Towards the Correct Measurement of Thermal Conductivity of Ionic Melts and Nanofluids †
Abstract
1. Introduction
- -
- Unsteady state or transient methods, in which the full Equation (4) is used and the principal measurement is the temporal history of the fluid temperature (transient hot-wire, transient hot strip, the interferometric technique adapted to states near the critical point etc.);
- -
- Steady state techniques, for which and the equation reduces to , which can be integrated for a given geometry (parallel plates, concentric cylinders, etc.).
- Change of needs—From laboratory work to in-situ measurements,
- Change of paradigm—Fit for purpose instead of best uncertainty,
- Change of financing priorities from state funding agencies—priority to industry driven/sponsored research,
- Decrease for users in industry of the added value of property data of good quality,
- The use and misuse of commercial equipment’s, with methods of measurement not adequate to the object systems.
2. Methods of Measuring Thermal Conductivity of Ionic Melts and Nanofluids
- -
- The transient hot wire technique was identified as the best technique for obtaining standard reference data (certification of reference materials).
- -
- It is an absolute technique, with a working equation and a complete set of corrections reflecting the departure from the ideal model, where the principal variables are measured with a high degree of accuracy. It is accepted by the scientific community as a primary method, the top of the traceability chain for this physical quantity.
- -
- The liquids proposed by IUPAC (toluene, benzene, and water) as primary standards for the measurement of thermal conductivity were measured with this technique with an accuracy of 1% or better.
- -
- It has been extended, from its original version, to its application at high temperatures (correction for radiation effects), for conducting liquids and melts.
- -
- High temperature measurements (molten salts).
- -
- Radiation must be corrected or minimized, and convection eliminated.
- -
- Electrical insulation of measuring probes indispensable.
- -
- Use of current commercial instrumentation, such as transient hot-probes and transient hot-strips/disks, based on adaptations of the heat source immersed in the fluid exact behaviour, sacrifice accuracy at the cost of instrumentation and ease of handling of probes and samples. The accountability of methods must be proven.
- -
- Many of the results presented in the bibliography were taken with commercial instrumentation, and not properly validated.
- -
- In the limited context of hot wire, for instance, it is common to use just one wire, sometimes shorter than the model requires (end effects), which directly affects the accuracy of the measurements.
- -
- The contact between the bare metallic wire and the conducting liquid provides a secondary path for the flow of current in the cell and the heat generation in the wire cannot be defined unambiguously.
- -
- Polarization of the liquid occurs at the surface of the wire, producing an electrical double-layer.
- -
- The electrical measuring system (an automatic Wheatstone bridge) that detects the changes in the voltage signals in the wire is affected by the combined resistance /capacitance effect, caused by the dual path conduction.
- -
- Insulated wires (necessity to cover the wire with materials or insulating anodizing coatings to protect it during the measurements) or polarized techniques are required [11].
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nieto de Castro, C.A. Absolute measurements of the viscosity and thermal conductivity of fluids. JSME Int. J. Ser. II 1988, 31, 387–401. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John and Wiley and Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Wakeham, W.A.; Nagashima, A.; Sengers, J.V. Measurement of the Transport Properties of Fluids: Experimental Thermodynamics; Blackwell Scientific Pubs: Oxford, UK, 1991; Volume III. [Google Scholar]
- Nieto de Castro, C.A.; Li, S.F.Y.; Nagashima, A.; Trengove, R.D.; Wakeham, W.A. Standard reference data for the thermal conductivity of liquids. J. Phys. Chem. Ref. Data 1986, 15, 1073. [Google Scholar] [CrossRef]
- Mardolcar, U.V.; Nieto de Castro, C.A. The measurement of thermal conductivity at high temperatures. High Temp. High Press. 1992, 24, 551. [Google Scholar]
- Assael, M.J.; Goodwin, A.R.H.; Vesovic, V.; Wakeham, W.A. Experimental Thermodynamics Volume IX: Advances in Transport Properties of Fluids; Especially Chapter 5; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Nunes, V.M.B.; Queirós, C.S.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A. Molten salts as engineering fluids—A review: Part I. Molten alkali nitrates. Appl. Energy 2016, 183, 603–611. [Google Scholar] [CrossRef]
- Janz, G.J.; Allen, C.B.; Bansal, N.P.; Murphy, R.M.; Tomkins, R.P.T. Physical Properties Data Compilations Relevant to Energy Storage; NSRDS-NBS 61; Part II; USA Department of Commerce: Washington, DC, USA, 1979.
