Thermodynamic Analysis of Kalina Based Power and Cooling Cogeneration Cycle Employed Once Through Configuration
Abstract
1. Introduction
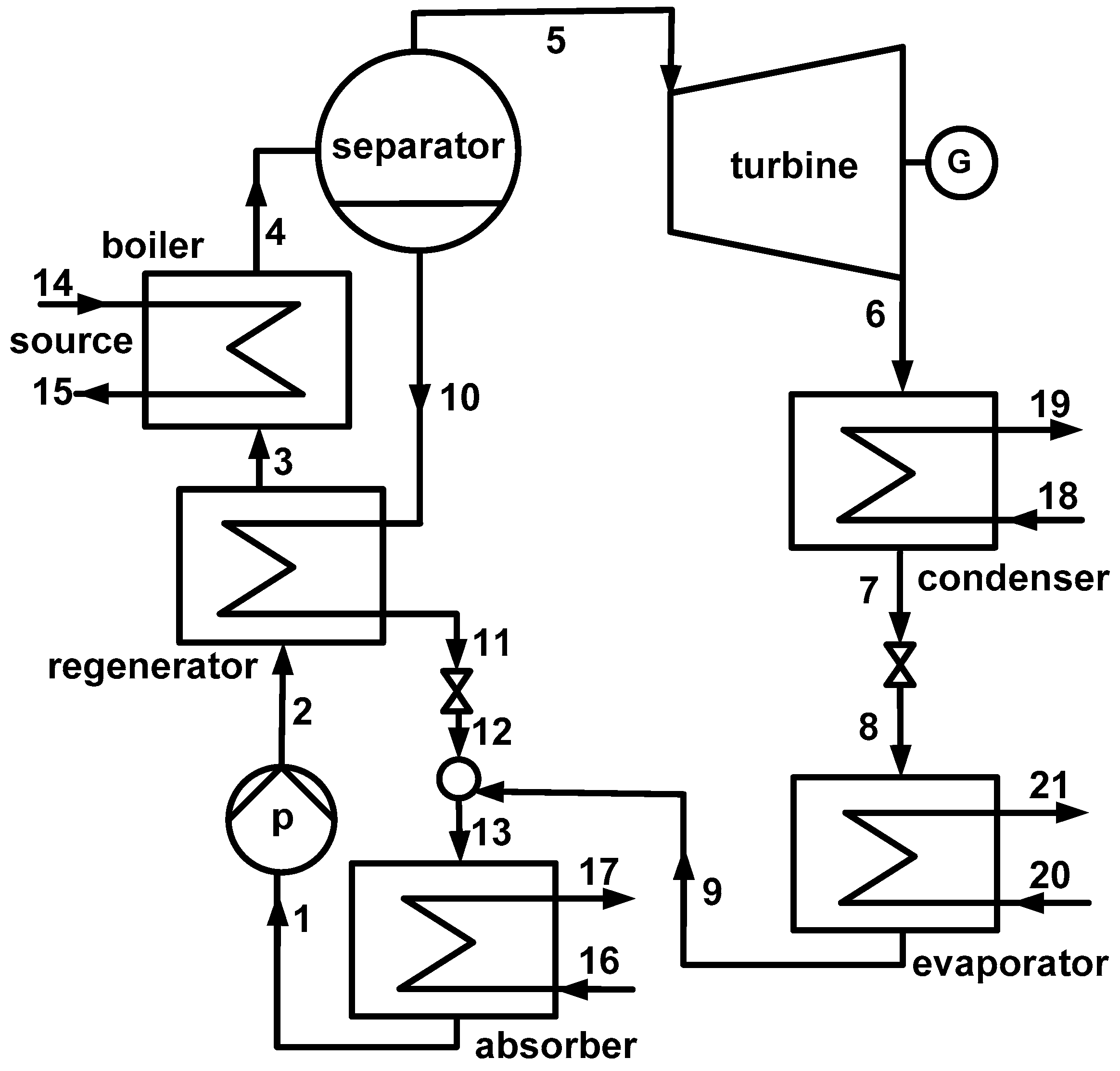
2. System Description
3. System Modeling
