Techno-Economic Analysis (TEA) of Different Pretreatment and Product Separation Technologies for Cellulosic Butanol Production from Oil Palm Frond
Abstract
:1. Introduction
2. Materials and Methods
2.1. Software Tools
2.2. Techno-Economic Analysis (TEA)
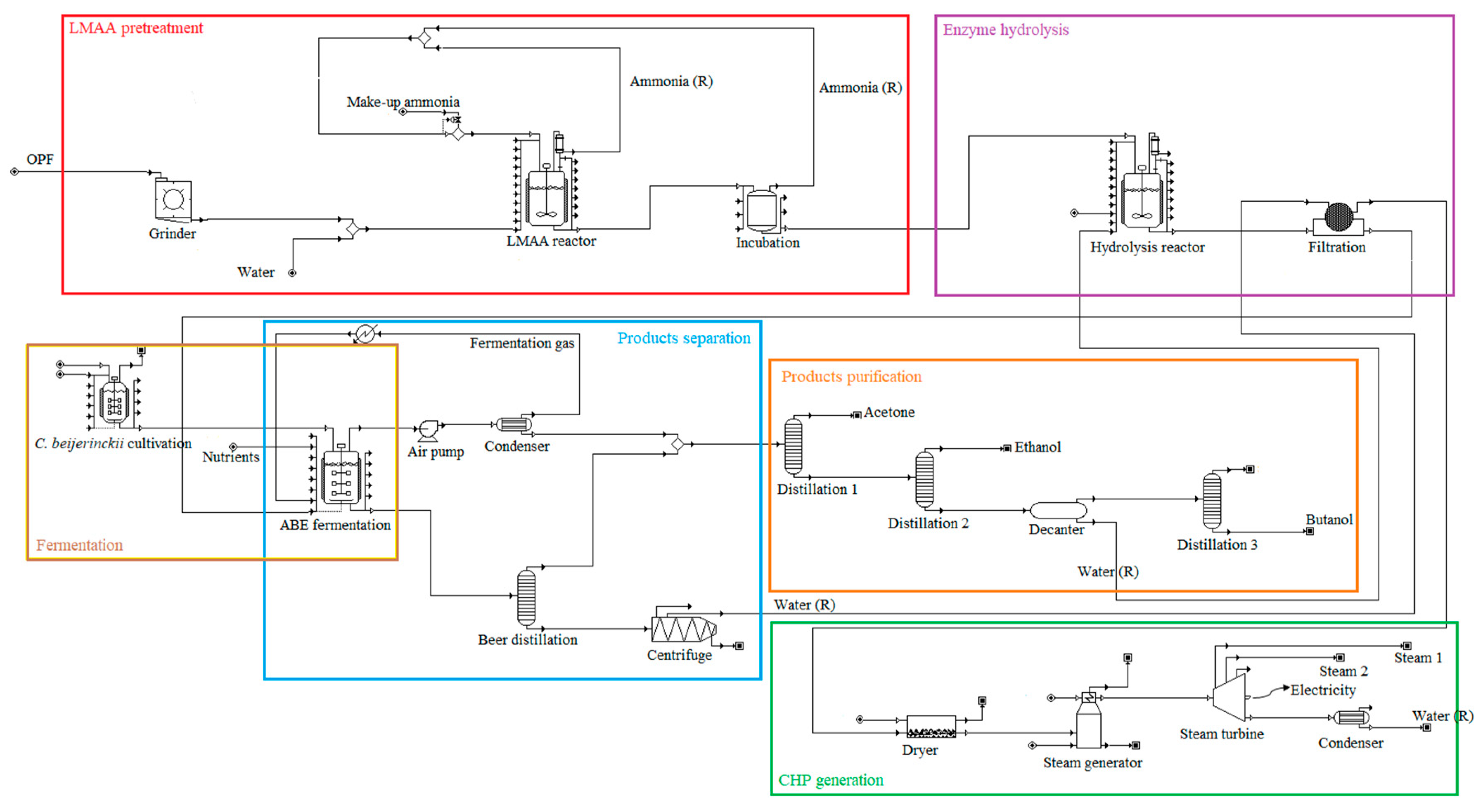
2.3. Pretreatment Approaches
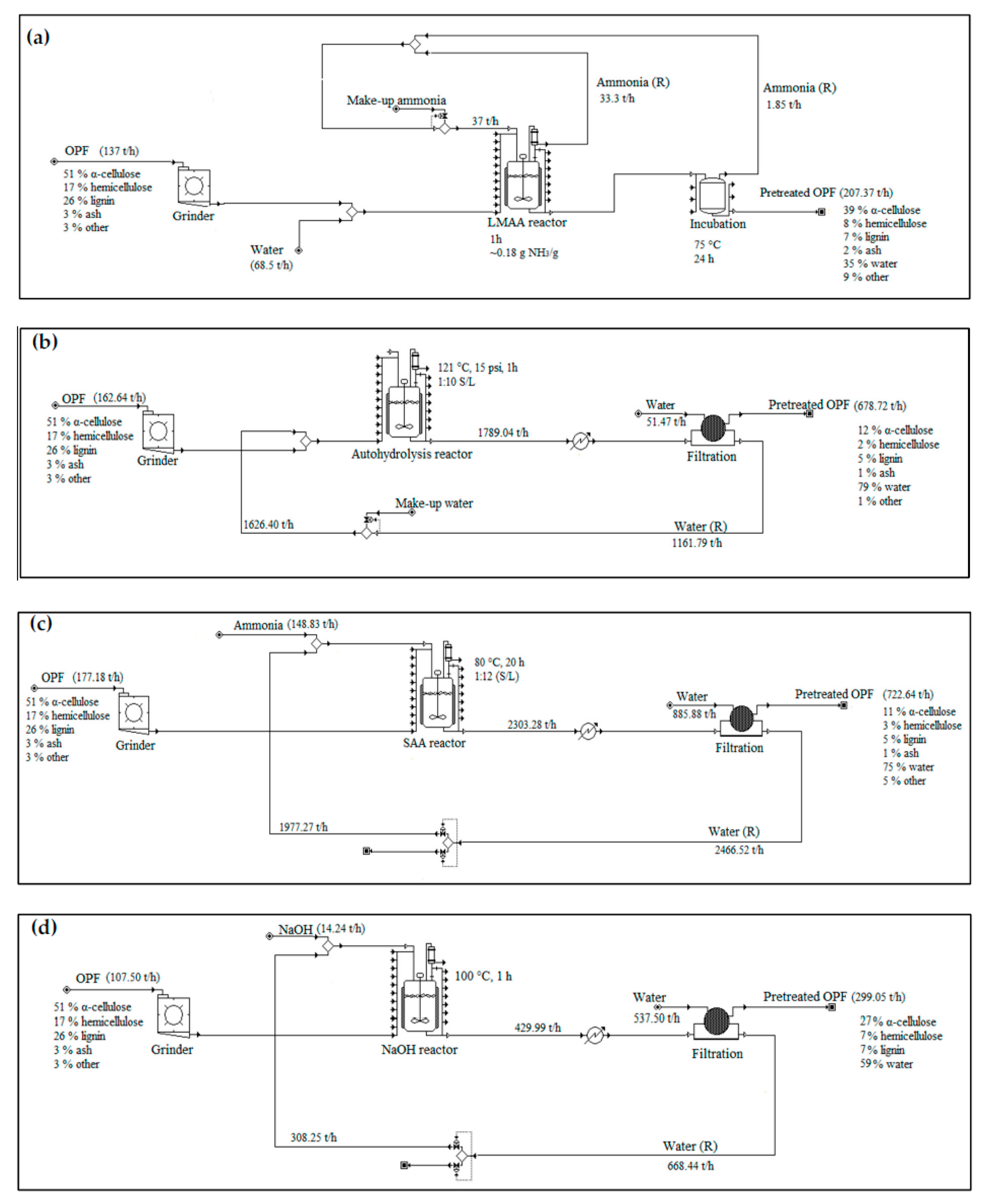
2.3.1. Low-Moisture Anhydrous Ammonia (LMAA) Pretreatment
2.3.2. Autohydrolysis Pretreatment
2.3.3. Soaking in Aqueous Ammonia (SAA) Pretreatment
2.3.4. Soaking in Sodium Hydroxide (NaOH) Pretreatment
2.4. Enzyme Hydrolysis and ABE Fermentation
2.5. Product Separation Approaches and Downstream Processing
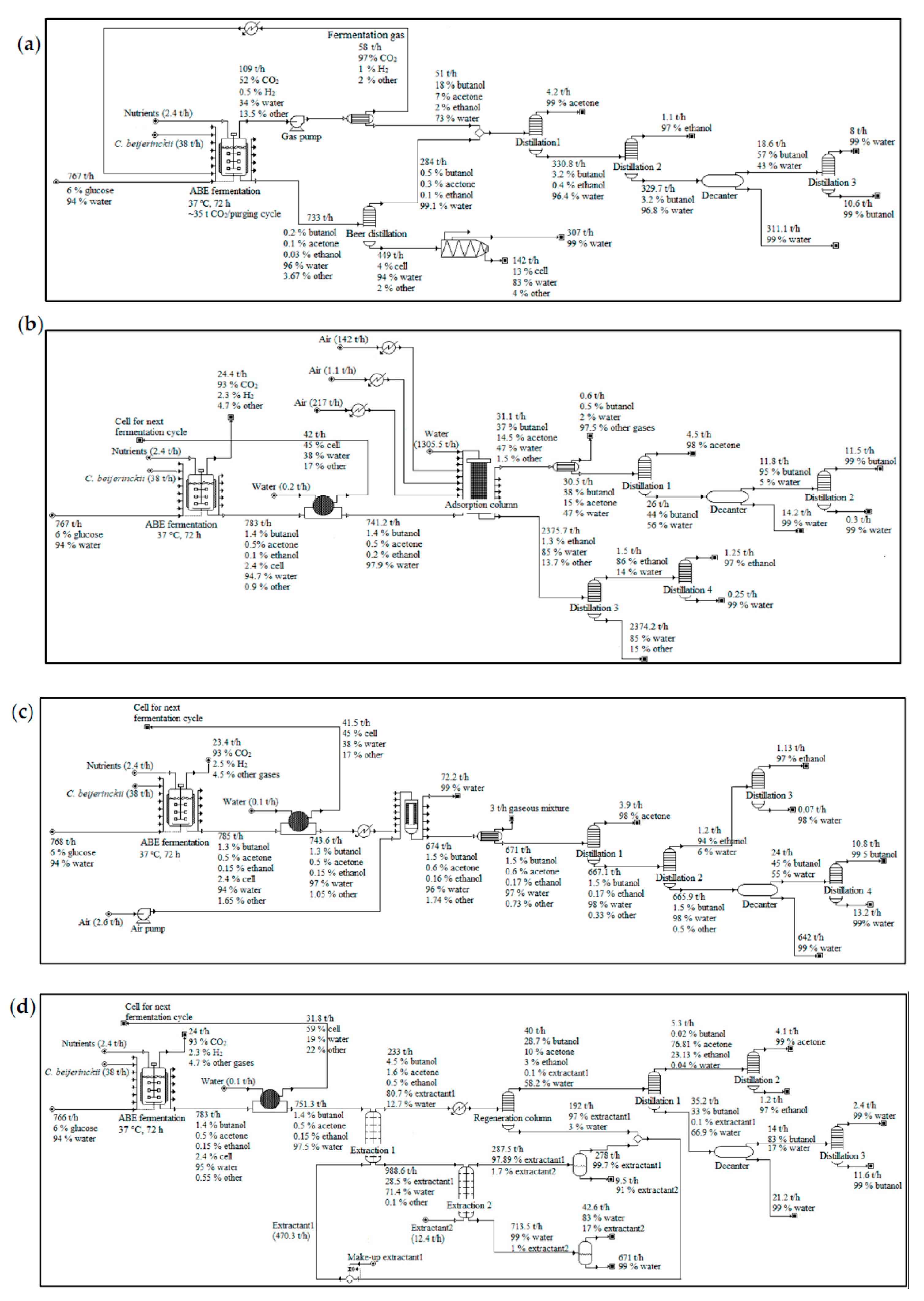
2.5.1. In Situ Stripping
2.5.2. Dual Extraction
2.5.3. Adsorption
2.5.4. Membrane Pervaporation
2.6. Power, Heating, and Cooling Requirement
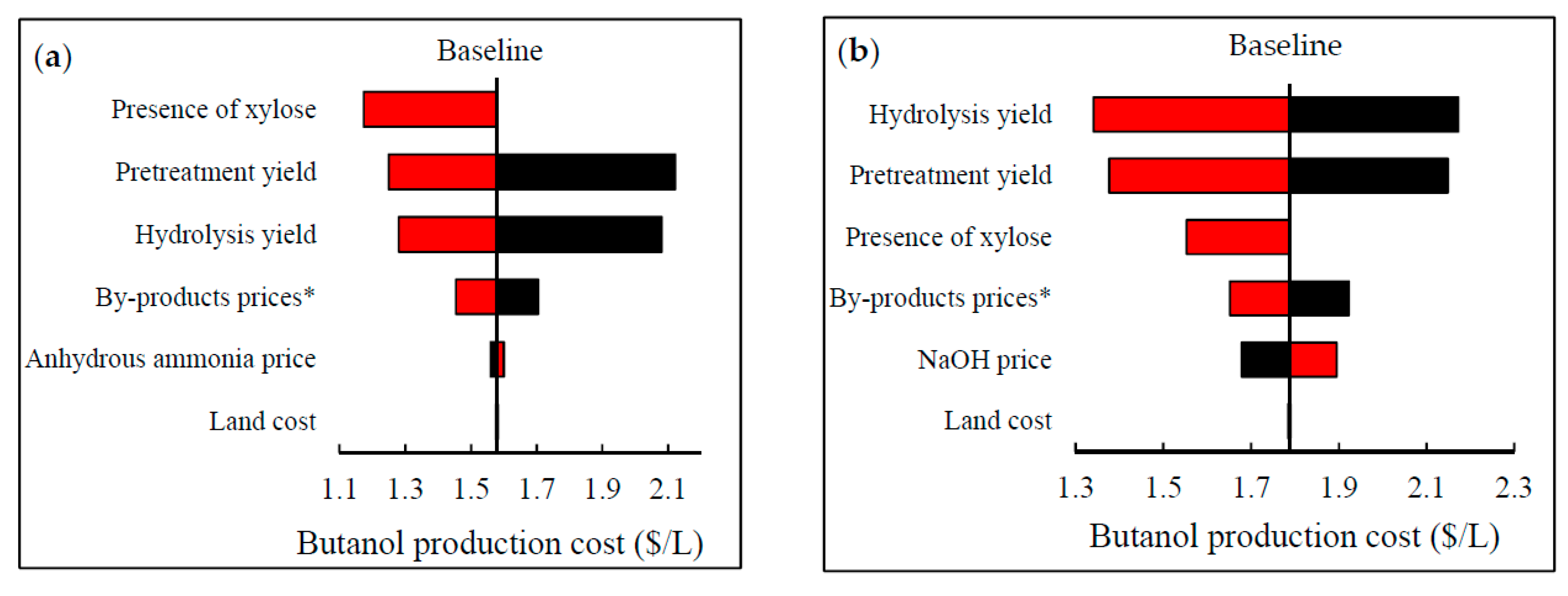
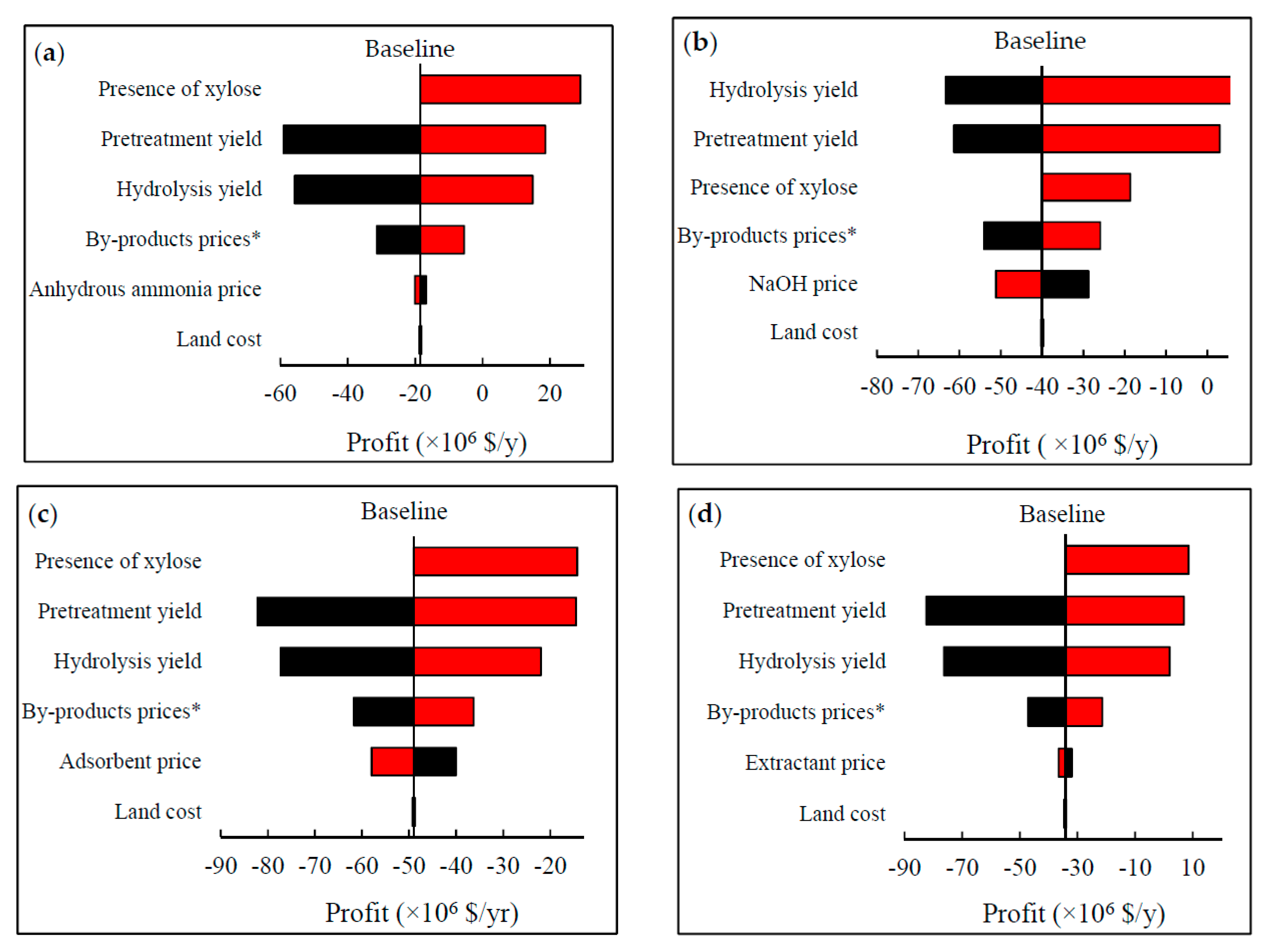
2.7. Sensitivity Analysis
2.8. Assumptions and Limitations of the Study
3. Results and Discussions
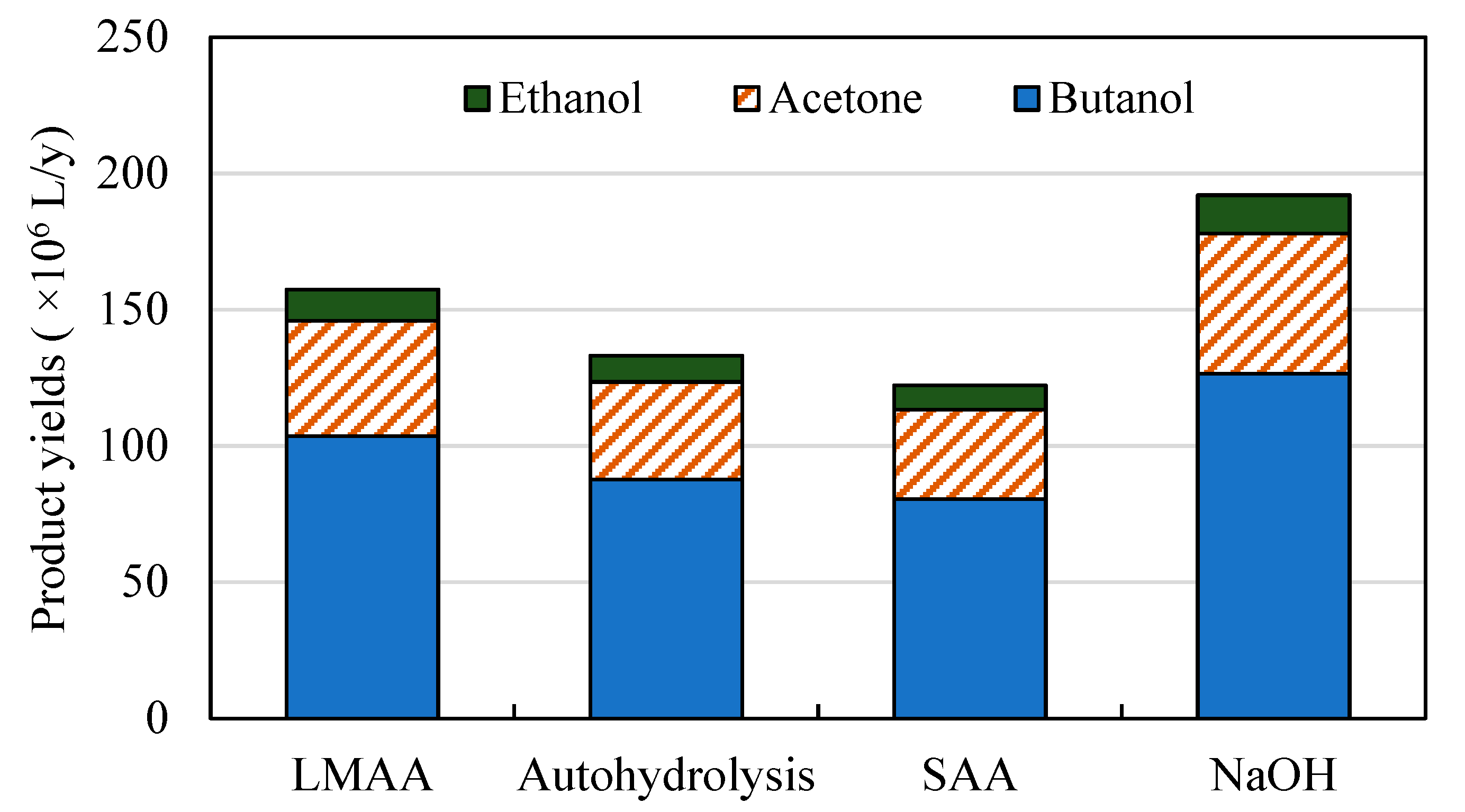
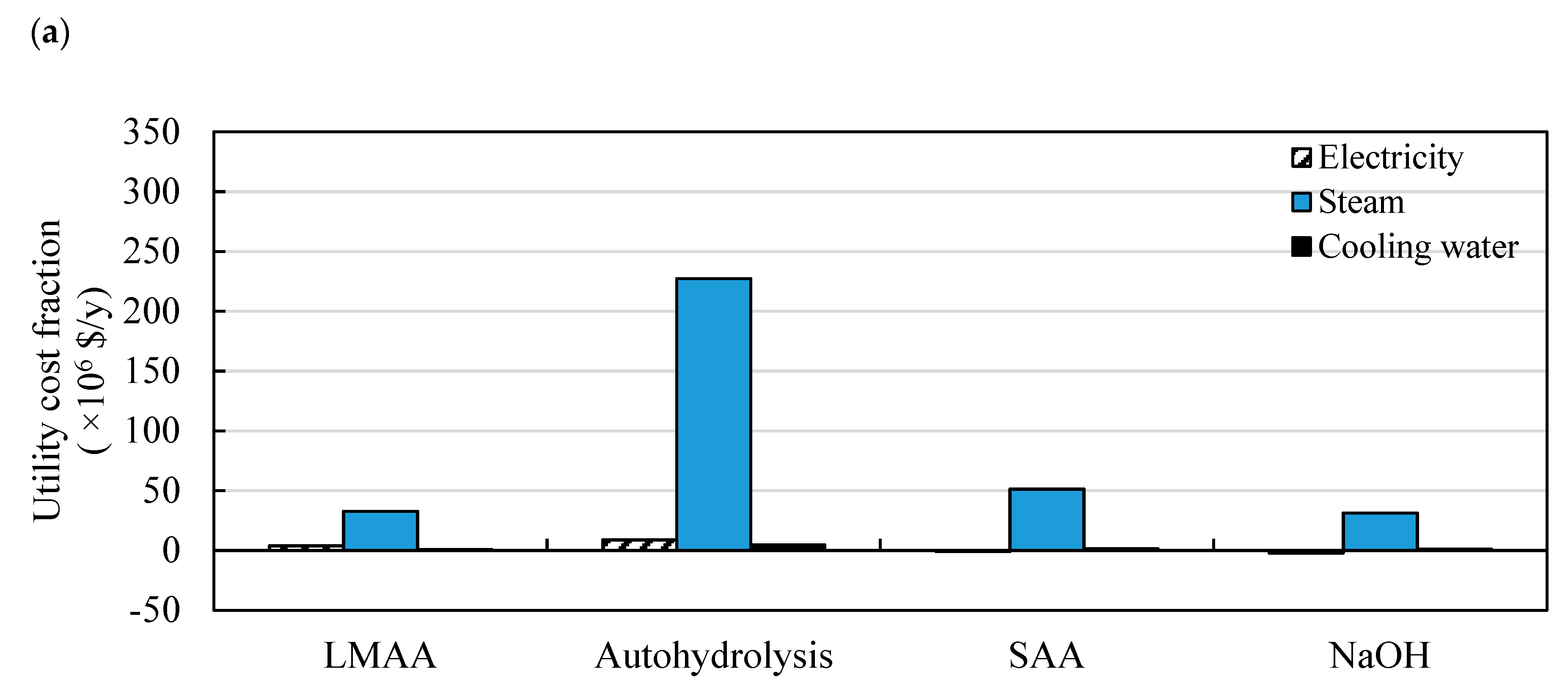
3.1. Comparison of Different Pretreatment Technologies