- Nagasaka, Y.; Nagashima, A. The Thermal Conductivity of Molten NaNO3 and KNO3. Int. J. Thermophys. 1991, 12, 769–781. [Google Scholar] [CrossRef]
- Nunes, V.M.B.; Lourenço, M.J.V.; Santos, F.J.V.; Matos Lopes, M.L.S.; Nieto de Castro, C.A. Accurate Measurement of Physico-Chemical Properties on Ionic Liquids and Molten Salts. In Ionic Liquids and Molten Salts: Never the Twain; Seddon, K.E., Gaune-Escard, M., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2010; pp. 229–263. [Google Scholar]
- Nieto de Castro, C.A.; Vieira, S.I.C.; Lourenço, M.J.V.; Sohel Murshed, S.M. Understanding Stability, Measurements, and Mechanisms of Thermal Conductivity of Nanofluids. J. Nanofluids 2017, 6, 804–811. [Google Scholar] [CrossRef]
- Tada, Y.; Harada, M.; Tanigaki, M.; Egushi, W. Laser flash method for measuring thermal conductivity of liquids-Application to low thermal conductivity liquids. Rev. Sci. Instrum. 1978, 49, 1305–1314. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, S.D.; Kang, S.; Shin, S.H.; Kim, J.H.; Bang, I.C. Feasibility study on molten gallium with suspended nanoparticles for nuclear coolant. Nucl. Eng. Des. 2012, 247, 147–159. [Google Scholar] [CrossRef]
- Kraft, K.; Lopes, M.M.; Leipertz, A. Thermal-diffusivity and thermal-conductivity of toluene by photon-correlation spectroscopy—A test of the accuracy of the method. Int. J. Thermophys. 1995, 16, 423–432. [Google Scholar] [CrossRef]
- Nagasaka, Y.; Nakazawa, N.; Nagashima, A. Experimental determination of the thermal conductivity of molten alkali halides by the forced Rayleigh scattering method. I. Molten LiCl, NaCl, KCl, RbCl and CsCl. Int. J. Thermophys. 1992, 13, 555–574. [Google Scholar] [CrossRef]
- Frez, C.; Diebold, G.J.; Tran, C.D.; Yu, S. Determination of the thermal diffusivities, thermal conductivities, and sound speeds of room-temperature ionic liquids by the transient grating technique. J. Chem. Eng. Data 2006, 51, 1250–1255. [Google Scholar] [CrossRef]
- Bobbo, S.; Fidele, L. Experimental methods for the characterization of thermophysical properties of nanofluids. In Heat Transfer Enhancement with Nanofluids; Bianco, V., Manca, O., Nardini, S., Vafai, K., Eds.; CRC Press: Boca Raton, FL, USA, 2015; Chapter 4. [Google Scholar]
- Nieto de Castro, C.A. State of Art in Liquid Thermal Conductivity Standards. In Proceedings of the Invited Communication to BIPM-CCT-WG9 Meeting, Bratislava, Slovak Republic, 9 September 2005. [Google Scholar]
