Comparative Analysis of On-Board Methane and Methanol Reforming Systems Combined with HT-PEM Fuel Cell and CO2 Capture/Liquefaction System for Hydrogen Fueled Ship Application
Abstract
1. Introduction
- (1)
- Steam reforming, HT-PEMFC, and CO2 capture/liquefaction systems are simultaneously considered. Heat integration and recovery were implemented for practical comparison. Excess heat from the HT-PEMFC and steam reforming system were used in the CO2 capture system.
- (2)
- For the steam methane reforming-based system, liquefied natural gas (LNG) was used as primary fuel because it is the most cost effective for ship storage of natural gas and has been well proved in LNG fueled-ship applications.
- (3)
- For CO2 liquefaction, the steam methane reforming-based system used the cold energy of LNG, whereas the steam methanol reforming-based system has a separate refrigeration cycle.
- (1)
- To develop methane and methanol steam reforming systems combined with HT-PEMFC and CO2 capture/liquefaction systems suitable for the reference ship.
- (2)
- To carry out exergy and energy analyses for the developed integrated systems to assess the energy efficiency, exergy efficiency, and exergy destruction of components within each system.
- (3)
- To evaluate the overall fuel cost and overall space required for storage of the liquefied CO2 and primary fuels.
- (4)
- To carry out parametric studies with varying operating conditions, such as the S/C ratio, operating temperature of the reforming process, and CO2 capture ratio.
