Catalytic Conversion of Palm Oil to Bio-Hydrogenated Diesel over Novel N-Doped Activated Carbon Supported Pt Nanoparticles
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterisation
2.3. Catalytic Deoxygenation Testing
2.4. Product Analysis
3. Results & Discussion
3.1. Deoxygenation of Palm Oil
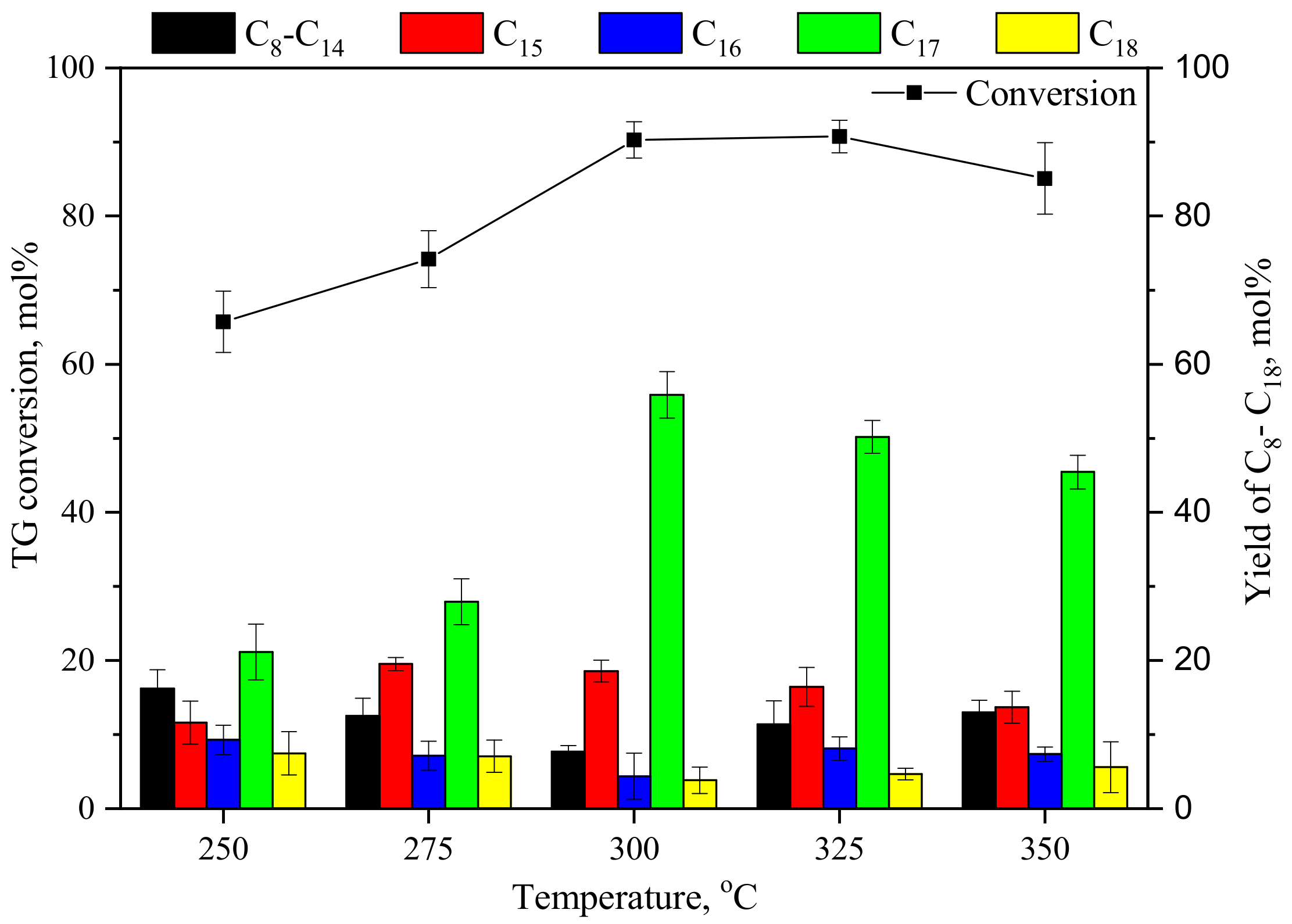
