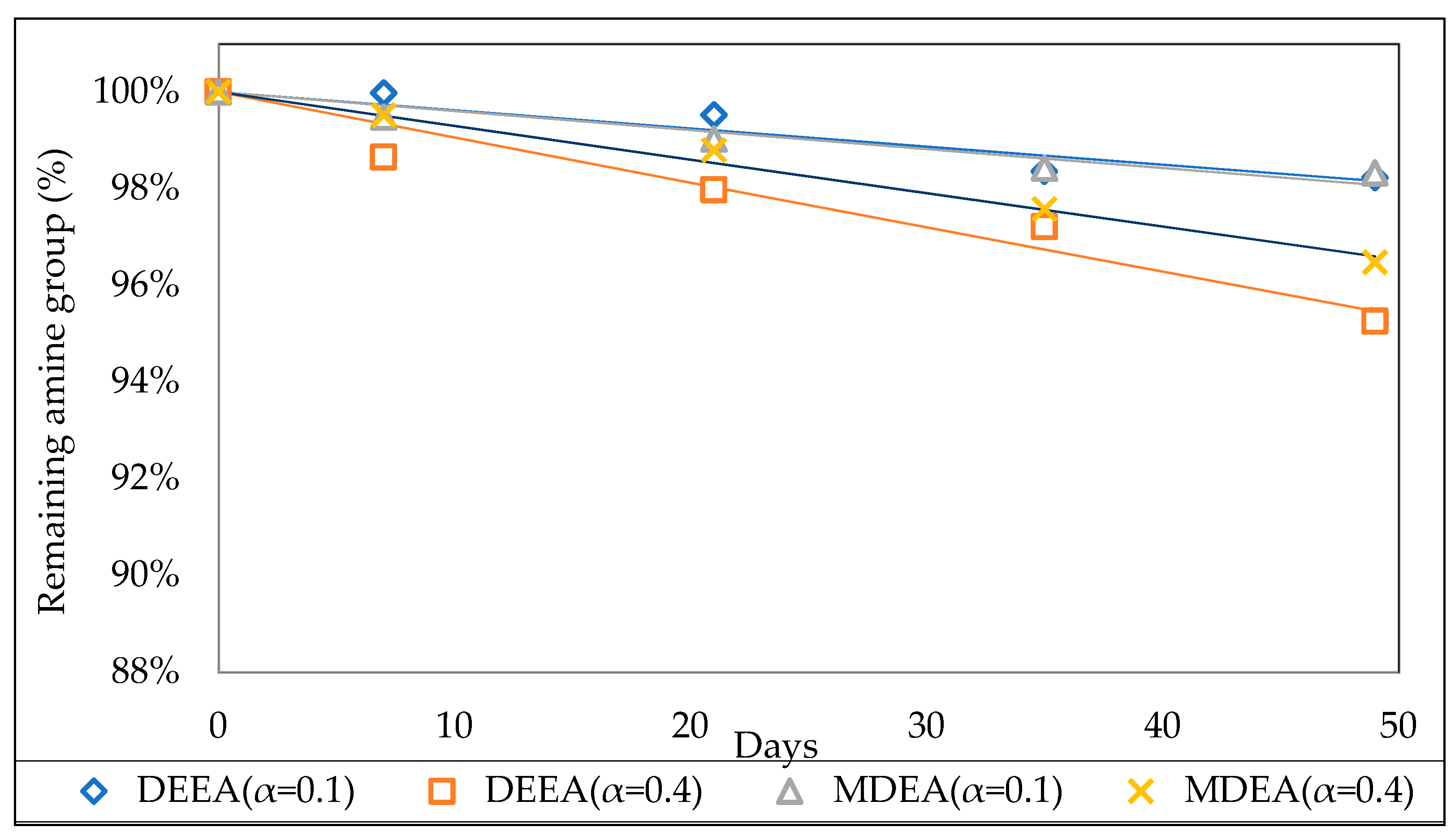
3.2.1. Effect of the Initial Amine Concentration
3.2.2. Effect of Initial CO2 Loading
3.3. Corrosion
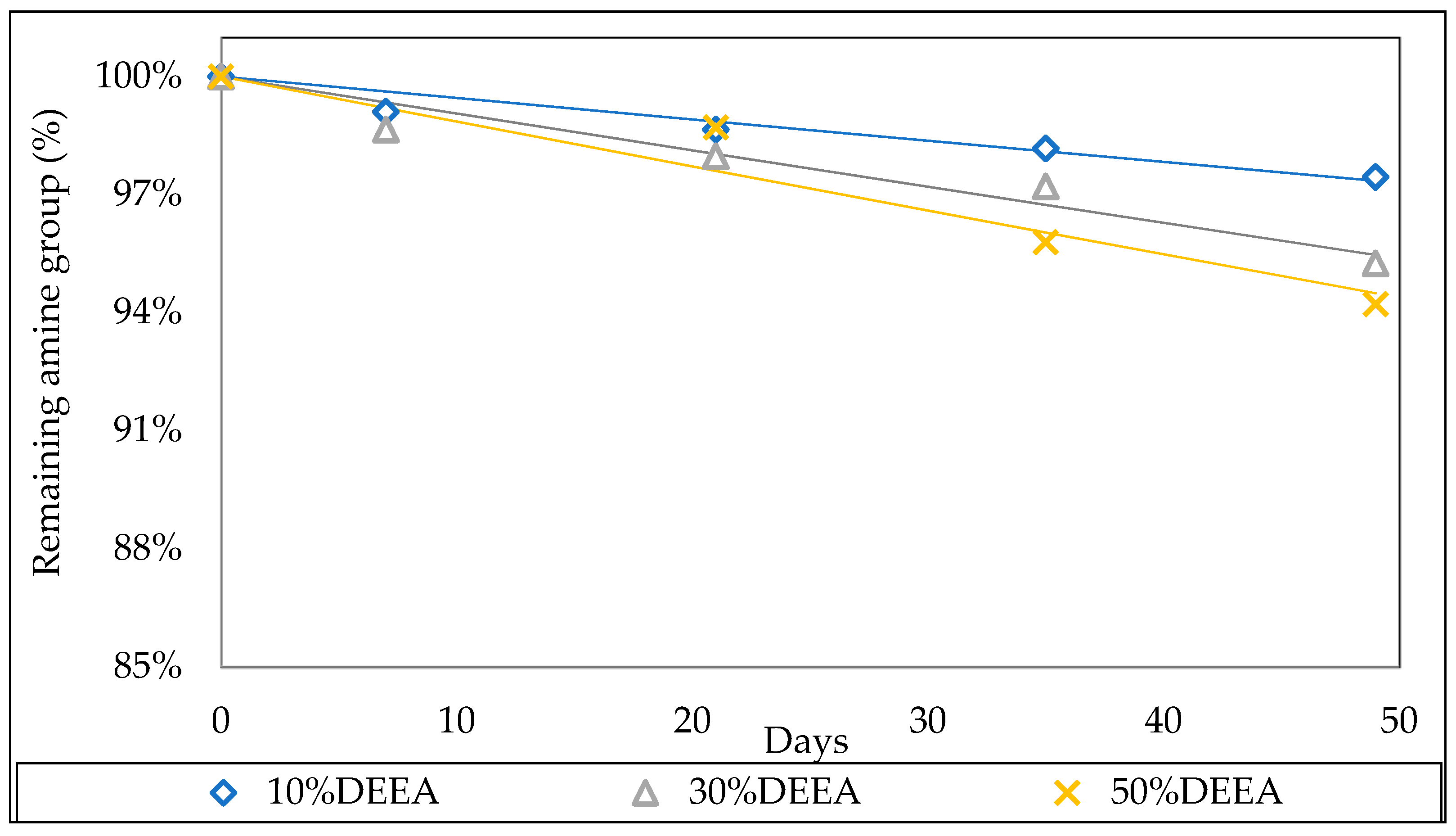
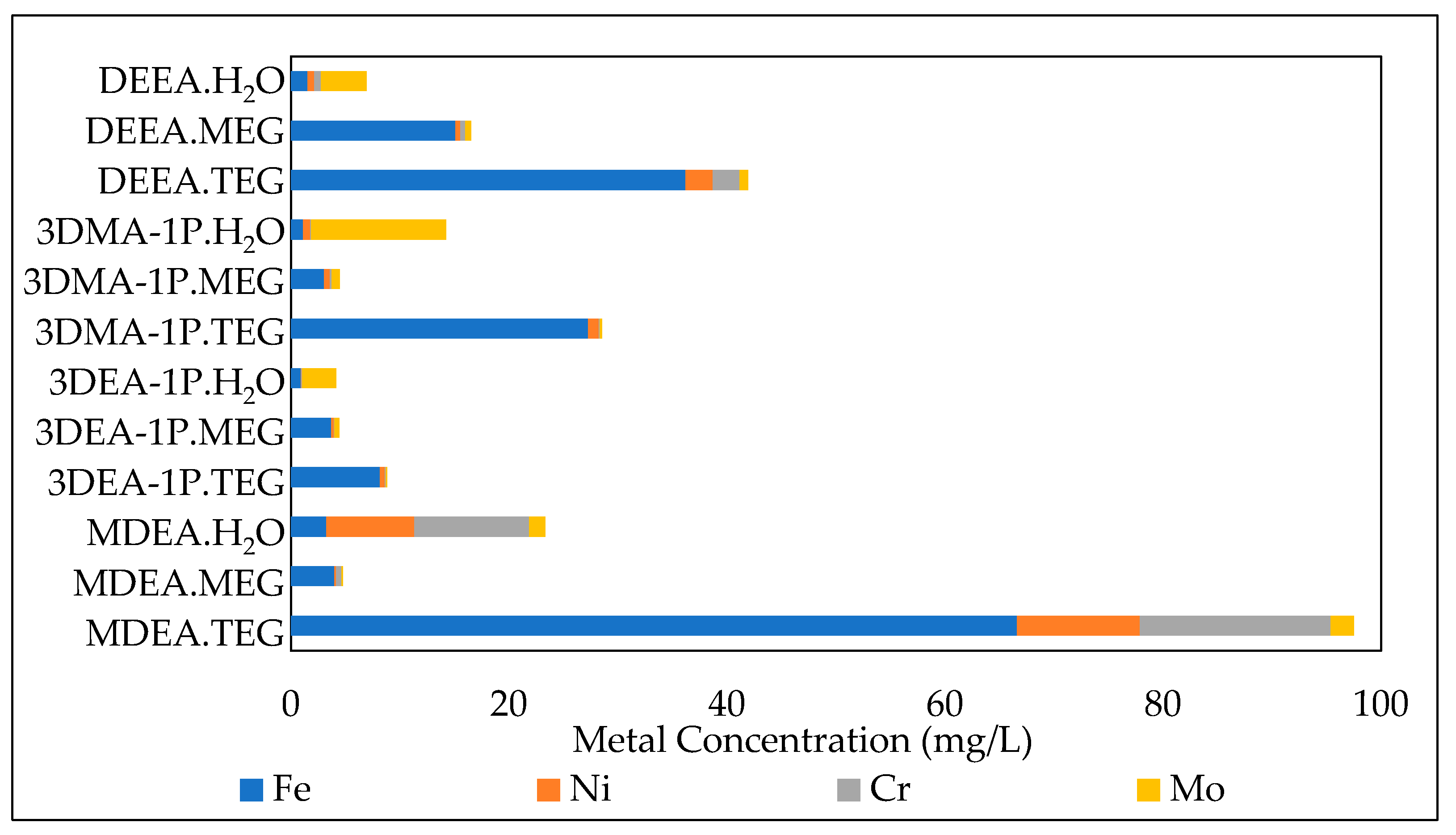
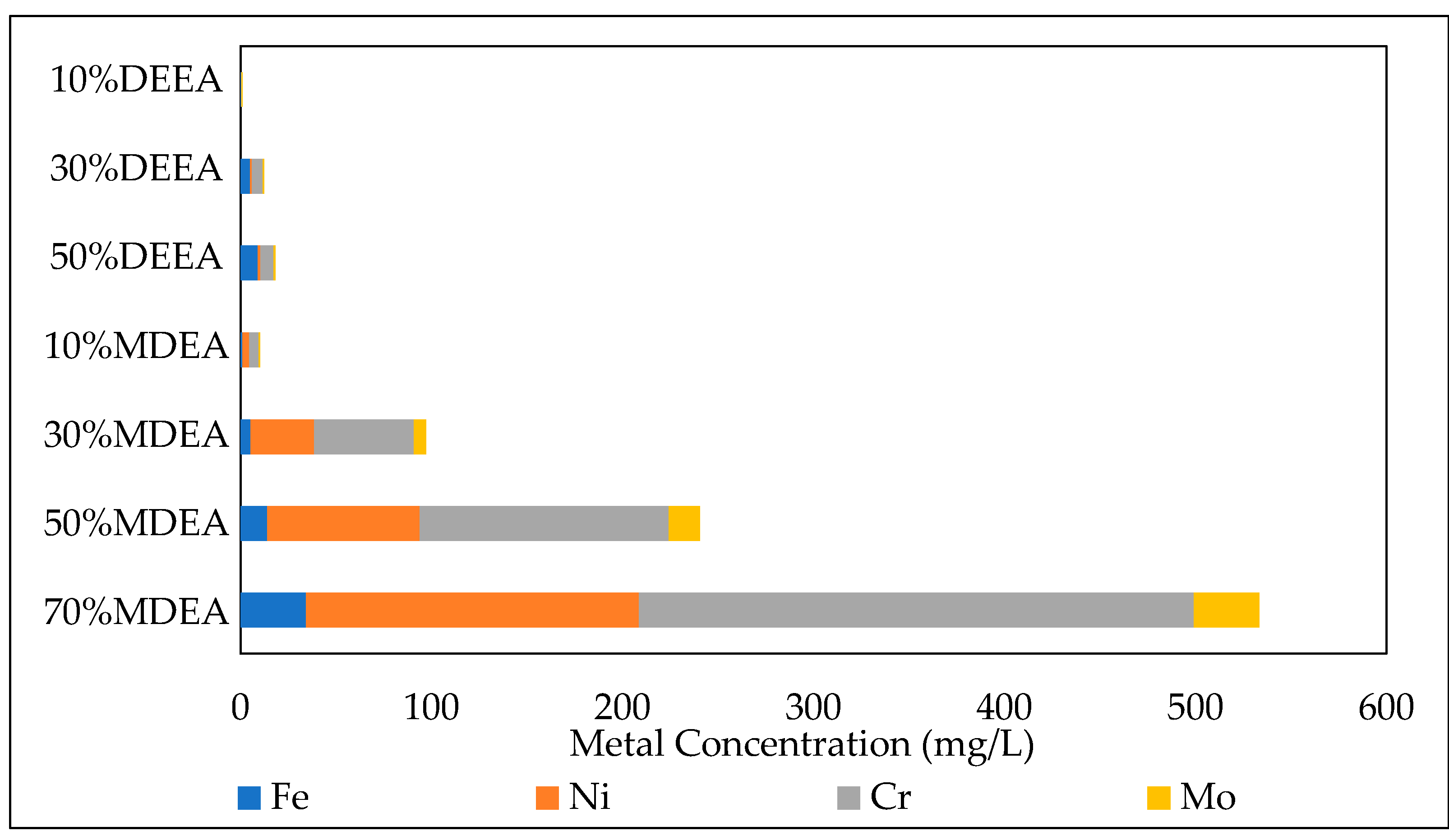
3.3.1. Effect of the initial amine concentration
3.3.2. Effect of Initial CO2 Loading
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Name | CO2 Loading (α) | Remaining Amine Group Concentration (mole/L) after Time (days) | ||||
|---|---|---|---|---|---|---|
| 0 | 7 | 21 | 35 | 49 | ||
| DEEA.H2O | 0.1 | 2.5005 | 2.4999 | 2.4886 | 2.4594 | 2.4561 |
| 3DMA-1P.H2O | 0.1 | 2.9098 | 2.8992 | 2.8935 | 2.8755 | 2.8716 |
| 3DEA-1P.H2O | 0.1 | 2.1932 | 2.1871 | 2.1833 | 2.1730 | 2.1641 |
| MDEA.H2O | 0.1 | 2.4178 | 2.4051 | 2.3942 | 2.3796 | 2.3772 |
| MDEA.H2O(Glass) | 0.1 | 2.4178 | 2.4022 | - | 2.3812 | - |
| DEEA.MEG | 0.1 | 2.5044 | 2.4871 | 2.4761 | 2.4212 | 2.4130 |
| 3DMA-1P.MEG | 0.1 | 2.9063 | 2.9052 | 2.9038 | 2.9025 | 2.9014 |
| 3DEA-1P.MEG | 0.1 | 2.2519 | 2.2318 | 2.2209 | 2.1922 | 2.1869 |
| MDEA.MEG | 0.1 | 2.4167 | 2.3426 | 2.2768 | 2.2162 | 2.1854 |
| MDEA.MEG(Glass) | 0.1 | 2.4167 | 2.3497 | 2.3161 | 2.2639 | 2.2371 |
| DEEA.TEG | 0.1 | 2.4816 | 2.4714 | 2.4628 | 2.4447 | 2.4378 |
| 3DMA-1P.TEG | 0.1 | 2.8995 | 2.8876 | 2.8856 | 2.8562 | 2.8295 |
| 3DEA-1P.TEG | 0.1 | 2.2387 | 2.2254 | 2.2153 | 2.2058 | 2.1987 |
| MDEA.TEG | 0.1 | 2.4297 | 2.3973 | 2.3367 | 2.2537 | 2.2183 |
| MDEA.TEG(Glass) | 0.1 | 2.4297 | 2.4121 | 2.3697 | 2.3543 | 2.2748 |
| DEEA.H2O | 0.4 | 0.8141 | 0.8068 | 0.8031 | 0.7992 | 0.7933 |
| DEEA.H2O | 0.4 | 2.4062 | 2.3737 | 2.3574 | 2.3390 | 2.2919 |
| DEEA.H2O | 0.4 | 3.9907 | - | 3.9392 | 3.8226 | 3.7596 |
| MDEA.H2O | 0.4 | 0.7779 | 0.7756 | 0.7743 | 0.7697 | 0.7658 |
| MDEA.H2O | 0.4 | 2.3081 | 2.2970 | 2.2802 | 2.2522 | 2.2266 |
| MDEA.H2O | 0.4 | 3.8348 | 3.8001 | 3.7076 | 3.6184 | 3.5348 |