- (1)
- The system is steady.
- (2)
- Any pressure losses are negligible.
- (3)
- The isentropic efficiencies of the pump and turbines are constant.
- (4)
- The fluid exits the condenser and the absorber in a saturated liquid state.
- (5)
- The pinch temperature differences in the heat exchangers are set at the prescribed values.
4. Results and Discussion
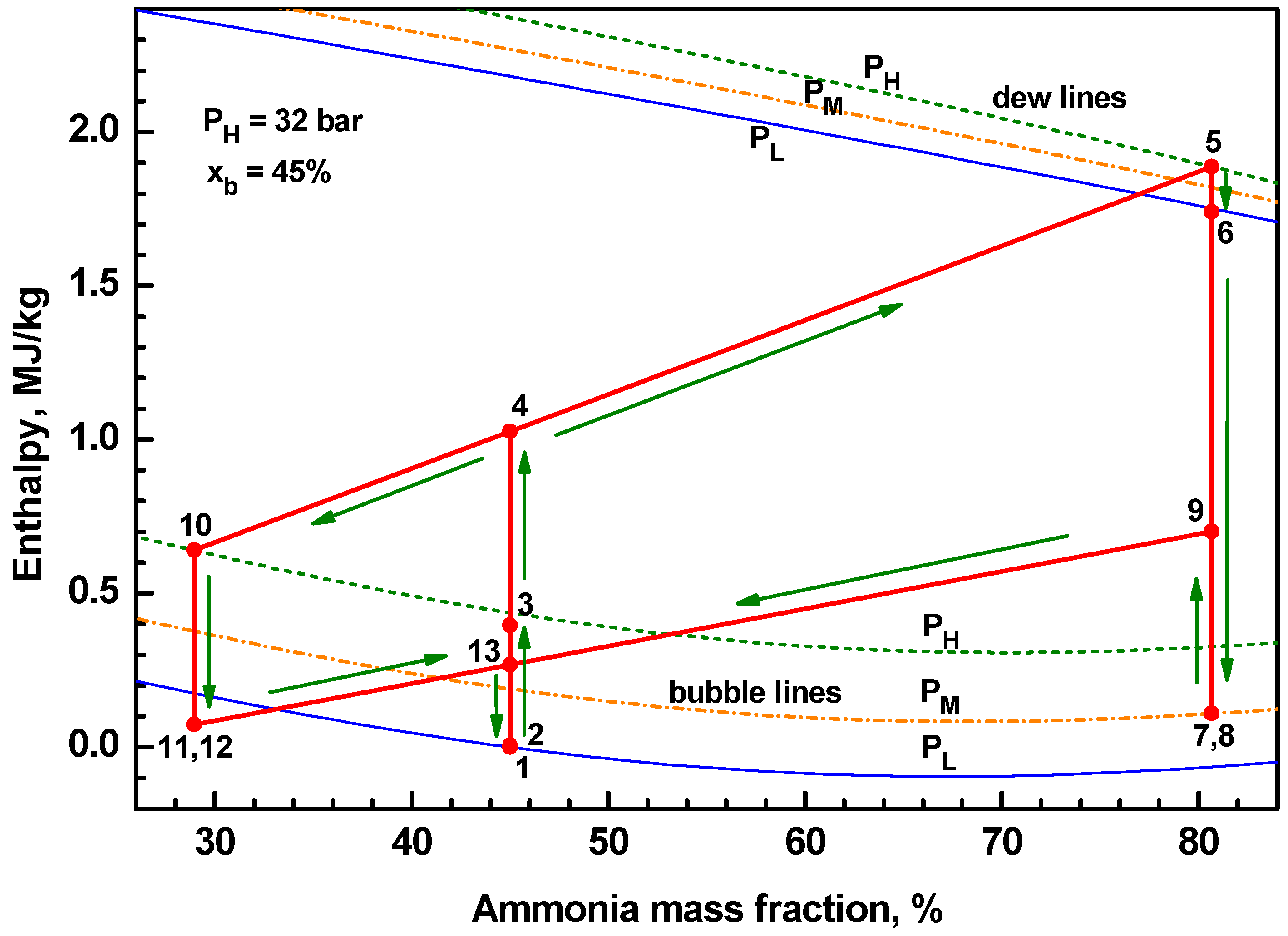
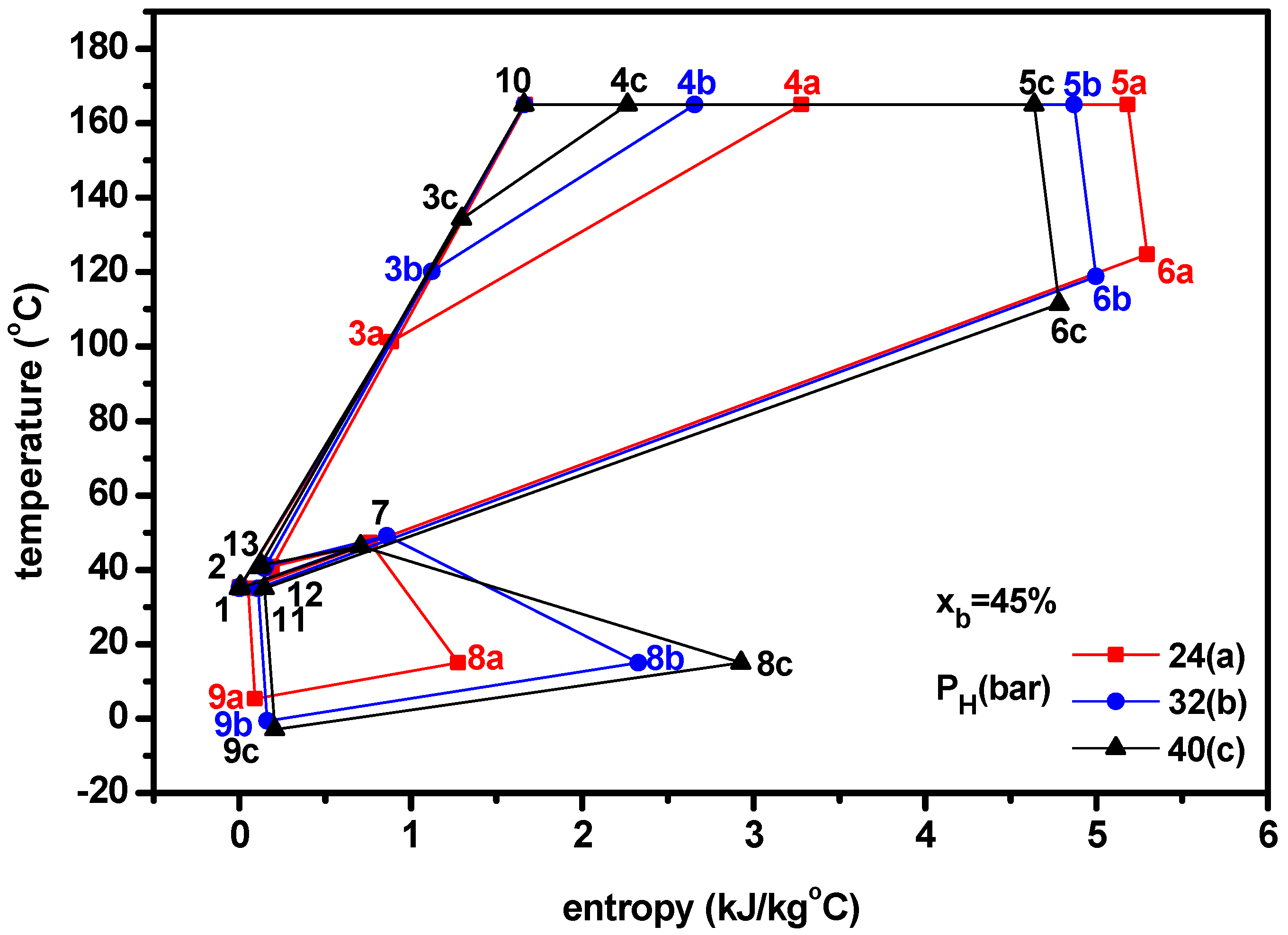
