Abstract
Environmental impact assessment is a crucial aspect of biofuels production to ensure that the process generates emissions within the designated limits. In typical cellulosic biofuel production process, the pretreatment and downstream processing stages were reported to require a high amount of chemicals and energy, thus generating high emissions. Cellulosic butanol production while using low moisture anhydrous ammonia (LMAA) pretreatment was expected to have a low chemical, water, and energy footprint, especially when the process was combined with more efficient downstream processing technologies. In this study, the quantification of environmental impact potentials from cellulosic butanol production plants was conducted with modeled different pretreatment and product separation approaches. The results have shown that LMAA pretreatment possessed a potential for commercialization by having low energy requirements when compared to the other modeled pretreatments. With high safety measures that reduce the possibility of anhydrous ammonia leaking to the air, LMAA pretreatment resulted in GWP of 5.72 kg CO2 eq./L butanol, ecotoxicity potential of 2.84 × 10−6 CTU eco/L butanol, and eutrophication potential of 0.011 kg N eq./L butanol. The lowest energy requirement in biobutanol production (19.43 MJ/L), as well as better life-cycle energy metrics performances (NEV of 24.69 MJ/L and NER of 2.27) and environmental impacts potentials (GWP of 3.92 kg N eq./L butanol and ecotoxicity potential of 2.14 × 10−4 CTU eco/L butanol), were recorded when the LMAA pretreatment was combined with the membrane pervaporation process in the product separation stage.
1. Introduction
The Intergovernmental Panel on Climate Change (IPCC) has stressed the importance of switching to the renewable energy source to mitigate the current climate change problem [1]. One of the renewable energies, biofuels, are widely consumed in the transportation sector and are considered to be one of the most viable methods for decarbonization [2]. The main advantage of using biofuels is its environmental friendly characteristic; generating emissions less than the conventional fuels (gasoline), throughout its life cycle (resource production, resource extraction, products production, and products utilization stages) [3,4,5]. For cellulosic biofuels, especially those that are produced from agricultural waste materials, the emissions from land preparation and plantation often eliminated or reduced, generating a better environmental footprint than the first-generation biofuels [6,7].
The low emissions of cellulosic biofuels has been attractive, and more study has been conducted in recent years focusing on the environmental evaluation of biofuels [8,9,10,11,12,13]. In the United States (U.S.), under the Renewable Fuel Standard (RFS) program, each type of biofuel must achieve a specific reduction of greenhouse gas (GHG) emissions. For instance, to be classified as cellulosic biofuels, 60% GHG emissions reduction from the 2005 petroleum baseline values must be achieved [14]. It shows the importance of quantifying emissions that are associated with a specific biofuel.