| MDEA.H2O | 0.4 | 5.3424 | 5.2131 | 4.9166 | 4.7757 | 4.5688 |
| Name | Unloaded Amine Weight (%) | CO2 Loading (α) | Fe (mg/L) | Ni (mg/L) | Cr (mg/L) | Mo (mg/L) |
|---|---|---|---|---|---|---|
| DEEA.H2O | 30 | 0.1 | 1.55 | 0.62 | 0.62 | 4.17 |
| 3DMA-1P.H2O | 30 | 0.1 | 1.13 | 0.66 | 0.07 | 12.36 |
| 3DEA-1P.H2O | 30 | 0.1 | 1.15 | 0.06 | 0.11 | 3.15 |
| MDEA.H2O | 30 | 0.1 | 3.24 | 8.08 | 10.53 | 1.49 |
| DEEA.MEG | 30 | 0.1 | 15.11 | 0.44 | 0.44 | 0.56 |
| 3DMA-1P.MEG | 30 | 0.1 | 3.06 | 0.53 | 0.14 | 0.74 |
| 3DEA-1P.MEG | 30 | 0.1 | 3.70 | 0.22 | 0.03 | 0.36 |
| MDEA.MEG | 30 | 0.1 | 3.99 | 0.16 | 0.51 | 0.10 |
| DEEA.TEG | 30 | 0.1 | 36.21 | 2.48 | 2.48 | 0.76 |
| 3DMA-1P.TEG | 30 | 0.1 | 27.28 | 0.98 | 0.08 | 0.22 |
| 3DEA-1P.TEG | 30 | 0.1 | 8.18 | 0.46 | 0.07 | 0.12 |
| MDEA.TEG | 30 | 0.1 | 66.64 | 11.25 | 17.53 | 2.12 |
| DEEA.H2O | 10 | 0.4 | 0.33 | 0.01 | 0.26 | 0.37 |
| DEEA.H2O | 30 | 0.4 | 5.06 | 0.91 | 5.69 | 0.64 |
| DEEA.H2O | 50 | 0.4 | 9.11 | 1.46 | 6.91 | 0.84 |
| MDEA.H2O | 10 | 0.4 | 1.13 | 3.54 | 4.83 | 0.82 |
| MDEA.H2O | 30 | 0.4 | 5.42 | 33.24 | 52.27 | 6.31 |
| MDEA.H2O | 50 | 0.4 | 14.10 | 79.80 | 130.50 | 16.07 |
| MDEA.H2O | 70 | 0.4 | 34.48 | 174.17 | 290.49 | 34.39 |
References
- Amosa, M.; Mohammed, I.; Yaro, S. Sulphide scavengers in oil and gas industry–A review. Nafta 2010, 61, 85–92. [Google Scholar]
- Jalili, A.H.; Shokouhi, M.; Maurer, G.; Zoghi, A.T.; Ahari, J.; Forsat, K. Measuring and modelling the absorption and volumetric properties of CO2 and H2S in the ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate. J. Chem. Thermodyn. 2019, 131, 544–556. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Chapter 11—Absorption of Water Vapor by Dehydrating Solutions. In Gas Purification, 5th ed.; Kohl, A.L., Nielsen, R.B., Eds.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 946–1021. [Google Scholar]