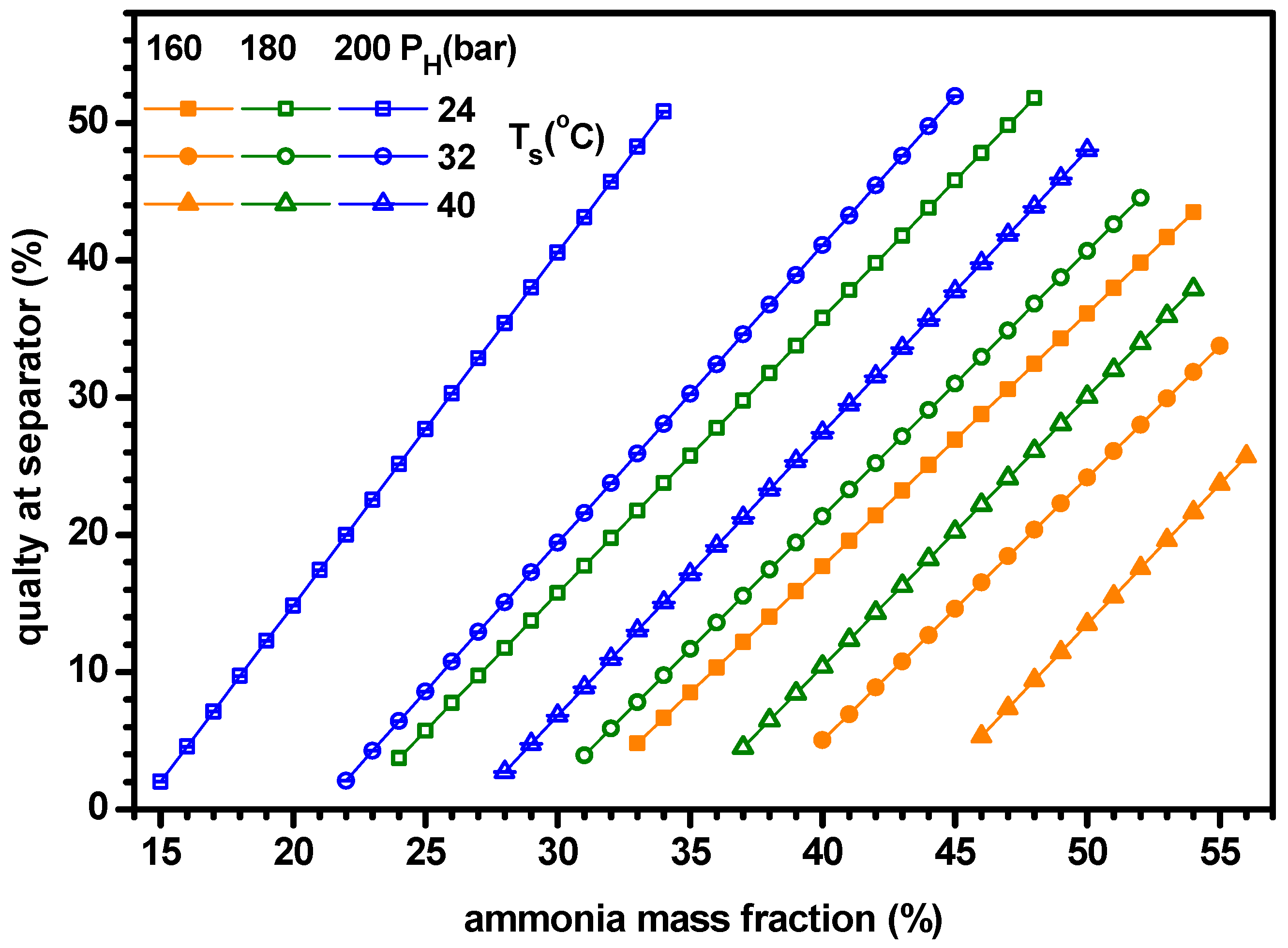
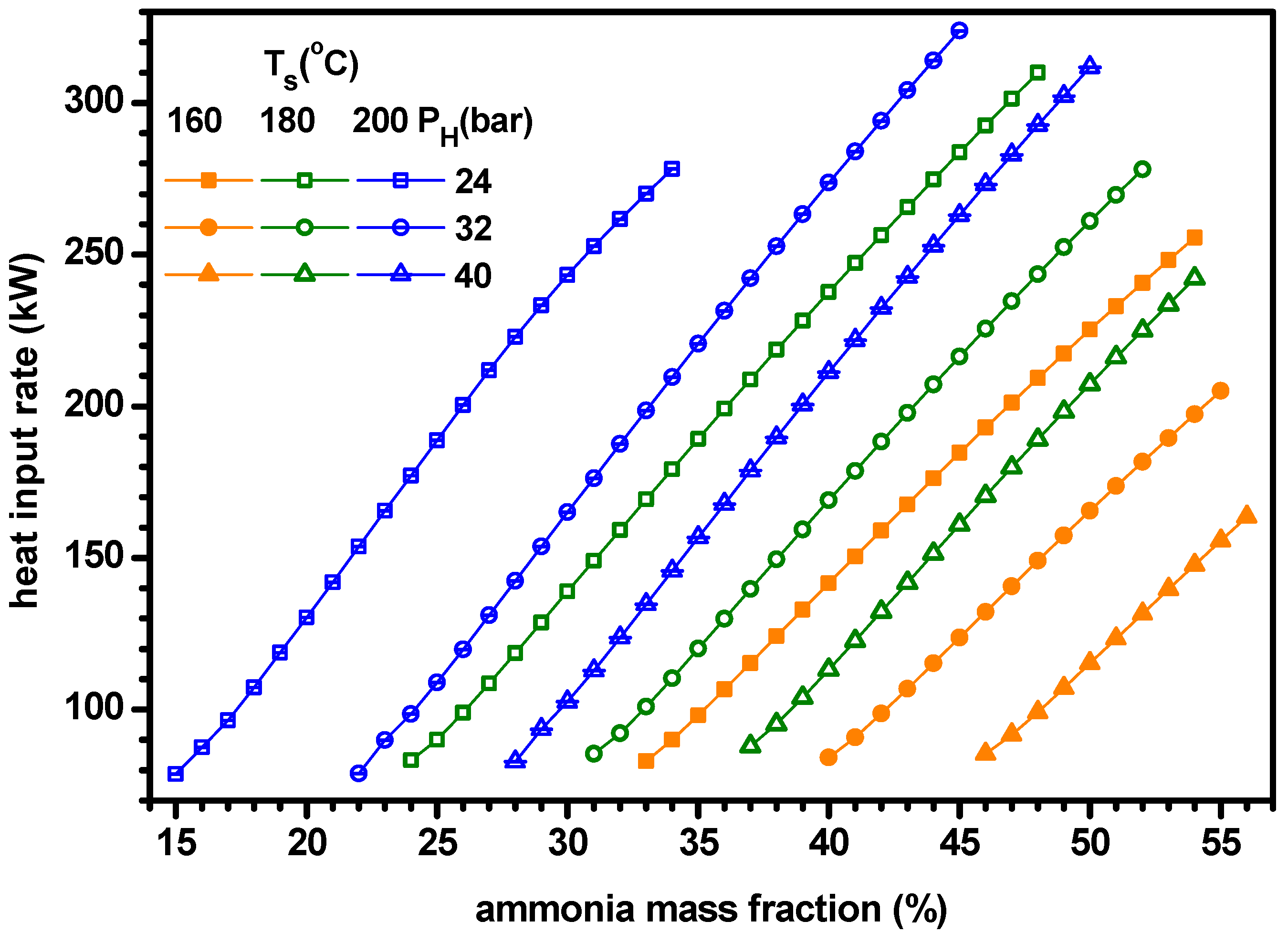
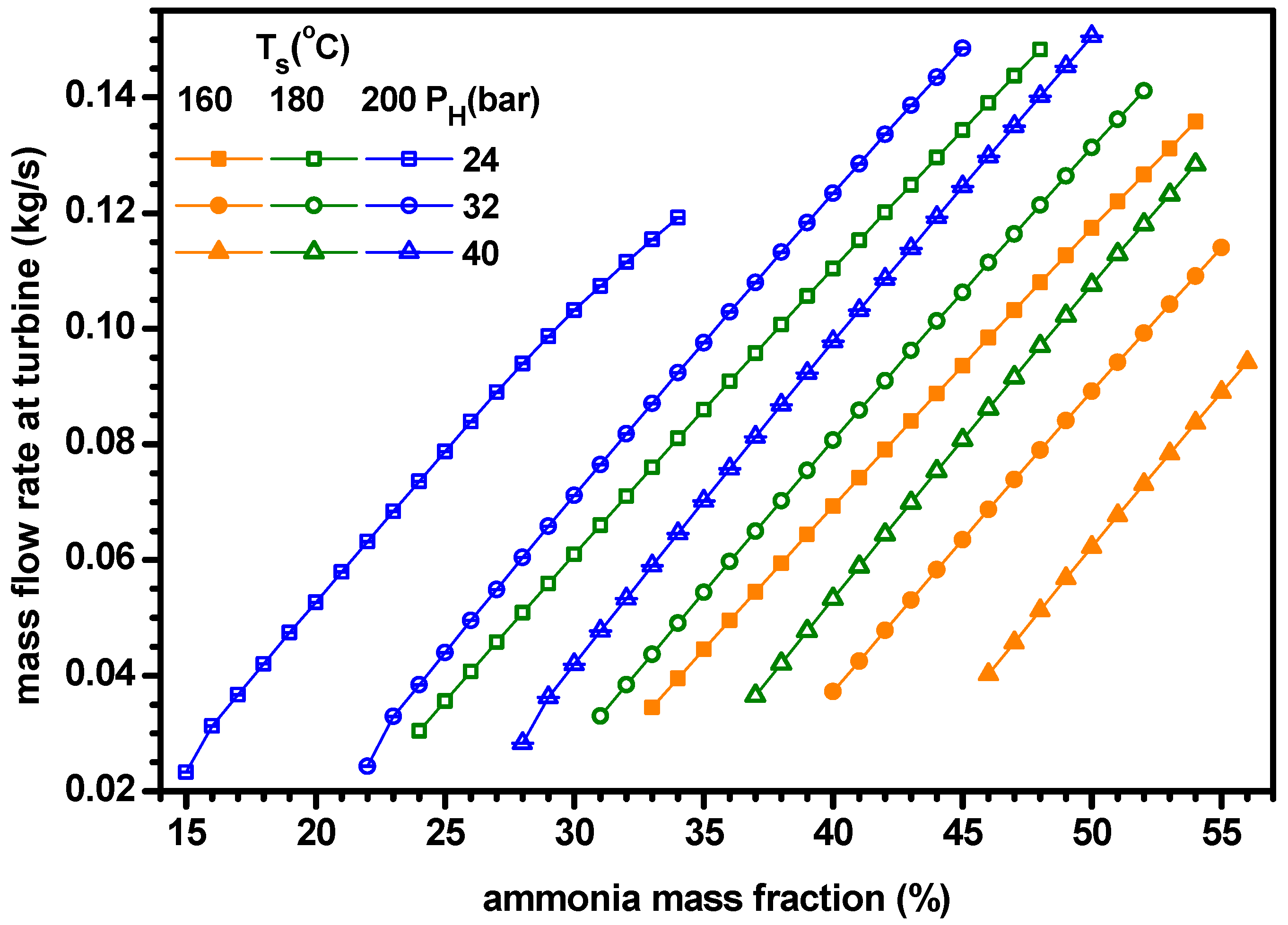
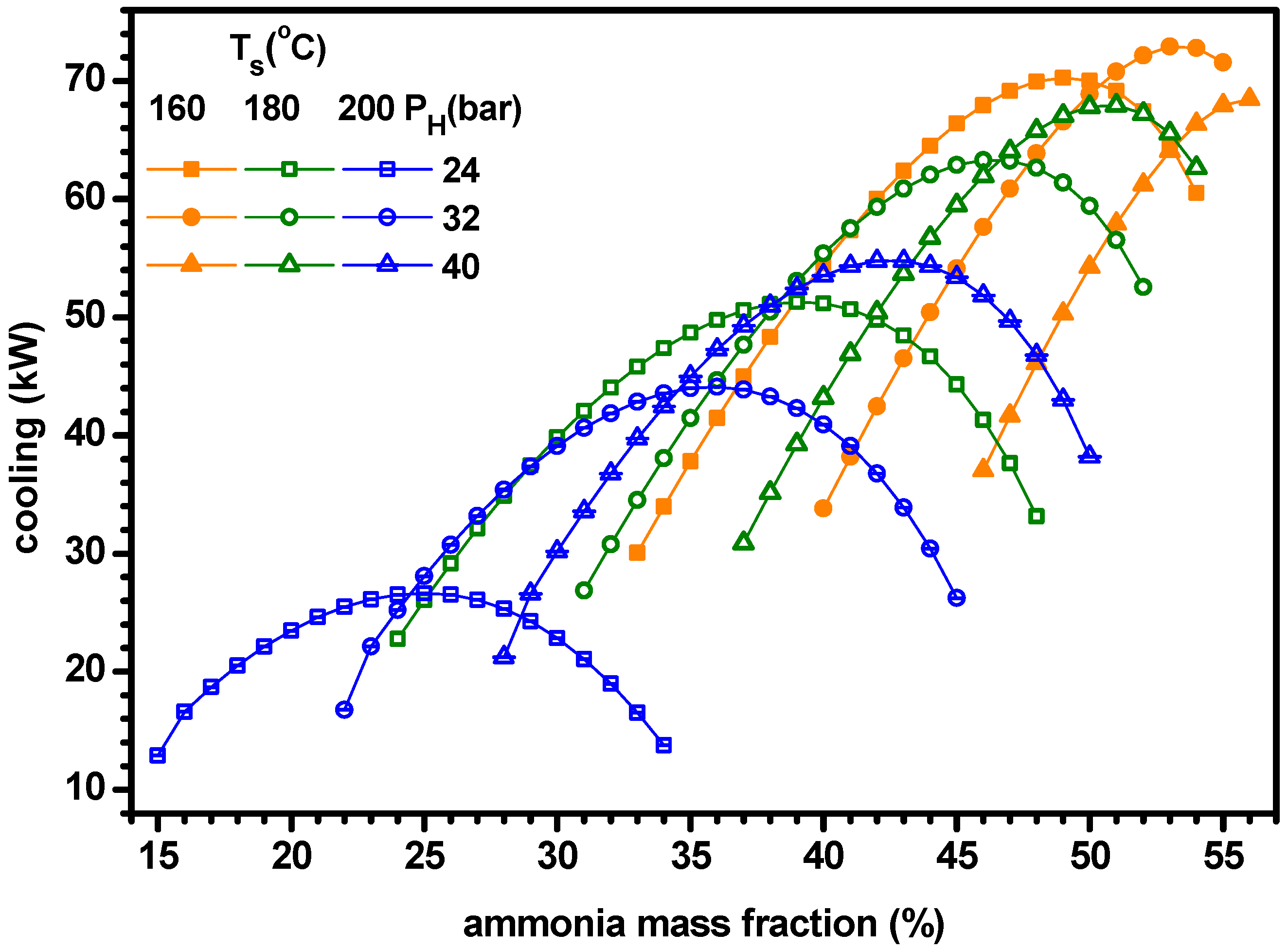
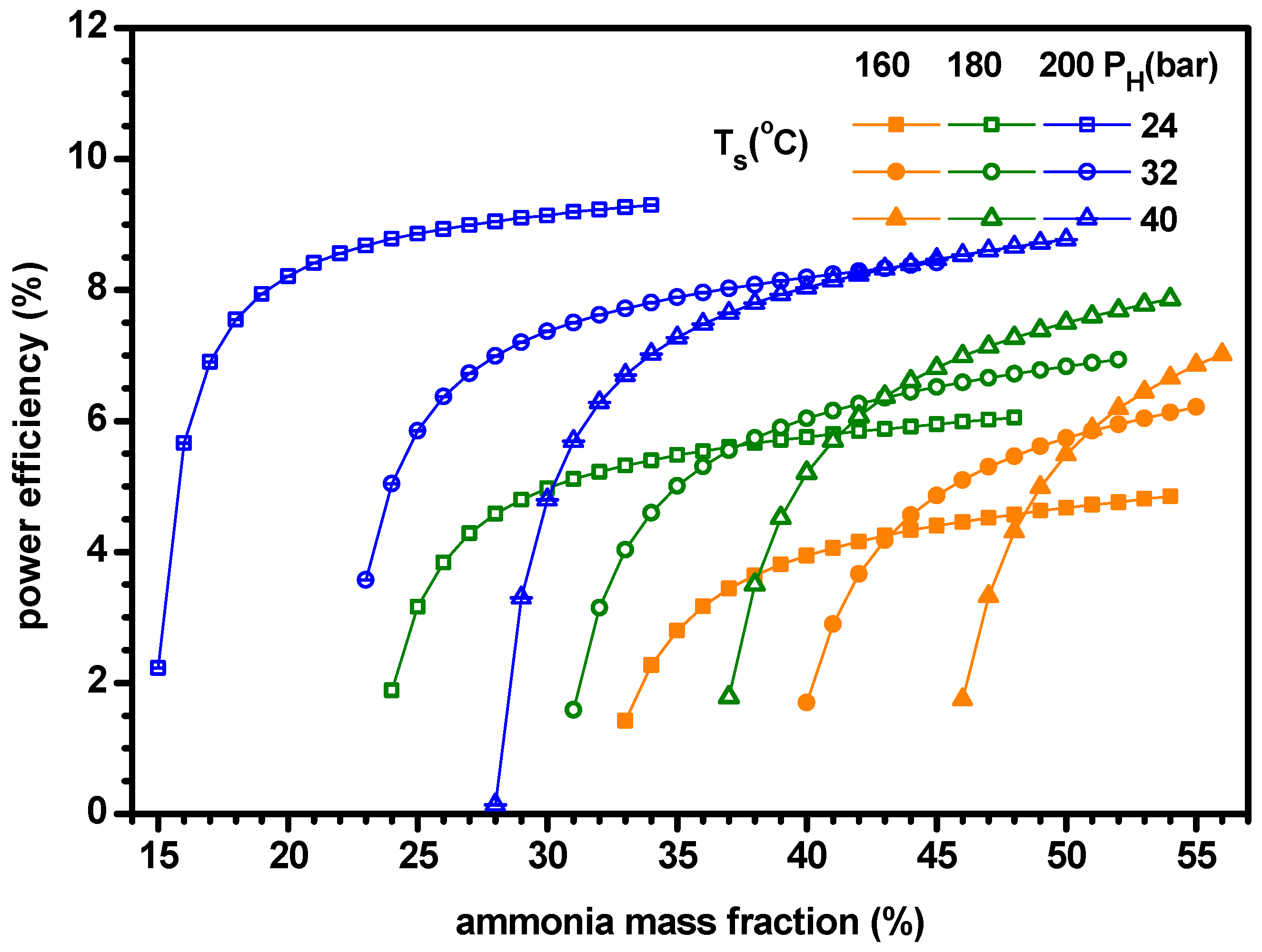
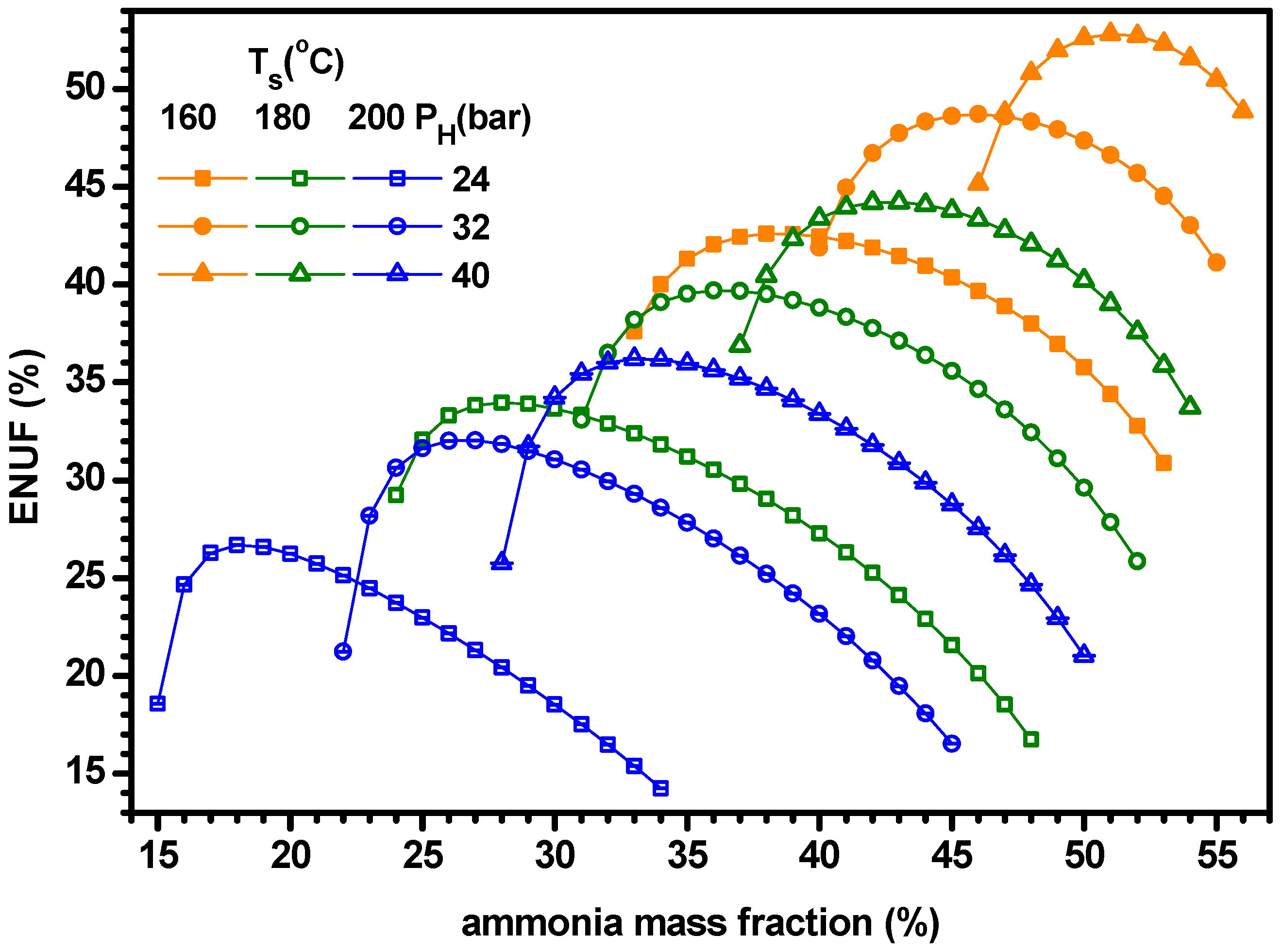