In the cellulosic biofuels production, the pretreatment stage is critical in ensuring a high yield of products. Some pretreatment approaches are energy-intensive and utilize a high amount of chemicals and water, causing substantial impacts to the environment. In addition to that, the conventional downstream processing approach for biofuels production (distillation) is also energy-intensive, which further increases the carbon dioxide (CO2) footprint. LCA on cellulosic biofuels production plant was mostly conducted while using dilute sulfuric acid (DA) pretreatment and ammonia fiber explosion (AFEX) pretreatment [10,15,16]. Other highly potential pretreatment approaches are still lacking environmental impacts performance data, thus limiting its feasibility indication for commercial application.
AFEX pretreatment was reported to give more adverse environmental impacts potential than the DA pretreatment, due to the emitted ammonia and high lignin solubilization that led to low lignin that is usable for energy generation [15,16]. Nevertheless, Cronin et al. [15] found that ethanol yield from AFEX-treated material was higher than those that were given by DA-treated material. DA pretreatment has the benefit of requiring a short reaction time, as well as the availability and low cost of the acid. However, the corrosive nature of the acid and generation of a high amount of inhibitory compounds (i.e., furfural and hydroxymethylfurfural) that impaired the growth of microorganisms in the fermentation process were the main problems in DA pretreatment [17,18]. Alkali pretreatments, on the other hand, were proven to produce no inhibitory compounds [19,20,21]. In addition to that, alkali pretreatments other than AFEX, such as low moisture anhydrous ammonia (LMAA) and soaking in alkali, have a low lignin solubilization effect and they were conducted at process conditions milder than those of AFEX [22]. LMAA pretreatment also reported having low water requirements, and the pretreatment agent could easily be recycled, because it is in a gaseous state [23]. These features are highly attractive for replacing widely used DA pretreatment, with the primary aim of finding a more environmentally friendly process without compromising the yield (biofuels).
Better results are expected (lower CO2 footprint) when low energy downstream processing is adopted. Previous works mentioned that coupling other technologies for product separation with conventional distillation process have succeeded in reducing up to 75% of the total energy requirement [24,25]. The different separation principles utilized for facilitating product separation include membrane-based, gas-liquid equilibrium, and liquid-liquid equilibrium. This study focused on the environmental impact assessment of biobutanol, which is a more attractive gasoline substitute than bioethanol. When compared to the ethanol-water mixture, the needs of a pre-concentrate step before the distillation process is more critical for the butanol-water mixture, because butanol and water form azeotrope mixture at low butanol concentration and the resulting azeotrope mixture has a boiling point that is much higher than that of ethanol-water azeotrope [24]. By the application of new product separation methods, such as in-situ stripping, adsorption, pervaporation, and solvent extraction, pre-concentration of products are achieved, which reduces the capital for decanting equipment before butanol distillation and also reducing the extent (i.e., equipment size and time) of other downstream processes (ethanol and acetone distillation).