2. System Description
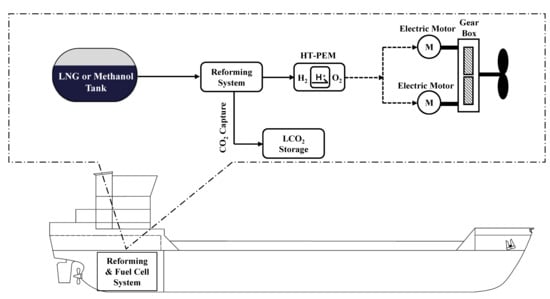
2.1. Reference Ship Description
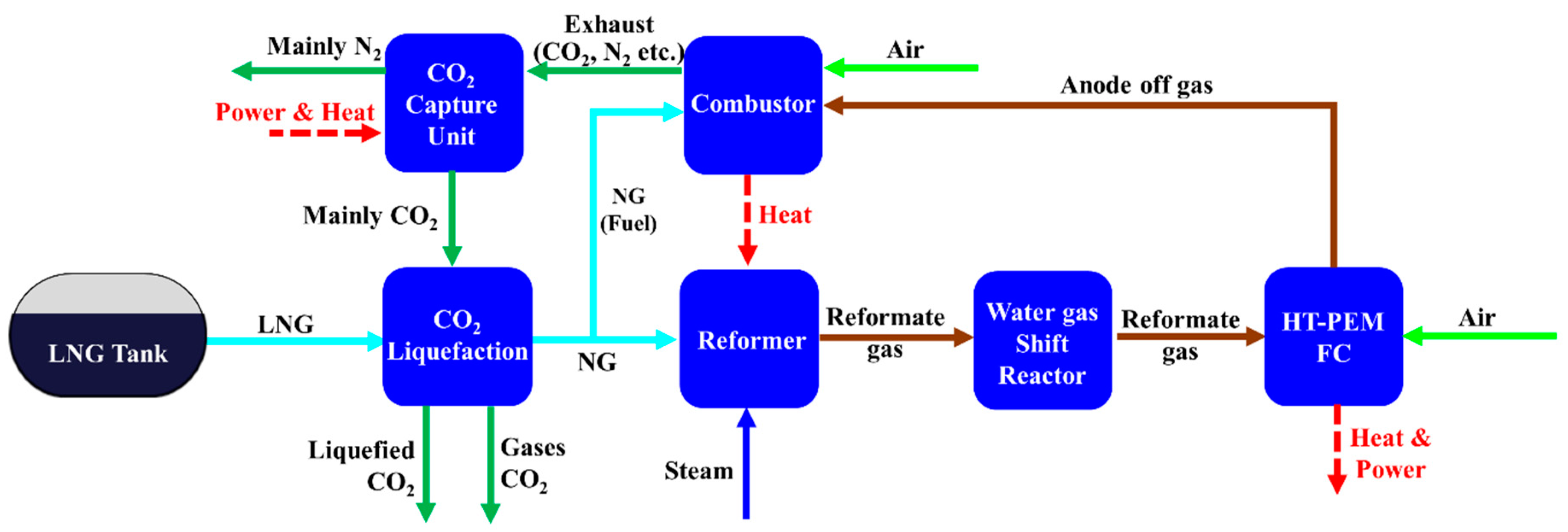
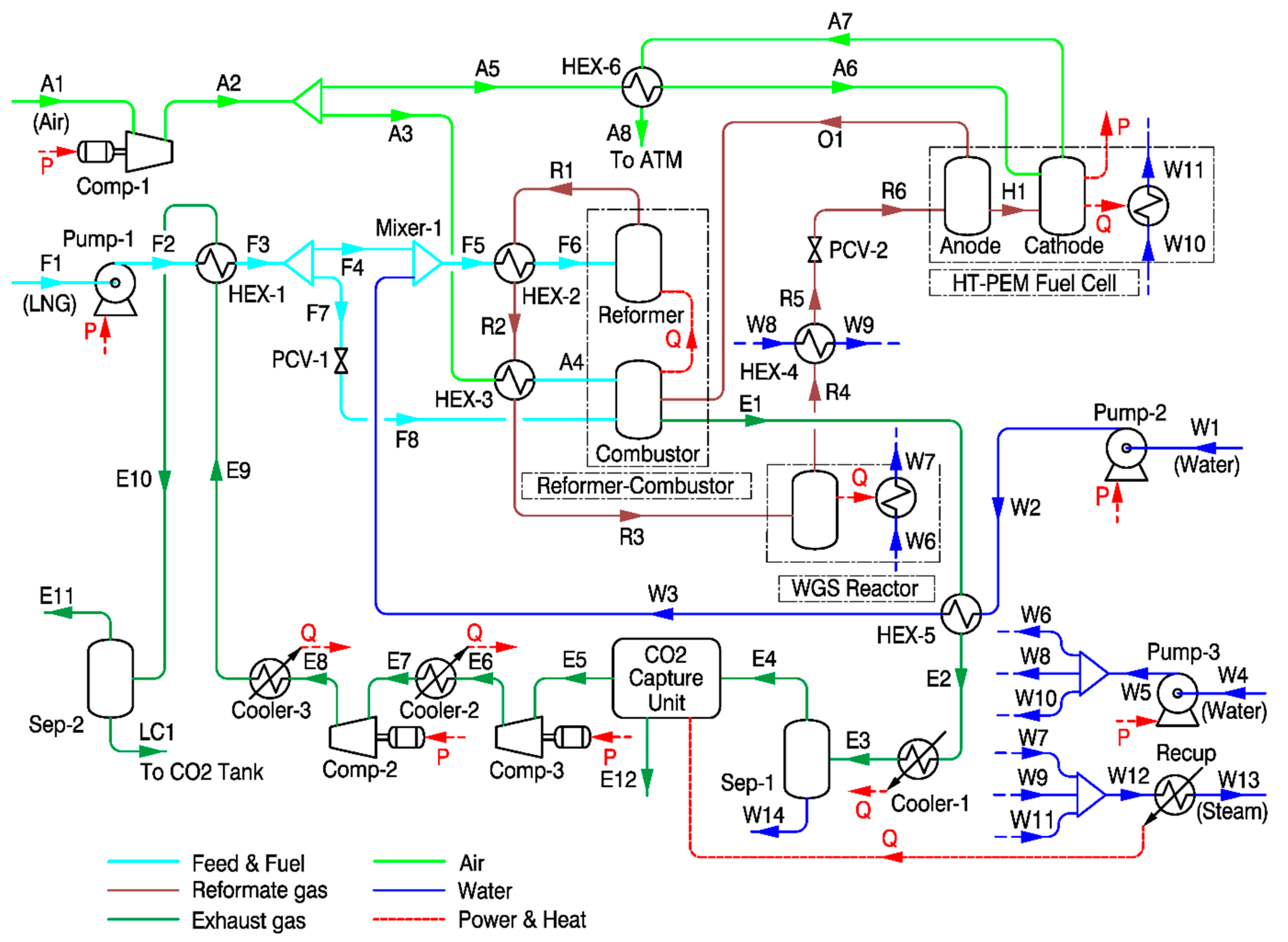
2.2. Description of Steam Methane Reforming-Based System
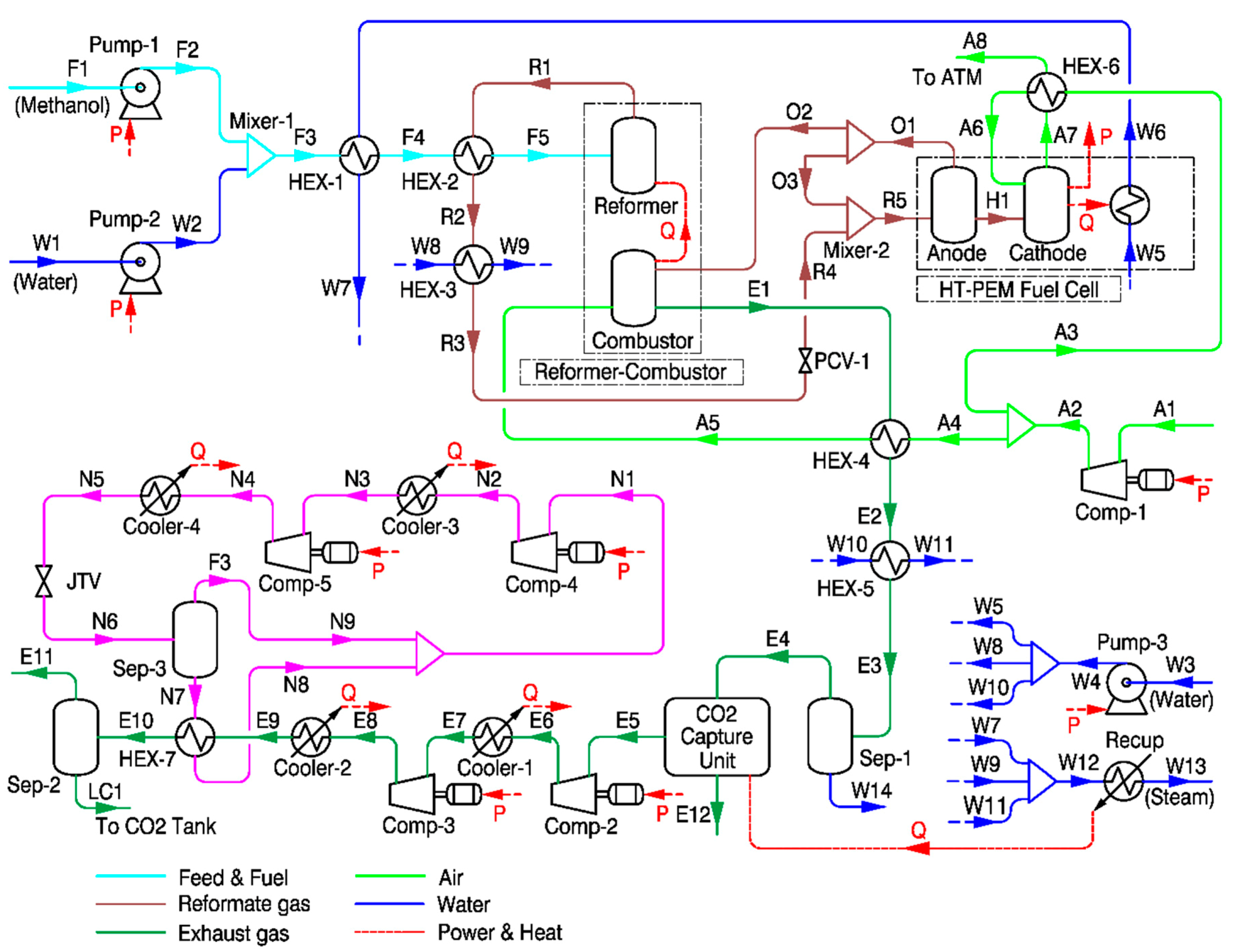
2.3. Description of Steam Methanol Reforming-Based System
3. System Simulation and Assumptions
- The simulations are implemented in a steady state and are not suitable for start-up operations.
- The composition of air is considered to be 79% N2 and 21% O2 on a mole basis.
- For simplicity, LNG is represented by pure, liquefied CH4.
- The reaction time is considered long enough to achieve phase and chemical equilibrium.
- The reformate gases exiting the reformer are at the reformer temperature.
- Heat and pressure losses are assumed to be negligible in all operational units.
- Complete fuel oxidation is assumed in the combustor.
- In the CO2 capture unit, only heat consumption is considered because the power consumption at cooling pumps, solvent pumps, and other devices is relatively small.
- The heat ejected from coolers is not recovered.
4. Performance Evaluation
4.1. Energy Analysis of the Integrated Systems
4.2. Exergy Analysis of the Integrated Systems
5. Results and Discussion
5.1. Energy and Exergy Analyses
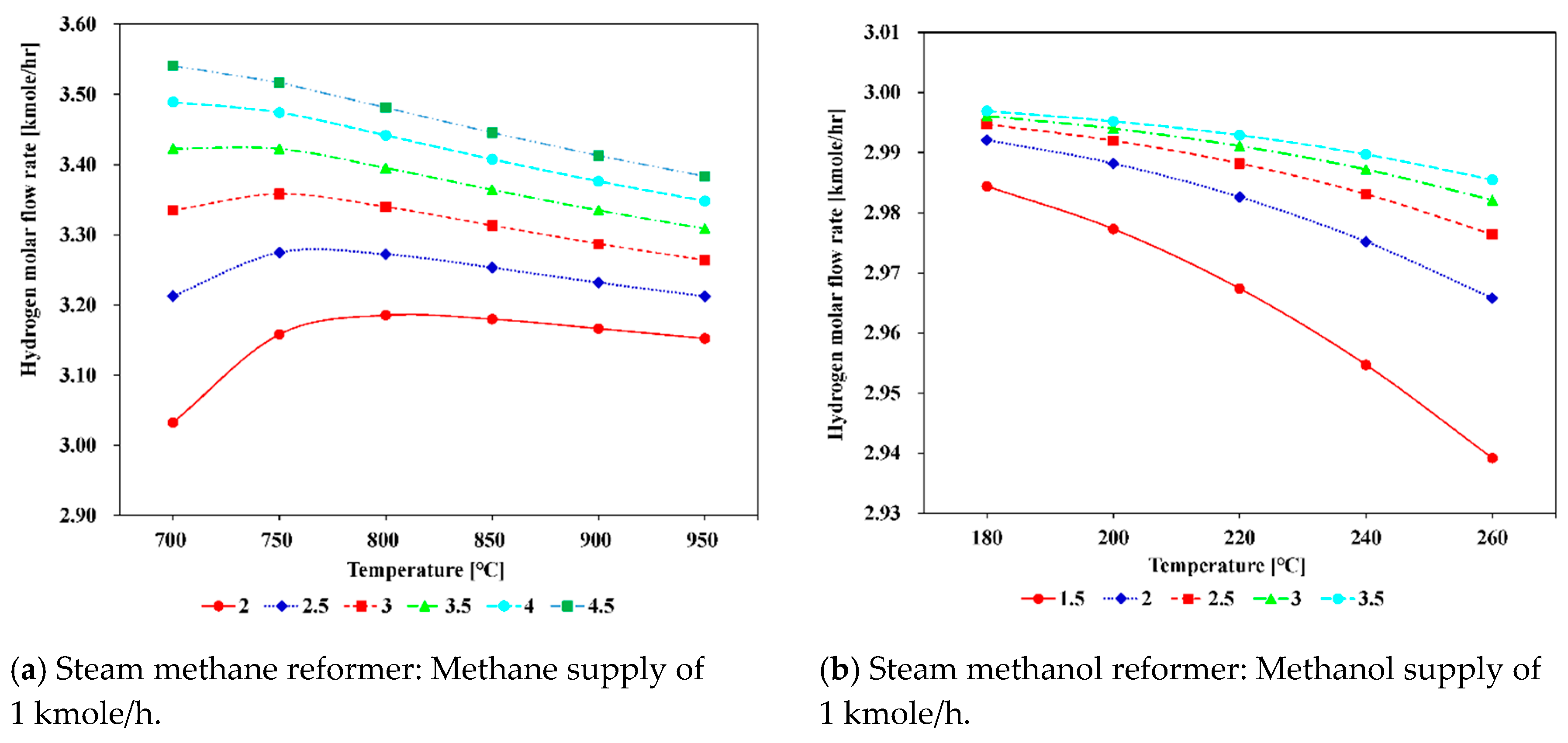
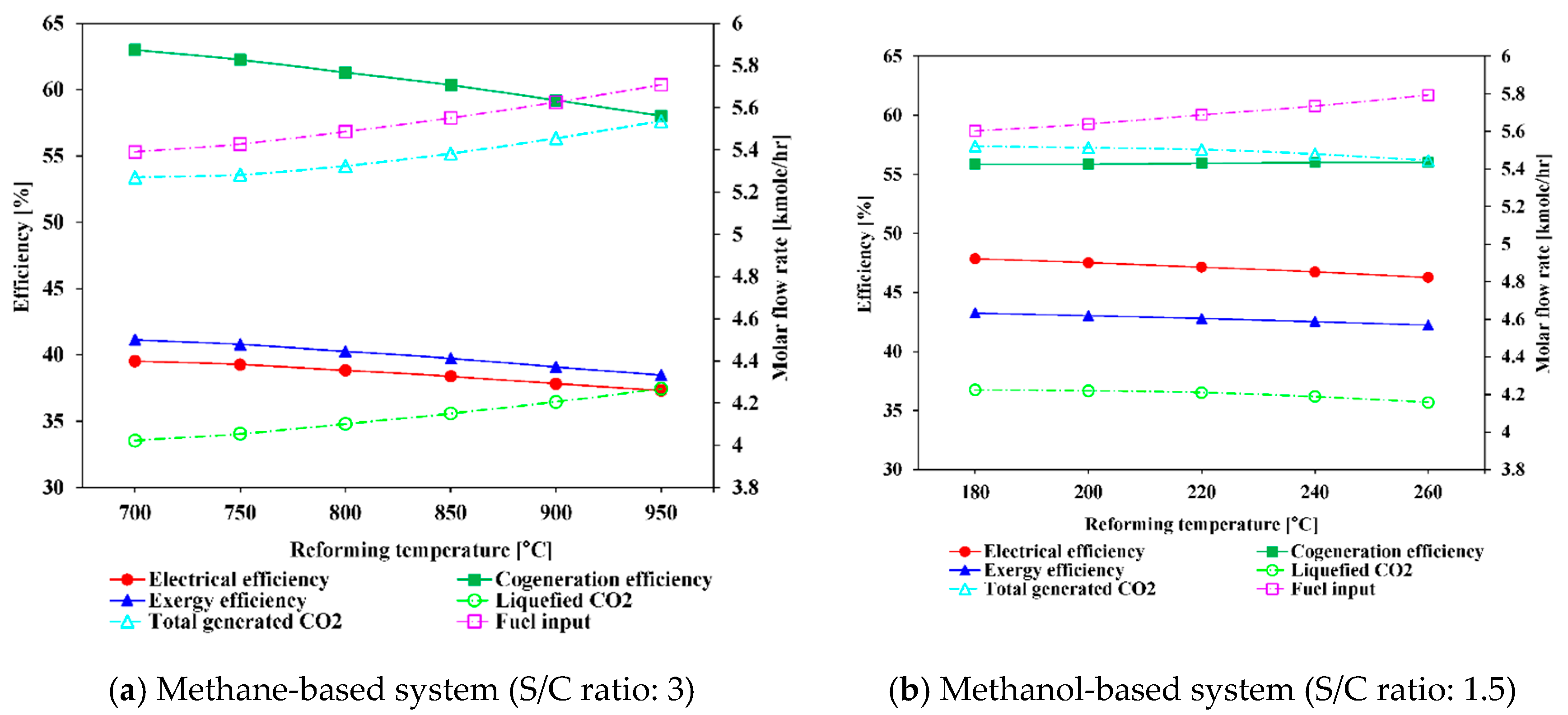
5.1.1. Effect of Varying Reforming Temperature
5.1.2. Effect of Varying Steam to Carbon Ratio