- Assael, M.J.; Nieto de Castro, C.A.; Roder, H.M.; Wakeham, W.A. Experimental Chemical Thermodynamics. In Measurement of the Transport Properties of Fluids; Wakeham, W.A., Nagashima, A., Sengers, J.V., Eds.; Blackwells: Oxford, UK, 1991; Volume 2, Chapter 7. [Google Scholar]
- Ramires, M.L.V.; Nieto de Castro, C.A. Uncertainty and Performance of the Transient Hot Wire Method. In Proceedings of the Tempmeko 2001, 8th International Symposium on Temperature and Thermal Measurements in Industry and Science, Berlin, Germany, 19–21 June 2002; Fellmuth, B., Seidel, J., Scholz, G., Eds.; Vde Verlag GmbH: Berlin, Germany, 2002; Volume 2, pp. 1181–1886. [Google Scholar]
- Beirão, S.G.S.; Ramires, M.L.V.; Dix, M.; Nieto de Castro, C.A. A New Instrument for the Measurement of Thermal Conductivity of Fluids. Int. J. Thermophys. 2006, 27, 1018–1041. [Google Scholar] [CrossRef]
- Carslaw, H.S.; Jaeger, J.C. Conduction of Heat in Solids; Oxford University Press: London, UK, 1959; p. 256. [Google Scholar]
- Healy, J.; de Groot, J.J.; Kestin, J. The Theory of the Transient Hot-wire Method for Measuring Thermal Conductivity. Physica 1976, 82, 392–408. [Google Scholar] [CrossRef]
- Nieto de Castro, C.A.; Taxis, B.; Roder, H.M.; Wakeham, W.A. Thermal Diffusivity Measurement by the Transient Hot-Wire Technique: A Reappraisal. Int. J. Thermophys. 1988, 9, 293–316. [Google Scholar] [CrossRef]
- Nagasaka, Y.; Nagashima, A. Absolute Measurement of the Thermal Conductivity of Electrically Conducting Liquids by The Transient Hot-Wire Method. J. Phys. E Sci. Instrum. 1981, 14, 1435–1440. [Google Scholar] [CrossRef]
- Fisher, J. Zur Bestimmung der Wärmeleitfähigkeit und der Temperaturleitfähigkeit aus dem Ausgleichvorgang beim Schleiermacherschen Meßrohrverfahren und beim Plattenverfahren. Ann. Phys. 1939, 34, 669–688. [Google Scholar] [CrossRef]
- Nieto de Castro, C.A. Medida da Condutibilidade Térmica de Hidrocarbonetos Líquidos pelo Método do Fio Aquecido. Ph.D. Thesis, Instituto Superior Técnico, UTL, Portugal, 1977. [Google Scholar]
- Goldstein, R.J.; Briggs, D.G. Transient Free Convection about Vertical Plates and Circular Cylinders. J. Heat Trans. 1964, 86, 490–500. [Google Scholar] [CrossRef]
- De Groot, S.R.; Kestin, J.; Sookiazian, H. Instrument to measure the thermal conductivity of gases. Physica 1974, 75, 454–482. [Google Scholar] [CrossRef]