A combination of better pretreatment methods with updated downstream processing approaches would likely give not only better economic performance, but also better environmental impacts potential for cellulosic biofuels production. To date, there are no fundamental environmental impacts evaluation data available that are related to butanol production from oil palm frond (OPF). Such data are especially crucial to countries with oil palm as the major agricultural plant, where OPF is produced in abundance throughout the year. These data are also essential for the further development effort of this biomass material. Therefore, in this study, LCA was conducted on different pretreatment and product separation approaches for butanol production from OPF.
2. Methodology
2.1. Software Tools
The life cycle assessment (LCA) was conducted in the Excel spreadsheet while using process data that were extracted from SuperPro Designer V.9.0 (Intelligen, Inc., Scotch Plains, NJ, USA) simulation results.
2.2. System Boundary and Functional Unit
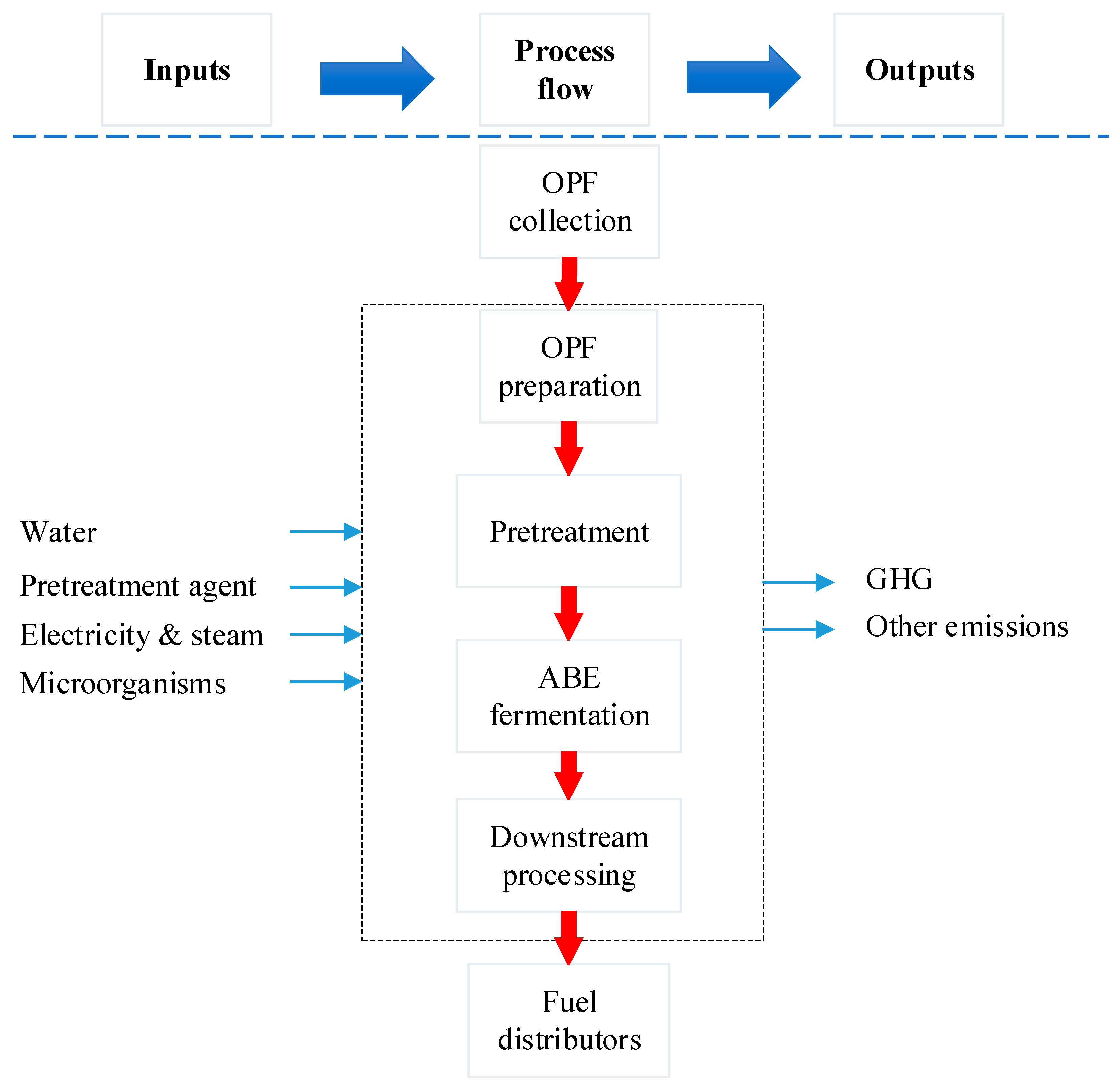
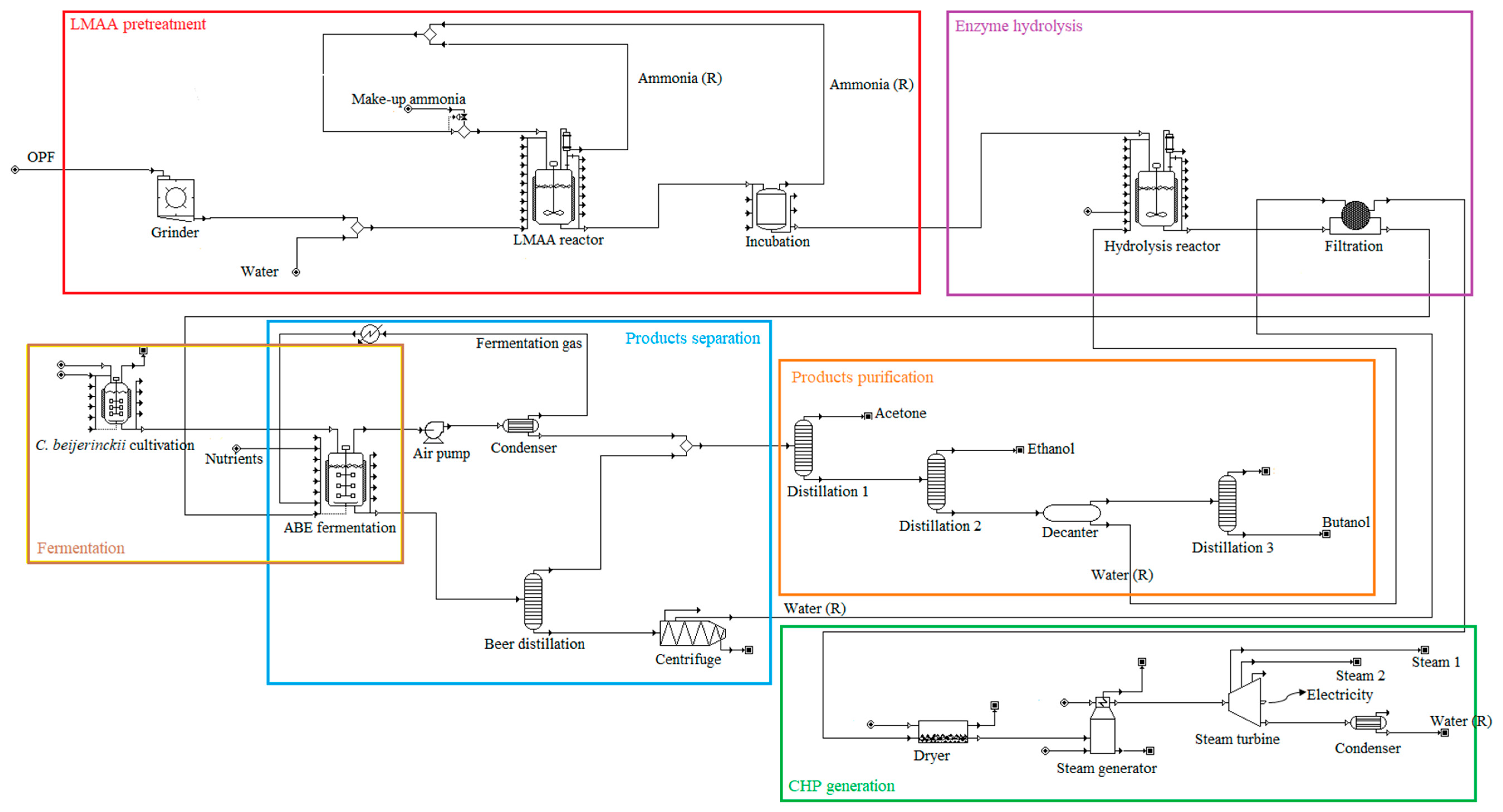
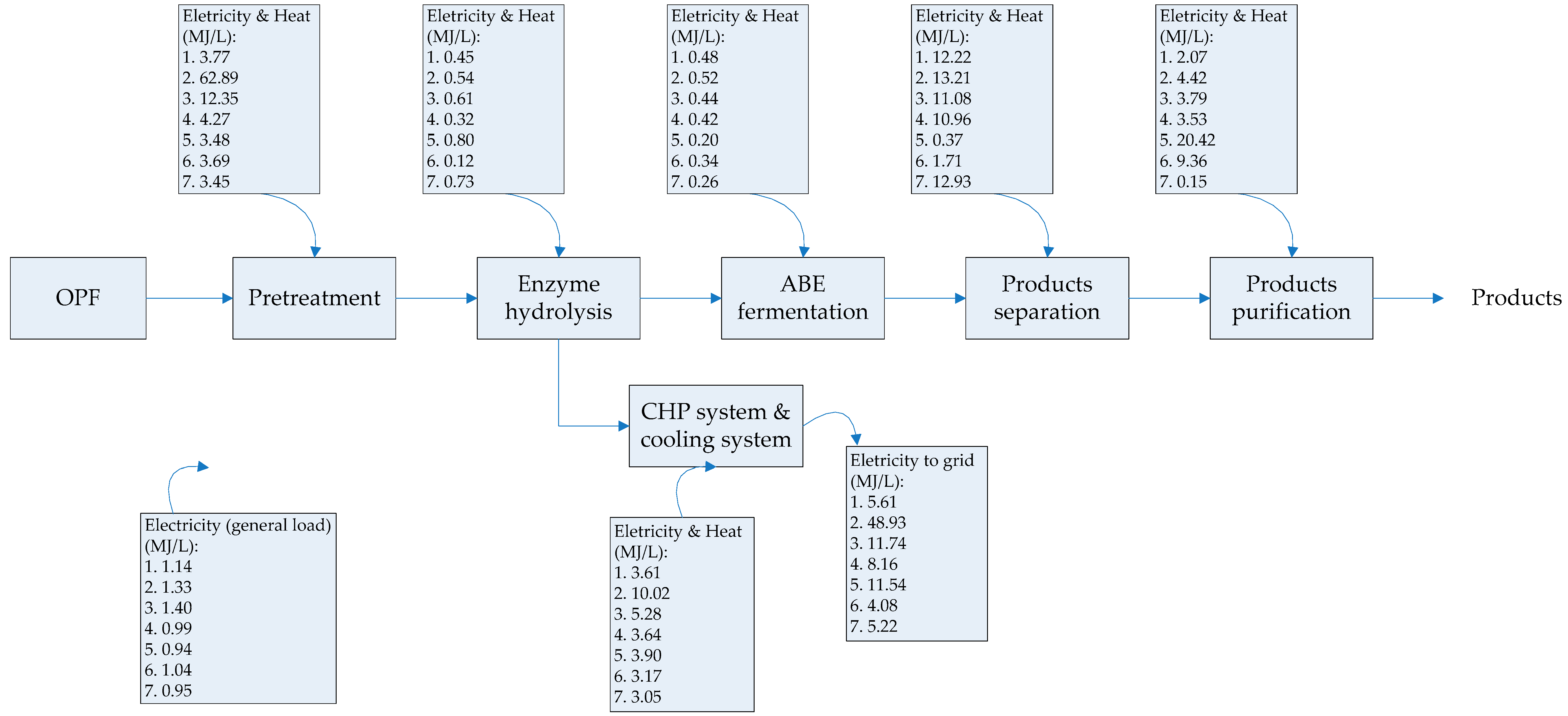
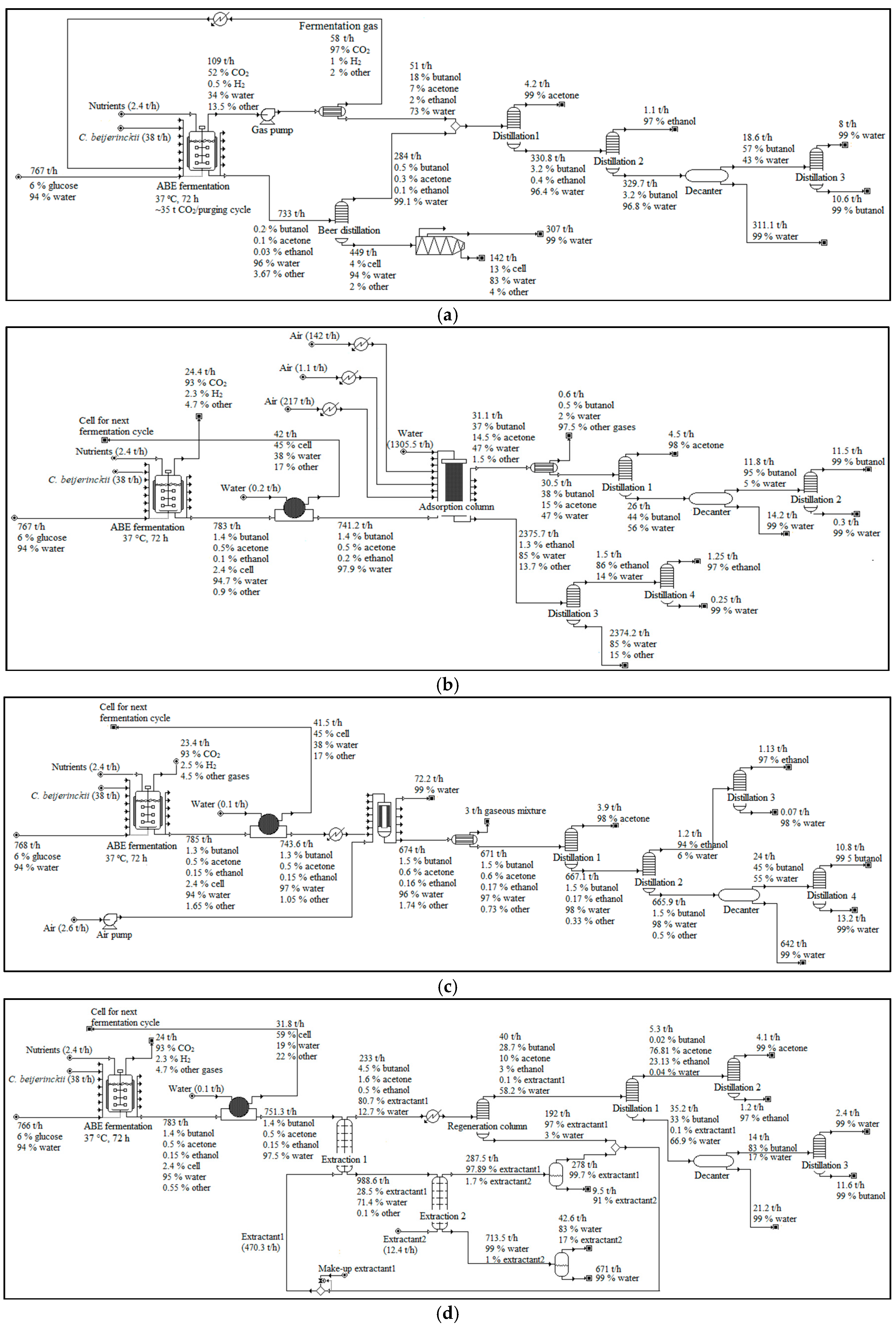
The scope of the study was gate-to-gate analysis, which includes feedstocks pretreatment, ABE (Acetone Butanol Ethanol) fermentation, and downstream processing as a system boundary (Figure 1 and Figure 2). 1 L butanol was the functional unit used. Figure 3, Figure 4 and Figure 5 show the mass and energy balances from the simulation of this work [23]. The objectives of this study were to determine the environmental impacts associated with butanol production from OPF and establish the baseline data for LCA of LMAA pretreatment.
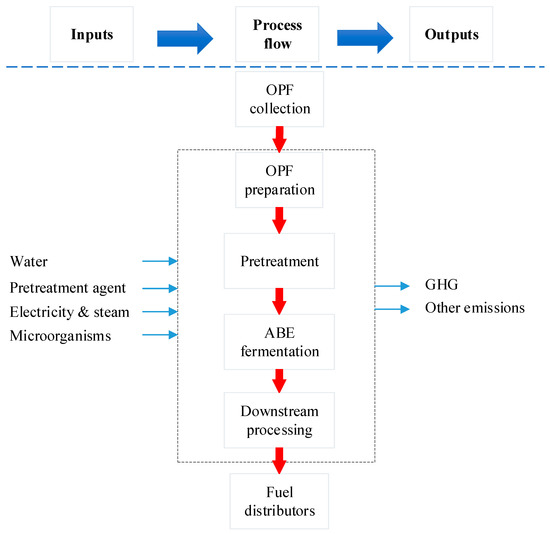
Figure 1.
Gate-to-gate system boundary applied in the assessment. OPF = oil palm frond; ABE = Acetone Butanol Ethanol.
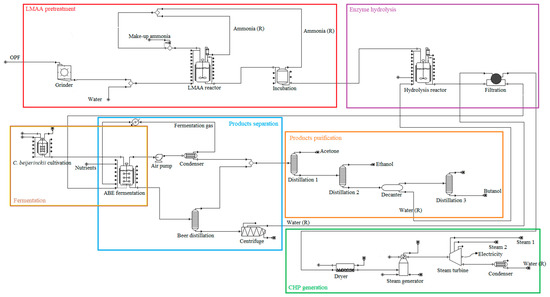
Figure 2.
Major unit operations in the simulated biobutanol production plant. Different colored boxes denote different production stages; (R) = recycle.
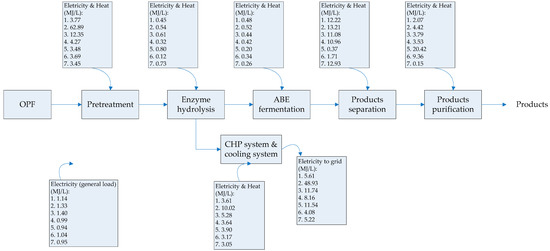
Figure 3.
Energy required in each processing stage for butanol production. Numbering in boxes indicates various pretreatment or product separation steps. 1. low moisture anhydrous ammonia (LMAA) pretreatment/in-situ stripping; 2. autohydrolysis pretreatment; 3. soaking in aqueous ammonia (SAA) pretreatment; 4. NaOH pretreatment; 5. adsorption; 6. pervaporation; 7. dual extraction. The energy requirement/generated were assessed based on 1 L butanol. Products produced are butanol, acetone, and ethanol. OPF = oil palm frond; ABE = Acetone Butanol Ethanol; CHP = combined heat and power.