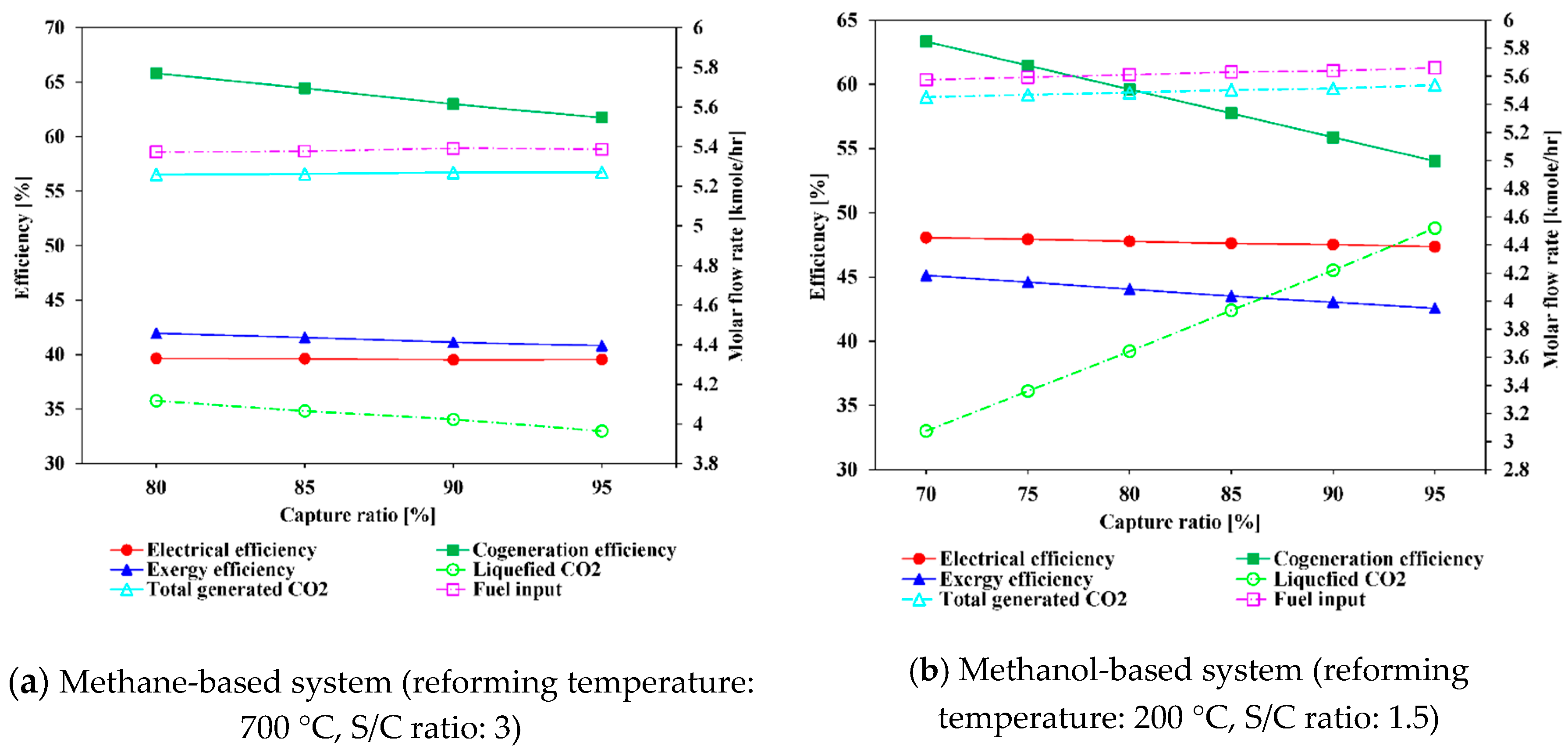
5.1.3. Effect of Varying CO2 Capture Ratio
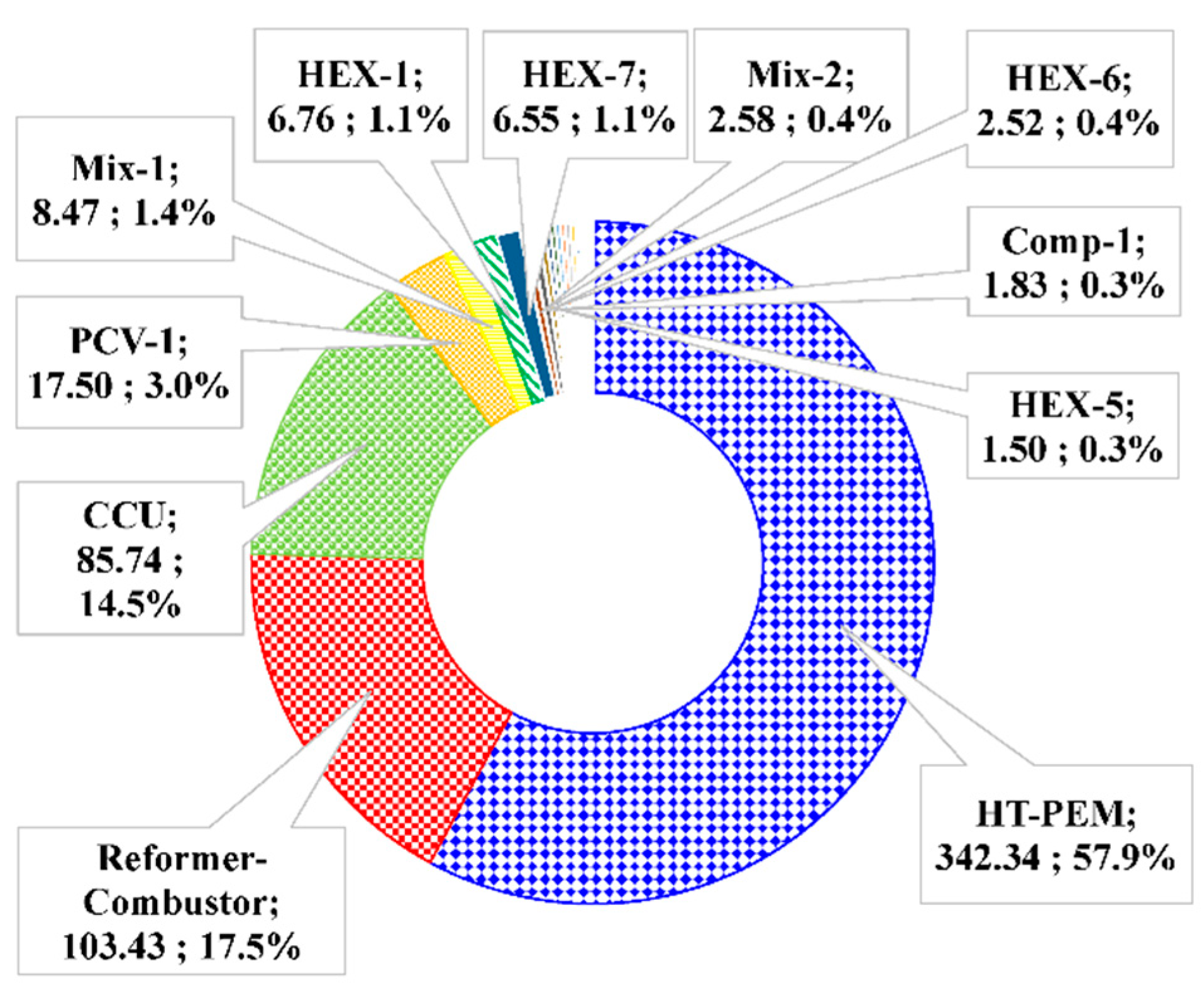
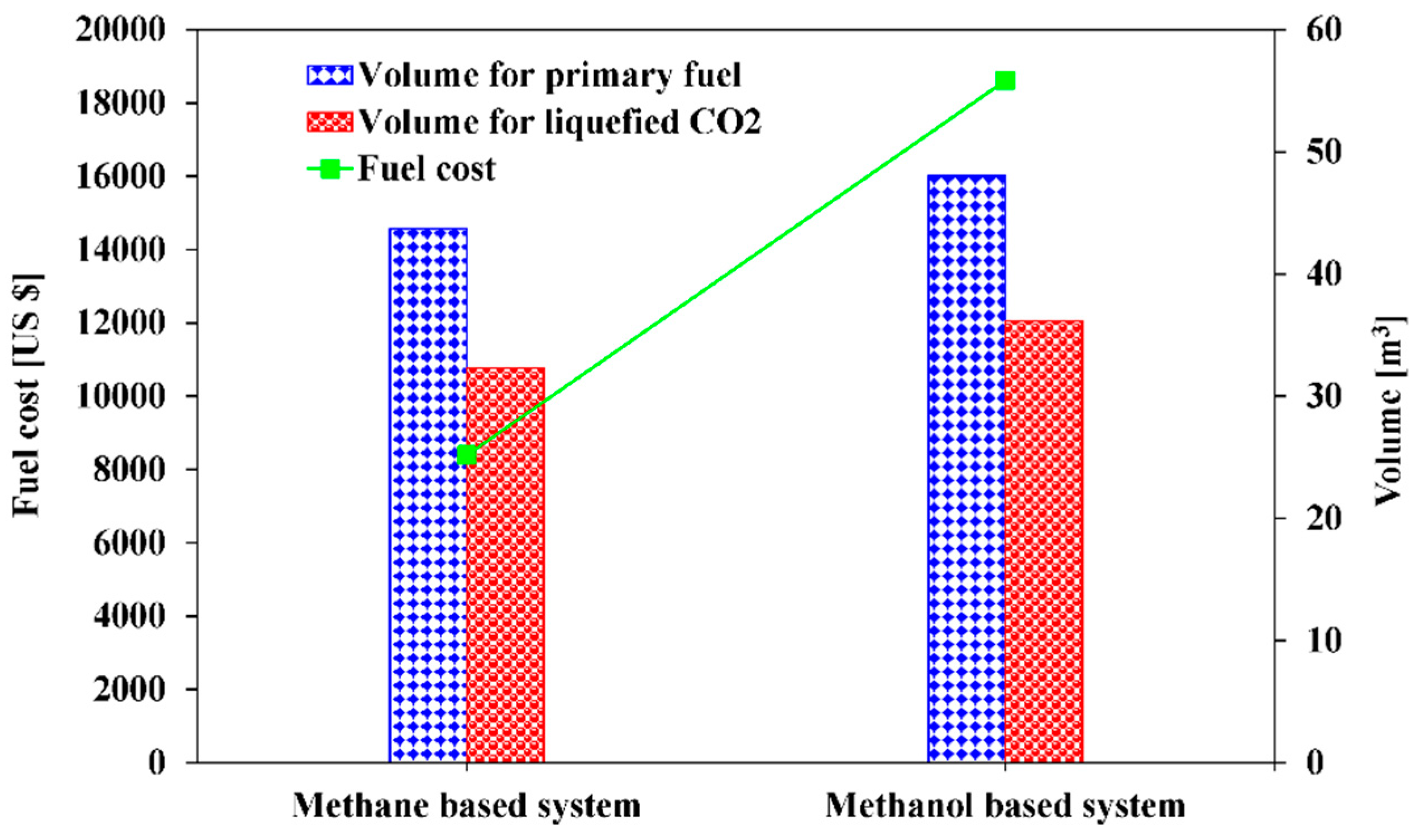
5.2. Space and Operational Cost
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Symbols | |
| e | Specific exergy, kJ/kg |
| Exergy flow rate, kW | |
| s | Specific entropy, kJ/kg·°C |
| h | Specific enthalpy, kJ/kg |
| P | Pressure, bar |
| T | Temperature, °C |
| h | Time, h |
| Mass flow rate, kg/h | |
| Molar flow rate, kmole/h | |
| Vc | Output voltage |
| Fuel utilization factor | |
| Q | Heat rate, kW |
| Density, kg/m3 | |
| Abbreviations | |
| LHV | Lower heating value |
| LNG | Liquefied natural gas |
| CCU | Carbon capture unit |
| MEA | Monoethanolamine |
| HT-PEMFC | High temperature proton-exchange membrane fuel cell |
| LT-PEMFC | Low temperature proton-exchange membrane fuel cell |
| OPEX | Operation expenditure |
| WGS | Water gas shift |
| HFO | Heavy fuel oil |
| CCS | Carbon capture and storage |
References
- International Maritime Organization. Third IMO Greenhouse Gas Study. Available online: http://www.imo.org/en/OurWork/Environment/PollutionPrevention/AirPollution/Pages/Greenhouse-Gas-Studies-2014.aspx (accessed on 30 December 2019).
- DNV GL. Maritime Forecast to 2050, Energy Transition Outlook 2018. Available online: https://eto.dnvgl.com/2018/#Energy-Transition-Outlook-2018 (accessed on 30 December 2019).