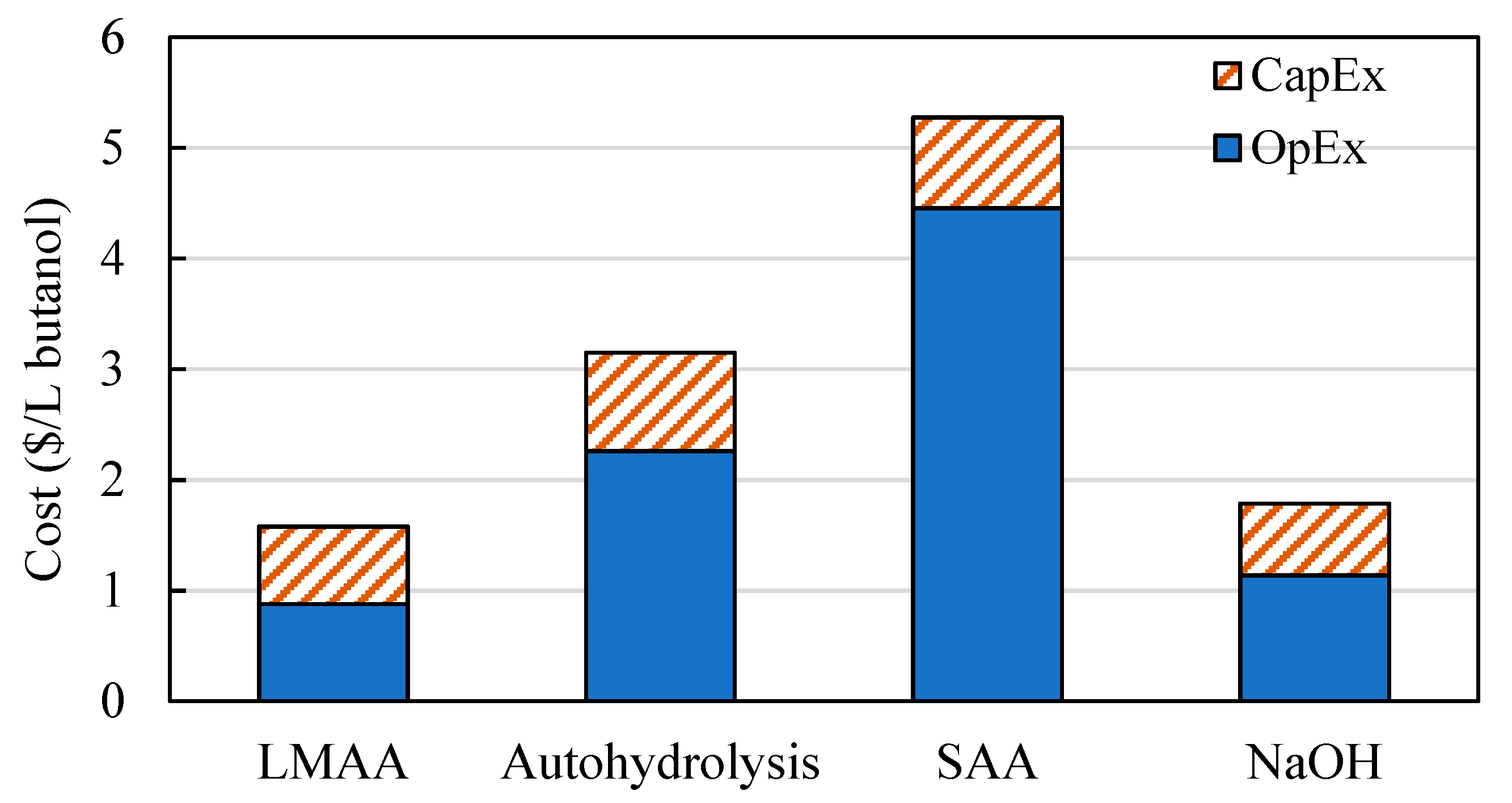
3.1.1. Production Costs of Butanol
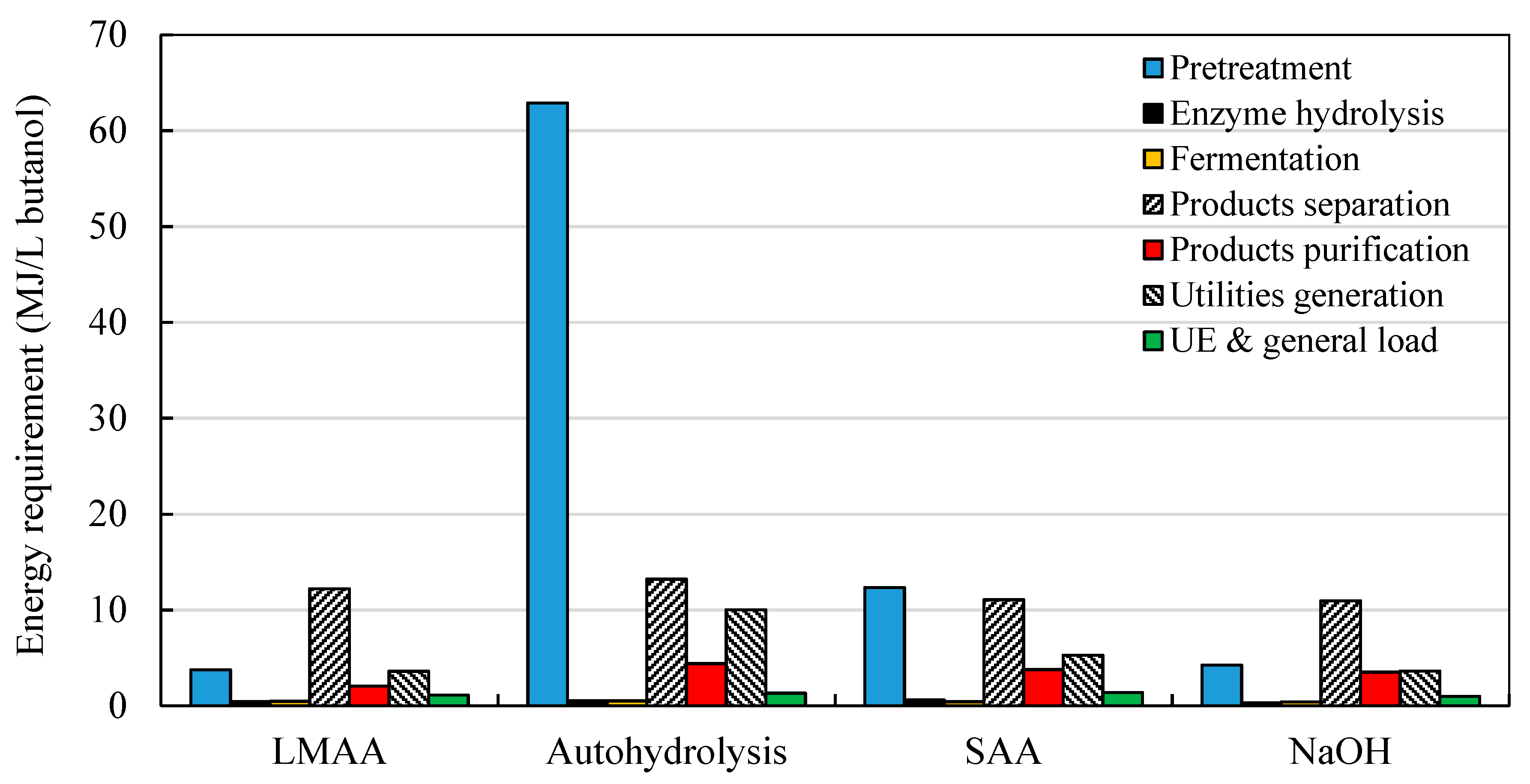
3.1.2. Energy Requirements
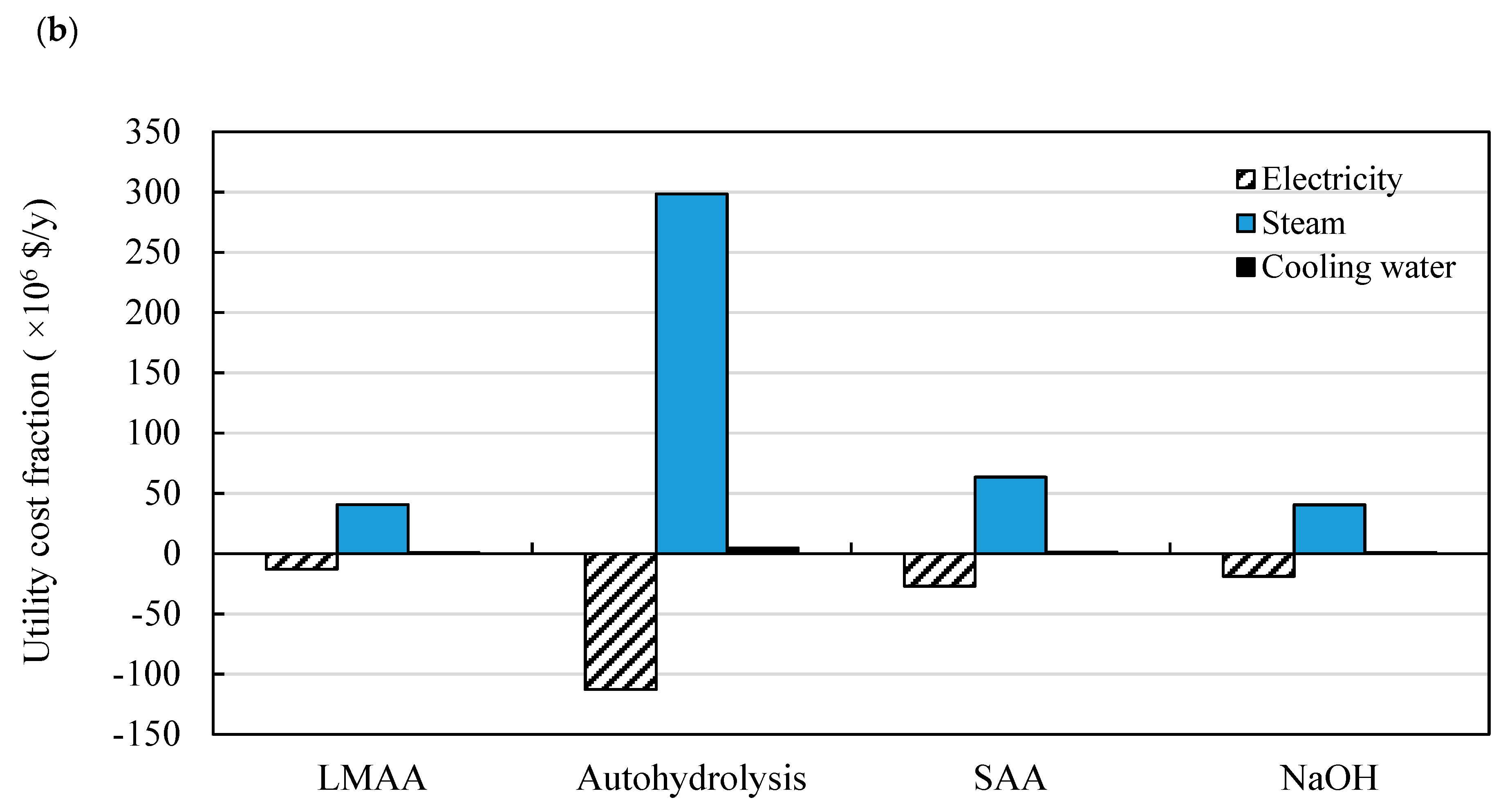
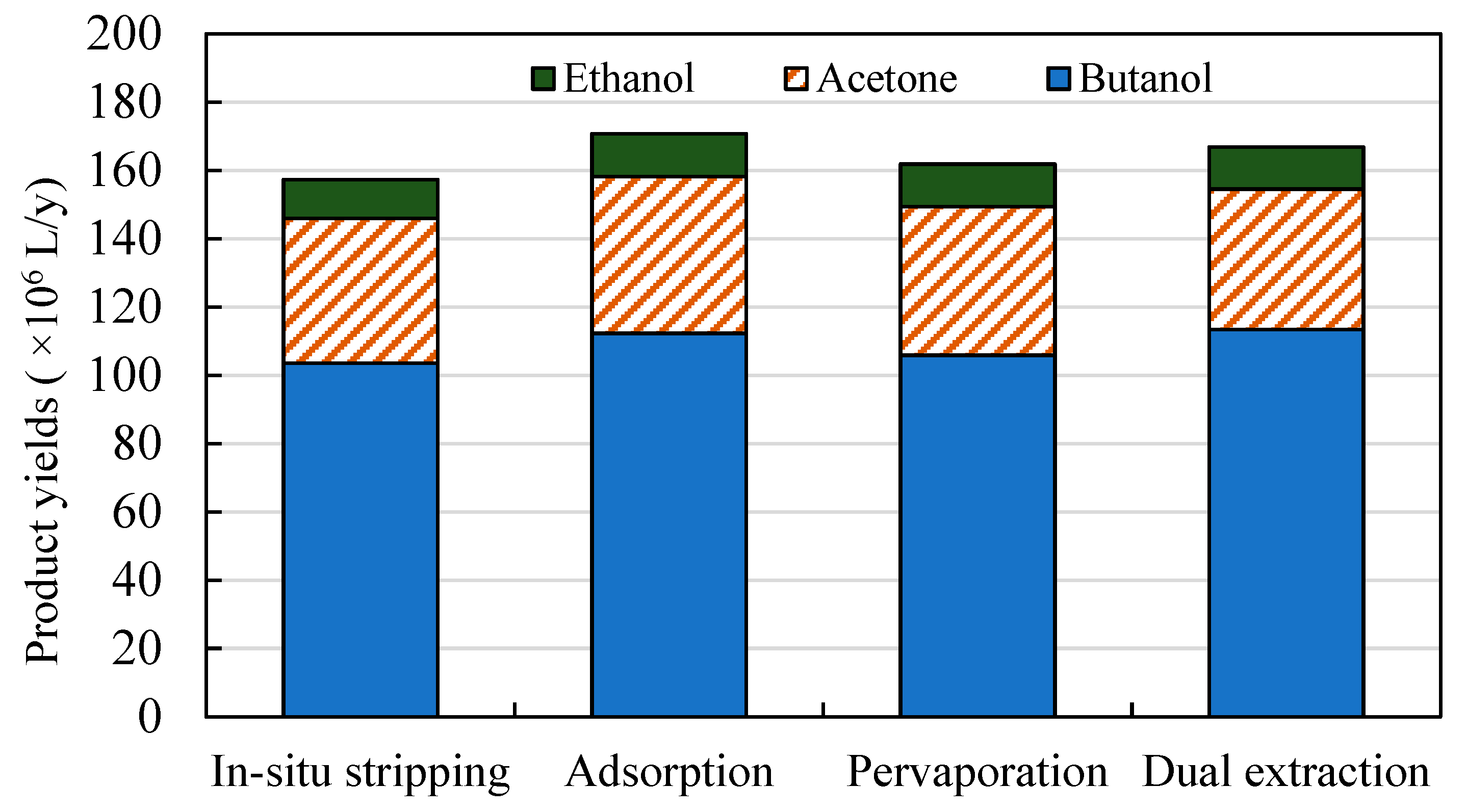
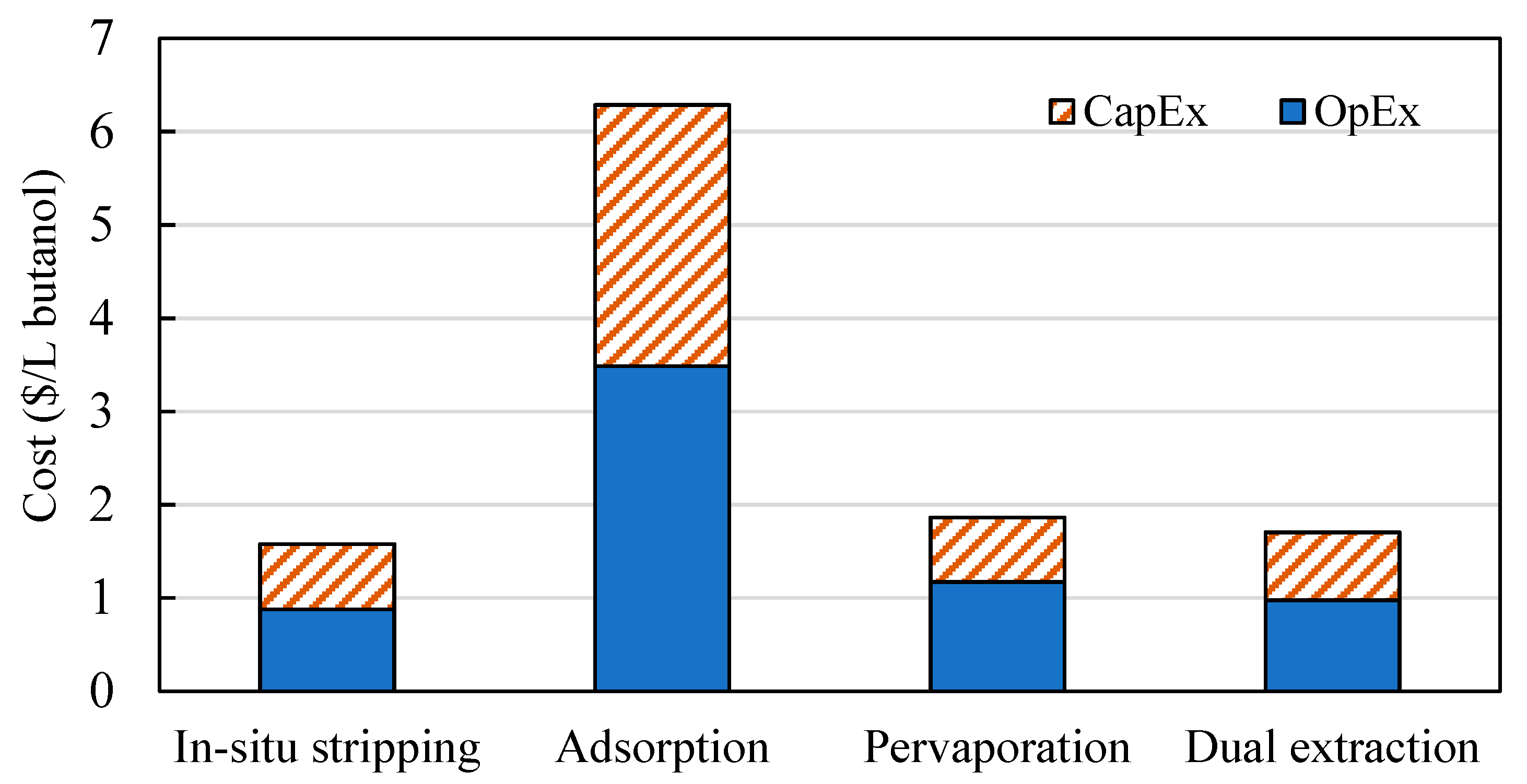
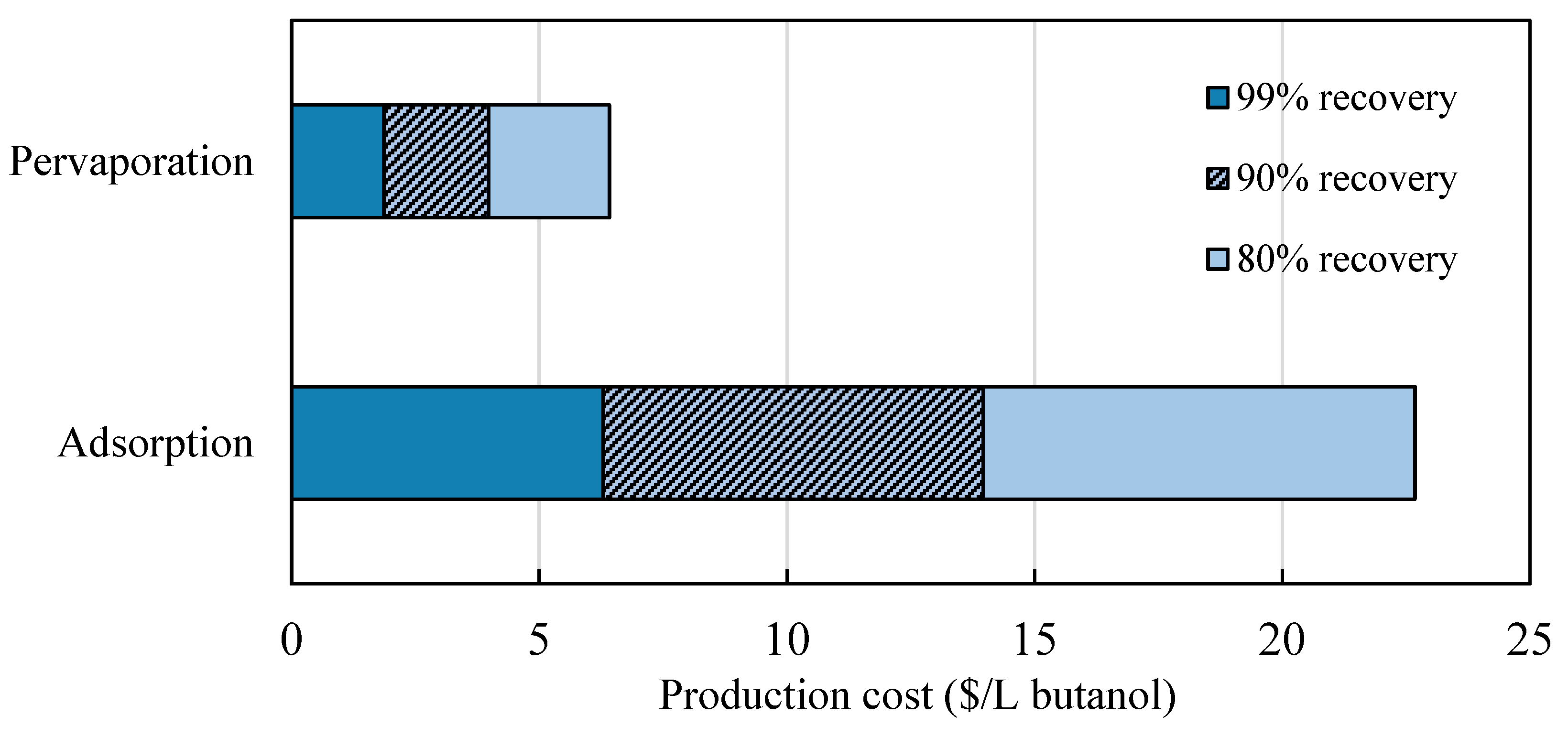
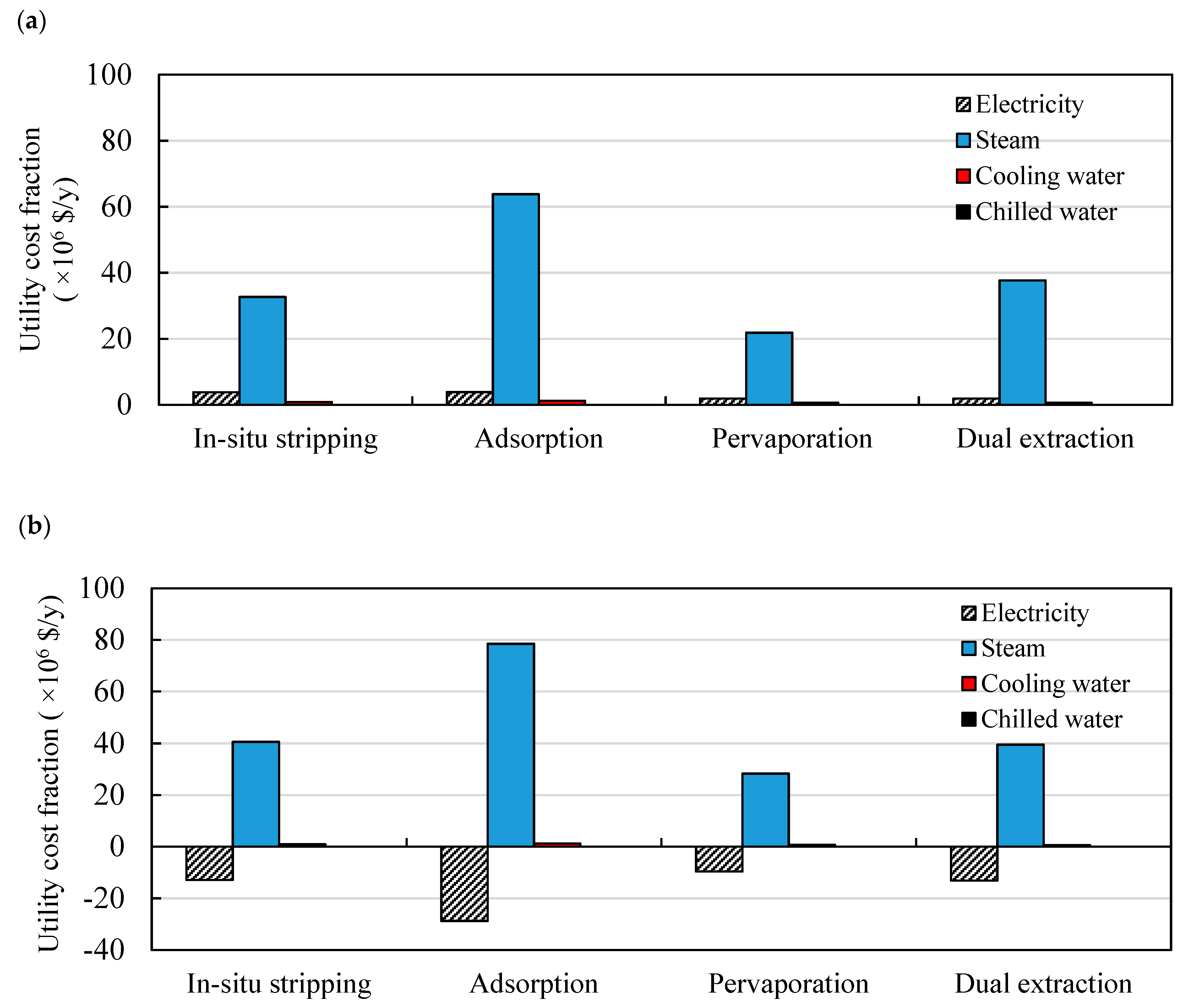
3.2. Comparison of Different Product Recovery Technologies
3.2.1. Production Costs of Butanol
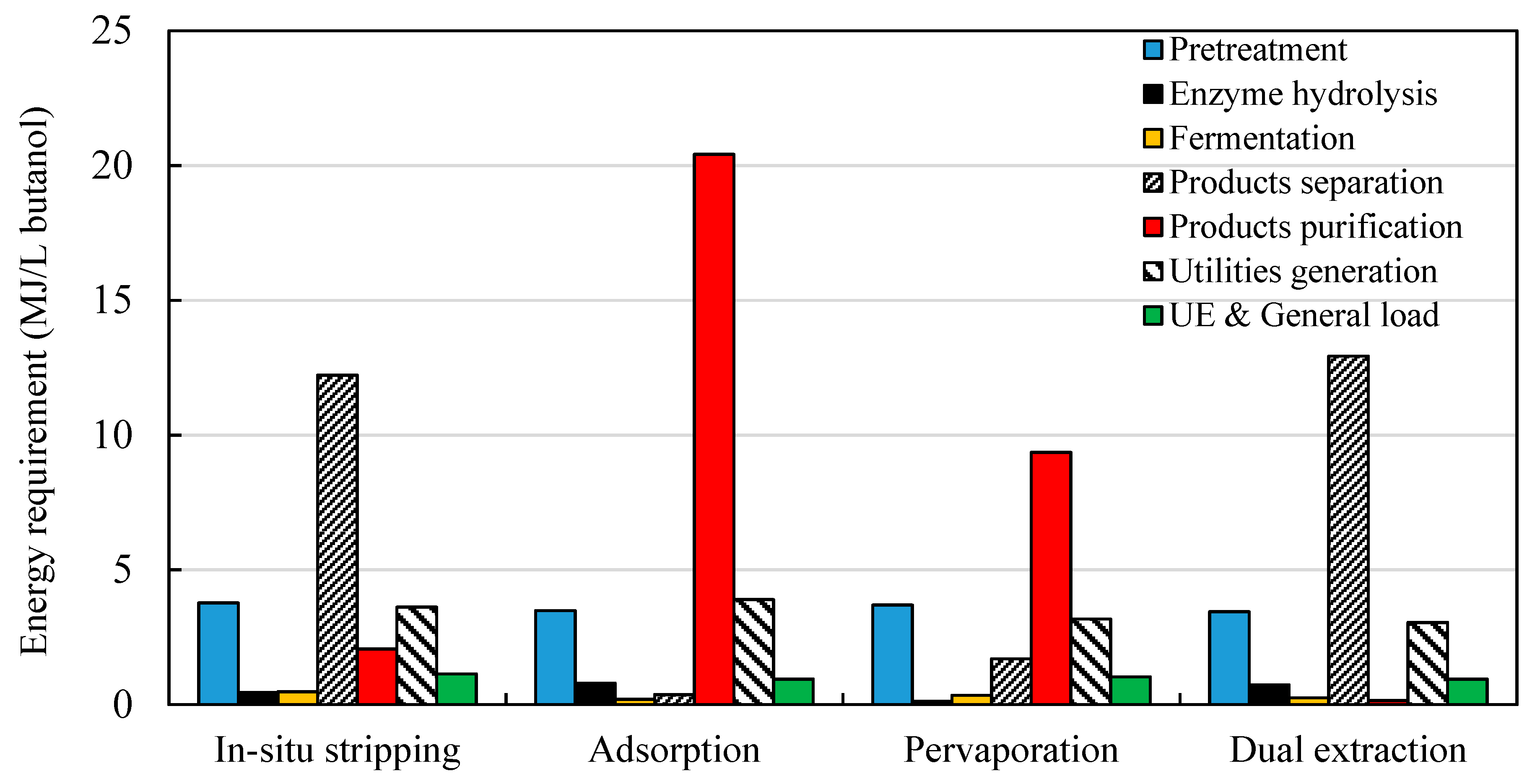
3.2.2. Energy Requirements
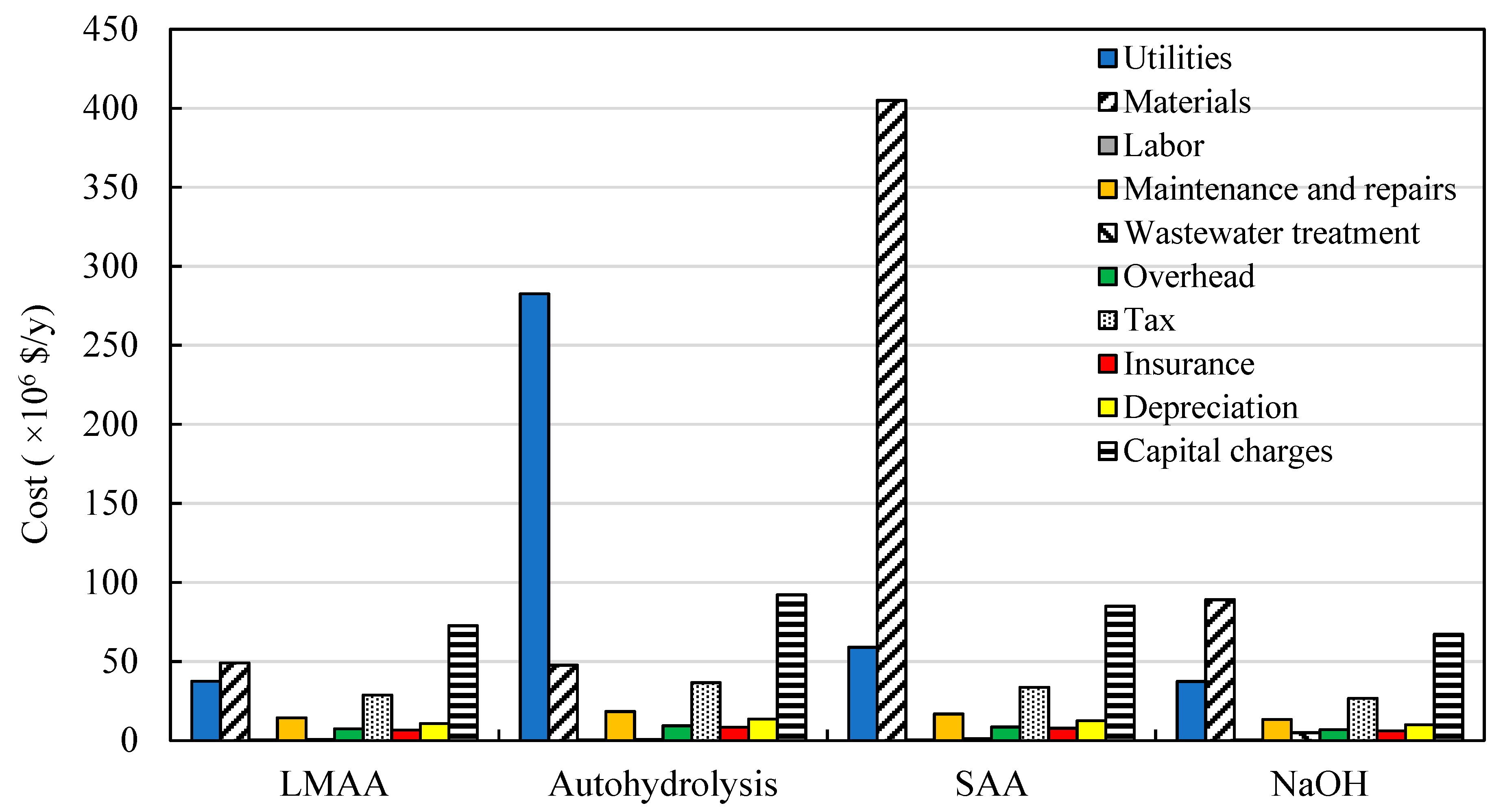
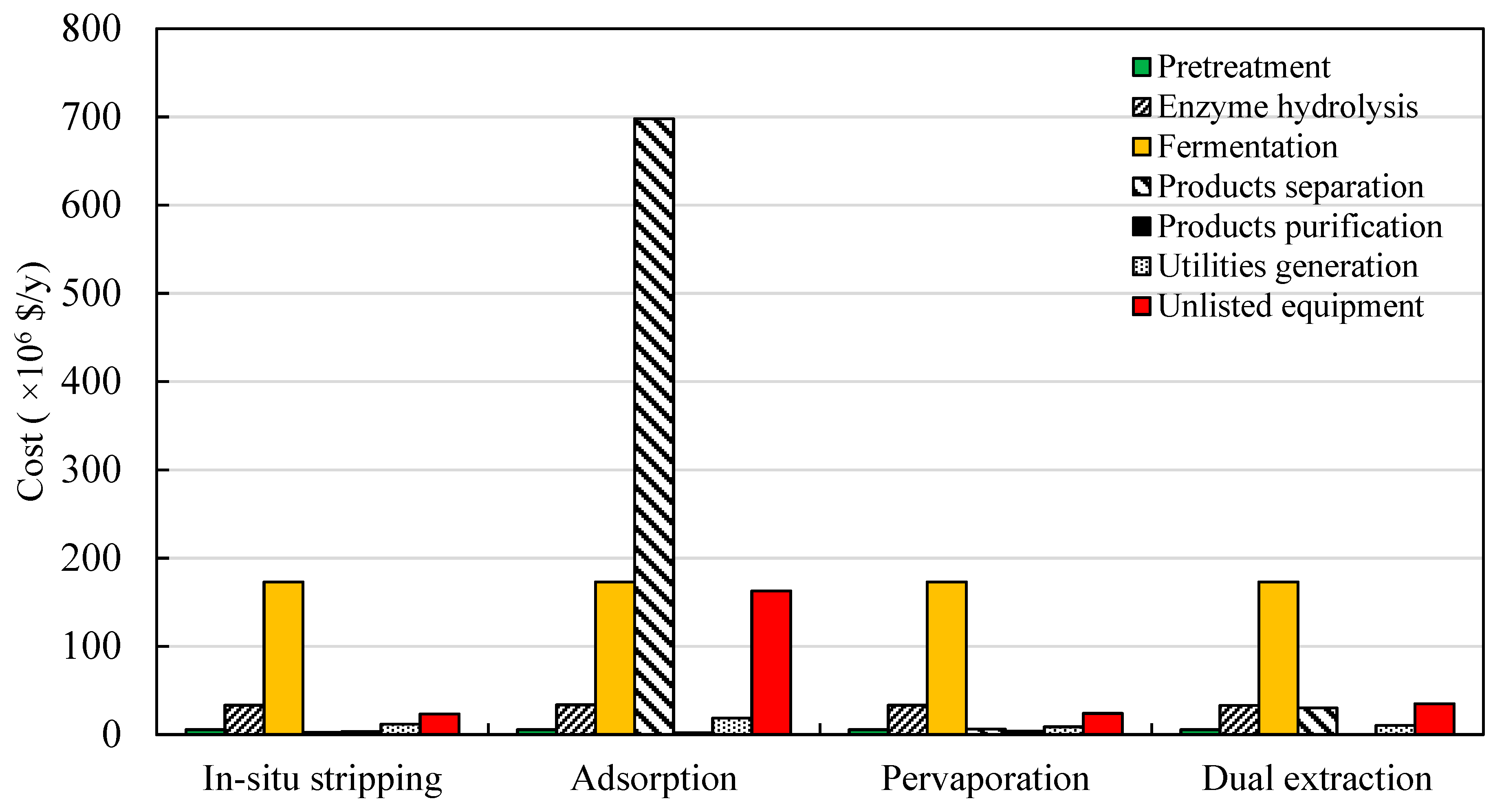
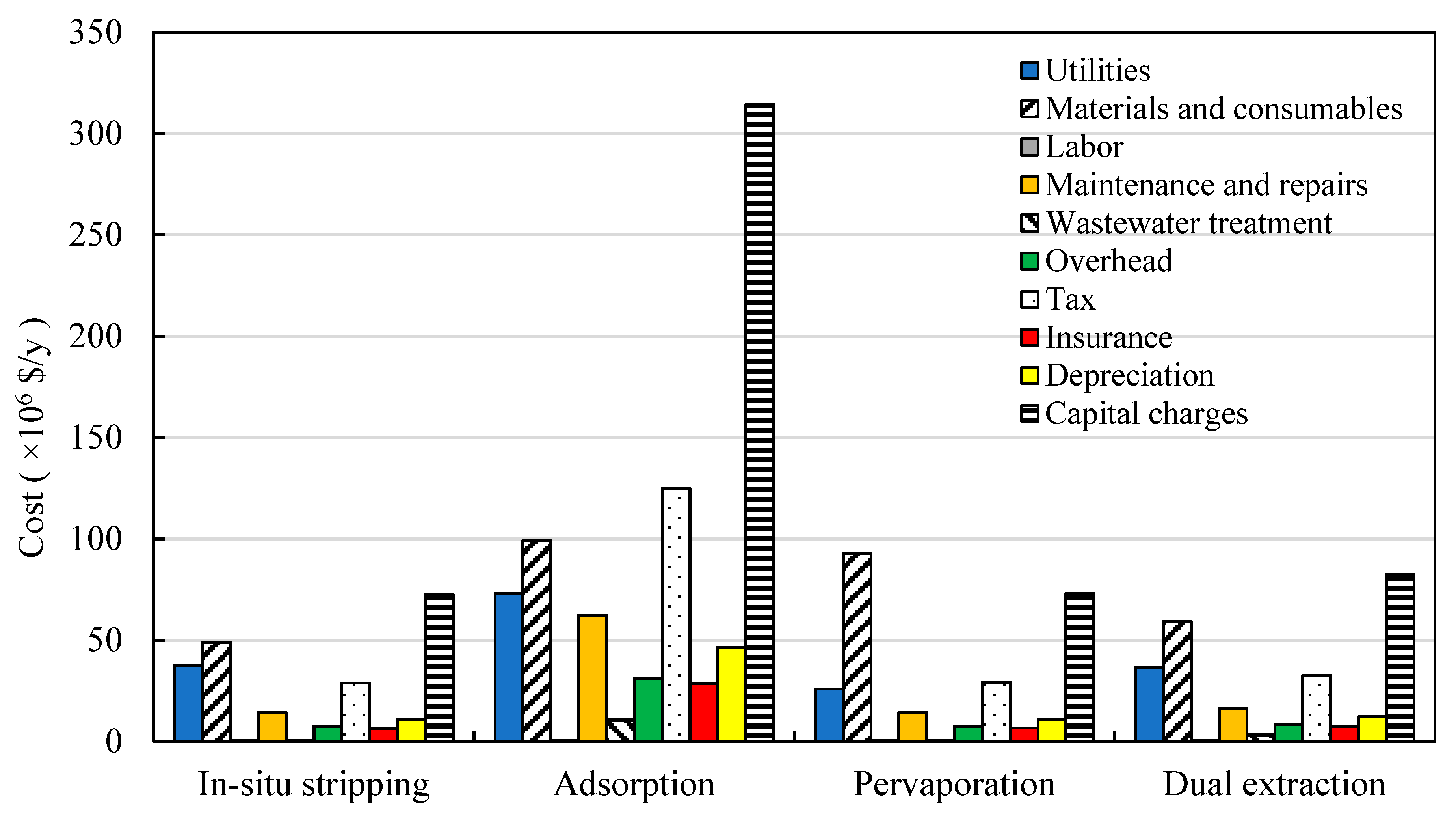
3.3. Production Costs Distributions
3.4. Uncertainties in the Simulated Models
3.5. Limitations and Future Scope for Research
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abdi, H.K.; Alanazi, K.F.; Rohani, A.S.; Mehrani, P.; Thibault, J. Economic Comparison of a Continuous ABE Fermentation with and without the Integration of an in Situ Vacuum Separation Unit. Can. J. Chem. Eng. 2016, 94, 833–843. [Google Scholar] [CrossRef]
- Adom, F.; Dunn, B.J.; Han, J. GREET Pretreatment Module; Argonne National Lab.: Argonne, IL, USA, 2014. [Google Scholar]