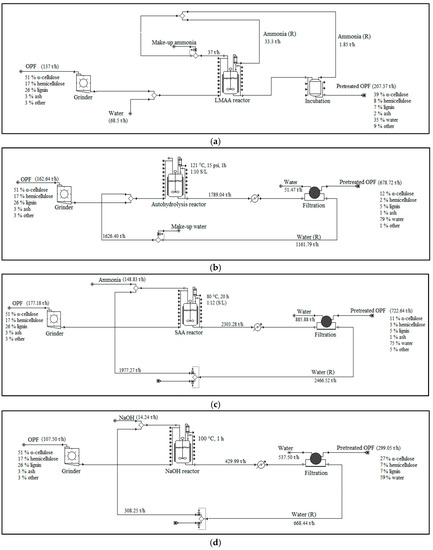
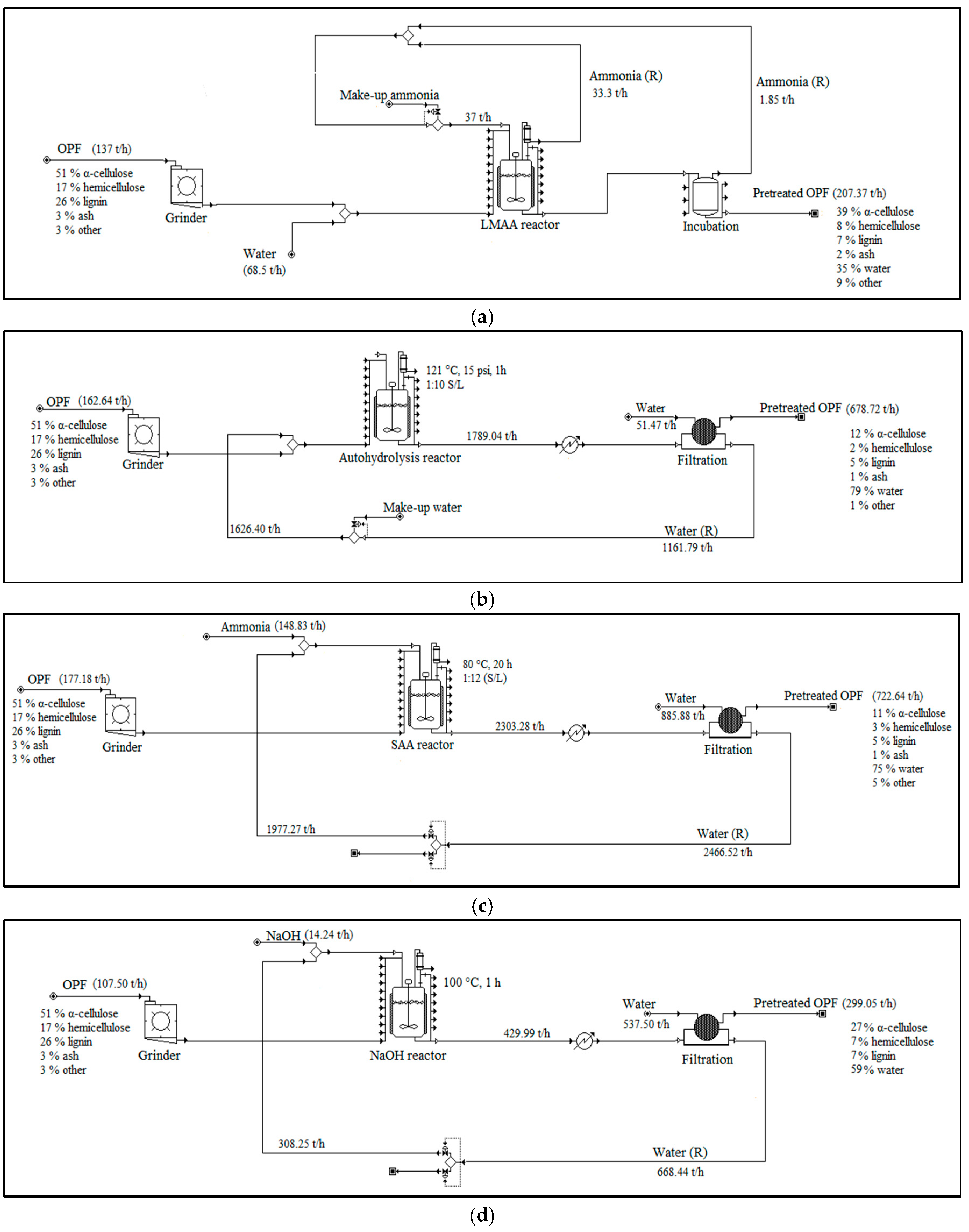
Figure 4.
Pretreatment set-ups used in the simulation models. Note that this figure only includes major unit operations. More detailed plant set-ups were actually used in the simulations. (a) LMAA pretreatment; (b) autohydrolysis pretreatment; (c) SAA pretreatment; (d) NaOH pretreatment; (R) = recycle.
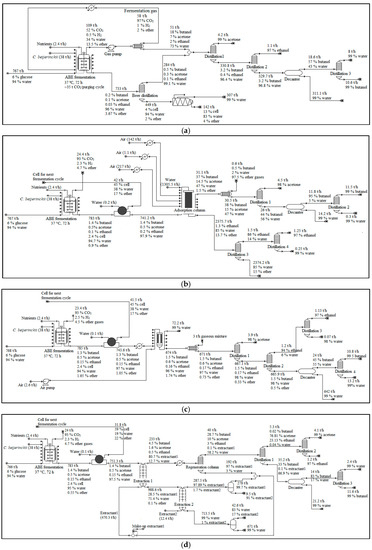
Figure 5.
Product separation and purification set-up used in the simulation models. (a) in-situ stripping; (b) adsorption-distillation; (c) pervaporation-distillation; (d) dual extraction-distillation. Note that this figure only includes major unit operations. More detailed plant set-ups were actually used in the simulations.
2.3. Life-Cycle Inventory
Table 1 summarizes the life cycle inventory emissions to air data from each model that were simulated in this study. These data were either extracted from SuperPro Designer simulation software or manually calculated while using the given formulae.

Table 1.
Inventory of life-cycle emissions (to air) data for different cellulosic butanol production models.
2.4. Production Stages
2.4.1. Pretreatment
Different pretreatment approaches were primarily selected to serve as a comparison to the performance of LMAA pretreatment, which was the main pretreatment method investigated in this study. The selected pretreatments were from available works on OPF pretreatments, which includes autohydrolysis pretreatment, soaking in aqueous ammonia (SAA) pretreatment, and soaking in sodium hydroxide (NaOH) pretreatment. LMAA was conducted based on works from [19,26]. No emission of ammonia (NH3) to the environment was assumed in LMAA pretreatment. Nevertheless, separate impacts assessment was also conducted on 5% and 9% emissions of NH3 to the environment. The small percentage of NH3 that was retained in the feedstock was not affecting the pH of the system and, hence, the neutralization procedure was not conducted [27].
Autohydrolysis pretreatment was modeled according to [28]. No chemical pretreatment agent was used in autohydrolysis pretreatment. Water stream exiting the autohydrolysis reactor was cooled before being recycled to the next cycle of autohydrolysis process.
SAA pretreatment was modeled according to [20]. Aqueous ammonia that was used in the pretreatment was highly diluted and not easy to recycle. The aqueous ammonia leaving the pretreatment unit was treated, and no fraction was assumed to be emitted.
NaOH pretreatment was simulated according to [21]. The NaOH solution leaving the pretreatment process was highly diluted. The stream was treated and no emitted fraction was assumed.
For all of the models, with the assumptions that all aqueous waste streams were appropriately treated and not leaked to the environment, the environmental impacts were assessed being primarily based on electricity and steam generation, with a small fraction of the emitted components.
2.4.2. Enzyme Hydrolysis and ABE Fermentation
The treated OPF was hydrolyzed while using cellulase enzyme according to [29]. After the hydrolysis process, the hydrolysate mixture was separated from the fibers residue and brought to the closed fermenter, while the fibers residues were used for combined heat and power (CHP) generation. The ABE fermentation was conducted following the works from [30,31]. There were no emissions from the hydrolysis process. However, the ABE fermentation process emitted a substantial amount of carbon dioxide (CO2) and hydrogen (H2).
2.4.3. Downstream Processing
The product separation from the fermented mixture was conducted while using several different technologies, namely in-situ stripping, dual extraction, membrane pervaporation, and adsorption. LMAA was used in the pretreatment section in each simulation while comparing different product separation technologies. The density of each butanol, acetone, and ethanol was 810 kg/m3, 783 kg/m3, and 789 kg/m3, respectively [32]. The subsequent processes of the downstream processing were simulated according to [33], with modification being based on the stream compositions.
In-situ stripping model development and simulation was according to work from [34,35]. In-situ stripping involves the use of fermentation gases to separate the products from the fermentation broth. The fermentation gases were compressed and then heated to 35 °C before being purged into the bottom of the fermenter. The stripping cycles run at fixed interval throughout the fermentation, and the stripping gases were released to the environment once the fermentation has completed (72 h). The retired fermentation broth that contained unstripped products went through beer distillation according to [36] for the separation of the remaining products. The mixture of products was then processed in a series of distillation columns to separate and purify acetone, ethanol, and butanol. A decanter unit was installed before the butanol distillation column to separate most of the water. The light-phase from the decanter unit will go through butanol distillation column, while the heavy-phase that was mostly water was recycled.
The dual extraction model development and simulation was based on work from [37]. The fermentation broth was undergoing a filtration unit to allow for microorganisms recycling before going through extraction-1, where most of the acetone, butanol, and ethanol were separated. The organic stream proceeds to regeneration column to recover the extractant-1. The aqueous phase from extraction-1 then underwent extraction-2, where the remaining extractant-1 was removed before recycling the broth back to the fermenter. Both of the extraction processes were conducted at 37 °C and 1 bar.
2-methyl-1-hexanol (non-biocompatible) and cyclopentane (biocompatible) were the extractants used for extraction-1 and extraction-2, respectively. The mixture of products from the regeneration column underwent distillation, where the lowest boiling point components (acetone and ethanol) were separated from the butanol-water mixture. The acetone-ethanol mixture then underwent distillation to purify each of the acetone and ethanol. A decanter was used to generate two phases, in which the lighter phase (butanol) will proceed to distillation for purification, because of the high water content in the water-butanol mixture. Some of the extractant-1 and extractant-2 were unrecovered and ended in the aqueous waste stream, however, assumed to be appropriately treated. No emission from the extractants was assumed.
The adsorption model development and simulation was according to [38,39]. Activated carbon (AC) F-400 was used as the adsorbent. Fermentation broth from fermenter undergone filtration to allow for cell recycling. The cell-free mixture was loaded into the adsorption column, which is selective towards butanol and acetone. An optimistic condition was assumed (~99% separation). Hot air (150 °C) was purged into the adsorption column to desorb the acetone and butanol, and to regenerate the adsorbent. The stripped air containing acetone and butanol was condensed before going through a series of distillation. Approximately 0.70%, 0.02%, and 0.08% of acetone, butanol, and water, respectively, were emitted during this process. Column equilibration was conducted while using water at room temperature before the next cycle of the adsorption process. The butanol- and acetone-depleted solution from the adsorption column proceed to the ethanol distillation. The entire aqueous waste stream was assumed to be appropriately treated.