- Initial IMO Strategy on the Reduction of GHG Emissions From Ships. Resolution MEPC. Available online: http://www.imo.org/en/MediaCentre/HotTopics/GHG/Pages/default.aspx (accessed on 30 December 2019).
- Zhu, M.; Fai, K.; Wei, J.; Li, K.X. Impact of maritime emissions trading system on fleet deployment and mitigation of CO2 emission. Transp. Res. Part D 2018, 62, 474–488. [Google Scholar] [CrossRef]
- Van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef]
- Balcombe, P.; Brierley, J.; Lewis, C.; Skatvedt, L.; Speirs, J.; Hawkes, A.; Staffell, I. How to decarbonise international shipping: Options for fuels, technologies and policies. Energy Convers. Manag. 2019, 182, 72–88. [Google Scholar] [CrossRef]
- Tronstad, T.; Astrand, H.H.; Haugom, G.P.; Langfeldt, L. Study on the Use of Fuel Cells in Shipping. Available online: http://www.emsa.europa.eu/component/flexicontent/download/4545/2921/23.html (accessed on 30 December 2019).
- Jaggi, V.; Jayanti, S. A conceptual model of a high-efficiency, stand-alone power unit based on a fuel cell stack with an integrated auto-thermal ethanol reformer. Appl. Energy 2013, 110, 295–303. [Google Scholar] [CrossRef]
- Romero-Pascual, E.; Soler, J. Modelling of an HTPEM-based micro-combined heat and power fuel cell system with methanol. Int. J. Hydrogen Energy 2014, 39, 4053–4059. [Google Scholar] [CrossRef]
- Hydrogen Analysis Resource Center. Lower and Higher Heating Values of Fuels. Available online: https://h2tools.org/hyarc/hydrogen-data/lower-and-higher-heating-values-hydrogen-and-other-fuels (accessed on 30 December 2019).