- Kurkijärvi, A.J.; Melin, K.; Lehtonen, J. Comparison of Reactive Distillation and Dual Extraction Processes for the Separation of Acetone, Butanol, and Ethanol from Fermentation Broth. Ind. Eng. Chem. Res. 2016, 55, 1952–1964. [Google Scholar] [CrossRef]
- Outram, V.; Lalander, C.A.; Lee, J.G.M.; Davies, E.T.; Harvey, A.P. Applied in Situ Product Recovery in ABE Fermentation. Biotechnol. Prog. 2017, 33, 563–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, K.; Tabatabaei, M.; Horváth, I.S.; Kumar, R. Recent Trends in Acetone, Butanol, and Ethanol (ABE) Production. Biofuel Res. J. 2015, 8, 301–308. [Google Scholar] [CrossRef]
- Maiti, S.; Gallastegui, G.; Sarma, S.J.; Brar, S.K.; Le Bihan, Y.; Drogui, P.; Buelna, G.; Verma, M. A Re-Look at the Biochemical Strategies to Enhance Butanol Production. Biomass Bioenergy 2016, 94, 187–200. [Google Scholar] [CrossRef]
- Friedl, A. Downstream Process Options for the ABE Fermentation. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [Green Version]
- Vane, L.M. Separation Technologies for the Recovery and Dehydration of Alcohols from Fermentation Broths. Biofuels Bioprod. Biorefin. 2008, 2, 553–588. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Microbial Production of a Biofuel (Acetone-Butanol-Ethanol) in a Continuous Bioreactor: Impact of Bleed and Simultaneous Product Removal. Bioprocess Biosyst. Eng. 2013, 36, 109–116. [Google Scholar] [CrossRef]
- Huang, H.J.; Ramaswamy, S.; Liu, Y. Separation and Purification of Biobutanol during Bioconversion of Biomass. Sep. Purif. Technol. 2014, 132, 513–540. [Google Scholar] [CrossRef]
- Abdehagh, N.; Tezel, F.H.; Thibault, J. Separation Techniques in Butanol Production: Challenges and Developments. Biomass Bioenergy 2014, 60, 222–246. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Butanol Fermentation Research: Upstream and Downstream Manipulations. Chem. Rec. 2004, 4, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Oudshoorn, A.; Van Der Wielen, L.A.M.; Straathof, A.J.J. Assessment of Options for Selective 1-Butanol Recovery from Aqueous Solution. Ind. Eng. Chem. Res. 2009, 48, 7325–7336. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, E.; Quiroz-Ramírez, J.J.; Segovia-Hernández, J.G.; Hernández, S.; Bonilla-Petriciolet, A. Process Alternatives for Biobutanol Purification: Design