The pervaporation model development and simulation followed the work from [40]. Filtration was conducted before the process to recycle the cells. Silicone membrane was used in the process. In this simulation, an optimistic yield was assumed for the pervaporation membrane, where it was capable of achieving ~99% separation efficiencies for all products. Compressed air was introduced to remove the diffused products from the membrane surface, which then condensed and collected. Approximately 0.13%, 0.01%, 0.04%, 0.01%, 0.002%, and 0.02% of acetone, butanol, ethanol, acetic acid, butyric acid, and water, respectively, were emitted during this process. The products-free broth was then recycled to the fermenter. The mixture of products was processed in a series of distillation columns to separate and purify acetone, ethanol, and butanol.
2.5. Heating and Cooling
The remaining lignin and other fibers residues (undigested cellulose and hemicellulose) that were separated from the fermentable sugar after enzyme hydrolysis were dried to approximately 10% moisture content and then used as a fuel for combined heat and power (CHP) generation system to generate steam and electricity. The Actual Rankine Cycle consisting of a boiler unit and multistage steam turbine was used and simulated while using SuperPro built-in expansion model. The generator was assumed to be 90% efficient.
The CHP system was supplemented with natural gas, because burning only fibers residues was not capable of generating enough steam for the production plant. This study also investigated the CHP system that was fueled by 100% biomass; fibers residues and oil palm empty fruit bunch (EFB) pellet. Table 1 tabulates the elemental compositions of lignin, cellulose, hemicellulose, natural gas, and EFB used in the simulation. Air and water at a pressure of 1 bar and 11 bar, respectively, at atmospheric temperature, entering the boiler at a rate calculated by the software. The generated high-pressure, high-temperature steam (45 bar, 257 °C) was expanded in a multistage steam turbine to produce electricity as well as steam with specific pressures. The emissions that were generated by burning each type of fuels were calculated while using the mentioned formula.
Cooling water for the plant was supplied from the on-site cooling tower. The cooling tower used air to cool water from 30 °C to 25 °C. The chilled water requirement for the plant was generated on-site through the electric cooling, which cooled the water from 10 °C to 5 °C.
2.6. Net Energy Value, Net Energy Ratio, and Fossil Energy Ratio
From the simulation, the energy that was required for the production of butanol was determined and then used for energy metrics calculation. The assessed energy metrics include net energy value (NEV), net energy ratio (NER), and fossil energy ratio (FER), which calculated based on Equations (1)–(3), respectively [41,42]. The energy content in butanol, acetone, and ethanol was assumed to be 27.95 MJ/L, 23.28 MJ/L, and 21.37 MJ/L, respectively [32]. The energy that was required for obtaining the feedstocks was not considered in the evaluation.
where: E = energy content; FEI = fossil energy input; and TEI = total energy input.
NEV = Ebutanol (MJ/L) + Eco-product (MJ/L) + Esold (MJ/L) − TEI (MJ/L)
NER = (Ebutanol (MJ/L) + Eco-product (MJ/L) + Esold (MJ/L))/TEI (MJ/L)
FER = (Ebutanol (MJ/L) + Eco-product (MJ/L) + Esold (MJ/L))/FEI (MJ/L)
2.7. Life-Cycle Impact Assessment (LCIA)
The carbon dioxide (CO2) emission from burning different fuels in the CHP system was calculated based on Equation (4), with 44/12 being the ratio of molecular weights of CO2 and carbon [43]. The emitted methane (CH4) and nitrous oxide (N2O) were estimated based on Equation (5) [43]. The emission factors used in the calculation were 1.0 g CH4/MMBtu and 0.1 g N2O/MMBtu for natural gas, while those for waste fibers were 32 g CH4/MMBtu and 4.2 g N2O/MMBtu. Table 2 shows the carbon content and higher heating value (HHV) of each fuel. These emissions were then categorized based on biogenic and non-biogenic. Fermentation gases and fibers residues burning in the CHP system contribute the biogenic emissions, while natural gas combustion in the CHP system contributes the non-biogenic emissions.
Emissions (CO2) = Massfuel (kg) × Fuel carbon content (%) × (44/12)
Emissions (CH4 or N2O) = Massfuel (kg) × HHVfuel (MJ/kg) × Emission factor (g/MMBtu)

Table 2.
Elemental compositions of fuels used for the boiler.
An environmental impacts assessment from the butanol production plant was conducted based on several different criteria, which include global warming potential (GWP) (kg CO2 eq.), acidification (kg SO2 eq.), eutrophication (kg N eq.), and ecotoxicity (CTUeco/kg) potentials. Only emissions that were generated from the plant were considered for the assessment of specific environmental impacts potential. All of the aqueous waste streams were assumed to be appropriately treated, thus not creating any environmental impacts.
The GWP for 100-year time horizon was calculated from electricity usage and steam generation. Ecotoxicity and eutrophication potentials were estimated from the emissions containing solvents products and N2 produced from the processes. The acidification potential was only calculated for the LMAA pretreatment model based on the varying NH3 emitted from the process. The impact calculation and assessments were done with the aid of the Tool for Reduction and Assessment of Chemical and other Environmental Impacts (TRACI) 2.1 database. GWP was calculated based on Equation (6), while the other impacts potentials were calculated by multiplying the mass of a specific emission with the TRACI 2.1 emission factors following Equation (7) [48].
where 25 and 298 are the GWP factor for CH4 and N2O, respectively.
where:
GWP (kg CO2 eq./L) = CO2 (kg/L) + (CH4 (kg/L) × 25) + (N2O (kg/L) × 298)
- = potential impact of all chemicals (x) for a specific impact category (i);
- = characterization factor of chemical (x) emitted to media (m) for impact category (i); and,
- = mass of chemical (x) emitted to media (m).
3. Results and Discussions
3.1. Process Yield
Each of the plant set-ups (i.e., different pretreatment and different product separation technologies) was used for the simulation of biobutanol production (Figure 3, Figure 4 and Figure 5). The same product separation method (in-situ stripping) was applied when different pretreatment were modeled. The same pretreatment (LMAA) was used when different product separation were simulated. A fixed amount of OPF (1.09 × 106 t) was used in each simulated models. The results of the simulation were presented elsewhere [23], as briefly summarized in Table 3.

Table 3.
Products yield from different simulated plant set-up.
The analysis also found that the biobutanol production cost that was produced while using LMAA pretreatment was the lowest, being mainly contributed by its low operating cost (utilities, materials, labor, maintenance and repairs, waste treatment, overhead, tax, insurance, depreciation, and capital charges costs). The huge gap in butanol production costs between LMAA and those of SAA and autohydrolysis pretreatment were due to the differences in the materials cost and utility cost.
3.2. Life-Cycle Energy Metrics
Table 4 and Table 5 show the life-cycle energy metrics comparisons for cellulosic butanol production simulated while using different pretreatment and product separation technologies, respectively. The fossil energy input was coming from natural gas combustion for heat and power generation in the CHP system. In general, the variation of electricity that was required by all models was small as compared to the variation of heat needed between models.

Table 4.
Energy metrics of pretreatment technologies simulated.

Table 5.
Energy metrics of product separation technologies simulated.
Among all of the pretreatment technologies evaluated, the autohydrolysis pretreatment model recorded the highest heat and electricity requirements because of the high amount of feedstock loading (hence increased milling work) and operated at a higher temperature than the other pretreatments. In fulfilling the high demand for energy, the CHP system in autohydrolysis model also generated a high amount of electricity that was sold to the grid. As mentioned in [23], SAA pretreatment has the lowest efficiency and it required the highest amount of feedstock when compared to other pretreatments. Therefore, the SAA model was also generated the highest amount of fibers residues for the CHP system. The other pretreatment processes modeled have approximately similar total energy requirements.
When comparing different product separation technologies, in-situ stripping model recorded the highest electricity requirement (7.20 MJ/L), while the adsorption model recorded the highest heat requirement (23.44 MJ/L). The highest total energy requirement was recorded by the adsorption model (30.11 MJ/L), while the pervaporation process required the least energy (19.43 MJ/L). In general, all of the simulated models produced the same amount of fibers residues that were used to fuel the CHP system. However, varying products yield from each model rendered a different value of energy per unit butanol (Table 4). Similar to the earlier simulations, those required the highest amount of heat had the highest amount of excess electricity sold to the grid. As elaborated in [23], the adsorption process needed a high amount of heat in the products purification section due to the high volume of water that is available in the process.