- Vessels, M. Fuel Cell Applications for Marine Vessels Why Fuel Cells Make Sense. Available online: https://info.ballard.com/fuel-cell-applications-for-marine-vessels?hsCtaTracking=8b99d2e2-4e14-44a2-b88d-9cda7551cd89%7Cdef386da-b1b5-49ff-983b-9cdcf065b567 (accessed on 30 December 2019).
- Nerem, T. Assessment of Marine Fuels in a Fuel Cell on a Cruise Vessel. Available online: https://ntnuopen.ntnu.no/ntnu-xmlui/bitstream/handle/11250/2564492/19196_FULLTEXT.pdf?sequence=1&isAllowed=y (accessed on 30 December 2019).
- Sattler, G. Fuel cells going on-board. J. Power Sources 2000, 86, 61–67. [Google Scholar] [CrossRef]
- Welaya, Y.M.A.; El Gohary, M.M.; Ammar, N.R. Steam and partial oxidation reforming options for hydrogen production from fossil fuels for PEM fuel cells. Alex. Eng. J. 2012, 51, 69–75. [Google Scholar] [CrossRef]
- e4ships FUEL CELLS IN MARINE APPLICATIONS. Available online: https://www.e4ships.de/app/download/13416971890/e4ships_Brochure_engl_2016.pdf?t=1568291372 (accessed on 30 December 2019).
- Rajasekhar, D.; Narendrakumar, D. Fuel Cell Technology for Propulsion and Power Generation of Ships: Feasibility Study on Ocean Research Vessel Sagarnidhi. J. Ship. Ocean Eng. 2020, 5, 219–228. [Google Scholar]
- Saito, N. The Maritime Commons: Digital Repository of the World. The Economic Analysis of Commercial Ships with Hydrogen Fuel Cell through Case Studies. Available online: https://commons.wmu.se/cgi/viewcontent.cgi?article=1617&context=all_dissertations (accessed on 30 December 2019).
- Han, J.; Charpentier, J.F.; Tang, T. State of the Art of Fuel Cells for Ship Applications. In Proceedings of the 2012 IEEE International Symposium on Industrial Electronics, Hangzhou, China, 28–31 May 2012. [Google Scholar]
- Alvarez, C.; Fern, C.; Carral, L. Analysing the possibilities of using fuel cells in ships. Int. J. Hydrogen Energy 2015, 41, 2853–2866. [Google Scholar] [CrossRef]
- Leo, T.J.; Durango, J.A.; Navarro, E. Exergy analysis of PEM fuel cells for marine applications. Energy 2010, 35, 1164–1171. [Google Scholar] [CrossRef]
- Faungnawakij, K.; Kikuchi, R.; Eguchi, K. Thermodynamic evaluation of methanol steam reforming for hydrogen production. J. Power Sources 2006, 161, 87–94. [Google Scholar] [CrossRef]
- Chen, B.; Liao, Z.; Wang, J.; Yu, H.; Yang, Y. Exergy analysis and CO2 emission evaluation for steam methane reforming. Int. J. Hydrogen Energy 2012, 37, 3191–3200. [Google Scholar] [CrossRef]
- Simpson, A.P.; Lutz, A.E. Exergy analysis of hydrogen production via steam methane reforming. Int. J. Hydrogen Energy 2007, 32, 4811–4820. [Google Scholar] [CrossRef]
- Alhamdani, Y.A.; Hassim, M.H.; Ng, R.T.L.; Hurme, M. The estimation of fugitive gas emissions from hydrogen production by natural gas steam reforming. Int. J. Hydrogen Energy 2017, 42, 9342–9351. [Google Scholar] [CrossRef]
- Authayanun, S.; Saebea, D.; Patcharavorachot, Y.; Arpornwichanop, A. Effect of different fuel options on performance of high-temperature PEMFC (proton exchange membrane fuel cell) systems. Energy 2014, 68, 989–997. [Google Scholar] [CrossRef]
- Arsalis, A.; Nielsen, M.P.; Kær, S.K. Modeling and parametric study of a 1 kWe HT-PEMFC-based residential micro-CHP system. Int. J. Hydrogen Energy 2011, 36, 5010–5020. [Google Scholar] [CrossRef]
- Herdem, M.S.; Farhad, S.; Hamdullahpur, F. Modeling and parametric study of a methanol reformate gas-fueled HT-PEMFC system for portable power generation applications. Energy Convers. Manag. 2015, 101, 19–29. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Mousavi Ehteshami, S.M.; Chan, S.H. Techno-Economic Study of Hydrogen Production via Steam Reforming of Methanol, Ethanol, and Diesel. Energy Technol. Policy 2014, 1, 15–22. [Google Scholar] [CrossRef]
- Zhu, L.; Li, L.; Fan, J. A modified process for overcoming the drawbacks of conventional steam methane reforming for hydrogen production: Thermodynamic investigation. Chem. Eng. Res. Des. 2015, 104, 792–806. [Google Scholar] [CrossRef]
- Ye, L.; Jiao, K.; Du, Q.; Yin, Y. Exergy analysis of high-temperature proton exchange membrane fuel cell systems. Int. J. Green Energy 2015, 12, 917–929. [Google Scholar] [CrossRef]
- Ishihara, A.; Mitsushima, S.; Kamiya, N.; Ota, K.I. Exergy analysis of polymer electrolyte fuel cell systems using methanol. J. Power Sources 2004, 126, 34–40. [Google Scholar] [CrossRef]
- Wiese, W.; Emonts, B.; Peters, R. Methanol steam reforming in a fuel cell drive system. J. Power Sources 1999, 84, 187–193. [Google Scholar] [CrossRef]
- Lotrič, A.; Sekavčnik, M.; Pohar, A.; Likozar, B.; Hočevar, S. Conceptual design of an integrated thermally self-sustained methanol steam reformer—High-temperature PEM fuel cell stack manportable power generator. Int. J. Hydrogen Energy 2017, 42, 16700–16713. [Google Scholar] [CrossRef]
- TNO; Maritime Knowledge Centre. TU Delft Framework CO2 Reduction in Shipping. Available online: https://www.mkc-net.nl/library/documents/893/download/ (accessed on 30 December 2019).
- Voldsund, M.; Jordal, K.; Anantharaman, R. Hydrogen production with CO2 capture. Int. J. Hydrogen Energy 2016, 41, 4969–4992. [Google Scholar] [CrossRef]
- Ferrara, G.; Lanzini, A.; Leone, P.; Ho, M.T.; Wiley, D.E. Exergetic and exergoeconomic analysis of post-combustion CO2 capture using MEA-solvent chemical absorption. Energy 2017, 130, 113–128. [Google Scholar] [CrossRef]
- Bhown, A.S.; Freeman, B.C. Analysis and status of post-combustion carbon dioxide capture technologies. Environ. Sci. Technol. 2011, 45, 8624–8632. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, L. Performance analysis of a feasible technology for power and high-purity hydrogen production driven by methane fuel. Appl. Therm. Eng. 2015, 75, 103–114. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, L.; Jiang, P.; Li, L.; Liu, H. Comparative exergy analysis of chemical looping combustion thermally coupled and conventional steam methane reforming for hydrogen production. J. Clean. Prod. 2016, 131, 247–258. [Google Scholar] [CrossRef]
- Oh, S.Y.; Binns, M.; Cho, H.; Kim, J.K. Energy minimization of MEA-based CO2 capture process. Appl. Energy 2016, 169, 353–362. [Google Scholar] [CrossRef]
- Mangalapally, H.P.; Notz, R.; Hoch, S.; Asprion, N.; Sieder, G.; Garcia, H.; Hasse, H. Pilot plant experimental studies of post combustion CO2 capture by reactive absorption with MEA and new solvents. Energy Procedia 2009, 1, 963–970. [Google Scholar] [CrossRef]
- Abu-Zahra, M.R.M.; Schneiders, L.H.J.; Niederer, J.P.M.; Feron, P.H.M.; Versteeg, G.F. CO2 capture from power plants. Part I. A parametric study of the technical performance based on monoethanolamine. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Romeo, L.M.; Bolea, I.; Escosa, J.M. Integration of power plant and amine scrubbing to reduce CO2 capture costs. Appl. Therm. Eng. 2008, 28, 1039–1046. [Google Scholar] [CrossRef]
- Goo, S.; Bong, G.; Jun, C.; Min, J. Chemical Engineering Research and Design Optimal design and operating condition of boil-off CO2 re-liquefaction process, considering seawater temperature variation and compressor discharge. Chem. Eng. Res. Des. 2017, 124, 29–45. [Google Scholar]
- Feenstra, M.; Monteiro, J.; van den Akker, J.T.; Abu-Zahra, M.R.M.; Gilling, E.; Goetheer, E. Ship-based carbon capture onboard of diesel or LNG-fuelled ships. Int. J. Greenh. Gas Control 2019, 85, 1–10. [Google Scholar] [CrossRef]
- Berstad, D.; Anantharaman, R.; Nekså, P. Low-temperature CO2 capture technologies—Applications and potential. Int. J. Refrig. 2013, 36, 1403–1416. [Google Scholar] [CrossRef]
- Minnehan, J.J.; Pratt, J.W. Practical Application Limits of Fuel Cells and Batteries for Zero Emission Vessels. Available online: https://energy.sandia.gov/wp-content/uploads/2017/12/SAND2017-12665.pdf (accessed on 30 December 2019).