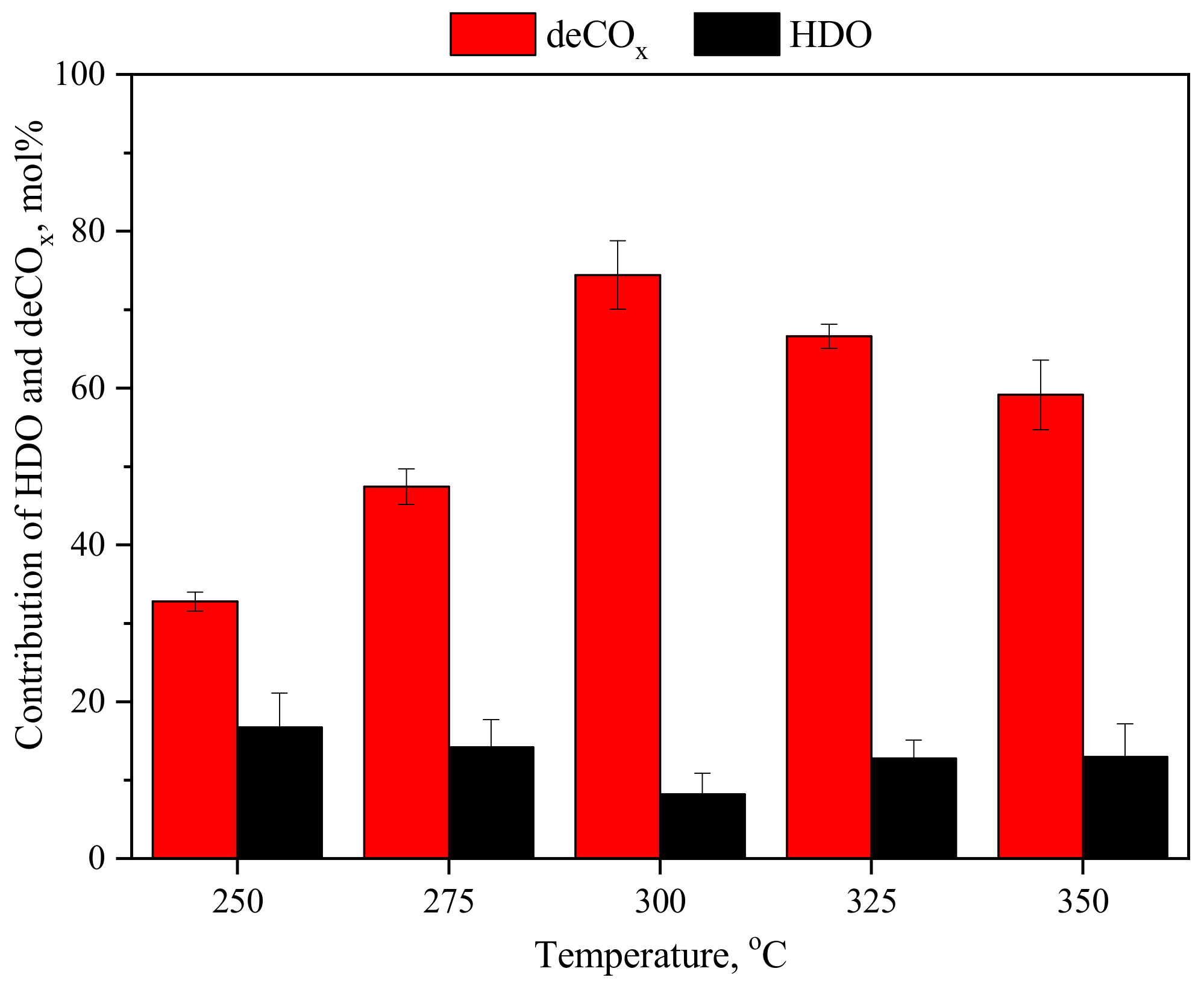
3.1.1. Effect of Temperature
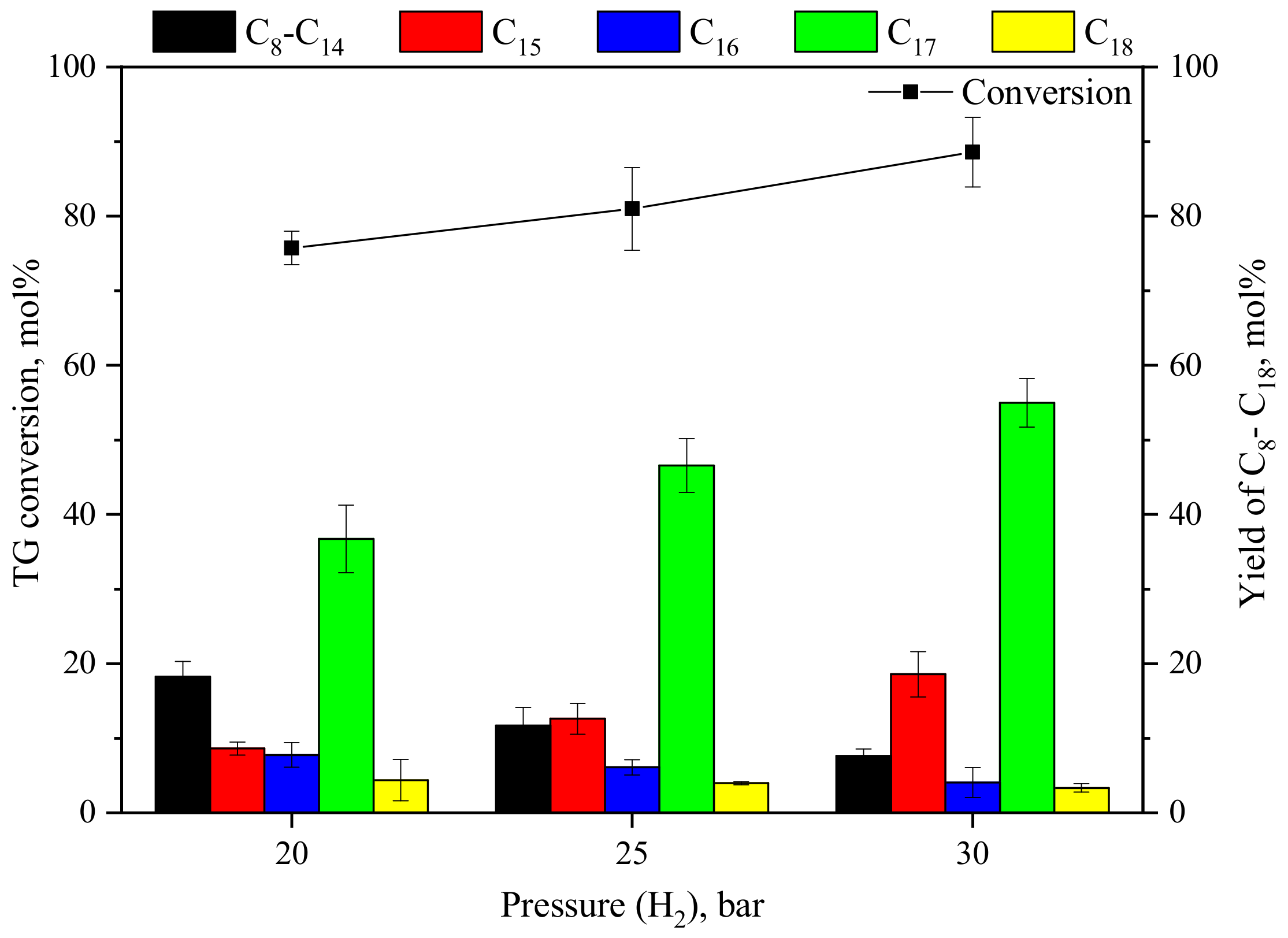
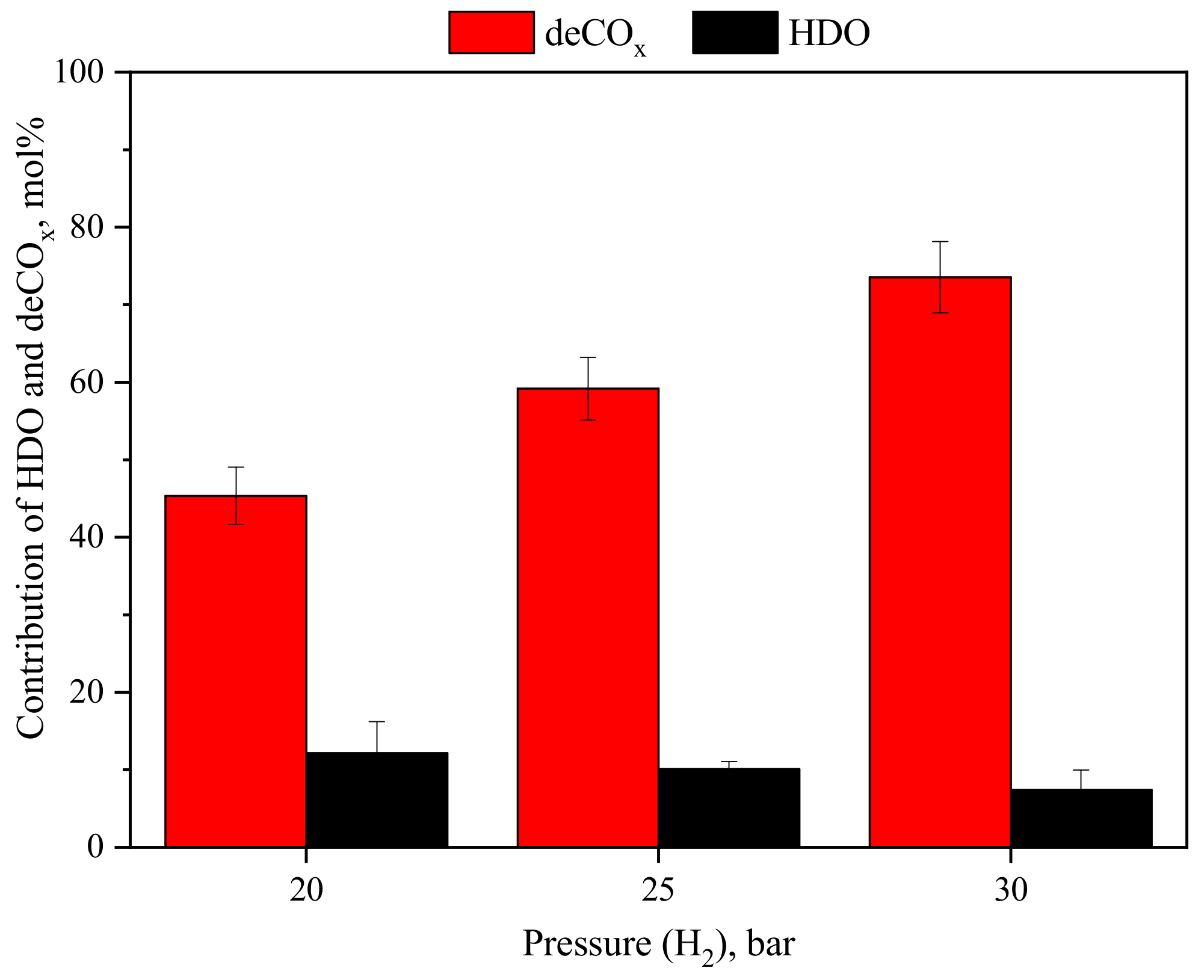
3.1.2. Effect of H2 Pressure
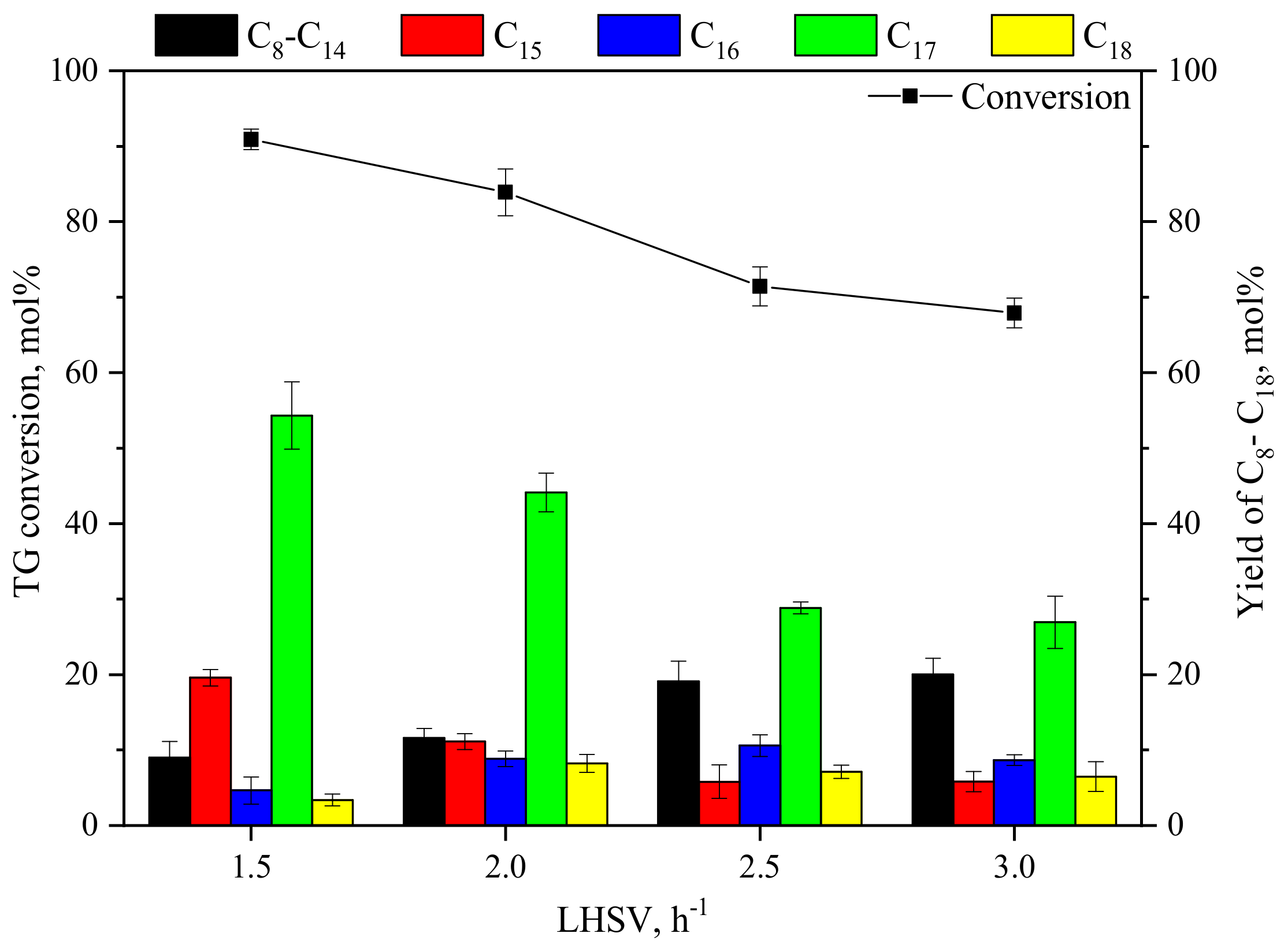
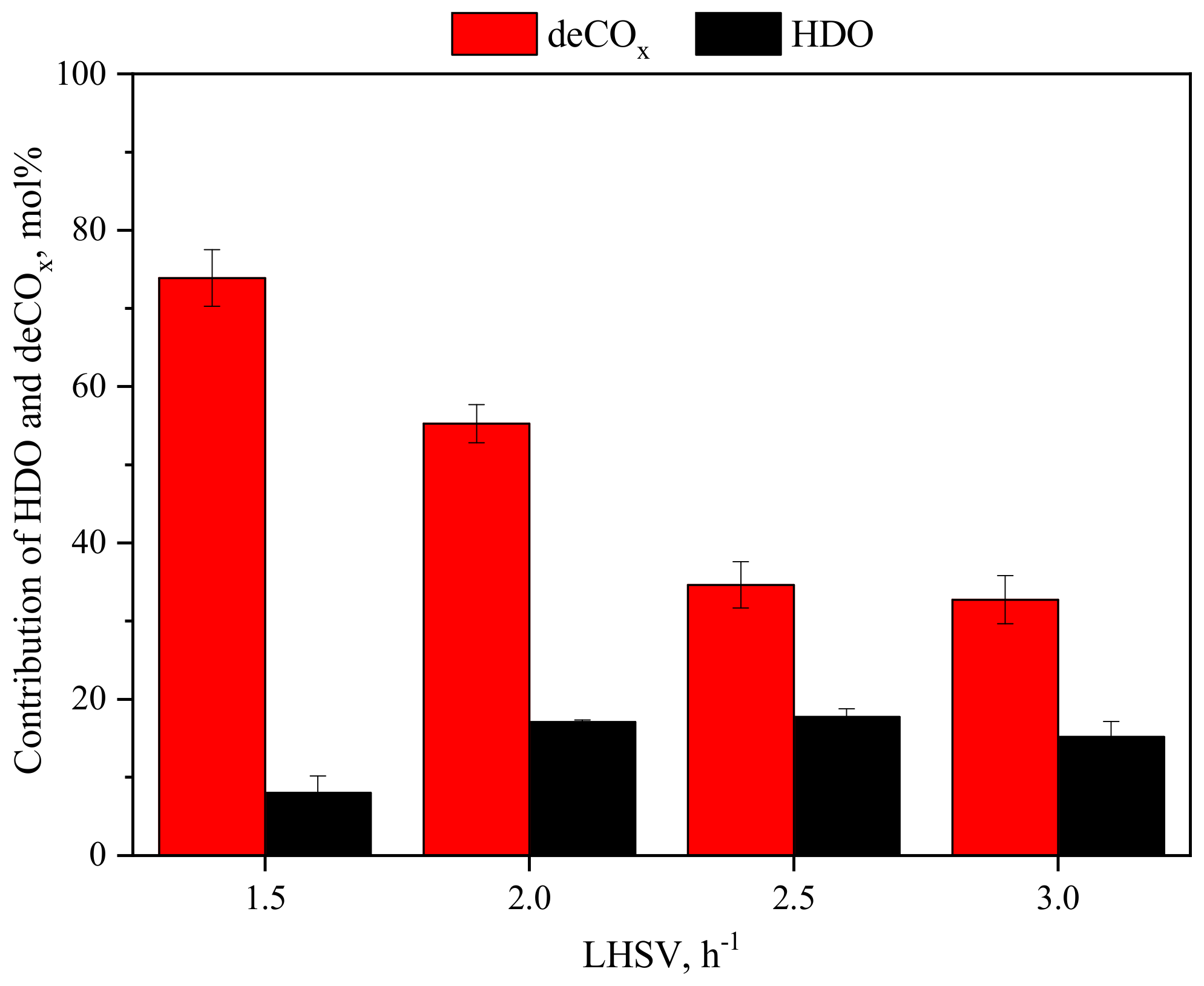
3.1.3. Effect of LHSV
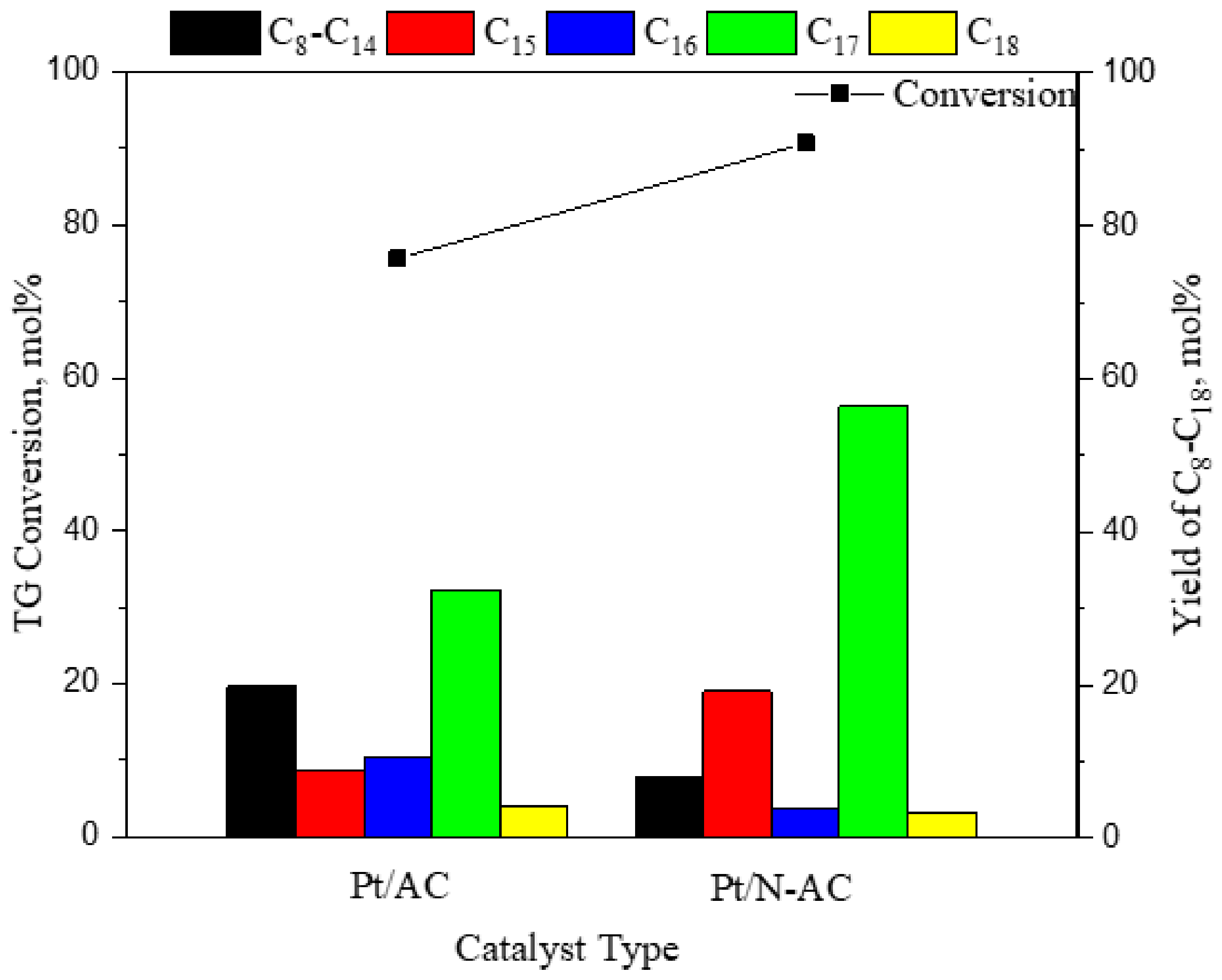
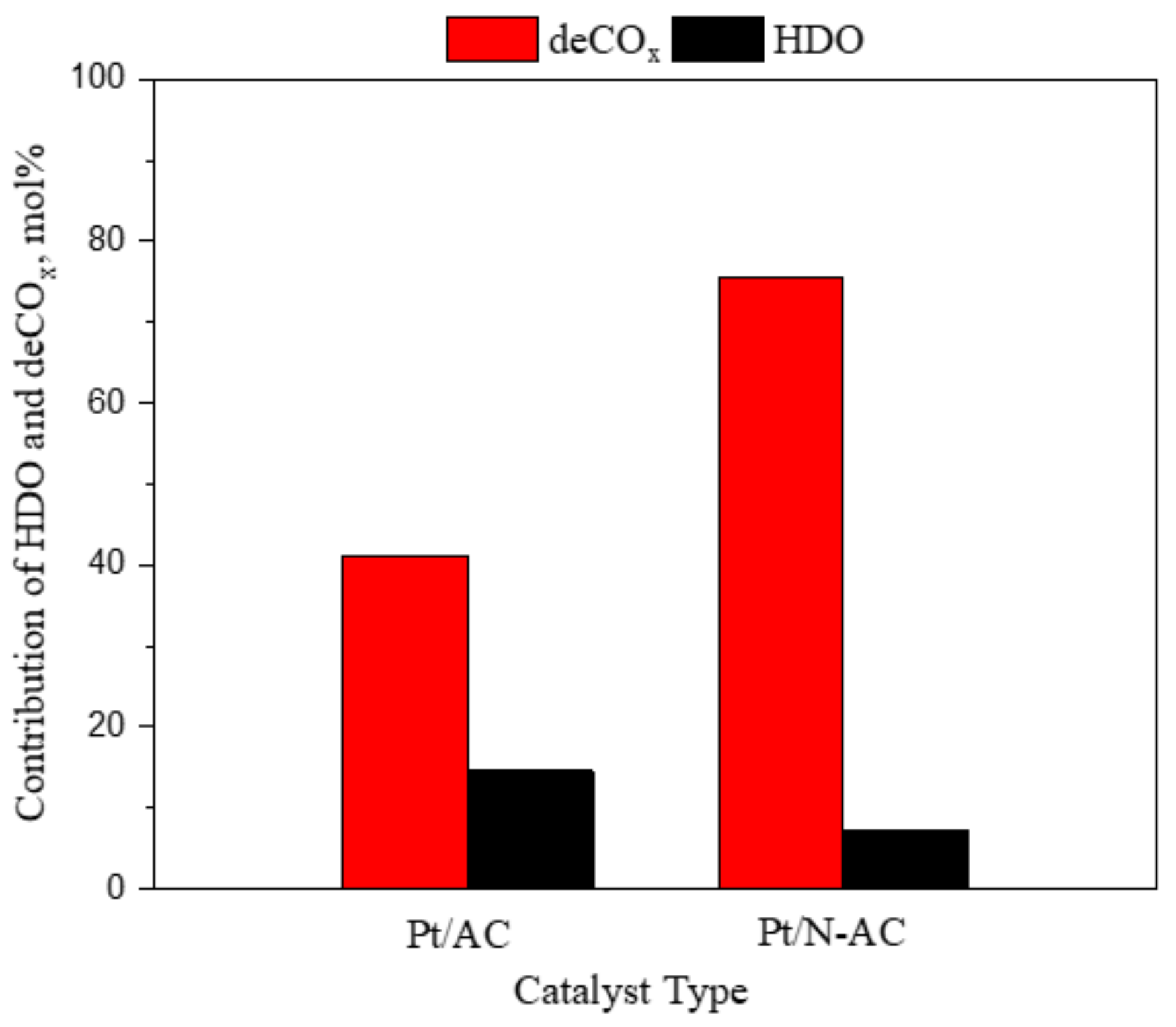