- Kohl, A.L.; Nielsen, R.B. Chapter 2—Alkanolamines for Hydrogen Sulfide and Carbon Dioxide Removal. In Gas Purification, 5th ed.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 40–186. [Google Scholar]
- Netusil, M.; Ditl, P. Comparison of three methods for natural gas dehydration. J. Nat. Gas Chem. 2011, 20, 471–476. [Google Scholar] [CrossRef]
- Hutchinson, A.J. Process for Treating Gases. U.S. Patent US2177068 A, 1939. [Google Scholar]
- McCartney, E.R. Gas Purification and Dehydration Process. U.S. Patent US2435089 A, 28 June 1948. [Google Scholar]
- Chapin, W.F. Purification and Dehydration of Gases. U.S. Patent US2518752 A, 28 June 1950. [Google Scholar]
- Chowdhury, F.A.; Yamada, H.; Higashii, T.; Goto, K.; Onoda, M. CO2 capture by tertiary amine absorbents: A performance comparison study. Ind. Eng. Chem. Res. 2013, 52, 8323–8331. [Google Scholar] [CrossRef]
- Shoukat, U.; Fytianos, G.; Knuutila, H.K. Thermal stability and corrosion studies of amines for combined acid gas removal and hydrate control for subsea gas treatment systems. In Techno-Ocean 2016: Return to the Oceans; IEEE: Kobe, Japan, 2016; pp. 176–180. [Google Scholar]
- Barth, D.; Tondre, C.; Delpuech, J.J. Kinetics and mechanisms of the reactions of carbon dioxide with alkanolamines: A discussion concerning the cases of MDEA and DEA. Chem. Eng. Sci. 1984, 39, 1753–1757. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Kenig, E.Y. CO2-Alkanolamine Reaction Kinetics: A Review of Recent Studies. Chem. Eng. Technol. 2007, 30, 1467–1474. [Google Scholar] [CrossRef]
- Chi, S.; Rochelle, G.T. Oxidative degradation of monoethanolamine. Ind. Eng. Chem. Res. 2002, 41, 4178–4186. [Google Scholar] [CrossRef]