- Hajjaji, N.; Pons, M.N.; Houas, A.; Renaudin, V. Exergy analysis: An efficient tool for understanding and improving hydrogen production via the steam methane reforming process. Energy Policy 2012, 42, 392–399. [Google Scholar] [CrossRef]
- Liquefied Natural Gas (LNG). Operations Consistent Methodology for Estimating Greenhouse Gas Emissions. Available online: https://www.api.org/~/media/Files/EHS/climate-change/api-lng-ghg-emissions-guidelines-05-2015.pdf (accessed on 30 December 2019).
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef]
- Tzanetis, K.F.; Martavaltzi, C.S.; Lemonidou, A.A. Comparative exergy analysis of sorption enhanced and conventional methane steam reforming. Int. J. Hydrogen Energy 2012, 37, 16308–16320. [Google Scholar] [CrossRef]
- Nalbant, Y.; Colpan, C.O.; Devrim, Y. Energy and exergy performance assessments of a high temperature-proton exchange membrane fuel cell based integrated cogeneration system. Int. J. Hydrogen Energy 2019, 1–11. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Abdollahzadeh, M.; Sousa, J.M.; Boaventura, M.; Mendes, A. Modelling of a high-temperature polymer electrolyte membrane fuel cell integrated with a methanol steam reformer cell. Appl. Energy 2017, 202, 6–19. [Google Scholar] [CrossRef]
- Boaventura, M.; Sander, H.; Friedrich, K.A.; Mendes, A. The influence of CO on the current density distribution of high temperature polymer electrolyte membrane fuel cells. Electrochim. Acta 2011, 56, 9467–9475. [Google Scholar] [CrossRef]
- Ersoz, A.; Olgun, H.; Ozdogan, S. Simulation study of a proton exchange membrane (PEM) fuel cell system with autothermal reforming. Energy 2006, 31, 1490–1500. [Google Scholar] [CrossRef]
- Zohrabian, A.; Mansouri, M.; Soltanieh, M. Techno-economic evaluation of an integrated hydrogen and power co-generation system with CO2 capture. Int. J. Greenh. Gas Control 2016, 44, 94–103. [Google Scholar] [CrossRef]
- Haydary, J. Chemical Process Design and Simulation: Aspen Plus and Aspen Hysys Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 1119089115. [Google Scholar]
- Simpson, A.P.; Simon, A.J. Second law comparison of oxy-fuel combustion and post-combustion carbon dioxide separation. Energy Convers. Manag. 2007, 48, 3034–3045. [Google Scholar] [CrossRef]
- Andreasen, S.J.; Vang, J.R.; Kær, S.K. High temperature PEM fuel cell performance characterisation with CO and CO2 using electrochemical impedance spectroscopy. Int. J. Hydrogen Energy 2011, 36, 9815–9830. [Google Scholar] [CrossRef]
- Devrim, Y.; Albostan, A.; Devrim, H. Experimental investigation of CO tolerance in high temperature PEM fuel cells. Int. J. Hydrogen Energy 2018, 43, 18672–18681. [Google Scholar] [CrossRef]
- Oh, K.; Ju, H. Temperature dependence of CO poisoning in high-temperature proton exchange membrane fuel cells with phosphoric acid-doped polybenzimidazole membranes. Int. J. Hydrogen Energy 2015, 40, 7743–7753. [Google Scholar] [CrossRef]
- Jo, A.; Oh, K.; Lee, J.; Han, D.; Kim, D.; Kim, J.; Kim, B.; Kim, J.; Park, D.; Kim, M.; et al. Modeling and analysis of a 5 kWe HT-PEMFC system for residential heat and power generation. Int. J. Hydrogen Energy 2017, 42, 1698–1714. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A.; McDonald, M.S. Fuel Cell Systems Explained; J. Wiley: Chichester, UK, 2003; Volume 2. [Google Scholar]
- Authayanun, S.; Hacker, V. Energy and exergy analyses of a stand-alone HT-PEMFC based trigeneration system for residential applications. Energy Convers. Manag. 2018, 160, 230–242. [Google Scholar] [CrossRef]
- Tsatsaronis, G. Definitions and nomenclature in exergy analysis and exergoeconomics. Energy 2007, 32, 249–253. [Google Scholar] [CrossRef]
- Hinderink, A.P.; Kerkhof, F.P.J.M.; Lie, A.B.K.; De Swaan Arons, J.; Van Der Kooi, H.J. Exergy analysis with a flowsheeting simulator—I. Theory; calculating exergies of material streams. Chem. Eng. Sci. 1996, 51, 4693–4700. [Google Scholar] [CrossRef]
- Mert, S.O.; Ozcelik, Z.; Dincer, I. Comparative assessment and optimization of fuel cells. Int. J. Hydrogen Energy 2015, 40, 7835–7845. [Google Scholar] [CrossRef]
- Obara, S.; Tanno, I. Exergy analysis of a regional-distributed PEM fuel cell system. Int. J. Hydrogen Energy 2008, 33, 2300–2310. [Google Scholar] [CrossRef]
- Lee, H.; Shao, Y.; Lee, S.; Roh, G.; Chun, K.; Kang, H. Analysis and assessment of partial re-liquefaction system for liquefied hydrogen tankers using liquefied natural gas (LNG)and H2 hybrid propulsion. Int. J. Hydrogen Energy 2019, 44, 15056–15071. [Google Scholar] [CrossRef]
- BP Statistical Review of World Energy Statistical Review of World. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-natural-gas.pdf (accessed on 30 December 2019).
- Methanex Monthly Average Regional Posted Contract Price History. Available online: https://www.methanex.com (accessed on 30 December 2019).