- Nagasaka, Y.; Nagashima, A. Simultaneous measurement of the thermal conductivity and thermal diffusivity of fluids. High Temp. High Press. 1989, 21, 363–371. [Google Scholar]
- Menashe, J.; Wakeham, W.A.; Bunsenges, B. The Thermal Conductivity of n-Nonane and n-Undecane at Pressures up to 500 MPa in the Temperature Range 35–90 °C. Phys. Chem. 1981, 85, 340–347. [Google Scholar] [CrossRef]
- Nieto de Castro, C.A.; Li, S.F.Y.; Maitland, G.C.; Wakeham, W.A. Thermal Conductivity of Toluene in the Temperature Range 35–90 °C at Pressures up to 600 MPa. Int. J. Thermophys. 1983, 4, 311–327. [Google Scholar] [CrossRef]
- Calado, J.C.G.; Fareleira, J.M.N.A.; Nieto de Castro, C.A.; Wakeham, W.A. Reference state in thermal conductivity measurements. Revista Virtual de Química 1984, 26, 173–176. [Google Scholar]
- Ramires, M.L.V.; Nieto de Castro, C.A.; Fareleira, J.M.N.A.; Wakeham, W.A. The Thermal Conductivity of Aqueous Sodium Chloride Solutions. J. Chem. Eng. Data 1994, 39, 186–190. [Google Scholar] [CrossRef]
- Beirão, S.G.S.; Ribeiro, A.P.C.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A. Thermal Conductivity of Humid Air. Int. J. Thermophys. 2012, 33, 1686–1703. [Google Scholar] [CrossRef]
- Antoniadis, K.D.; Tertsinidou, G.J.; Assael, M.J.; Wakeham, W.A. Necessary Conditions for Accurate, Transient Hot-Wire Measurements of the Apparent Thermal Conductivity of Nanofluids are Seldom Satisfied. Int. J. Thermophys. 2016, 37, 78. [Google Scholar] [CrossRef]
- Rutin, S.B.; Skripov, P.V. Comments on “The Apparent Thermal Conductivity of Liquids Containing Solid Particles of Nanometer Dimensions: A Critique” (Int. J. Thermophys. 2015, 36, 1367). Int. J. Thermophys. 2016, 37, 102. [Google Scholar] [CrossRef]
- Haselman, D.P.H. Can the Temperature Dependence of the Heat Transfer Coefficient of the Wire—Nanofluid Interface Explain the “Anomalous” Thermal Conductivity of Nanofluids Measured by the Hot-Wire Method? Int. J. Thermophys. 2018, 39, 109. [Google Scholar] [CrossRef]
- França, J.M.P.; Nieto de Castro, C.A.; Pádua, A.A.H. Molecular Interactions and Thermal Transport in Ionic Liquids with Carbon Nanomaterials. Phys. Chem. Chem. Phys. 2017, 19, 17075–17087. [Google Scholar] [CrossRef]