and Optimization. Ind. Eng. Chem. Res. 2015, 54, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Baral, N.R.; Shah, A. Comparative Techno-Economic Analysis of Steam Explosion, Dilute Sulfuric Acid, Ammonia Fiber Explosion and Biological Pretreatments of Corn Stover. Bioresour. Technol. 2017, 232, 331–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, C.G.; Nghiem, N.P.; Hicks, K.B.; Kim, T.H. Pretreatment of Corn Stover Using Low-Moisture Anhydrous Ammonia (LMAA) Process. Bioresour. Technol. 2011, 102, 10028–10034. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Senske, G.E.; Kim, T.H. Pretreatment of Corn Stover by Low Moisture Anhydrous Ammonia (LMAA) in a Pilot-Scale Reactor and Bioconversion to Fuel Ethanol and Industrial Chemicals. Appl. Biochem. Biotechnol. 2016, 179, 111–125. [Google Scholar] [CrossRef]
- Díaz, V.H.G.; Tost, G.O. Techno-Economic Analysis of Extraction-Based Separation Systems for Acetone, Butanol, and Ethanol Recovery and Purification. Bioresour. Bioprocess. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, N.; Hughes, S.; Maddox, I.S.; Cotta, M.A. Energy-Efficient Recovery of Butanol from Model Solutions and Fermentation Broth by Adsorption. Bioprocess Biosyst. Eng. 2005, 27, 215–222. [Google Scholar] [CrossRef]