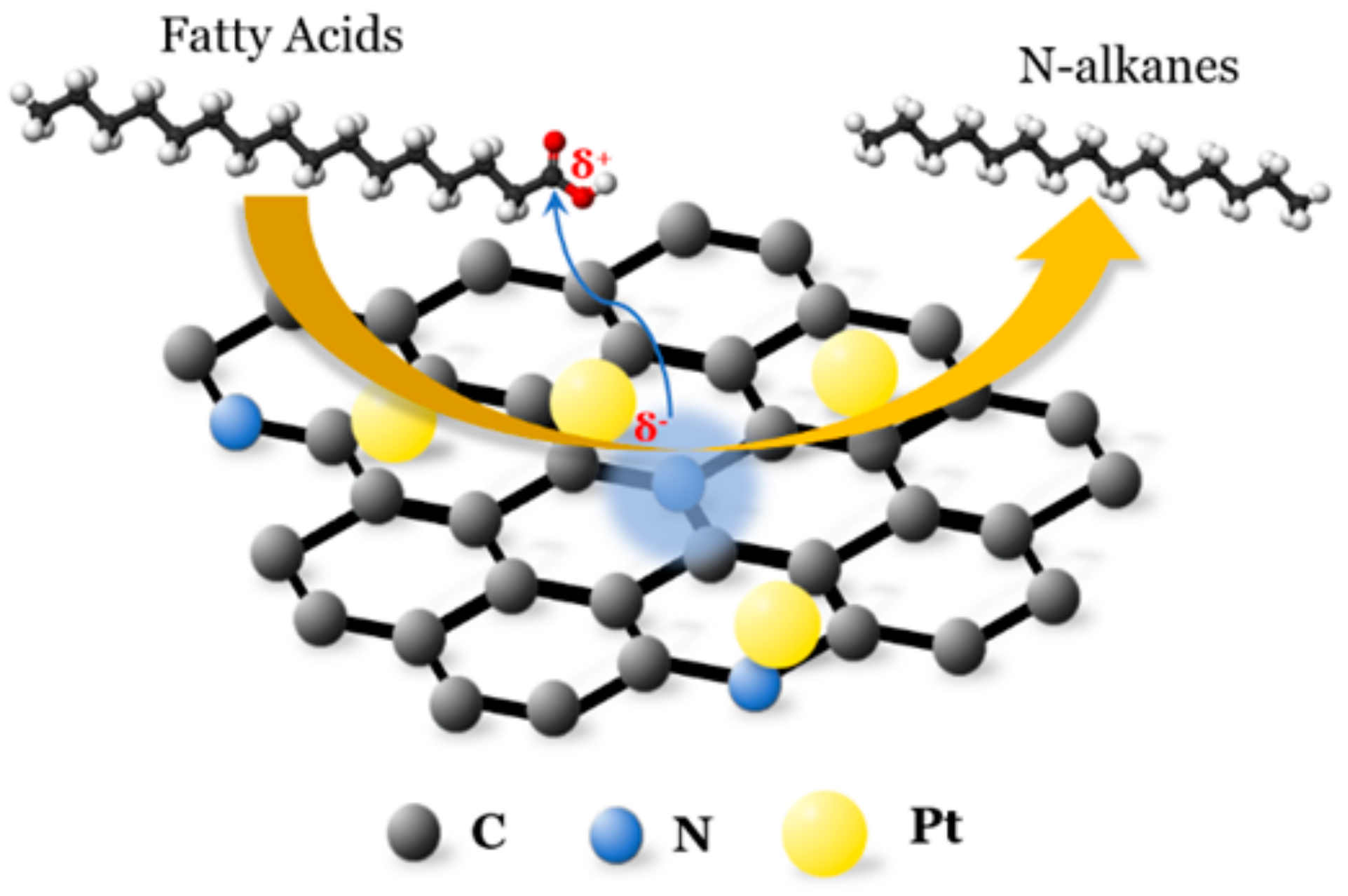
3.1.4. Effect of Catalysts
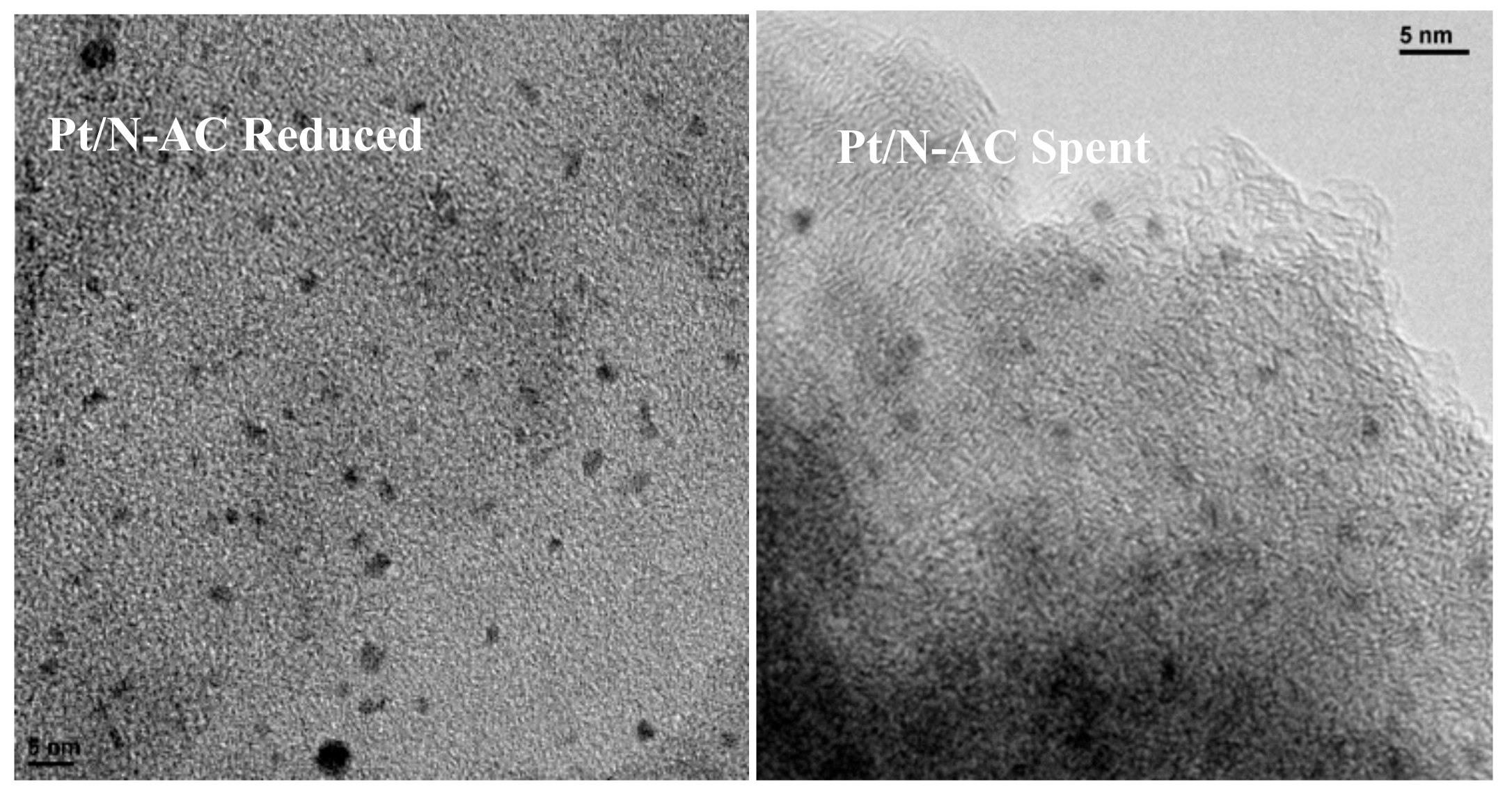
3.2. Characterisation of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WBA Global Bioenergy Statistics 2018. Available online: https://worldbioenergy.org/ (accessed on 7 September 2019).