- Vevelstad, S.J.; Grimstvedt, A.; Elnan, J.; da Silva, E.F.; Svendsen, H.F. Oxidative degradation of 2-ethanolamine: The effect of oxygen concentration and temperature on product formation. Int. J. Greenh. Gas Control 2013, 18 (Suppl. SC), 88–100. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Yusoff, R.; Si Ali, B.; Islam, M.N.; Chakrabarti, M.H. Degradation studies of amines and alkanolamines during sour gas treatment process. Int. J. Phys. Sci. 2011, 6, 5883–5896. [Google Scholar]
- Gouedard, C.; Picq, D.; Launay, F.; Carrette, P.L. Amine degradation in CO2 capture. I. A review. Int. J. Greenh. Gas Control 2012, 10, 244–270. [Google Scholar] [CrossRef]
- Da Silva, E.F.; Lepaumier, H.; Grimstvedt, A.; Vevelstad, S.J.; Einbu, A.; Vernstad, K.; Svendsen, H.F.; Zahlsen, K. Understanding 2-ethanolamine degradation in postcombustion CO2 capture. Ind. Eng. Chem. Res. 2012, 51, 13329–13338. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, S.; Chen, C. Thermal degradation of monoethanolamine in CO2 capture with acidic impurities in flue gas. Ind. Eng. Chem. Res. 2012, 51, 2539–2547. [Google Scholar] [CrossRef]
- Zoannou, K.S.; Sapsford, D.J.; Griffiths, A.J. Thermal degradation of monoethanolamine and its effect on CO2 capture capacity. Int. J. Greenh. Gas Control 2013, 17, 423–430. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [Green Version]
- Freeman, S.A.; Davis, J.; Rochelle, G.T. Degradation of aqueous piperazine in carbon dioxide capture. Int. J. Greenh. Gas Control 2010, 4, 756–761. [Google Scholar] [CrossRef]
- Davoudi, M.; Safadoust, A.R.; Akbar Mansoori, S.A.; Mottaghi, H.R. The impurities effect on thermal degradation and corrosivity of amine solution in South Pars gas sweetening plants. J. Nat. Gas Sci. Eng. 2014, 19, 116–124. [Google Scholar] [CrossRef]
- Sandengen, K.; Kaasa, B.; Østvold, T. pH Measurements in Monoethylene Glycol (MEG) + Water Solutions. Ind. Eng. Chem. Res. 2007, 46, 4734. [Google Scholar] [CrossRef]