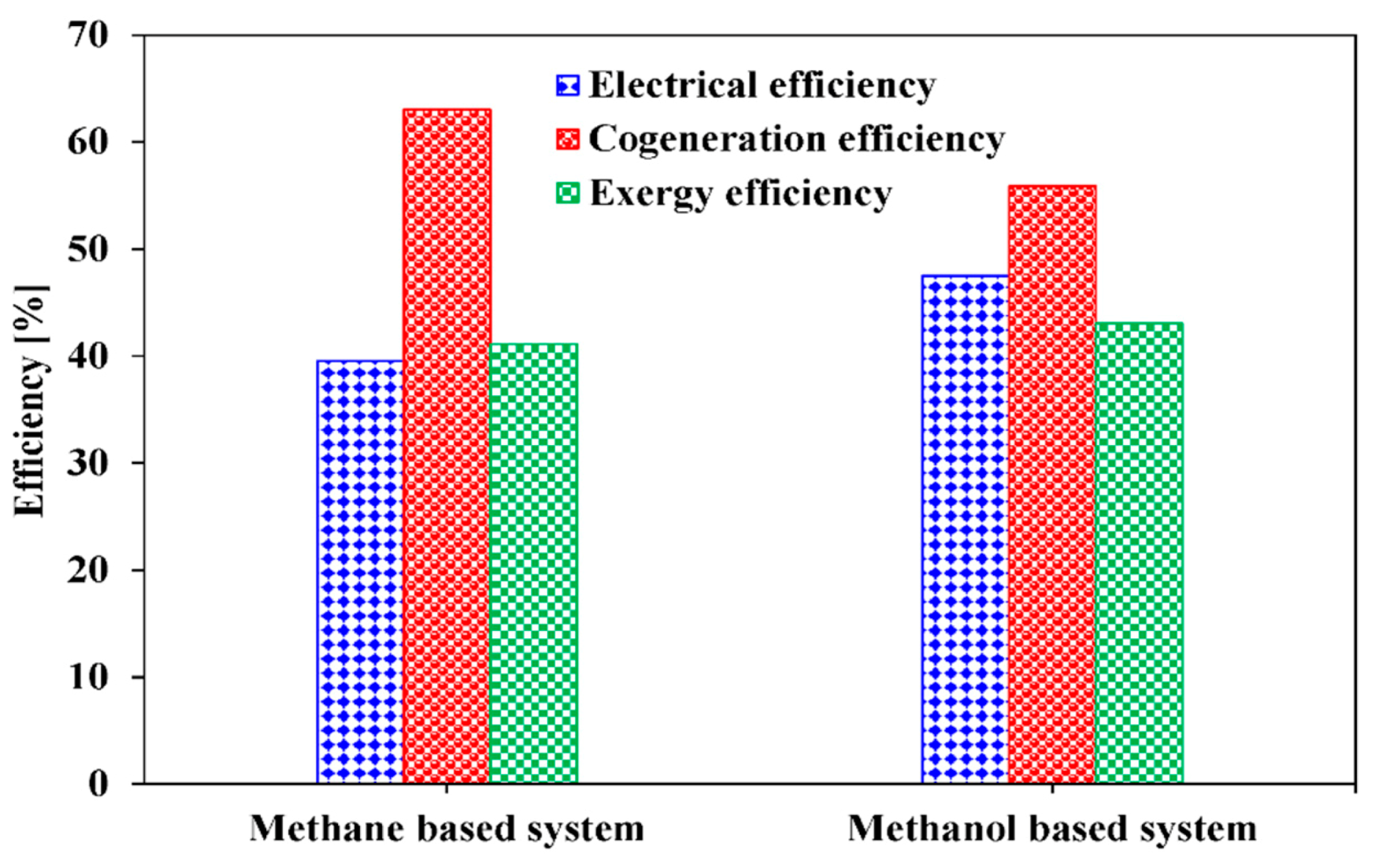
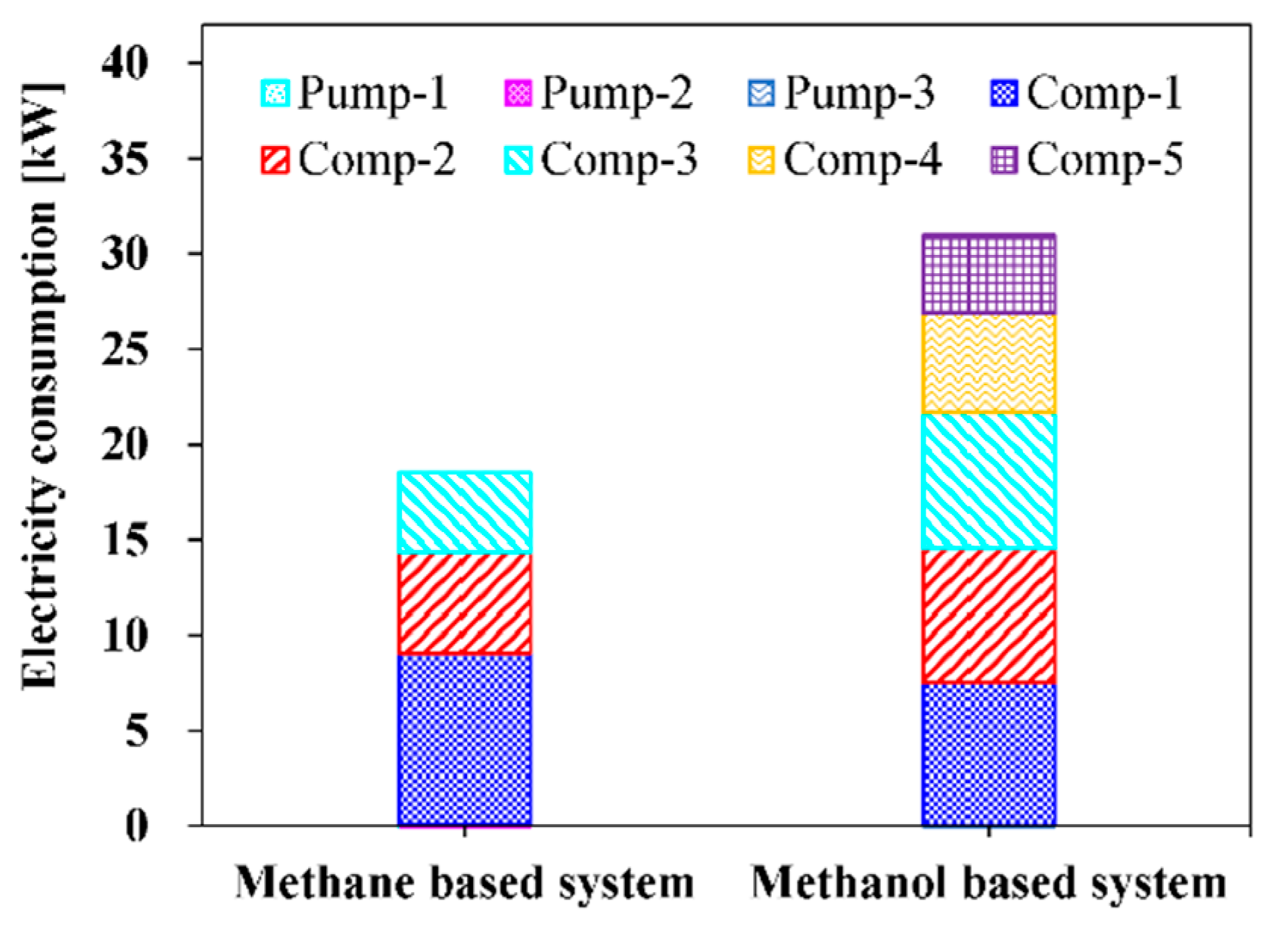
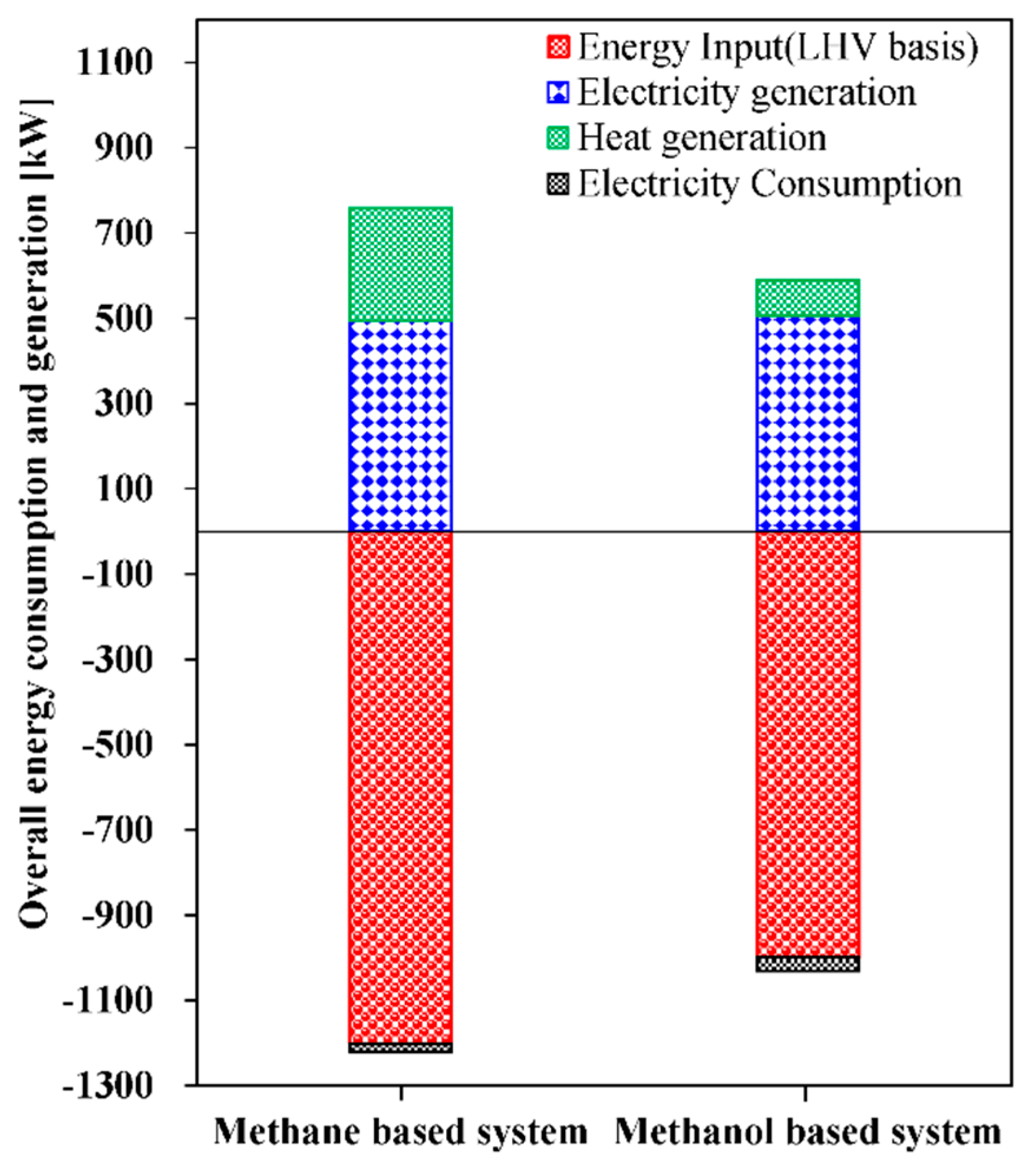
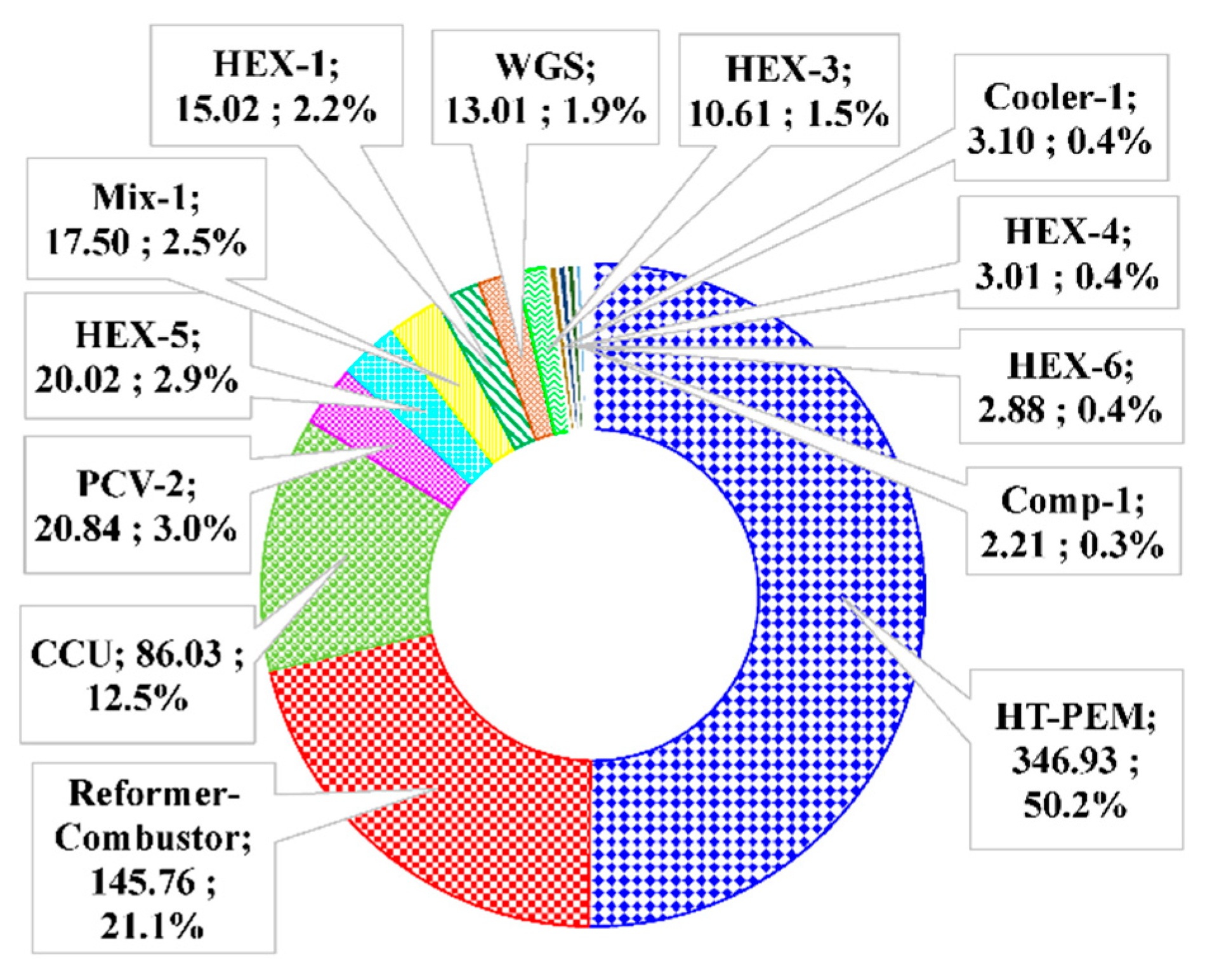

| Ref. | Primary Fuels | S/C Ratio | Pref >(Bar) | Tref >(°C) | Notes |
|---|---|---|---|---|---|
| [23] | Methane | 3.2 | 10 | 700 | Purpose of paper was to evaluate performance of hydrogen production via steam methane reforming. |
| [30] | 4 | 25 | 900 | Steam methane reforming system was modeled and reformer in simulation was developed using a Gibbs equilibrium model in Aspen Plus. For CO2 capture, MEA scrubbing process was applied as black box model. | |
| [31] | 3 | 1 | 700 | Steam methane reforming system integrated with HT-PEMFC was simulated and performance was evaluated by exergy analysis. | |
| [32] | Methanol | 1.2 | - | 350 | Steam methanol reforming system integrated with PEMFC was simulated and performance was evaluated by exergy analysis. |
| [33] | 1.5 | 3.8 | 260 | Steam methanol reforming system was experimented and the obtained results were used for simulation of power train integrated system. | |
| [34] | 1–2 | - | 240–300 | Steam methanol reforming system integrated with HT-PEMFC was simulated. Parametric study with varying S/C ratio, Tref, reformate composition, etc. were implemented. |
| Specifications | Values |
|---|---|
| Type | General Cargo |
| Overall length | 120 m |
| Beam | 13 m |
| Deadweight | 3000 tonnage |
| Main engine power | 3800 kW |
| Maximum speed | 14 knots |
| Average speed | 7 knots |
| Total voyage time | 209 h |
| Load factor | 0.125 |
| Average shaft power | 475 kW |
| Unit Name | Parameter | Values | |
|---|---|---|---|
| Steam Methane Reforming-Based System | Steam Methanol Reforming-Based System | ||
| Steam reformer | Operating temperature | 700 °C [23,31] | 200 °C [34] |
| Operating pressure | 3 bar | 3 bar | |
| S/C ratio | 3 [31,53] | 1.5 [27] | |
| WGS reactor | Operating temperature | 250 °C [54] | − |
| Operating pressure | 1.1 bar | − | |
| Combustor | Operating temperature | 800 °C | 300 °C |
| Operating pressure | 1.1 bar | 1.1 bar | |
| Air-fuel ratio | 1.05 [30,41] | 1.05 [27] | |