- Abdehagh, N.; Tezel, F.H.; Thibault, J. Adsorbent Screening for Biobutanol Separation by Adsorption: Kinetics, Isotherms and Competitive Effect of Other Compounds. Adsorption 2013, 19, 1263–1272. [Google Scholar] [CrossRef]
- Abdehagh, N.; Gurnani, P.; Tezel, F.H.; Thibault, J. Adsorptive Separation and Recovery of Biobutanol from ABE Model Solutions. Adsorption 2015, 21, 185–194. [Google Scholar] [CrossRef]
- Van Hecke, W.; Hofmann, T.; De Wever, H. Pervaporative Recovery of ABE during Continuous Cultivation: Enhancement of Performance. Bioresour. Technol. 2013, 129, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xiao, Z.; Tang, X.; Cui, H.; Zhang, J.; Li, W.; Ying, C. Acetone-Butanol-Ethanol Fermentation in a Continuous and Closed-Circulating Fermentation System with PDMS Membrane Bioreactor. Bioresour. Technol. 2013, 128, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, W.; Vandezande, P.; Claes, S.; Vangeel, S.; Beckers, H.; Diels, L.; De Wever, H. Integrated Bioprocess for Long-Term Continuous Cultivation of Clostridium Acetobutylicum Coupled to Pervaporation with PDMS Composite Membranes. Bioresour. Technol. 