- Fu, J.; Lu, X.; Savage, P.E. Hydrothermal decarboxylation and hydrogenation of fatty acids over Pt/C. ChemSusChem 2011, 4, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Srifa, A.; Faungnawakij, K.; Itthibenchapong, V.; Assabumrungrat, S. Roles of monometallic catalysts in hydrodeoxygenation of palm oil to green diesel. Chem. Eng. J. 2015, 278, 249–258. [Google Scholar] [CrossRef]
- Ramya, G.; Sudhakar, R.; Joice, J.A.I.; Ramakrishnan, R.; Sivakumar, T. Liquid hydrocarbon fuels from jatropha oil through catalytic cracking technology using AlMCM-41/ZSM-5 composite catalysts. Appl. Catal. A Gen. 2012, 433, 170–178. [Google Scholar] [CrossRef]
- Kubička, D.; Horáček, J.; Setnička, M.; Bulanek, R.; Zukal, A.; Kubičková, I. Effect of support-active phase interactions on the catalyst activity and selectivity in deoxygenation of triglycerides. Appl. Catal. B Environ. 2014, 145, 101–107. [Google Scholar] [CrossRef]
- Murzin, D.Y.; Kubickova, I.; Snåre, M.; Mäki-Arvela, P.; Myllyoja, J. Method for the Manufacture of Hydrocarbons; Patent and Trademark Office: Washington, DC, USA, 2009.