NEV, NER, and FER were calculated to assess the energy performance of the processes. A positive NEV value is desired for biofuel to be a substitute for fossil fuel, because it means that more energy was generated than consumed [49]. Among pretreatment technologies evaluated, NaOH pretreatment model recorded the highest NEV (23.87 MJ/L), followed by LMAA, SAA, and autohydrolysis pretreatment models (Table 4). When comparing different product separation technologies, the pervaporation model recorded the highest NEV (24.69 MJ/L), followed by dual extraction, in-situ stripping, and adsorption model (Table 5). As a comparison, Tao et al. [49] recorded an NEV of 90 MJ/GGE (~15.00 MJ/L) for n-butanol produced from corn stover, and Morey et al. [41] reported NEV of 30.50 MJ/L for ethanol produced from corn stover.
For NER, a value that is higher than 1.0 is desired, which indicates the positive energy balance [42]. NER value for NaOH pretreatment model was the largest (1.99) as compared to other pretreatment technologies, while the pervaporation model recorded the highest NER value among all of the product separation approaches (2.27). Only the autohydrolysis pretreatment model had NER lower than 1.0, which indicated that it used higher energy for production process than the energy available in the products.
Baral et al. [50], who treated corn stover for butanol production recorded NER of 1.78, 1.80, 1.73, 0.41, and 3.69 from the steam explosion, DA, AFEX, ionic liquid, and biological pretreatments, respectively. When only considering chemical pretreatment methods (DA and AFEX), their NER values were not far different from those that were recorded in this study. It could serve as a baseline value for this study, although they were assessing different pretreatment methods.
Another comparison on the NER value was from those recorded by [49], which were 2.8 and 2.2 for corn stover-based n-butanol and isobutanol, respectively, higher than almost all NER values that were recorded in this study. However, their NER values without electricity displacement credits were 1.5 and 1.4 for n-butanol and isobutanol, respectively, which were similar to those that were recorded in almost all models in this study before the inclusion of electricity credits (1.68, 0.43, 1.14, 1.65, 1.68, 1.32, 2.06, and 1.80 for LMAA, autohydrolysis, SAA, NaOH, in-situ stripping, adsorption, pervaporation, and dual extraction models respectively—data not shown in the results table). It might indicate that their production plant had generated a greater amount of excess electricity than those that were generated in this study, probably because of the low consumption, or because of the high requirement of process steam that at the same time produced a high amount of electricity. In general, the NER value of cellulosic butanol cannot compete with the NER value of the cellulosic ethanol. Cronin et al. [15] reported an NER value in the range of 4.96–9.00 for cellulosic ethanol that was produced from AFEX- and DA-treated corn stover, switchgrass, and miscanthus.
FER measures the degree of renewability of the produced butanol. The larger the FER indicates that the process generated a considerable output of renewable energy in comparison to the low amount of fossil energy input. FER for NaOH pretreatment model was the greatest (6.47) when compared to the other pretreatments, while FER for the pervaporation model was the highest (7.24) among other product separation technologies evaluated. No available study has provided any FER value of cellulosic butanol for comparison purposes.
Nevertheless, direct comparison with cellulosic ethanol showed agreement with the FER value that was obtained in this study; Morey et al. [41] reported FER of 5.31 for corn stover ethanol, while [51] estimated an FER value of cellulosic ethanol in the range of 4.4–6.6 [52]. These FER values are far better than that of gasoline, which is 0.8. In general, there was no direct relationship between NEV, NER, and FER, i.e., those that recorded the highest NEV did not necessarily have the highest NER and FER. Nevertheless, all of the evaluated technologies have recorded values that were almost similar to available works. The LMAA pretreatment model recorded neither the best nor the lowest values of all energy metrics evaluated, which suggests its prospect for commercial application.
3.3. Process Emissions
All of the energy metrics evaluated above did not show any relationship with the emissions that were generated by the process, i.e., a process that has the highest energy metrics values does not necessarily have a specific amount (highest or lowest) of emissions. Table 6 and Table 7 tabulate the results of gate-to-gate process emissions from butanol production plant simulated while using different pretreatment and product separation technologies, respectively. The evaluated emissions include CO2, N2O, and CH4, which then differentiated between biogenic and non-biogenic. In general, the process with a high-energy requirement recorded high emissions, which were mostly generated from the energy generation process (CHP system).

Table 6.
Feedstocks consumed, water consumed, and emissions from different pretreatment models simulated.

Table 7.
Water consumed and emissions from different product separation models simulated.
Baral et al. [50] conducted an LCA on the steam explosion, sulfuric acid, ammonia fiber explosion, ionic liquid, and biological pretreatments for butanol production from corn stover. They estimated biogenic CO2 between 5.21–11.46 kg CO2/L butanol, which was only overlapped with the LMAA pretreatment model in this study (10.32 kg CO2/L butanol). However, when comparing different product separation methods, the biogenic CO2 emission of the process recorded much lower results—10.32, 8.30, 8.98, and 8.34 kg CO2/L butanol for in-situ stripping, adsorption, pervaporation, and dual extraction models, respectively. The higher CO2 emission recorded in in-situ stripping model was mainly due to the longer fermentation process in this model because of the immediate solvents-removal effect.
According to the IPCC, only non-biogenic CO2 emissions should be considered as greenhouse gas (GHG) emissions, which eventually contribute towards an increase in global warming potential (GWP). The biogenic CO2 is not considered as GHG emission, because, throughout plants’ life, they are conducting a photosynthesis process by taking CO2 from the atmosphere [49]. The biogenic CO2 emissions fractions in most of the simulated models were higher than that of non-biogenic, because of the large fraction of CO2 generated during the fermentation process and the waste fibers burning in the CHP generation system.
The case was different for N2O and CH4 emissions, in which both biogenic and non-biogenic fractions from these emissions were considered as GHG emissions, because growing plants have no abilities to utilize those gases throughout its life. The higher biogenic N2O and CH4 emissions than those of non-biogenic came from the combustion of fibers residues in the CHP system. In all simulation cases, the amount of natural gas that was required for energy generation was multiple degrees higher than the available amount of fibers residues. It indicated that burning biomass in CHP generating higher emission than burning fossil fuel (natural gas). By using fibers residues as the fuel for CHP system, the cost that is associated with energy generation could be reduced [23]. However, it was creating more emissions.
3.4. Environmental Impacts
3.4.1. Global Warming Potential (GWP)
Table 8 tabulates the GWP that was calculated from the emissions data in Table 6 and Table 7. Among all of the pretreatment models, LMAA had the lowest GWP (5.72 kg CO2 eq. /L butanol), while the lowest GWP among all product separation models was recorded from pervaporation model (3.92 kg CO2 eq. /L butanol). By subtracting the electricity sold to the grid in the calculation, 50%, 71%, 86%, and more than 100% reduction in GWP were recorded for LMAA, autohydrolysis, SAA, and NaOH pretreatment models, respectively. While the in-situ stripping, adsorption, pervaporation, and dual extraction product separation model recorded GWP reduction of 50%, 62%, 57%, and 53%, respectively, when the electricity credits were counted.

Table 8.
Global warming potentials (GWP) of simulated cellulosic butanol production plants.
As a comparison, Tao et al. [49] recorded 29% and 35% GHG emissions reduction after the inclusion of electricity displacement credit for isobutanol and n-butanol production, respectively. Another available study on LCA of OPF-based biofuel recorded a GWP of 3.84 kg CO2 equivalent/ kg bioethanol produced (value has excluded the plant cultivation stage that was included in their study) [53].
3.4.2. Other Environmental Impacts Potential
Table 9 shows the ecotoxicity and eutrophication potentials of all the simulated models. In general, ecotoxicity potential was very low for all models, with only decimals different between each. On the other hand, the eutrophication potentials showed slightly larger values, with the autohydrolysis pretreatment model and pervaporation model recording the highest value among all of the pretreatment and product separation models, respectively.

Table 9.
Ecotoxicity and eutrophication potentials of simulated cellulosic butanol production plants.
Ecotoxicity is the measure of the toxic effect of chemical compounds on human and the whole ecosystems [48]. The eutrophication impact is the enrichment of nutrients (nitrogen and phosphorus) in the ecosystem. Phosphorus could have a more negative impact on freshwater, while nitrogen could give a negative effect on freshwater and air. Impaired breathing ability, limited visibility, and altered plant growth are among the effects of nitrogen to air [54,55].
LMAA pretreatment had slightly higher ecotoxicity and eutrophication impact potentials than the other alkali pretreatments assessed. Besides, the degree of eutrophication potential was increased if a small fraction of the NH3 used was emitted, and it also caused acidification potential to occur (Table 10). It suggests that, to avoid severe environmental impacts from LMAA pretreatment, efficient unit operation enabling no pretreatment agent (NH3) escape from the system and allowing for maximum NH3 recycling must be in place, along with rigorous safety measures. The acidification impact potential indicates the increase in acidity of the local environment. Increased air acidification could cause damage to building, plant life, and ecosystems [48].

Table 10.
Environmental impacts comparison from LMAA pretreatment model with varying anhydrous ammonia emitted.