- Davoudi, M.; Heidari, Y.; Safadoost, A.; Samieirad, S. Chemical injection policy for internal corrosion prevention of South Pars sea-pipeline: A case study. J. Nat. Gas Sci. Eng. 2014, 21, 592. [Google Scholar] [CrossRef]
- AlHarooni, K.; Pack, D.; Iglauer, S.; Gubner, R.; Ghodkay, V.; Barifcani, A. Analytical Techniques for Analyzing Thermally Degraded Monoethylene Glycol with Methyl Diethanolamine and Film Formation Corrosion Inhibitor. Energy Fuels 2016, 30, 10937–10949. [Google Scholar] [CrossRef]
- Khorrami, M.R.; Raeissi, K.; Shahban, H.; Torkan, M.A.; Saatchi, A. Corrosion behavior of carbon steel in carbon dioxide-loaded activated methyl diethanol amine solution. Corrosion 2008, 64, 124–130. [Google Scholar] [CrossRef]
- Zheng, L.; Landon, J.; Matin, N.S.; Thomas, G.A.; Liu, K. Corrosion mitigation via a pH stabilization method in monoethanolamine-based solutions for post-combustion CO2 capture. Corros. Sci. 2016, 106, 281. [Google Scholar] [CrossRef] [Green Version]
- Fytianos, G.; Ucar, S.; Grimstvedt, A.; Hyldbakk, A.; Svendsen, H.F.; Knuutila, H.K. Corrosion and degradation in MEA based post-combustion CO2 capture. Int. J. Greenh. Gas Control 2016, 46, 48–56. [Google Scholar] [CrossRef]
- Xiang, Y.; Yan, M.; Choi, Y.-S.; Young, D.; Nesic, S. Time-dependent electrochemical behavior of carbon steel in MEA-based CO2 capture process. Int. J. Greenh. Gas Control 2014, 30 (Suppl. SC), 125–132. [Google Scholar]
- Nordsveen, M.; Nešić, S.; Nyborg, R.; Stangeland, A. A Mechanistic Model for Carbon Dioxide Corrosion of Mild Steel in the Presence of Protective Iron Carbonate Films—Part 1: Theory and Verification. Corrosion 2003, 59, 443–456. [Google Scholar] [CrossRef] [Green Version]
- AlHarooni, K.; Pack, D.; Iglauer, S.; Gubner, R.; Ghodkay, V.; Barifcani, A. Effects of Thermally Degraded Monoethylene Glycol with Methyl Diethanolamine and Film-Forming Corrosion Inhibitor on Gas Hydrate Kinetics. Energy Fuels 2017, 31, 6397–6412. [Google Scholar] [CrossRef]