| HT-PEMFC | Fuel utilization factor | 0.83 [31,55] | |
| Cathode stoichiometric ratio | 2 [31,55] | ||
| Operating temperature | 160 °C [31] | ||
| Operating pressure | 1.1 bar | ||
| Output voltage per cell | 0.637 V [56] | ||
| Current density | 0.2 A cm−2 [56] | ||
| CO2 capture unit [38] | Solvent | MEA | |
| Feed gas temperature | 40 °C | ||
| Steam temperature | 130 °C | ||
| Specific reboiler duty | 5112 kJ/kg CO2 | ||
| CO2 Capture ratio | 90% | ||
| CO2 Liquefaction system | Refrigerant | LNG | NH3 |
| Liquefaction condition | −8.5 °C at 7 bar | −29.75 °C at 14 bar | |
| Total emitted CO2 to ATM (Stream E11 and E12) | 55.83 kg/h | ||
| Compressors | Polytropic efficiency | 75% | |
| Pump | Adiabatic efficiency | 85% | |
| Converter | Efficiency | 98% [57] | |
| Heat exchangers | Min. temperature approach | Heat exchangers for CO2 liquefaction: 3 °C [46] Other heat exchangers: 10 °C [41] | |
| Net electrical power (AC) | 475 (±0.2) kW | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Jung, I.; Roh, G.; Na, Y.; Kang, H. Comparative Analysis of On-Board Methane and Methanol Reforming Systems Combined with HT-PEM Fuel Cell and CO2 Capture/Liquefaction System for Hydrogen Fueled Ship Application. Energies 2020, 13, 224. https://doi.org/10.3390/en13010224
Lee H, Jung I, Roh G, Na Y, Kang H. Comparative Analysis of On-Board Methane and Methanol Reforming Systems Combined with HT-PEM Fuel Cell and CO2 Capture/Liquefaction System for Hydrogen Fueled Ship Application. Energies. 2020; 13(1):224. https://doi.org/10.3390/en13010224
Chicago/Turabian StyleLee, Hyunyong, Inchul Jung, Gilltae Roh, Youngseung Na, and Hokeun Kang. 2020. "Comparative Analysis of On-Board Methane and Methanol Reforming Systems Combined with HT-PEM Fuel Cell and CO2 Capture/Liquefaction System for Hydrogen Fueled Ship Application" Energies 13, no. 1: 224. https://doi.org/10.3390/en13010224
APA StyleLee, H., Jung, I., Roh, G., Na, Y., & Kang, H. (2020). Comparative Analysis of On-Board Methane and Methanol Reforming Systems Combined with HT-PEM Fuel Cell and CO2 Capture/Liquefaction System for Hydrogen Fueled Ship Application. Energies, 13(1), 224. https://doi.org/10.3390/en13010224