Ofori-Boateng & Lee [53] recorded 0.13 kg SO2 eq., 0.13 kg N eq., and 0.04 CTUeco of acidification, eutrophication, and ecotoxicity potentials, respectively, for each kg of ethanol produced. The LMAA pretreatment model in this study potentially generated a higher acidification impact potential if the pretreatment unit operations were not properly sealed, causing NH3 to leak into the air. There was no previous LCA study on butanol production from OPF for a more appropriate comparison.
3.5. Effect of 100% Biomass-Fueled CHP to the GWP Performance
The biobutanol production plants have to rely on the electricity displacement credit to achieve a substantial reduction of GWP by burning natural gas in addition to the fibers residues in the CHP system (Table 8). By fueling the CHP system with only biomass, the EFB pellet was used as a replacement for natural gas, and the production plants generated only biogenic emissions (Table 11). Although burning biomass emitted higher CO2, N2O, and CH4 when compared to burning natural gas, the total GWP of the plants were substantially lower than those using natural gas in CHP system since biogenic CO2 is not considered in GWP impact calculation.

Table 11.
Biogenic emissions from cellulosic butanol production plants employing 100% biomass-fueled combined heat and power (CHP) system.
The well-to-tank GHG emissions of gasoline were reported in the range of 0.016–0.026 kg CO2 eq./MJ butanol [56,57,58]. The GWP profiles that were recorded while using this option (100% biomass-fueled CHP), for most of the pretreatment and product separation approaches were below those values (Table 12), potentially giving total lower GHG emissions when further including the fuel products combustion, which could indicate the environmental preservation potential of the biobutanol produced.

Table 12.
Global warming potentials (GWP) of cellulosic butanol production plants employing 100% biomass-fueled CHP system.
3.6. Applications, limitations, and Future Scope of Research
The assessed environmental impact potentials could be useful baseline data for a decision on butanol biorefinery development. In the U.S., most of the works on cellulosic butanol production are done by retrofitting the existing bioethanol plant [24]. In contrast, the countries where OPF or other oil palm biomass available in abundance are mostly developing countries where the effort in any green energy development is starting at a slower pace. Without any available commercial plant currently running, these data could potentially help the government or industry to get the whole idea about biobutanol without having to primarily rely on foreign expertise and investment.
The environmental impact potentials that were assessed in this work were conducted with the exclusion of the localized factor, such as emission area and potency of stressor [48]. Therefore, the obtained values of impact potentials are not site-specific. Nevertheless, they could be used as a preliminary assessment or decision-making tool in any potential site for the biobutanol refinery development. Future assessment of the more accurate environmental impacts potential should be conducted if any common site-specific conditions are known. Other than that, future research should be focused on Monte Carlo simulation to accurately determine the changes of impacts potential, along with the changes in energy consumption, specific emissions, or other important factors.
4. Conclusions
The results have shown that the LMAA pretreatment emissions performance was comparable to the conventional chemical pretreatment methods that were evaluated in this work. With the benefit of conducted without increased temperature, the LMAA pretreatment model recorded the lowest energy requirement when compared to other pretreatment models. Further study of the combination of LMAA pretreatment with different product separation approaches for butanol biorefinery has shown that the pervaporation process required the lowest energy as compared to other processes and, hence, it also recorded the lowest GWP. While taking the economic benefit into account, the dual extraction recovery process shows better potential for commercial application due to its low production cost and considerably low environmental impacts potential.
Author Contributions
N.M. conducted the modeling and wrote the original manuscript. K.A.R. directed the research and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Intergovernmental Panel on Climate Change. Summary for Policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development and Effort to Eradicate Poverty; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018. [Google Scholar]
- Wise, M.; Muratori, M.; Kyle, P. Biojet Fuels and Emissions Mitigation in Aviation: An Integrated Assessment Modeling Analysis. Transp. Res. Part D Transp. Environ. 2017, 52, 244–253. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels Securing the Planet’s Future Energy Needs. Energy Convers. Manag. 2009, 50, 2239–2249. [Google Scholar] [CrossRef]
- International Renewable Energy Agency. Road Transport: The Cost of Renewable Solutions; International Renewable Energy Agency: Washington, DC, USA, 2013. [Google Scholar]
- Luo, L.; van der Voet, E.; Huppes, G. Life Cycle Assessment and Life Cycle Costing of Bioethanol from Sugarcane in Brazil. Renew. Sustain. Energy Rev. 2009, 13, 1613–1619. [Google Scholar] [CrossRef]
- Warner, E.; Inman, D.; Kunstman, B.; Bush, B.; Vimmerstedt, L.; Peterson, S.; Macknick, J.; Zhang, Y. Modeling Biofuel Expansion Effects on Land Use Change Dynamics. Environ. Res. Lett. 2013, 8, 015003. [Google Scholar] [CrossRef]
- Dunn, J.B.; Mueller, S.; Kwon, H.; Wang, M.Q. Land-Use Change and Greenhouse Gas Emissions from Corn and Cellulosic Ethanol. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Jangam, S.V.; Akhtar, S.; Sasmito, A.P.; Mujumdar, A.S. Advances in Biofuel Production from Oil Palm and Palm Oil Processing Wastes: A Review. Biofuel Res. J. 2016, 9, 332–346. [Google Scholar] [CrossRef]
- Baral, N.R.; Slutzky, L.; Shah, A.; Ezeji, T.C.; Cornish, K.; Christy, A. Acetone-Butanol-Ethanol Fermentation of Corn Stover: Current Production Methods, Economic Viability and Commercial Use. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Comparative Techno-Economic Analysis of Steam Explosion, Dilute Sulfuric Acid, Ammonia Fiber Explosion and Biological Pretreatments of Corn Stover. Bioresour. Technol. 2017, 232, 331–343. [Google Scholar] [CrossRef]
- Kumneadklang, S.; Larpkiattaworn, S.; Niyasom, C.; O-Thong, S. Bioethanol Production from Oil Palm Frond by Simultaneous Saccharification and Fermentation. Energy Procedia 2015, 79, 784–790. [Google Scholar] [CrossRef]
- Olupot, P.W.; Candia, A.; Menya, E.; Walozi, R. Characterization of Rice Husk Varieties in Uganda for Biofuels and Their Techno-Economic Feasibility in Gasification. Chem. Eng. Res. Des. 2016, 107, 63–72. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Senske, G.E.; Kim, T.H. Pretreatment of Corn Stover by Low Moisture Anhydrous Ammonia (LMAA) in a Pilot-Scale Reactor and Bioconversion to Fuel Ethanol and Industrial Chemicals. Appl. Biochem. Biotechnol. 2016, 179, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. EPA Lifecycle Analysis of Greenhouse Gas Emissions from Renewable Fuels; Environmental Protection Agency: Washington, DC, USA, 2009.