- Chakma, A.; Meisen, A. Methyl-Diethanolamine Degradation - Mechanism and Kinetics. Can. J. Chem. Eng. 1997, 75, 861–871. [Google Scholar] [CrossRef]
- Lepaumier, H.; Picq, D.; Carrette, P.L. New amines for CO2 Capture. I. mechanisms of amine degradation in the presence of CO2. Ind. Eng. Chem. Res. 2009, 48, 9061–9067. [Google Scholar] [CrossRef]
- Closmann, F.; Rochelle, G.T. Degradation of aqueous methyldiethanolamine by temperature and oxygen Cycling. Energy Procedia 2011, 4, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Eide-Haugmo, I. Environmental Impacts and Aspects of Absorbents used for CO2 Capture. Ph.D. Thesis, Norwegian Univeristy of Science and Technology, Trondheim, Norway, 2011. [Google Scholar]
- Handojo, L.; Yudiyanto, M.D.; Susanti, R.F.; Yaswari, Y.; Raksajati, A.; Indarto, A. Non-oxidative thermal degradation of amines: GCMS/FTIR spectra analysis and molecular modeling. Sep. Sci. Technol. 2018, 53, 2259–2266. [Google Scholar] [CrossRef]
- Shoukat, U.; Baumeister, E.; Pinto, D.D.; Knuutila, H.K. Thermal stability and corrosion of tertiary amines in aqueous amine and amine-glycol-water solutions for combined acid gas and water removal. J. Nat. Gas Sci. Eng. 2019, 62, 26–37. [Google Scholar] [CrossRef]
- Shoukat, U.; Baumeister, E.; Knuutila, H.K. ATR-FTIR Model Development and Verification for Qualitative and Quantitative Analysis in MDEA–H2O–MEG/TEG–CO2 Blends. Energies 2019, 12, 3285. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Liang, Z.; Liao, H.; Idem, R.O. Thermal degradation of aqueous DEEA solution at stripper conditions for post-combustion CO2 capture. Chem. Eng. Sci. 2015, 135, 330–342. [Google Scholar] [CrossRef]
- Rossiter, W.J.; Brown, P.W.; Godette, M. The determination of acidic degradation products in aqueous ethylene glycol and propylene glycol solutions using ion chromatography. Sol. Energy Mater. 1983, 9, 267–279. [Google Scholar] [CrossRef]