2012, 111, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Negishi, H.; Sakaki, K.; Ikegami, T. Silicalite Pervaporation Membrane Exhibiting a Separation Factor of over 400 for Butanol. Chem. Lett. 2010, 39, 1312–1314. [Google Scholar] [CrossRef]
- Qureshi, N.; Blaschek, H.P. Production of Acetone Butanol Ethanol (ABE) by a Hyper-Producing Mutant Strain of Clostridium Beijerinckii BA101 and Recovery by Pervaporation. Biotechnol. Prog. 1999, 15, 594–602. [Google Scholar] [CrossRef]
- de Vrije, T.; Budde, M.; van der Wal, H.; Claassen, P.A.M.; López-Contreras, A.M. In Situ Removal of Isopropanol, Butanol and Ethanol from Fermentation Broth by Gas Stripping. Bioresour. Technol. 2013, 137, 153–159. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, J.; Liu, F.; Lu, C.; Yang, S.T.; Bai, F.W. Two-Stage in Situ Gas Stripping for Enhanced Butanol Fermentation and Energy-Saving Product Recovery. Bioresour. Technol. 2013, 135, 396–402. [Google Scholar] [CrossRef]
- Department of Agriculture Malaysia. Industrial Crops Statistics; Department of Agriculture Malaysia: Putrajaya, Malaysia, 2016.