- Tertsinidou, G.J.; Tsolakidou, C.M.; Pantzali, M.; Marc, J.; Assael Colla, L.; Fedele, L.; Bobbo, S.; Wakeham, W.A. New Measurements of the Apparent Thermal Conductivity of Nanofluids and Investigation of Their Heat Transfer Capabilities. J. Chem. Eng. Data 2017, 62, 491–507. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. Int. J. Refrig. 2012, 35, 1359–1366. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Fujii, M. Experimental Study on the Effective Thermal Conductivity and Thermal Diffusivity of Nanofluids. Int. J. Thermophys. 2006, 27, 569–580. [Google Scholar] [CrossRef]
- Duangthongsuk, W.; Wongwises, S. Measurement of temperature-dependent thermal conductivity and viscosity of TiO2—Water nanofluids. Exp. Therm. Fluid Sci. 2009, 33, 706–714. [Google Scholar] [CrossRef]
- Yoo, D.H.; Hong, K.S.; Yang, H.S. Study of thermal conductivity of nanofluids for the application of heat transfer fluids. Thermochim. Acta 2007, 455, 66–69. [Google Scholar] [CrossRef]
- Reddy, M.C.S.; Rao, V.V. Experimental studies on thermal conductivity of blends of ethylene glycol-water-based TiO2 nanofluids. Int. Commun. Heat Mass Transf. 2013, 46, 31–36. [Google Scholar] [CrossRef]
- Yiamsawasd, T.; Dalkilic, A.S.; Wongwises, S. Measurement of the thermal conductivity of titania and alumina nanofluids. Thermochim. Acta 2012, 545, 48–56. [Google Scholar] [CrossRef]
- Haghighi, E.B.; Saleemi, M.; Nikkam, N.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M.; Palm, B. Accurate basis of comparison for convective heat transfer in nanofluids. Int. Commun. Heat Mass Transf. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal conductivity of heterogeneous two-component systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Nunes, V.M.B.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A. Molten Alkali Carbonates as Alternative Engineering Fluids for High Temperature Applications. Appl. Energy 2019, 242, 1626–1633. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Lourenço, M.J.V.; Vieira, S.I.; Serra, J.M.; Nieto de Castro, C.A. New Portable Instrument for the Measurement of Thermal Conductivity in Gas Process Conditions. Rev. Sci. Instrum. 2016, 87, 065105. [Google Scholar] [CrossRef]
- Lourenço, M.J.V.; Serra, J.M.; Nunes, M.R.; Vallêra, A.M.; Nieto de Castro, C.A. Thin-Film Characterization for High Temperature Applications. Int. J. Thermophys. 1998, 19, 1253. [Google Scholar] [CrossRef]