- Campbell, J.M. Gas Conditioning and Processing; Campbell Petroleum Series: Norman, OK, USA, 1982. [Google Scholar]
- Grimstvedt, A.; da Silva, E.F.; Hoff, K.A. Thermal degradation of MEA, effect of temperature and CO2 loading. TCCS7 SINTEF Mater. Chem. 2013, 7465. [Google Scholar]
- Shoukat, U.; Pinto, D.D.D.; Knuutila, H.K. Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas. Processes 2019, 7, 160. [Google Scholar] [CrossRef] [Green Version]
- Ma’mun, S.; Svendsen, H.F.; Hoff, K.A.; Juliussen, O. Selection of new absorbents for carbon dioxide capture. Energy Convers. Manag. 2007, 48, 251–258. [Google Scholar] [CrossRef]
- Monteiro, J.G.M.S.; Pinto, D.D.D.; Zaidy, S.A.H.; Hartono, A.; Svendsen, H.F. VLE data and modelling of aqueous N,N-diethylethanolamine (DEEA) solutions. Int. J. Greenh. Gas Control 2013, 19, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Vevelstad, S.J.; Johansen, M.T.; Knuutila, H.; Svendsen, H.F. Oxygen and Temperature Effect on Formation of Degradation Compounds from MEA. Energy Procedia 2014, 63, 957–975. [Google Scholar] [CrossRef] [Green Version]
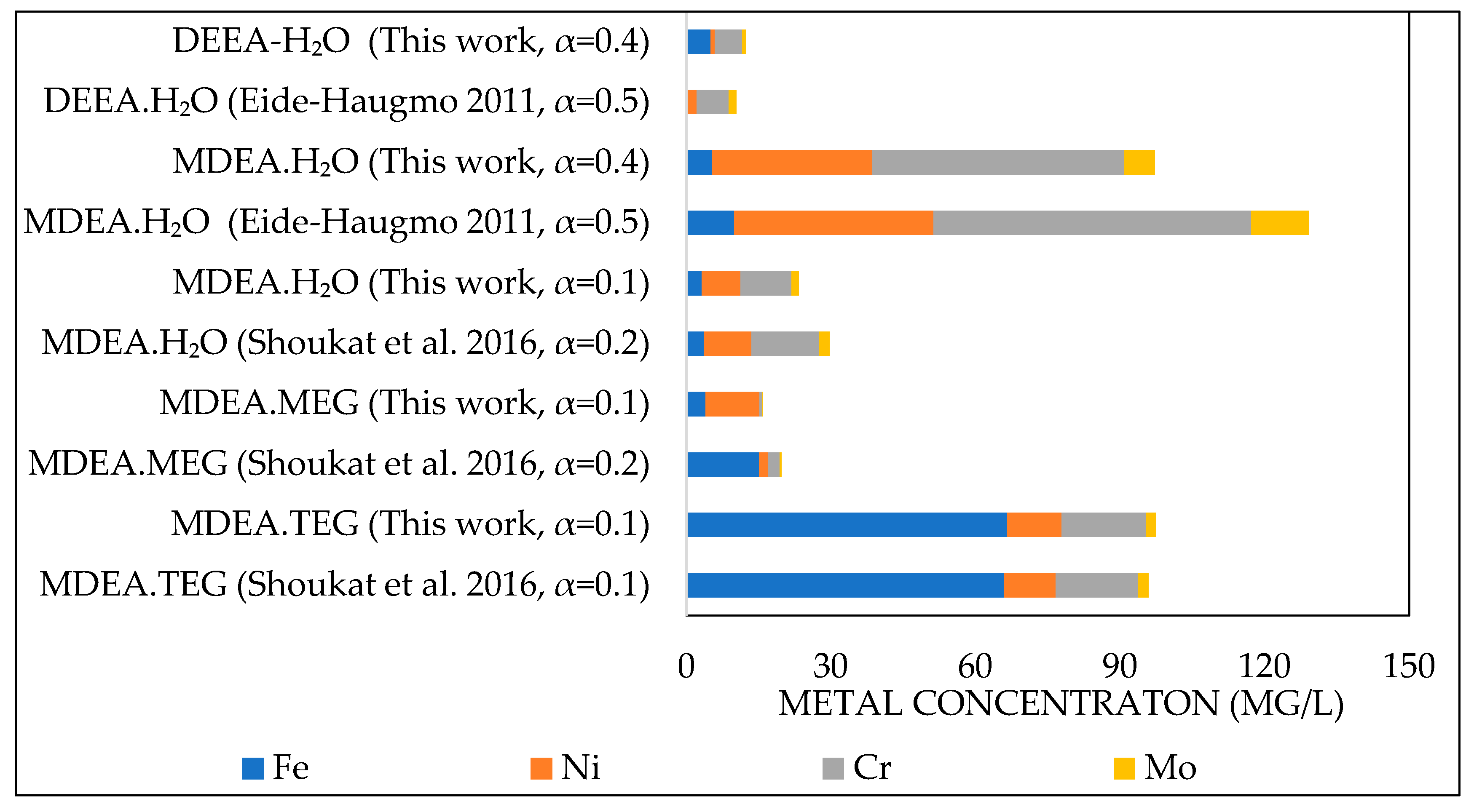



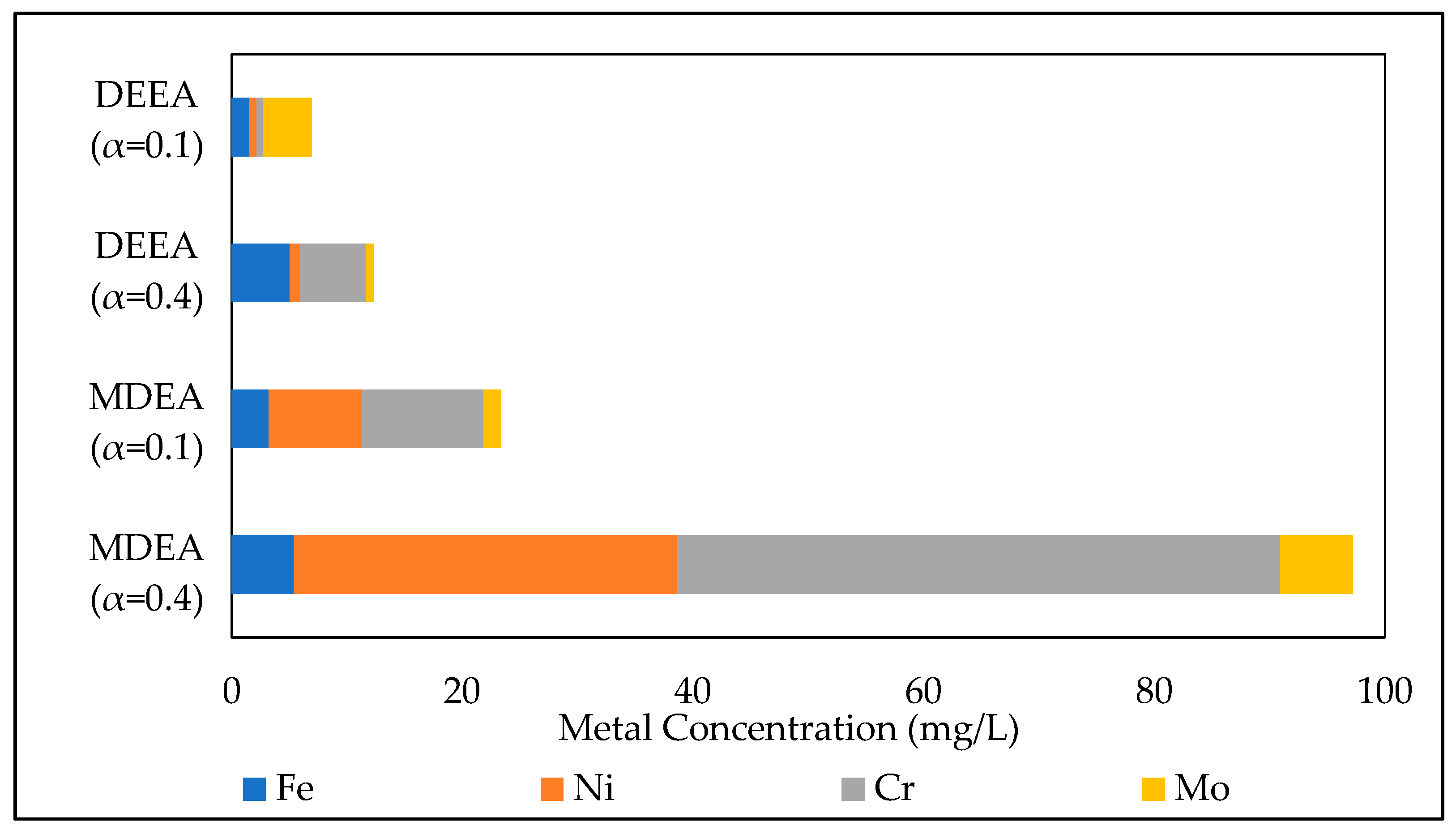
| Chemical | CAS | Purity | Molecular Weight (g/mol) |
|---|---|---|---|
| N-Methyldiethanolamine (MDEA) | 105-59-9 | ≥99.0% | 119.16 |
| 2-(Diethylamino)ethanol (DEEA) | 100-37-8 | ≥99.5% | 117.19 |
| 3-Dimethylamino-1-propanol (3DMA-1P) | 3179-63-3 | ≥99.0% | 103.16 |
| 3-Diethylamino-1-propanol (3DEA-1P) | 622-93-5 | ≥95.0% | 131.22 |
| Monoethylene glycol (MEG) | 107-21-1 | ≥99.5% | 62.07 |
| Triethylene glycol (TEG) | 112-27-6 | ≥99.8% | 150.17 |
| Carbon dioxide (CO2) | 124-38-9 | ≥99.9% | 44.01 |
| Name | Unloaded Amine wt.% | α (mol CO2/mol amine) | Time (days) | k × 10−4 (day−1) | R2 |
|---|---|---|---|---|---|
| DEEA.H2O | 30 | 0.1 | 49 | 3.792 | 0.910 |