- Cheng, M.; Rosentrater, K.A. Optimization of Low Moisture Anhydrous Ammonia (LMAA) Pretreatment for Corn Stover Enzymatic Digestibility during Hydrolysis Process. In Proceedings of the 2016 ASABE Annual International Meeting, Orlando, FL, USA, 17–20 July 2016. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Rosentrater, K.A. Small-Scale Low-Moisture Anhydrous Ammonia (LMAA) Pretreatment of Corn Stover. Biomass Bioenergy 2017, 97, 38–42. [Google Scholar] [CrossRef]
- Mahmud, N. Low Moisture Anhydrous Ammonia (LMAA) Pretreatment of Lignocellulosic Biomass and Assessments for Biobutanol Production. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2019. [Google Scholar]
- Sabiha-Hanim, S.; Noor, M.A.M.; Rosma, A. Effect of Autohydrolysis and Enzymatic Treatment on Oil Palm (Elaeis Guineensis Jacq.) Frond Fibres for Xylose and Xylooligosaccharides Production. Bioresour. Technol. 2011, 102, 1234–1239. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, S.; Yang, T.H.; Lee, H.J.; Seung, D.; Park, Y.-C.; Seo, J.-H.; Choi, I.-G.; Kim, K.H. Aqueous Ammonia Pretreatment, Saccharification, and Fermentation Evaluation of Oil Palm Fronds for Ethanol Production. Bioprocess Biosyst. Eng. 2012, 35, 1497–1503. [Google Scholar] [CrossRef]
- Sukri, S.S.M.; Rahman, R.A.; Illias, R.M.D.; Yaakob, H. Optimization of Alkaline Pretreatment Conditions of Oil Palm Fronds in Improving the Lignocelluloses Contents for Reducing Sugar Production. Rom. Biotechnol. Lett. 2014, 19, 9006–9018. [Google Scholar] [CrossRef] [Green Version]
- Triwahyuni, E.; Hariyanti, S.; Dahnum, D.; Nurdin, M.; Abimanyu, H. Optimization of Saccharification and Fermentation Process in Bioethanol Production from Oil Palm Fronds. Procedia Chem. 2015, 16, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Ezeji, T.C.; Blaschek, H.P. Fermentation of Dried Distillers’ Grains and Solubles (DDGS) Hydrolysates to Solvents and Value-Added Products by Solventogenic Clostridia. Bioresour. Technol. 2008, 99, 5232–5242. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; He, X.; Tan, E.C.D.; Zhang, M.; Aden, A. Comparative Techno-Economic Analysis and Reviews of n-Butanol Production from Corn Grain and Corn Stover. Biofuels Bioprod. Biorefin. 2014, 8, 342–361. [Google Scholar] [CrossRef]
- Qureshi, N.; Ezeji, T.C.; Ebener, J.; Dien, B.S.; Cotta, M.A.; Blaschek, H.P. Butanol Production by Clostridium Beijerinckii. Part I: Use of Acid and Enzyme Hydrolyzed Corn Fiber. Bioresour. Technol. 2008, 99, 5915–5922. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, M.; Liu, J.; Huo, H. Assessment of Potential Life-Cycle Energy and Greenhouse Gas Emission Effects from Using Corn-Based Butanol as a Transportation Fuel. Biotechnol. Prog. 2008, 24, 1204–1214. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Techno-Economic Analysis of Cellulosic Butanol Production from Corn Stover through Acetone-Butanol-Ethanol Fermentation. Energy Fuels 2016, 30, 5779–5790. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of Acetone, Butanol and Ethanol by Clostridium Beijerinckii BA101 and in Situ Recovery by Gas Stripping. World J. Microbiol. Biotechnol. 2003, 19, 595–603. [Google Scholar] [CrossRef]
- Águeda, V.I.; Delgado, J.A.; Uguina, M.A.; Sotelo, J.L.; García, Á. Column Dynamics of an Adsorption-Drying-Desorption Process for Butanol Recovery from Aqueous Solutions with Silicalite Pellets. Sep. Purif. Technol. 2013, 104, 307–321. [Google Scholar] [CrossRef]
- Hussin, M.H.; Samad, N.A.; Latif, N.H.A.; Rozuli, N.A.; Yusoff, S.B.; Gambier, F.; Brosse, N. Production of Oil Palm (Elaeis Guineensis) Fronds Lignin-Derived Non-Toxic Aldehyde for Eco-Friendly Wood Adhesive. Int. J. Biol. Macromol. 2018, 113, 1266–1272. [Google Scholar] [CrossRef]
- Demirbaş, A. Estimating of Structural Composition of Wood and Non-Wood Biomass Samples. Energy Sources 2005, 27, 761–767. [Google Scholar] [CrossRef]
- Morvay, Z.K.; Gvozdenac, D.D. Applied Industrial Eenergy and Environmental Management; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Haigh, K.F.; Petersen, A.M.; Gottumukkala, L.; Mandegari, M.; Naleli, K.; Görgens, J.F. Simulation and Comparison of Processes for Biobutanol Production from Lignocellulose via ABE Fermentation. Biofuels Bioprod. Biorefin. 2018, 12, 1023–1036. [Google Scholar] [CrossRef]
- Richardson. Richardson International Construction Factors ManualTM; Cost Data On Line, Inc.: Pahrump, NV, USA, 2008. [Google Scholar]
- Heinzle, E.; Biwer, A.P.; Cooney, C.L. Development of Sustainable Bioprocesses: Modelling and Assessment; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Brown, R.C.; Brown, T.R. Economics of Biorenewable Resources. In Biorenewable Resources: Engineering New Products from Agriculture, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 287–326. [Google Scholar]
- Utusan-Malaysia. Kilang Pelet Pelepah Sawit Untuk Ternakan. Available online: http://integratedfarm.blogspot.com/2009/07/kilang-pelet-pelepah-sawit-untuk.html (accessed on 8 September 2016).
- Schnitkey, G. Weekly Farm Economics: Fertilizer Prices Higher for 2019 Crop. Farmdoc Dly. 2018, 8, 178. [Google Scholar]
- You, Z.; Zhang, S.; Kim, H.; Chiang, P.-C.; Sun, Y.; Guo, Z.; Xu, H. Effects of Corn Stover Pretreated with NaOH and CaO on Anaerobic Co-Digestion of Swine Manure and Corn Stover. Appl. Sci. 2019, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, Z.; Hao, P. Investigation on Mechanical and Microwave Heating Characteristics of Asphalt Mastic Using Activated Carbon Powder as Electro-Magnetic Absorbing Materials. Constr. Build. Mater. 2019, 202, 692–703. [Google Scholar] [CrossRef]
- TNB-Malaysia. Pricing & Tariffs. Available online: http://www.tnb.com.my/residential/pricing-tariffs (accessed on 10 March 2018).
- SPAN-Malaysia. Water Tariff. Available online: https://www.span.gov.my/document/upload/AtBz79IrBNcxpXRh9R2SXYAcr1cAZ5oK.pdf (accessed on 10 March 2018).
- UHY. Doing Business in Malaysia; Urbach Hacker Young International Ltd.: Kuala Lumpur, Malaysia, 2015. [Google Scholar] [CrossRef] [Green Version]
- Richard, R. Kenyataan Media: Perintah Gaji Minimum 2016; Ministry of Human Resources Malaysia: Putrajaya, Malaysia, 2016.
- Mahmud, N.; Rosentrater, K.A. Techno-Economic Analysis of Low Moisture Anhydrous Ammonia (LMAA) Pretreatment for Butanol Production from Oil Palm Frond. Biomass Convers. Biorefin. 2019. [Google Scholar] [CrossRef]
- Yang, M.; Rosentrater, K.A. Techno-Economic Analysis (TEA) of Low-Moisture Anhydrous Ammonia (LMAA) Pretreatment Method for Corn Stover. Ind. Crops Prod. 2015, 76, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, M.; Kataoka, H.; Sueki, M.; Araki, K. Energy Saving Effect of Pervaporation Using Oleyl Alcohol Liquid Membrane in Butanol Purification. Bioprocess Eng. 1988, 3, 93–100. [Google Scholar] [CrossRef]

| Elements | Lignin (%) [44] | Cellulose (%) [45] | Hemicellulose (%) [45] | Natural Gas (%) [46] |
|---|---|---|---|---|
| Carbon | 58.45 | 44.40 | 45.50 | 75.85 |
| Hydrogen | 6.80 | 6.20 | 6.10 | 24.15 |
| Nitrogen | 1.12 | - | - | - |
| Oxygen | 33.63 | 49.40 | 48.40 | - |
| Capital Cost | |
| Installation | 39% × Ep |
| Instrument and Control | 26% × Ep |
| Piping | 10% × Ep |
| Electrical System | 31% × Ep |
| Building | 29% × Ep |
| Yard Improvement | 12% × Ep |
| Service | 55% × Ep |
| Total Installed Equipment Cost (TIEC) | |
| Engineering | 32% × Ep |
| Construction | 34% × Ep |
| Legal and Contractor Fees | 23% × Ep |
| Total Indirect Cost (TIC) | |
| Contingency | 10% × (TIEC + TIC) |
| Fixed Capital Investment (FCI) | (TIEC + TIC + contingency) × LF |
| Working Capital (WC) | 15% × FCI |
| Land | 6% × Ep |
| Total Project Investment (TPI) | FCI + WC + Land |
| Operating Cost | |
| Maintenance and Repairs | 1% × TPI |
| Materials | Unit cost of each materials × Total unit used |
| Labor Cost | Wage rate × No. of operator × Working hours |
| Utilities | Electricity cost + Heat cost + Cooling cost |
| Total Direct Operating Cost | |
| Overhead | 50% × (labor + maintenance and repairs) |
| Taxes | 2% × TPI |
| Insurance | 0.46% × TPI |
| Depreciation (D) | Straight line |
| Total Indirect Operating Cost | |
| Salvage | 15% |
| Capital Charges (CC) | TPI × i(1 + i)n/((1 + i)n − 1) |
| Annual Operating Cost | Total direct and indirect operating cost + CC + D |
| By-Product Credit | Amount by-product generated × Unit selling price |
| Net Annual Operating Cost | Annual operating cost—By-product credit |
| Unit Production Cost | Net annual operating cost/annual product |
| Assumptions | |
|---|---|
| Annual Working Hours | 7920 |
| OPF Price | $0.032/kg [51] |
| Cellulase Price | $5/kg [38] |
| Ammonia Solution Price | $300/t [38] |
| Anhydrous Ammonia Price | $512/t [52] |
| Sodium Hydroxide Price | $450/t [53] |
| 2-Methyl-1-Hexanol Price | ~$1900/t [3] * |
| Cyclopentane Price | ~$1150/t [3] * |
| Activated Carbon Price | $2000/t [54] |
| Silicone Membrane Price | $250/m2 |
| Electricity Price (to grid) | $0.08/kWh [55] |
| Water Price | $0.21/m3 [56] |
| Natural Gas Price | $0.004/ft3 |
| Annual Interest Rate | 0.08% [57] |
| Operator Wage Rate | $2.26/h [58] |
| Interest Year | 20 y |
| Equipment Lifetime | 20 y |
| Production Stages | Production Costs ($/L) | |||
|---|---|---|---|---|
| LMAA | Autohydrolysis | SAA | NaOH | |
| Pretreatment | 0.16 | 1.98 | 3.77 | 0.61 |
| Enzyme Hydrolysis | 0.11 | 0.11 | 0.11 | 0.10 |
| Fermentation | 0.53 | 0.54 | 0.50 | 0.49 |
| Product Separation | 0.21 | 0.42 | 0.20 | 0.19 |
| Product Purification | 0.04 | 0.15 | 0.08 | 0.07 |
| CHP System | 0.06 | 0.31 | 0.09 | 0.06 |
| Other | 1.09 | 1.42 | 1.34 | 1.02 |
| By-Product Credit | 0.63 | 1.77 | 0.81 | 0.74 |
| Net cost | 1.58 | 3.15 | 5.28 | 1.79 |
| Production Stages | Production Costs ($/L) | |||
|---|---|---|---|---|
| In situ Stripping | Adsorption | Pervaporation | Dual Extraction | |
| Pretreatment | 0.16 | 0.15 | 0.15 | 0.15 |
| Enzyme Hydrolysis | 0.11 | 0.11 | 0.10 | 0.10 |
| Fermentation | 0.53 | 0.50 | 0.51 | 0.48 |
| Product Separation | 0.21 | 2.25 | 0.45 | 0.38 |
| Product Purification | 0.04 | 0.48 | 0.14 | 0.01 |
| CHP System | 0.06 | 0.09 | 0.04 | 0.05 |
| Other | 1.09 | 3.47 | 1.07 | 1.10 |
| By-Product Credit | 0.63 | 0.76 | 0.61 | 0.58 |
| Net cost | 1.58 | 6.29 | 1.86 | 1.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, N.; Rosentrater, K.A. Techno-Economic Analysis (TEA) of Different Pretreatment and Product Separation Technologies for Cellulosic Butanol Production from Oil Palm Frond. Energies 2020, 13, 181. https://doi.org/10.3390/en13010181
Mahmud N, Rosentrater KA. Techno-Economic Analysis (TEA) of Different Pretreatment and Product Separation Technologies for Cellulosic Butanol Production from Oil Palm Frond. Energies. 2020; 13(1):181. https://doi.org/10.3390/en13010181
Chicago/Turabian StyleMahmud, Nazira, and Kurt A. Rosentrater. 2020. "Techno-Economic Analysis (TEA) of Different Pretreatment and Product Separation Technologies for Cellulosic Butanol Production from Oil Palm Frond" Energies 13, no. 1: 181. https://doi.org/10.3390/en13010181