- Lourenço, M.J.V.; Nieto de Castro, C.A. Measuring the Thermal Conductivity at High Temperatures. Instrumental Difficulties and Sensor Performance. In Proceedings of the Tempmeko 2001, 8th International Symposium on Temperature and Thermal Measurements in Industry and Science, Berlin, Germany, 19–21 June 2002; Fellmuth, B., Seidel, J., Scholz, G., Eds.; Vde Verlag GmbH: Berlin, Germany, 2002; Volume 2, pp. 989–995. [Google Scholar]
- Gustafsson, S.E.; Karawacki, E.; Khan, M.N. Transient hot-strip method for simultaneously measuring thermal conductivity and thermal diffusivity of solids and fluids. J. Phys. D Appl. Phys. 1979, 12, 1411–1421. [Google Scholar] [CrossRef]
- Gustafsson, S.E.; Karawacki, E.; Khan, M.N. Determination of the thermal-conductivity tensor and the heat capacity of insulating solids with the transientmethod. J. Appl. Phys. 1981, 52, 2596–2600. [Google Scholar] [CrossRef]
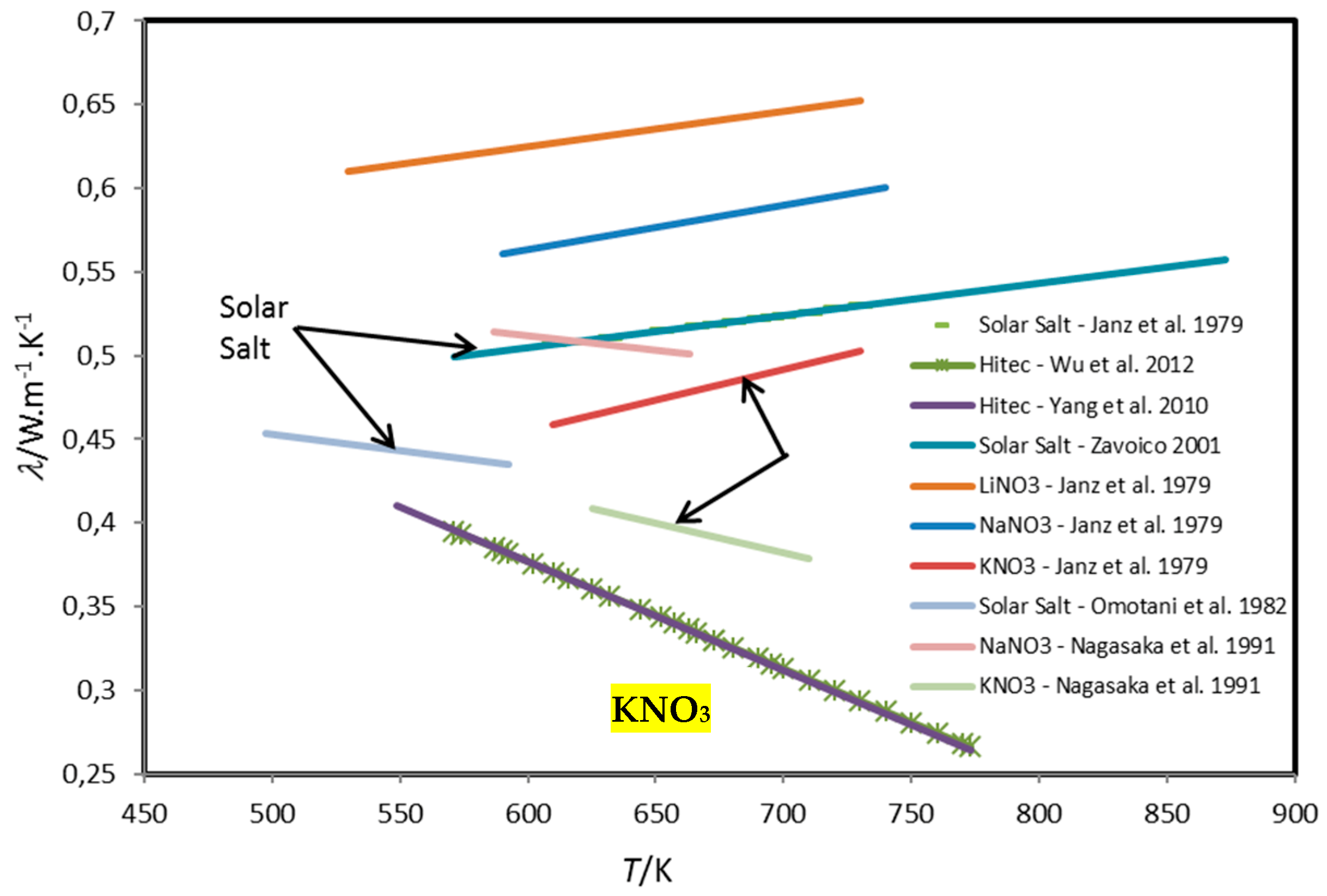
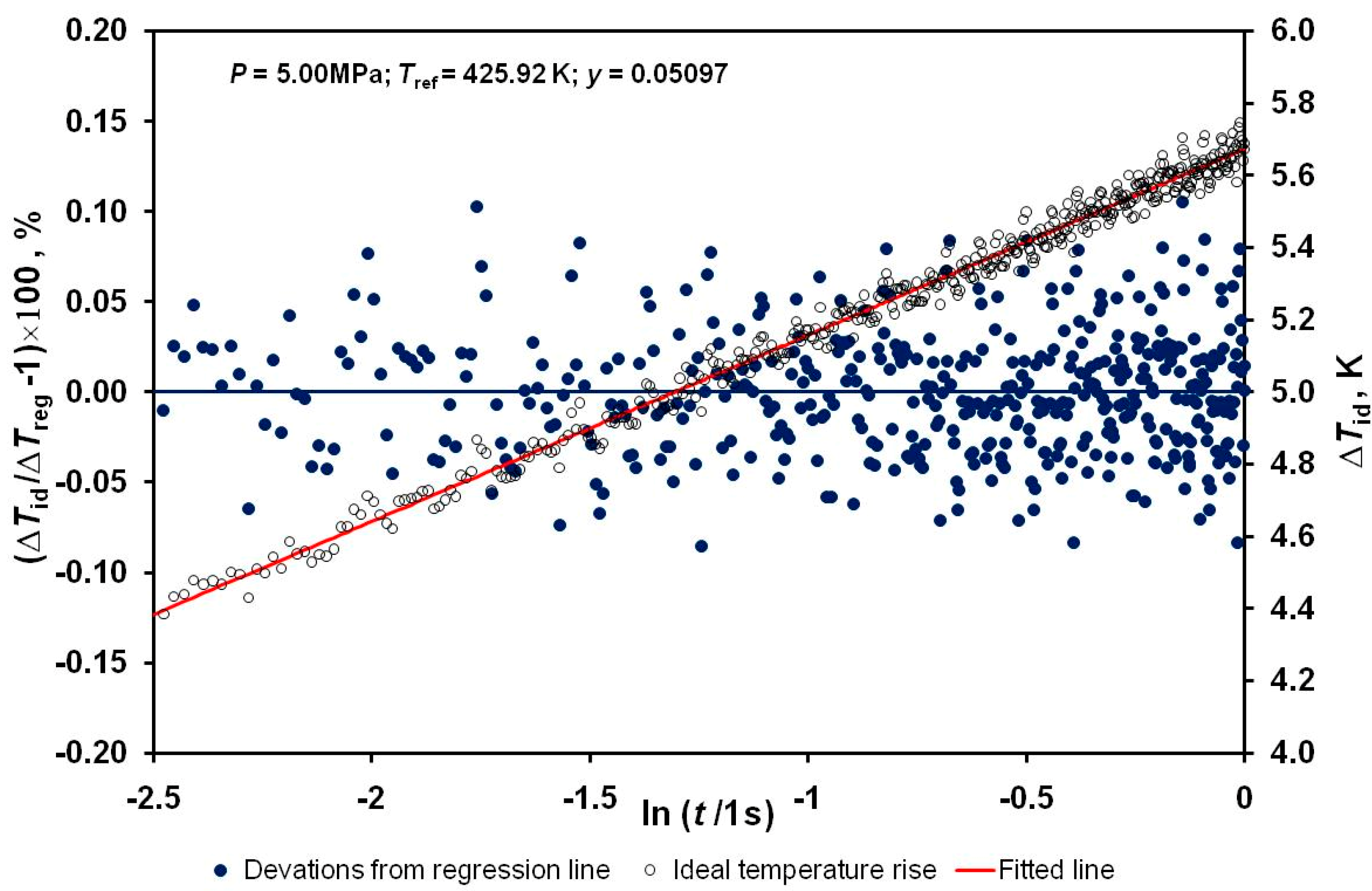

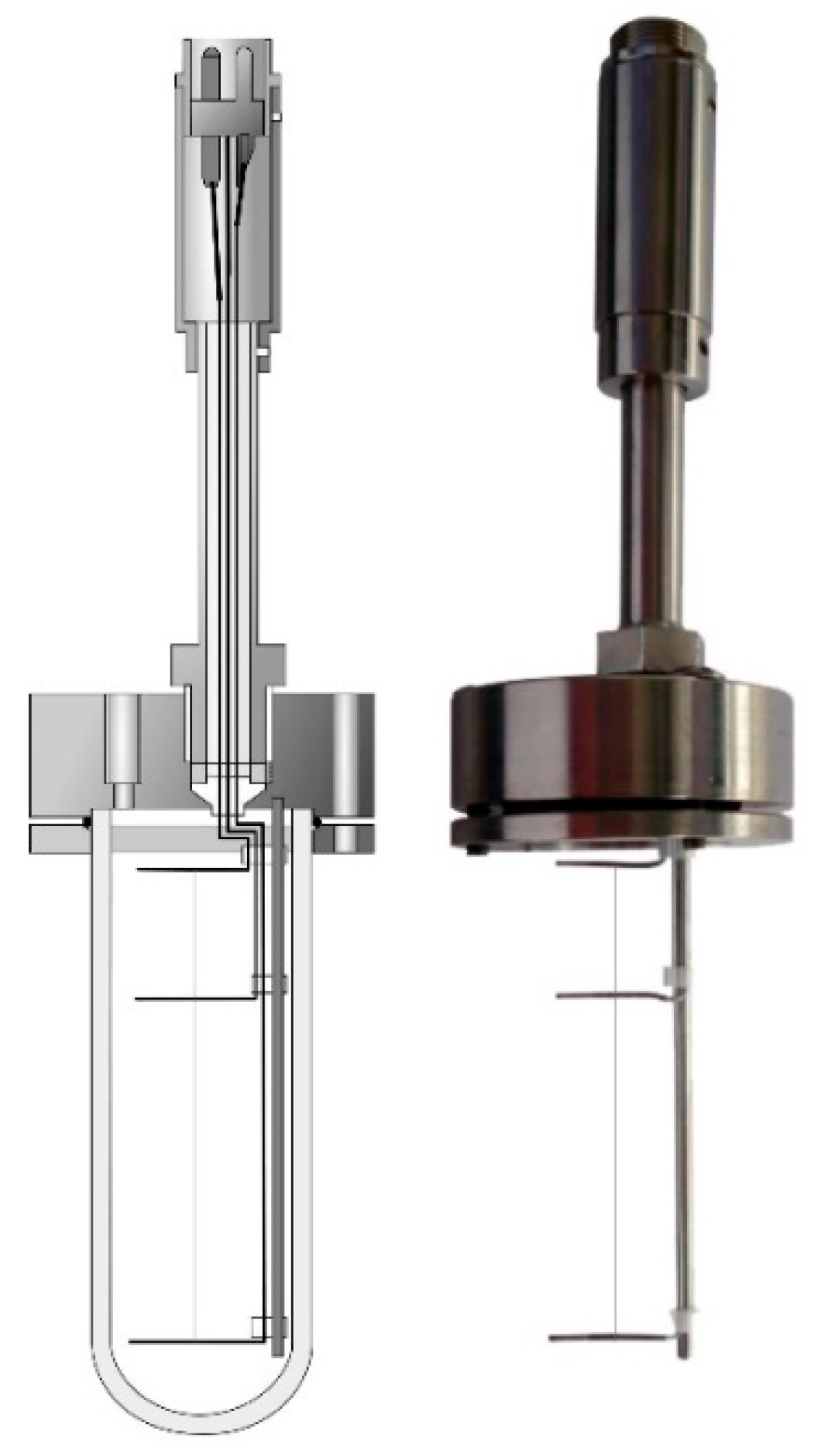
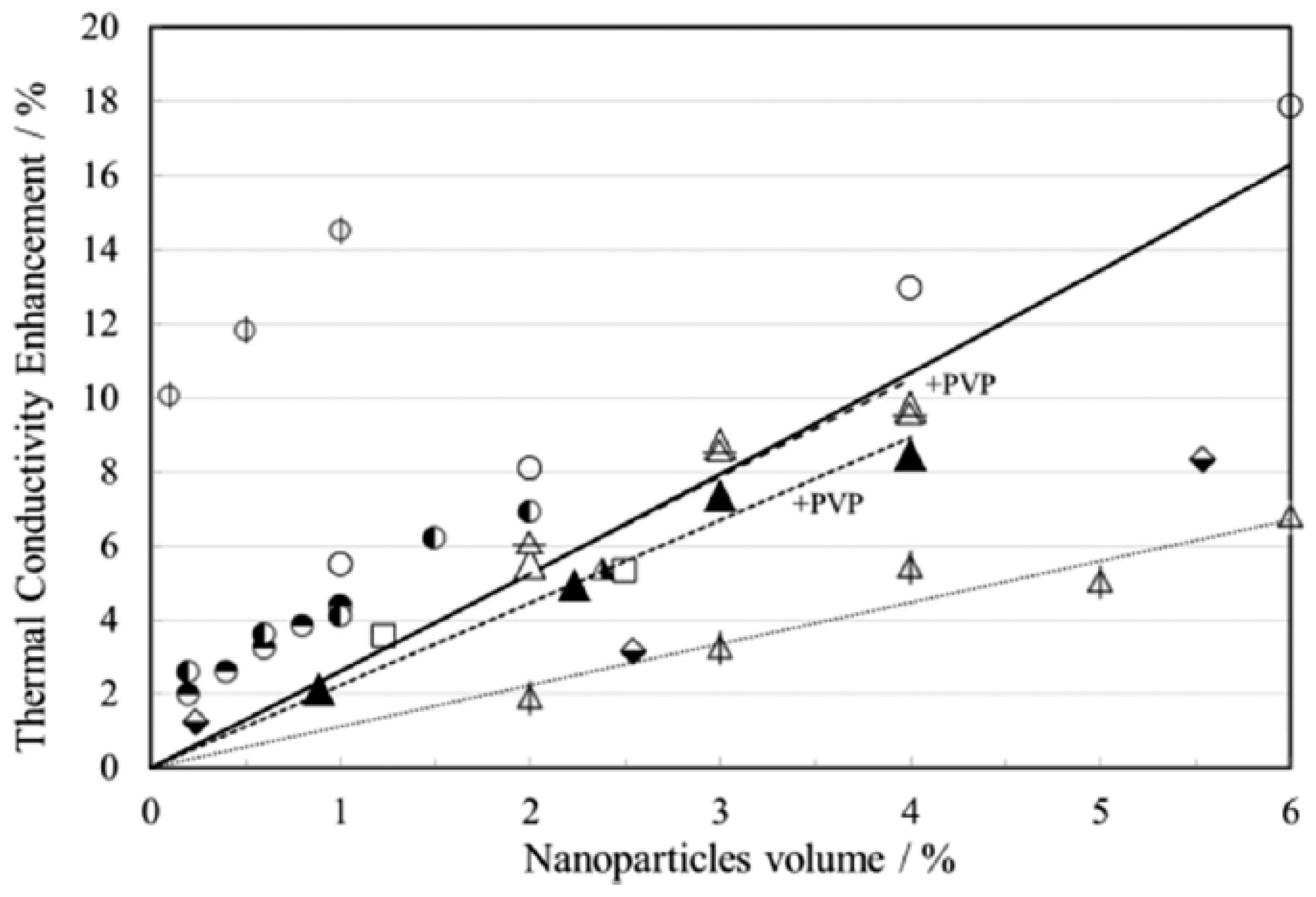
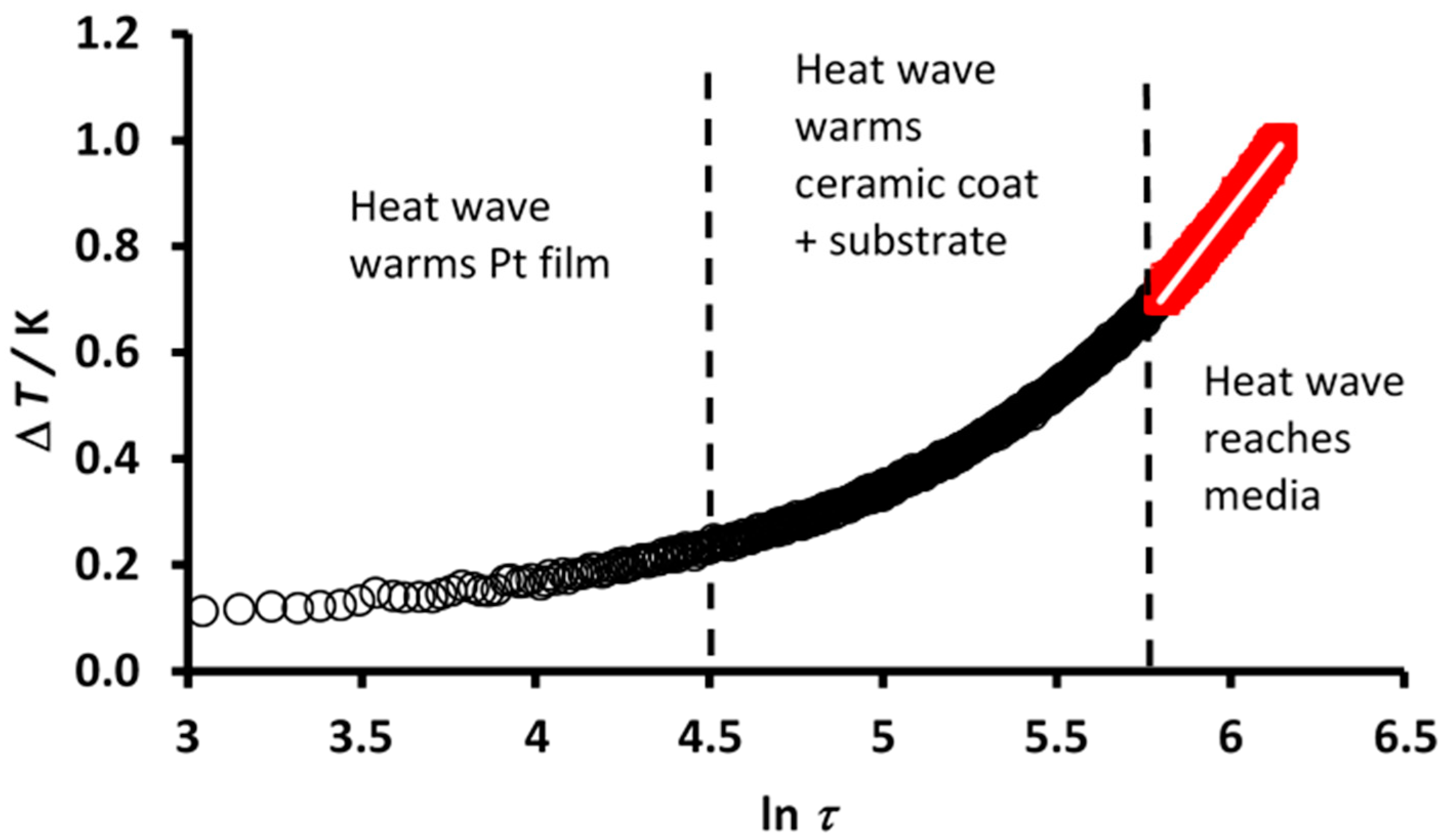
| Method | Type | Attainable Uncertainty 1 | Applicability to Ionic Melts | Applicability to Nanofluids | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Remarks | Yes | No | Remarks | |||
| Transient hot-wire for conducting liquids 2 | Primary | 1% | ✓ | The best method to be applied with classical designs | ✓ | The best method to be applied with classical designs | ||
| Transient hot-wire (bare wires) | Primary | 1% | ✓ | ✓ | Non-conducting base fluids and/or nanoparticles | |||
| Transient hot strip for conducting liquids (insulated strip) | Primary/Secondary | 2–3% | ✓ | Can be considered primary if the 3D heat transfer equation is solved for the geometries involved | ✓ | Can be considered primary if the 3D heat transfer equation is solved for the geometries involved | ||
| Transient Plane Source or Hot-Disk | Secondary | 3–5% | ✓ | Limited in temperature range | ✓ | Limited in temperature range | ||
| Steady-state Parallel Plates (Guarded Hot-Plates) | Secondary | 2–3% | ✓ | ✓ | Small spacing between plates might induce phase separation | |||
| Steady-state Concentric Cylinders | Secondary | 2–3% | ✓ | Small gaps. Guarded plates | ✓ | Small gaps must be used. | ||
| Laser Flash | Secondary | 3–5% | ✓ | Difficulties with originated waves in the liquid surface (Marangoni effects) | ✓ | Use low power lasers to avoid NP breakage and structure deployment | ||