- Ochoa-Hernández, C.; Yang, Y.; Pizarro, P.; Víctor, A.; Coronado, J.M.; Serrano, D.P. Hydrocarbons production through hydrotreating of methyl esters over Ni and Co supported on SBA-15 and Al-SBA-15. Catal. Today 2013, 210, 81–88. [Google Scholar] [CrossRef]
- Srifa, A.; Viriya-empikul, N.; Assabumrungrat, S.; Faungnawakij, K. Catalytic behaviors of Ni/γ-Al2O3 and Co/γ-Al2O3 during the hydrodeoxygenation of palm oil. Catal. Sci. Technol. 2015, 5, 3693–3705. [Google Scholar] [CrossRef]
- Hollak, S. Catalytic Deoxygenation of Fatty Acids and Triglycerides for Production of Fuels and Chemicals. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2014. [Google Scholar]
- Peng, B.; Zhao, C.; Kasakov, S.; Foraita, S.; Lercher, J.A. Manipulating catalytic pathways: Deoxygenation of palmitic acid on multifunctional catalysts. Chem. Eur. J. 2013, 19, 4732–4741. [Google Scholar] [CrossRef]
- Santillan-Jimenez, E.; Crocker, M. Catalytic deoxygenation of fatty acids and their derivatives to hydrocarbon fuels via decarboxylation/decarbonylation. J. Chem. Technol. Biotechnol. 2012, 87, 1041–1050. [Google Scholar] [CrossRef]
- Immer, J.G.; Lamb, H.H. Fed-batch catalytic deoxygenation of free fatty acids. Energy Fuels 2010, 24, 5291–5299. [Google Scholar] [CrossRef]
- Bertram, S. Heptadecaan: De wering van selenium op stearinzuur. Chem. Weekbl. 1936, 1, 457–459. [Google Scholar]
- Snåre, M.; Kubickova, I.; Mäki-Arvela, P.; Eränen, K.; Murzin, D.Y. Heterogeneous catalytic deoxygenation of stearic acid for production of biodiesel. Ind. Eng. Chem. Res. 2006, 45, 5708–5715. [Google Scholar] [CrossRef]
- Kiatkittipong, W.; Phimsen, S.; Kiatkittipong, K.; Wongsakulphasatch, S.; Laosiripojana, N.; Assabumrungrat, S. Diesel-like hydrocarbon production from hydroprocessing of relevant refining palm oil. Fuel Process. Technol. 2013, 116, 16–26. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Zhang, J.; Wang, Q. Tuning the decarboxylation selectivity for deoxygenation of vegetable oil over Pt-Ni bimetal catalysts via surface engineering. Catal. Sci. Technol. 2018, 8, 1126–1133. [Google Scholar] [CrossRef]
- Yang, L.; Carreon, M.A. Deoxygenation of palmitic and lauric acids over Pt/ZIF-67 membrane/zeolite 5A bead catalysts. ACS Appl. Mater. Interfaces 2017, 9, 31993–32000. [Google Scholar] [CrossRef] [PubMed]
- Na, J.-G.; Yi, B.E.; Han, J.K.; Oh, Y.-K.; Park, J.-H.; Jung, T.S.; Han, S.S.; Yoon, H.C.; Kim, J.-N.; Lee, H. Deoxygenation of microalgal oil into hydrocarbon with precious metal catalysts: Optimization of reaction conditions and supports. Energy 2012, 47, 25–30. [Google Scholar] [CrossRef]
- Alharbi, K.; Kozhevnikova, E.; Kozhevnikov, I. Hydrogenation of ketones over bifunctional Pt-heteropoly acid catalyst in the gas phase. Appl. Catal. A Gen. 2015, 504, 457–462. [Google Scholar] [CrossRef]
- Hengsawad, T.; Jindarat, T.; Resasco, D.E.; Jongpatiwut, S. Synergistic effect of oxygen vacancies and highly dispersed Pd nanoparticles over Pd-loaded TiO2 prepared by a single-step sol–gel process for deoxygenation of triglycerides. Appl. Catal. A Gen. 2018, 566, 74–86. [Google Scholar] [CrossRef]
- Grosso-Giordano, N.A.; Eaton, T.R.; Bo, Z.; Yacob, S.; Yang, C.-C.; Notestein, J.M. Silica support modifications to enhance Pd-catalyzed deoxygenation of stearic acid. Appl. Catal. B Environ. 2016, 192, 93–100. [Google Scholar] [CrossRef]
- Morgan, T.; Grubb, D.; Santillan-Jimenez, E.; Crocker, M. Conversion of triglycerides to hydrocarbons over supported metal catalysts. Top. Catal. 2010, 53, 820–829. [Google Scholar] [CrossRef]
- Liu, X.; Xu, L.; Xu, G.; Jia, W.; Ma, Y.; Zhang, Y. Selective hydrodeoxygenation of lignin-derived phenols to cyclohexanols or cyclohexanes over magnetic CoNx@ NC catalysts under mild conditions. ACS Catal. 2016, 6, 7611–7620. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, P.; Liao, C.; Zhang, Z.; Jin, S. Cobalt Nanoparticles Supported on Nitrogen-Doped Carbon: An Effective Non-Noble Metal Catalyst for the Upgrade of Biofuels. ChemSusChem 2018, 11, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Cao, Z.; Gu, D.; Pfänder, N.; Swertz, A.C.; Spliethoff, B.; Bongard, H.J.; Weidenthaler, C.; Schmidt, W.; Rinaldi, R. Nitrogen-Doped Ordered Mesoporous Carbon Supported Bimetallic PtCo Nanoparticles for Upgrading of Biophenolics. Angew. Chem. Int. Ed. 2016, 55, 8850–8855. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Wang, M.; Cai, W.; Cui, Y.; Gao, F.; Peng, L.; Chen, W.; Ding, W. Acid-resistant catalysis without use of noble metals: Carbon nitride with underlying nickel. ACS Catal. 2014, 4, 2536–2543. [Google Scholar] [CrossRef]
- Nie, R.; Yang, H.; Zhang, H.; Yu, X.; Lu, X.; Zhou, D.; Xia, Q. Mild-temperature hydrodeoxygenation of vanillin over porous nitrogen-doped carbon black supported nickel nanoparticles. Green Chem. 2017, 19, 3126–3134. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Wang, S.; Xu, G.; Wang, H.; Vlachos, D.G. Chemoselective Hydrodeoxygenation of Carboxylic Acids to Hydrocarbons over Nitrogen-Doped Carbon–Alumina Hybrid Supported Iron Catalysts. ACS Catal. 2019, 9, 1564–1577. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Rozmysłowicz, B.; Lestari, S.; Simakova, O.; Eränen, K.; Salmi, T.; Murzin, D.Y. Catalytic deoxygenation of tall oil fatty acid over palladium supported on mesoporous carbon. Energy Fuels 2011, 25, 2815–2825. [Google Scholar] [CrossRef]