4.1. Thermodynamic Performance with Varying Ammonia Fraction
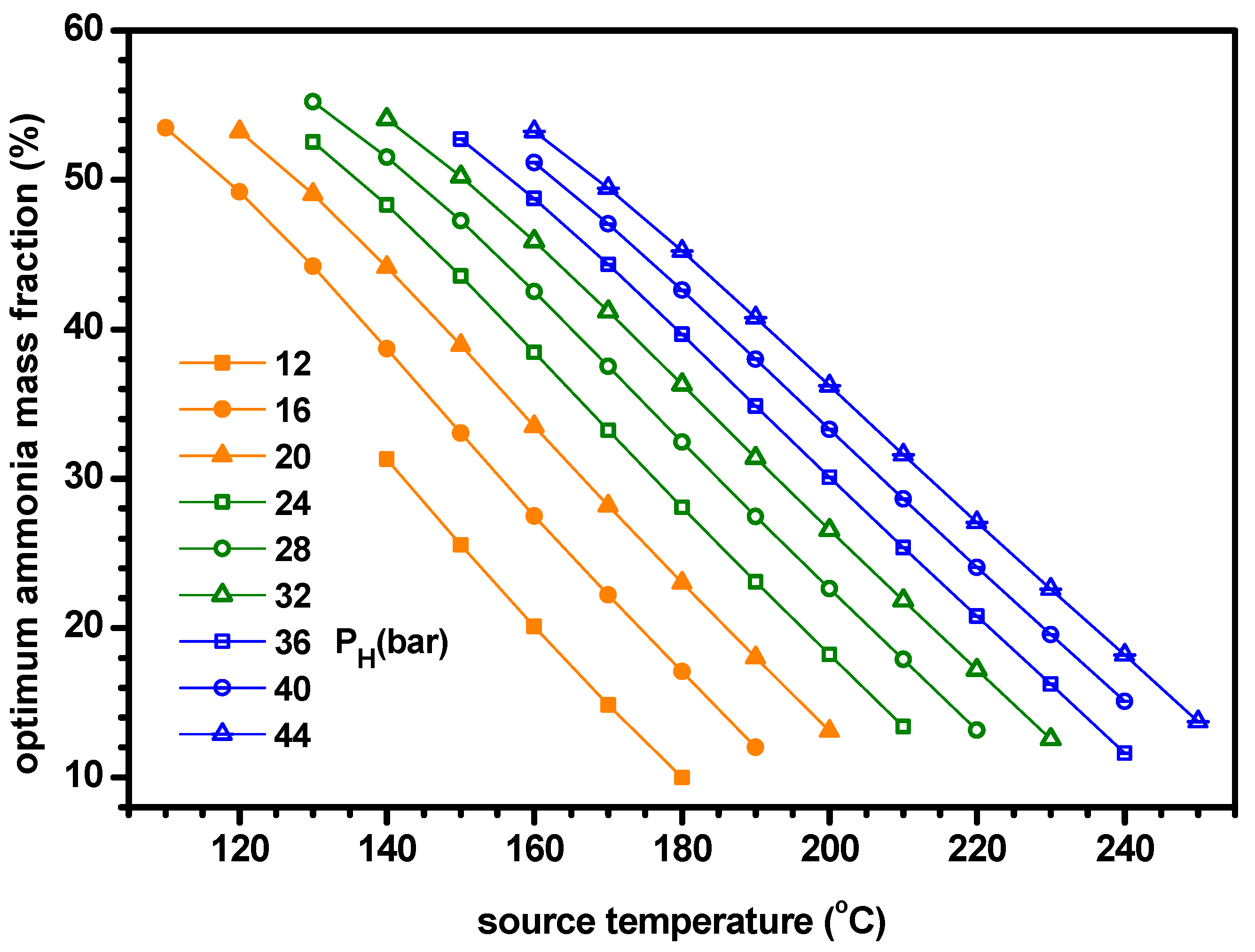
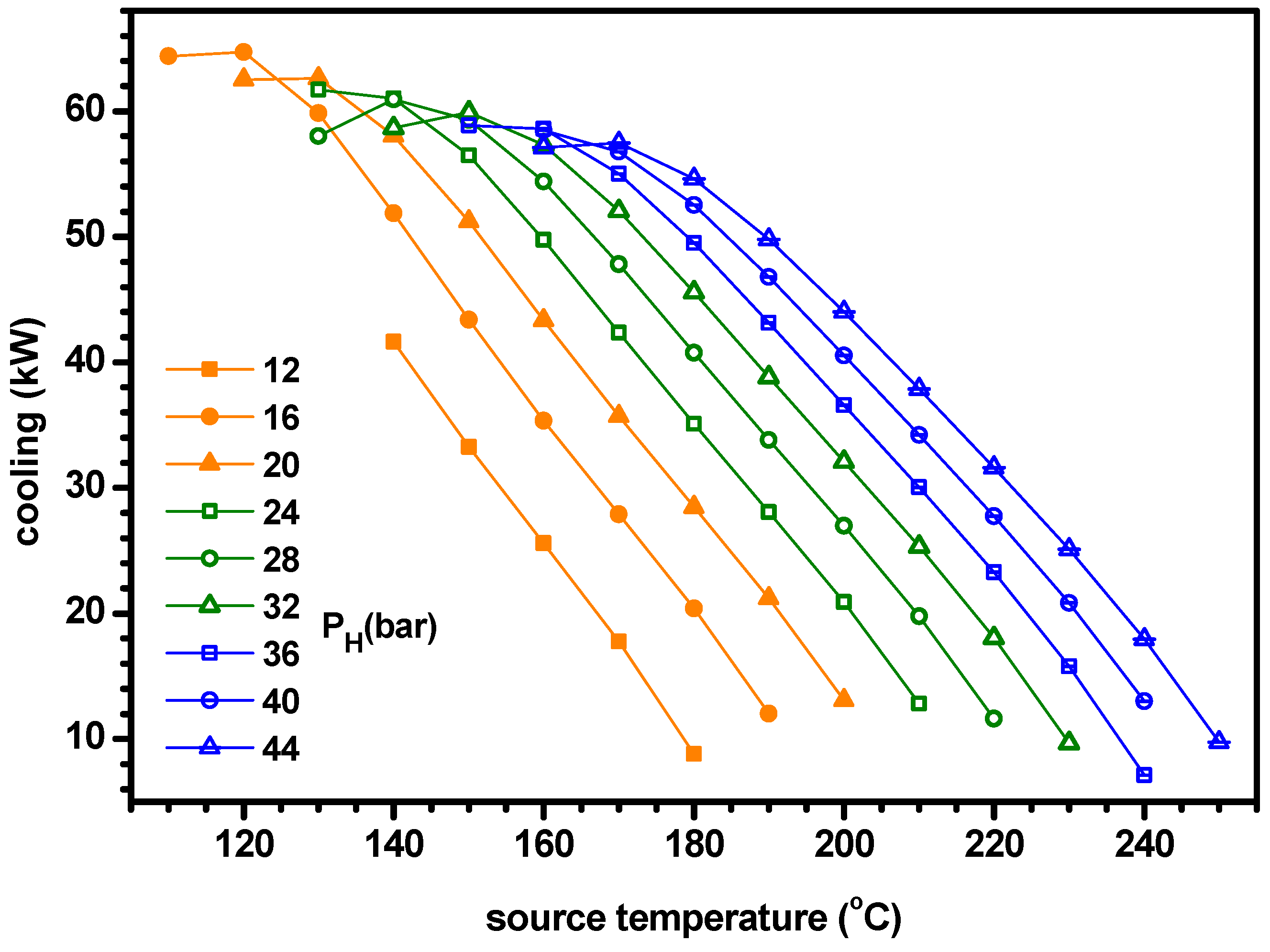
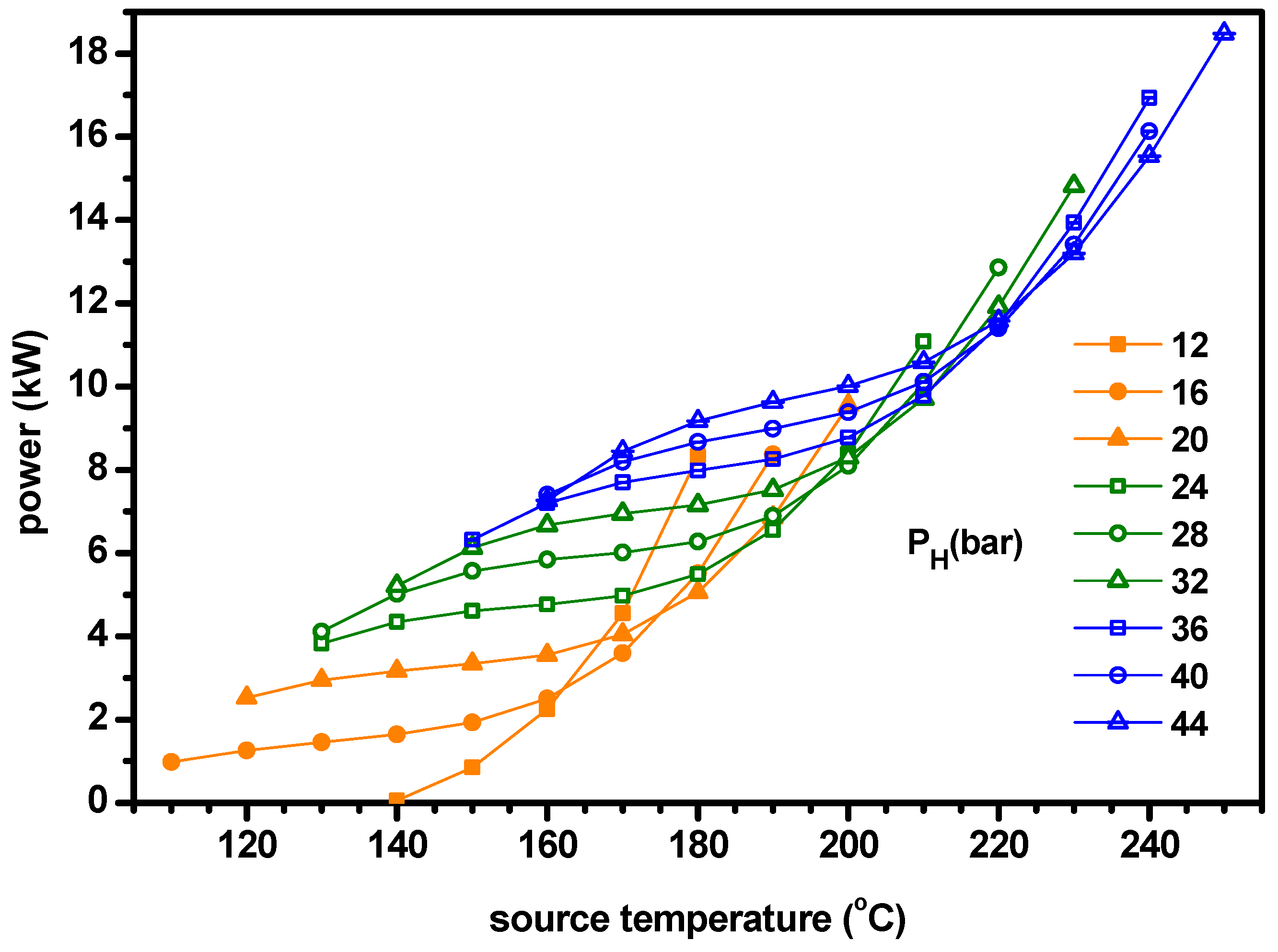
4.2. Thermodynamic Performance with Optimal Conditions of Ammonia Fraction
5. Conclusions
- (1)
- The temperature-entropy diagrams with varying ammonia fraction and separator pressure showed the important changes of system characteristics.