| Forced Rayleigh Scattering | Secondary/Relative | 3–5% | ✓ | Dyes necessary to enhance the signal compatible with molten salts or IL’s | ✓ | Dyes necessary to enhance the signal compatible with nanofluids. | ||
| Photon Correlation Spectroscopy | Secondary/Relative | 2–3% | ✓ | Optically transparent fluids. Not explored for measurements above 473 K. | ✓ | Optically transparent fluids. | ||
| Transient Grating | Secondary/Relative | 3–5% | ✓ | Needs a big improvement in the calibration. Not recommended for high quality work | ✓ | Needs a big improvement in the calibration. Not recommended for high quality work | ||
| 3ω Method | Secondary | 5% | ✓ | Needs improvement of the theory. However, alternating current, destroying the direction of the polarizing current, avoids current polarization | ✓ | Needs improvement of the theory. However, alternating current, destroying the direction of the polarizing current, avoids nanoparticle deposition and electrically insulation problems. | ||
| Correction | Physical Source | Expression | Reference | Recommendations |
|---|---|---|---|---|
| Wire physical properties | [22,23] | Apply always correction. | ||
| Outer boundary. Cell physical dimensions (b is cell wall diameter) | [23,26] | Apply always correction. Make design of cell to minimize it to 0.1% of . | ||
| Compression work (L is the wire length and V is the cell volume) | [23,24] | Negligible in liquid phases. For gases limits the operational zone of measurements to ρ > 40 kg·m−3. | ||
| Radial convection and viscous dissipation. Symbol w means wire properties. (αP is the isobaric thermal expansion coefficient of fluid and g the acceleration of gravity) | For gases and liquids (Δρ < ρ0) For liquids, expressions also graphical form, recording the vertical penetration depth of moving fluid front. | [23,24,27,28,29,30] | Avoid onset of convection in the measurements. Design measurement time to t < 5 s to render negligible in liquid phases. Wire(s) must be vertical. Avoid short wires. | |
| Radiation (s is the Stefan-Boltzmann constant, K is the a mean extinction coefficient for radiation and n the refractive index of the liquid) | Absorbing fluid | [23,31,32] | Always apply correction, dependent if fluid is transparent or absorbs radiation. K is difficult to obtain experimentally, but usually [30,32]. Introduces curvature in straight line and it is mostly important at high temperatures (molten salts). | |