| 3DMA-1P.H2O | 30 | 0.1 | 49 | 2.933 | 0.945 |
| 3DEA-1P.H2O | 30 | 0.1 | 49 | 2.659 | 0.981 |
| MDEA.H2O | 30 | 0.1 | 49 | 3.960 | 0.908 |
| MDEA.H2O (Glass) | 30 | 0.1 | 35 | 4.546 | 0.904 |
| DEEA.MEG | 30 | 0.1 | 49 | 7.998 | 0.938 |
| 3DMA-1P.MEG | 30 | 0.1 | 49 | 0.362 | 0.981 |
| 3DEA-1P.MEG | 30 | 0.1 | 49 | 6.634 | 0.930 |
| MDEA.MEG | 30 | 0.1 | 49 | 22.910 | 0.919 |
| MDEA.MEG (Glass) | 30 | 0.1 | 49 | 17.390 | 0.900 |
| DEEA.TEG | 30 | 0.1 | 49 | 3.853 | 0.974 |
| 3DMA-1P.TEG | 30 | 0.1 | 49 | 4.505 | 0.928 |
| 3DEA-1P.TEG | 30 | 0.1 | 49 | 4.043 | 0.914 |
| MDEA.TEG | 30 | 0.1 | 49 | 19.450 | 0.988 |
| MDEA.TEG (Glass) | 30 | 0.1 | 49 | 11.920 | 0.939 |
| DEEA.H2O | 10 | 0.4 | 49 | 5.483 | 0.916 |
| DEEA.H2O | 30 | 0.4 | 49 | 9.473 | 0.941 |
| DEEA.H2O | 50 | 0.4 | 49 | 11.561 | 0.935 |
| MDEA.H2O | 10 | 0.4 | 49 | 3.058 | 0.974 |
| MDEA.H2O | 30 | 0.4 | 49 | 7.061 | 0.990 |
| MDEA.H2O | 50 | 0.4 | 49 | 16.520 | 0.998 |
| MDEA.H2O | 70 | 0.4 | 49 | 32.810 | 0.986 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoukat, U.; Knuutila, H.K. Effect of Various Parameters on the Thermal Stability and Corrosion of CO2-Loaded Tertiary Amine Blends. Energies 2020, 13, 2626. https://doi.org/10.3390/en13102626
Shoukat U, Knuutila HK. Effect of Various Parameters on the Thermal Stability and Corrosion of CO2-Loaded Tertiary Amine Blends. Energies. 2020; 13(10):2626. https://doi.org/10.3390/en13102626
Chicago/Turabian StyleShoukat, Usman, and Hanna Katariina Knuutila. 2020. "Effect of Various Parameters on the Thermal Stability and Corrosion of CO2-Loaded Tertiary Amine Blends" Energies 13, no. 10: 2626. https://doi.org/10.3390/en13102626
APA StyleShoukat, U., & Knuutila, H. K. (2020). Effect of Various Parameters on the Thermal Stability and Corrosion of CO2-Loaded Tertiary Amine Blends. Energies, 13(10), 2626. https://doi.org/10.3390/en13102626