- (2)
- For a specified set of parameters, the system has both the lower and upper limits of ammonia fraction for proper operations of cogeneration. When ammonia fraction is too low, vapor is not supplied from the separator, while when the fraction is too high, the working fluid temperature entering evaporator becomes too high to chill the water.
- (3)
- As ammonia fraction or source temperature increases or separator pressure decreases, the quality of working fluid at separator, heat input rate and mass flow rate of turbine increase but the mass flow rate at boiler decreases.
- (4)
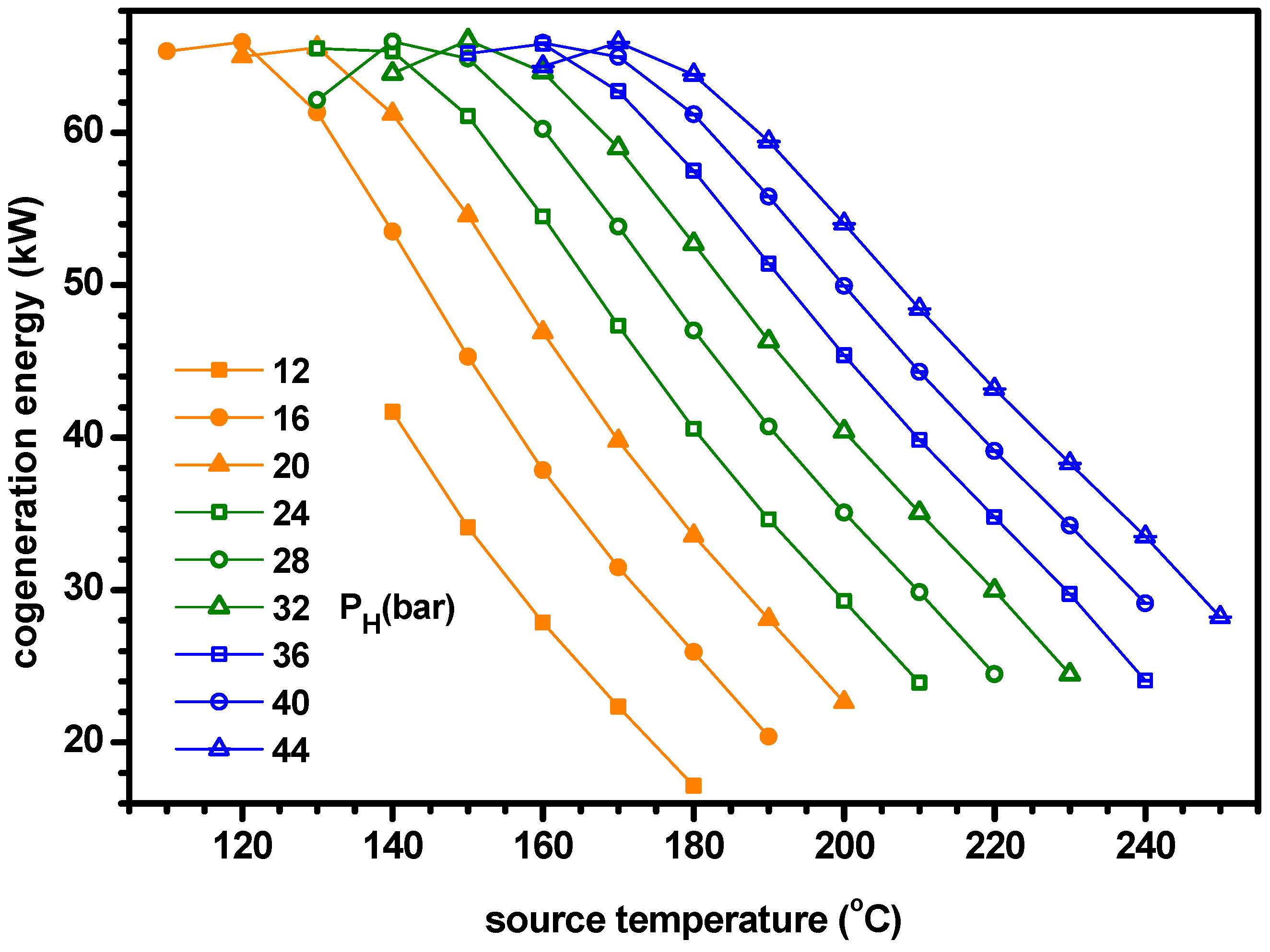
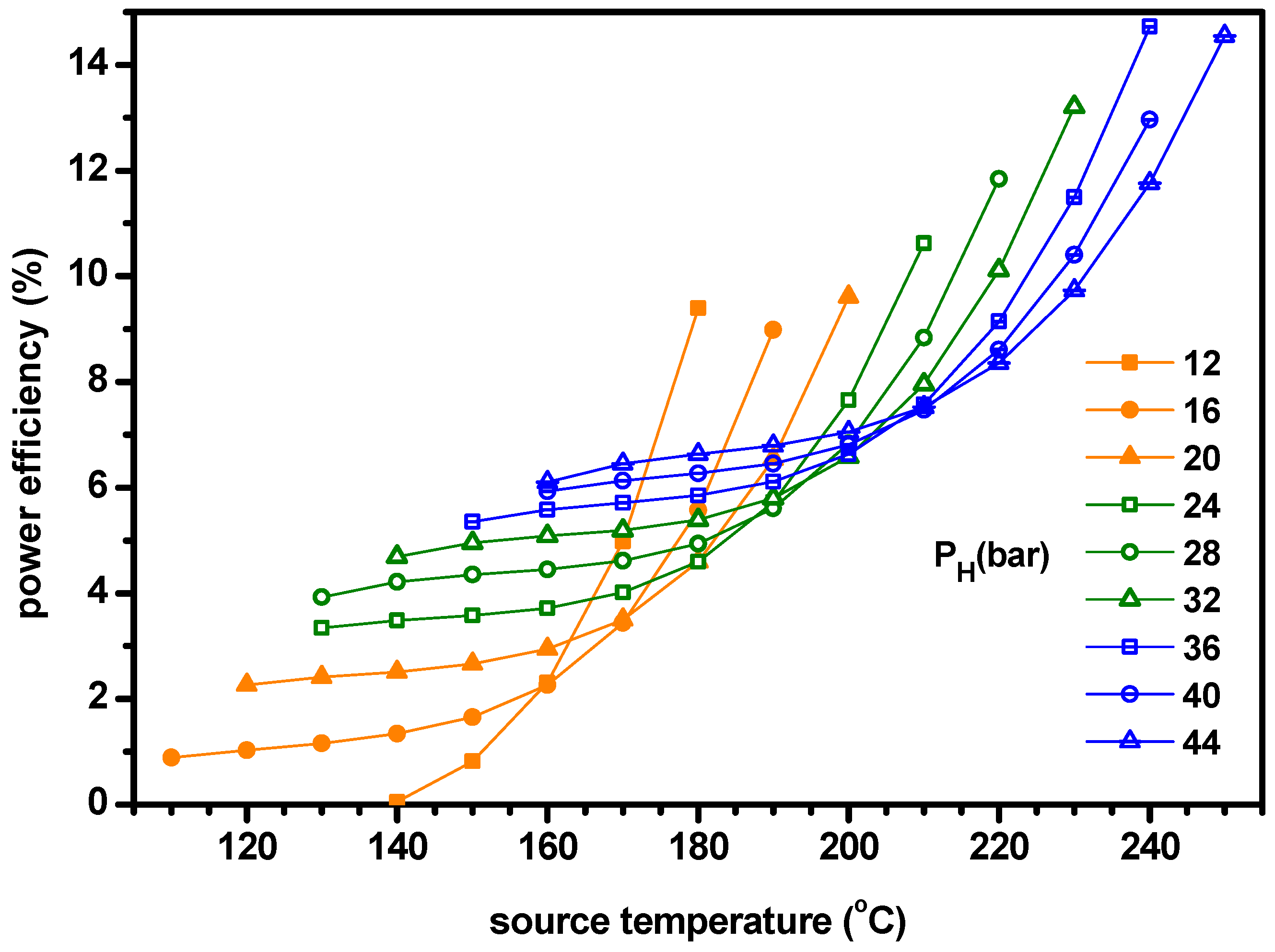
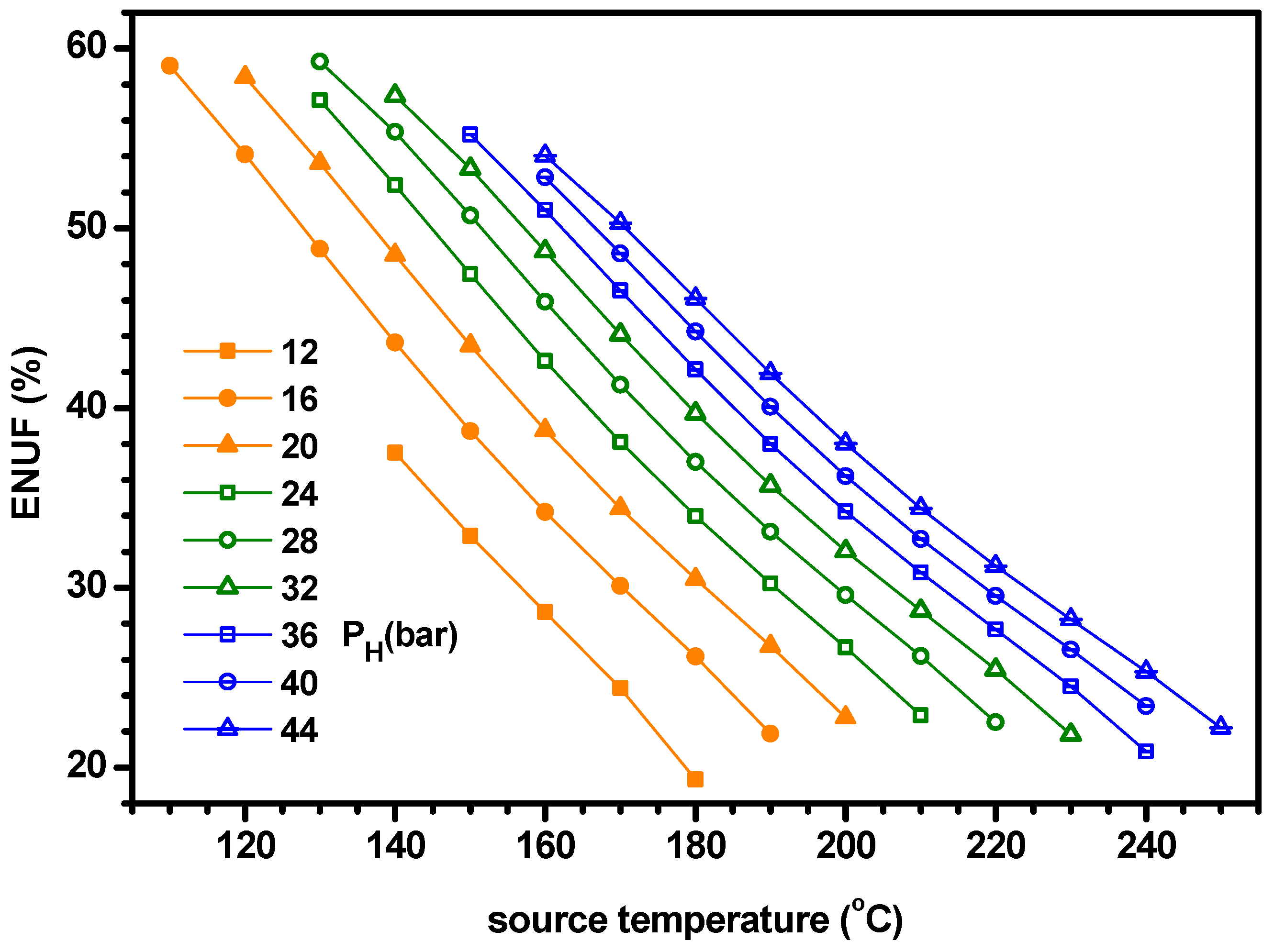
- The power increases with ammonia fraction but the cooling has a maximum for ammonia fraction. As the cooling is greater than the power, the cogeneration energy has a peak for ammonia fraction. Similarly, the power efficiency increases with ammonia fraction but the cooling efficiency and ENUF have a peak for ammonia fraction.
- (5)
- The optimum ammonia fraction for the maximum ENUF decreases with increasing source temperature or decreasing separator pressure. Under the conditions of optimal ammonia fractions, the power and power efficiency increase with the separator pressure but the cogeneration energy has a peak for the separator pressure. Under the optimal conditions, the ENUF increases as source temperature decreases or separator pressure increases.
- (6)