| Temperature jump | [23,29] | Important for gases. Work outside density range where it is not negligible (mean free path of the gas molecules Λ approaching wire diameter (microns). For nanofluids might play a role, but here with nanoparticles dimension. | ||
| Effect of variable fluid properties | [24,33] | Always apply correction. Valid for situations where fluid density during a measurement can be considered constant, as in the case of systems far from critical region. | ||
| Effect of variable fluid properties (fluids critical region) | ; ; | [30] | Always apply correction. For measurements in the critical region, the properties are extremely strong functions of temperature. | |
| Truncation error resulting from the expansion of exponential integral, Equation (8) | [30] | Rendered negligible by design. It decreases with time. | ||
| Effect of coating to insulate wires for electrically conducting liquids. Subscript 2 means coating properties. r0 is coating radius | [25,34] | Always apply correction. | ||
| Effect of variable fluid properties | [4,32,33] | Always apply correction. When the distribution of the measured temperature rises is not uniform, a more detailed expression is necessary [32]. | ||
| Effect of coating to insulate wires for electrically conducting liquids | [25,34] | Always apply correction. Remember to correct the expression for in , which has to be evaluated at the surface of the coat (r = r0). |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto de Castro, C.A.; Lourenço, M.J.V. Towards the Correct Measurement of Thermal Conductivity of Ionic Melts and Nanofluids. Energies 2020, 13, 99. https://doi.org/10.3390/en13010099
Nieto de Castro CA, Lourenço MJV. Towards the Correct Measurement of Thermal Conductivity of Ionic Melts and Nanofluids. Energies. 2020; 13(1):99. https://doi.org/10.3390/en13010099
Chicago/Turabian StyleNieto de Castro, Carlos A., and Maria José V. Lourenço. 2020. "Towards the Correct Measurement of Thermal Conductivity of Ionic Melts and Nanofluids" Energies 13, no. 1: 99. https://doi.org/10.3390/en13010099
APA StyleNieto de Castro, C. A., & Lourenço, M. J. V. (2020). Towards the Correct Measurement of Thermal Conductivity of Ionic Melts and Nanofluids. Energies, 13(1), 99. https://doi.org/10.3390/en13010099