- Cronin, K.R.; Runge, T.M.; Zhang, X.; Izaurralde, R.C.; Reinemann, D.J.; Sinistore, J.C. Spatially Explicit Life Cycle Analysis of Cellulosic Ethanol Production Scenarios in Southwestern Michigan. Bioenergy Res. 2017, 10, 13–25. [Google Scholar] [CrossRef]
- Adom, F.; Dunn, B.J.; Han, J. GREET Pretreatment Module; Argonne National Lab.: Argonne, IL, USA, 2014. [Google Scholar]
- Chaturvedi, V.; Verma, P. An Overview of Key Pretreatment Processes Employed for Bioconversion of Lignocellulosic Biomass into Biofuels and Value Added Products. 3 Biotech 2013, 3, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sheng, Y.; Zhang, L.; Song, J.; Cong, H.; Zhang, J. Biobutanol Production from Lignocellulosic Biomass: Prospective and Challenges. J. Bioremediat. Biodegrad. 2016, 7, 363. [Google Scholar] [CrossRef]
- Yang, M.; Rosentrater, K.A. Small-Scale Low-Moisture Anhydrous Ammonia (LMAA) Pretreatment of Corn Stover. Biomass Bioenergy 2017, 97, 38–42. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, S.; Yang, T.H.; Lee, H.J.; Seung, D.; Park, Y.-C.; Seo, J.-H.; Choi, I.-G.; Kim, K.H. Aqueous Ammonia Pretreatment, Saccharification, and Fermentation Evaluation of Oil Palm Fronds for Ethanol Production. Bioprocess Biosyst. Eng. 2012, 35, 1497–1503. [Google Scholar] [CrossRef]
- Sukri, S.S.M.; Rahman, R.A.; Illias, R.M.D.; Yaakob, H. Optimization of Alkaline Pretreatment Conditions of Oil Palm Fronds in Improving the Lignocelluloses Contents for Reducing Sugar Production. Rom. Biotechnol. Lett. 2014, 19, 9006–9018. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Mahmud, N. Low Moisture Anhydrous Ammonia (LMAA) Pretreatment of Lignocellulosic Biomass and Assessments for Biobutanol Production; Iowa State University: Ames, IA, USA, 2019. [Google Scholar]
- Huang, H.J.; Ramaswamy, S.; Liu, Y. Separation and Purification of Biobutanol during Bioconversion of Biomass. Sep. Purif. Technol. 2014, 132, 513–540. [Google Scholar] [CrossRef]
- Oudshoorn, A.; Van Der Wielen, L.A.M.; Straathof, A.J.J. Assessment of Options for Selective 1-Butanol Recovery from Aqueous Solution. Ind. Eng. Chem. Res. 2009, 48, 7325–7336. [Google Scholar] [CrossRef]
- Cheng, M.; Rosentrater, K.A. Optimization of Low Moisture Anhydrous Ammonia (LMAA) Pretreatment for Corn Stover Enzymatic Digestibility during Hydrolysis Process. In Proceedings of the 2016 ASABE Annual International Meeting, Orlando, Florida, USA, 17–20 July 2016. [Google Scholar] [CrossRef]
- Yoo, C.G.; Nghiem, N.P.; Hicks, K.B.; Kim, T.H. Pretreatment of Corn Stover Using Low-Moisture Anhydrous Ammonia (LMAA) Process. Bioresour. Technol. 2011, 102, 10028–10034. [Google Scholar] [CrossRef] [PubMed]
- Sabiha-Hanim, S.; Noor, M.A.M.; Rosma, A. Effect of Autohydrolysis and Enzymatic Treatment on Oil Palm (Elaeis Guineensis Jacq.) Frond Fibres for Xylose and Xylooligosaccharides Production. Bioresour. Technol. 2011, 102, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Triwahyuni, E.; Hariyanti, S.; Dahnum, D.; Nurdin, M.; Abimanyu, H. Optimization of Saccharification and Fermentation Process in Bioethanol Production from Oil Palm Fronds. Procedia Chem. 2015, 16, 141–148. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Blaschek, H.P. Fermentation of Dried Distillers’ Grains and Solubles (DDGS) Hydrolysates to Solvents and Value-Added Products by Solventogenic Clostridia. Bioresour. Technol. 2008, 99, 5232–5242. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Ezeji, T.C.; Ebener, J.; Dien, B.S.; Cotta, M.A.; Blaschek, H.P. Butanol Production by Clostridium Beijerinckii. Part I: Use of Acid and Enzyme Hydrolyzed Corn Fiber. Bioresour. Technol. 2008, 99, 5915–5922. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, M.; Liu, J.; Huo, H. Assessment of Potential Life-Cycle Energy and Greenhouse Gas Emission Effects from Using Corn-Based Butanol as a Transportation Fuel. Biotechnol. Prog. 2008, 24, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Baral, N.R.; Shah, A. Techno-Economic Analysis of Cellulosic Butanol Production from Corn Stover through Acetone-Butanol-Ethanol Fermentation. Energy Fuels 2016, 30, 5779–5790. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of Acetone, Butanol and Ethanol by Clostridium Beijerinckii BA101 and in Situ Recovery by Gas Stripping. World J. Microbiol. Biotechnol. 2003, 19, 595–603. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Microbial Production of a Biofuel (Acetone-Butanol-Ethanol) in a Continuous Bioreactor: Impact of Bleed and Simultaneous Product Removal. Bioprocess Biosyst. Eng. 2013, 36, 109–116. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, E.; Quiroz-Ramírez, J.J.; Segovia-Hernández, J.G.; Hernández, S.; Bonilla-Petriciolet, A. Process Alternatives for Biobutanol Purification: Design and Optimization. Ind. Eng. Chem. Res. 2015, 54, 351–358. [Google Scholar] [CrossRef]
- Kurkijärvi, A.J.; Melin, K.; Lehtonen, J. Comparison of Reactive Distillation and Dual Extraction Processes for the Separation of Acetone, Butanol, and Ethanol from Fermentation Broth. Ind. Eng. Chem. Res. 2016, 55, 1952–1964. [Google Scholar] [CrossRef]
- Abdehagh, N.; Gurnani, P.; Tezel, F.H.; Thibault, J. Adsorptive Separation and Recovery of Biobutanol from ABE Model Solutions. Adsorption 2015, 21, 185–194. [Google Scholar] [CrossRef]
- Águeda, V.I.; Delgado, J.A.; Uguina, M.A.; Sotelo, J.L.; García, Á. Column Dynamics of an Adsorption-Drying-Desorption Process for Butanol Recovery from Aqueous Solutions with Silicalite Pellets. Sep. Purif. Technol. 2013, 104, 307–321. [Google Scholar] [CrossRef]
- Qureshi, N.; Blaschek, H.P. Production of Acetone Butanol Ethanol (ABE) by a Hyper-Producing Mutant Strain of Clostridium Beijerinckii BA101 and Recovery by Pervaporation. Biotechnol. Prog. 1999, 15, 594–602. [Google Scholar] [CrossRef]
- Morey, R.V.; Tiffany, D.G.; Hatfield, D.L. Biomass for Electricity and Process Heat at Ethanol Plants. Appl. Eng. Agric. 2006, 22, 723–728. [Google Scholar] [CrossRef]
- Gupta, V.K.; Tuohy, M.G. Biofuel Technologies: Recent Developments; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Environmental Protection Agency. Direct Emissions from Stationary Combustion Sources; Environmental Protection Agency: Washington, DC, USA, 2016. [CrossRef]
- Hussin, M.H.; Samad, N.A.; Latif, N.H.A.; Rozuli, N.A.; Yusoff, S.B.; Gambier, F.; Brosse, N. Production of Oil Palm (Elaeis Guineensis) Fronds Lignin-Derived Non-Toxic Aldehyde for Eco-Friendly Wood Adhesive. Int. J. Biol. Macromol. 2018, 113, 1266–1272. [Google Scholar] [CrossRef]
- Demirbaş, A. Estimating of Structural Composition of Wood and Non-Wood Biomass Samples. Energy Sources 2005, 27, 761–767. [Google Scholar] [CrossRef]
- Morvay, Z.K.; Gvozdenac, D.D. Applied Industrial Eenergy and Environmental Management; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Nasrin, A.B.; Vijaya, S.; Loh, S.K.; Astimar, A.A.; Lim, W.S. Quality Compliance and Environmental Impact Assessment of Commercial Empty Fruit Bunch (EFB) Pellet Fuel in Malaysia. J. Oil Palm Res. 2017, 29, 570–578. [Google Scholar] [CrossRef]
- Bare, J.; Young, D.; Hopton, M. Tool for the Reduction and Assessment of Chemical and Other Environmental Impacts (TRACI) User’s Manual; Environmental Protection Agency: Washington, DC, USA, 2012.
- Tao, L.; Tan, E.C.D.; Mccormick, R.; Zhang, M.; Aden, A.; He, X.; Zigler, B.T. Techno-Economic Analysis and Life-Cycle Assessment of Cellulosic Isobutanol and Comparison with Cellulosic Ethanol and n-Butanol. Biofuels Bioprod. Biorefin. 2014, 8, 30–48. [Google Scholar] [CrossRef]
- Baral, N.R.; Quiroz-Arita, C.E.; Bradley, T.H. Probabilistic Lifecycle Assessment of Butanol Production from Corn Stover Using Different Pretreatment Methods. Environ. Sci. Technol. 2018, 52, 14528–14537. [Google Scholar] [CrossRef]
- Hammerschlag, R. Ethanol’s Energy Return on Investment: A Survey of the Literature 1990—Present. Environ. Sci. Technol. 2006, 40, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Keoleian, A.G. Biofuels; Center for Sustainable Systems: University of Michigan: Ann Arbor, MI, USA, 2018; Volume CSS08-09. [Google Scholar]
- Ofori-Boateng, C.; Lee, K.T. Sustainable Utilization of Oil Palm Fronds for Cellulosic Ethanol Production: Environmental Life Cycle Assessment. In WIT Transactions on Ecology and the Environment; WIT Press: Southampton, UK, 2013; Volume 179, pp. 859–869. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Nutrient Pollution—The Problem. Available online: https://www.epa.gov/nutrientpollution/problem (accessed on 3 April 2019).
- Fields, S. Global Nitrogen Cycle: Cycling out of Control. Environ. Health Perspect. 2004, 112, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Laurenzi, I.J.; Bergerson, J.A.; Motazedi, K. Life Cycle Greenhouse Gas Emissions and Freshwater Consumption Associated with Bakken Tight Oil. Proc. Natl. Acad. Sci. USA 2016, 1, E7672–E7680. [Google Scholar] [CrossRef]
- Elgowainy, A.; Han, J.; Cai, H.; Wang, M.; Forman, G.S.; Divita, V.B. Energy Efficiency and Greenhouse Gas Emission Intensity of Petroleum Products at U.S. Refineries. Environ. Sci. Technol. 2014, 48, 7612–7624. [Google Scholar] [CrossRef] [PubMed]
- Cooney, G.; Jamieson, M.; Marriott, J.; Bergerson, J.; Brandt, A.; Skone, T.J. Updating the U.S. Life Cycle GHG Petroleum Baseline to 2014 with Projections to 2040 Using Open-Source Engineering-Based Models. Environ. Sci. Technol. 2017, 51, 977–987. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).