- Lestari, S.; Mäki-Arvela, P.; Beltramini, J.; Lu, G.M.; Murzin, D.Y. Transforming triglycerides and fatty acids into biofuels. ChemSusChem 2009, 2, 1109–1119. [Google Scholar] [CrossRef]
- Hermida, L.; Abdullah, A.Z.; Mohamed, A.R. Deoxygenation of fatty acid to produce diesel-like hydrocarbons: A review of process conditions, reaction kinetics and mechanism. Renew. Sustain. Energy Rev. 2015, 42, 1223–1233. [Google Scholar] [CrossRef]
- Hellinger, M.; Carvalho, H.W.P.D.; Baier, S.; Gharnati, L.; Grunwaldt, J.D. Solvent Influence on the Hydrodeoxygenation of Guaiacol over Pt/SiO2 and Pt/H-MFI 90 Catalysts. Chem. Ing. Tech. 2015, 87, 1771–1780. [Google Scholar] [CrossRef]
- Pattanaik, B.P.; Misra, R.D. Effect of reaction pathway and operating parameters on the deoxygenation of vegetable oils to produce diesel range hydrocarbon fuels: A review. Renew. Sustain. Energy Rev. 2017, 73, 545–557. [Google Scholar] [CrossRef]
- Chen, N.; Gong, S.; Shirai, H.; Watanabe, T.; Qian, E.W. Effects of Si/Al ratio and Pt loading on Pt/SAPO-11 catalysts in hydroconversion of Jatropha oil. Appl. Catal. A Gen. 2013, 466, 105–115. [Google Scholar] [CrossRef]
- Kubičková, I.; Snåre, M.; Eränen, K.; Mäki-Arvela, P.; Murzin, D.Y. Hydrocarbons for diesel fuel via decarboxylation of vegetable oils. Catal. Today 2005, 106, 197–200. [Google Scholar] [CrossRef]
- Kaewmeesri, R.; Srifa, A.; Itthibenchapong, V.; Faungnawakij, K. Deoxygenation of waste chicken fats to green diesel over Ni/Al2O3: Effect of water and free fatty acid content. Energy Fuels 2015, 29, 833–840. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Gong, Y.; Zhang, P.; Li, H.; Wang, Y. Synthesis of palladium nanoparticles supported on mesoporous N-doped carbon and their catalytic ability for biofuel upgrade. J. Am. Chem. Soc. 2012, 134, 16987–16990. [Google Scholar] [CrossRef] [PubMed]
- Itthibenchapong, V.; Srifa, A.; Kaewmeesri, R.; Kidkhunthod, P.; Faungnawakij, K. Deoxygenation of palm kernel oil to jet fuel-like hydrocarbons using Ni-MoS2/γ-Al2O3 catalysts. Energy Convers. Manag. 2017, 134, 188–196. [Google Scholar] [CrossRef]
- Xia, W. Interactions between metal species and nitrogen-functionalized carbon nanotubes. Catal. Sci. Technol. 2016, 6, 630–644. [Google Scholar] [CrossRef]
| Fatty Acid | Structure * | Formula | Composition, wt.% |
|---|---|---|---|
| Myristic acid | C14:0 | C14H28O2 | 0.90 |
| Palmitic acid | C16:0 | C16H32O2 | 41.60 |
| Palmitoleic acid | C16:1 | C16H30O2 | 0.70 |
| Stearic acid | C18:0 | C18H36O2 | 2.10 |
| Oleic acid | C18:1 | C18H34O2 | 43.90 |
| Linoleic acid | C18:2 | C18H32O2 | 10.40 |
| Linolenic acid | C18:3 | C18H30O2 | 0.40 |
| Catalyst | Reactant | Reactor | T (°C) | P of H2 (bar) | LHSV (h−1) | H2/oil Ratio (cm3/cm3) | X (%) | HDO%, DeCOx% | Y (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1%Pt/AC | Refined palm olein | Fixed bed | 300 | 30 | 1.5 | 1000 | 76 | HDO% = 14.6%; DeCOx = 41.2% | 76 | This study |
| 1%Pt/N–C | Refined palm olein | Fixed bed | 300 | 30 | 1.5 | 1000 | 91 | HDO% = 7.1%; DeCOx = 75.5% | 91 | This study |
| 2%Pt/γ-Al2O3 | Refined palm olein 1 | Trickle-bed | 330 | 50 | 1 | 1000 | 95 | HDO ≈ 4%, DeCOx ≈ 27% | 31 | [3] |
| 5%Co/γ-Al2O3 | Refined palm olein | Trickle-bed | 330 | 50 | 1 | 1000 | 100 | HDO ≈ 52%, DeCOx ≈ 35% | 89 | [3] |
| 5%Pd/C | Degummed Palm oil (DPO) 2 | Batch | 400 | 40 | t = 1 h | - | ≈80 | - | 70 | [15] |
| 2.45%Ni9.4%Mo/γ-Al2O3 | DPO | Batch | 400 | 40 | t = 0.5 h | - | ≈80 | - | ≈50 | [15] |
| 4.95%Ni10.05%MoS2/γ-Al2O3 | Palm olein oil 3 | Trickle-bed | 330 | 50 | 1 | 1000 | 100 | - | ≈98 | [38] |
| 4.95%Ni10.05%MoS2/γ-Al2O3 | Refined kernel oil 4 | Trickle-bed | 330 | 50 | 1 | 1000 | 100 | HDO ≈ 60%, DeCOx ≈ 27% | ≈92 | [38] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Pastor-Pérez, L.; Villora-Pico, J.J.; Pastor-Blas, M.M.; Sepúlveda-Escribano, A.; Gu, S.; Charisiou, N.D.; Papageridis, K.; Goula, M.A.; Reina, T.R. Catalytic Conversion of Palm Oil to Bio-Hydrogenated Diesel over Novel N-Doped Activated Carbon Supported Pt Nanoparticles. Energies 2020, 13, 132. https://doi.org/10.3390/en13010132
Jin W, Pastor-Pérez L, Villora-Pico JJ, Pastor-Blas MM, Sepúlveda-Escribano A, Gu S, Charisiou ND, Papageridis K, Goula MA, Reina TR. Catalytic Conversion of Palm Oil to Bio-Hydrogenated Diesel over Novel N-Doped Activated Carbon Supported Pt Nanoparticles. Energies. 2020; 13(1):132. https://doi.org/10.3390/en13010132
Chicago/Turabian StyleJin, Wei, Laura Pastor-Pérez, Juan J. Villora-Pico, Mercedes M. Pastor-Blas, Antonio Sepúlveda-Escribano, Sai Gu, Nikolaos D. Charisiou, Kyriakos Papageridis, Maria A. Goula, and Tomas R. Reina. 2020. "Catalytic Conversion of Palm Oil to Bio-Hydrogenated Diesel over Novel N-Doped Activated Carbon Supported Pt Nanoparticles" Energies 13, no. 1: 132. https://doi.org/10.3390/en13010132
APA StyleJin, W., Pastor-Pérez, L., Villora-Pico, J. J., Pastor-Blas, M. M., Sepúlveda-Escribano, A., Gu, S., Charisiou, N. D., Papageridis, K., Goula, M. A., & Reina, T. R. (2020). Catalytic Conversion of Palm Oil to Bio-Hydrogenated Diesel over Novel N-Doped Activated Carbon Supported Pt Nanoparticles. Energies, 13(1), 132. https://doi.org/10.3390/en13010132