- Under the optimal conditions, the maximum cooling capacities and cogeneration energies are 64.7 kW and 66.0 kW for PH = 16 bar, 61.7 kW and 65.5 kW for PH = 24 bar, 59.9 kW and 66.1 kW for PH = 32 bar and 56.8 kW and 65.9 kW for PH = 40 bar, respectively. The optimum ammonia fraction and the ENUF can be correlated as a linear function with respect to the source temperature for a specified pressure as −A*TS+B. The values of A and B for PH = 16, 24, 32 and 40 in bar are 0.005181 and 1.103, 0.0004891 and 1.161, 0.004616 and 1.194 and 0.004510 and 1.243 for the optimum fraction and 0.004645 and 1.098, 0.0004276 and 1.110, 0.003950 and 1.108 and 0.003675 and 1.104 for ENUF, respectively.
- (7)
- The cogeneration energy and ENUF of the proposed system were significantly higher than power and thermal efficiency respectively of basic power generation cycle. As the proposed system is based on KCS-11 and is not employed with additional components such as rectifier and superheat, the system has a potential for efficient recovery of low-grade heat.
Funding
Conflicts of Interest
References
- Kim, K.H. Thermodynamic Performance and Optimization Analysis of a Modified Organic Flash Cycle for the Recovery of Low-Grade Heat. Energies 2019, 12, 442. [Google Scholar] [CrossRef]
- Lolos, P.A.; Rogdakis, E.D. A Kalina power cycle driven by renewable energy sources. Energy 2009, 34, 457–464. [Google Scholar] [CrossRef]
- Wagar, W.R.; Zamfirescu, C.; Dincer, I. Thermodynamic performance assessment of anammonia-water Rankine cycle for power and heat production. Energy Convers. Manag. 2010, 51, 2501–2509. [Google Scholar] [CrossRef]
- Kim, K.H.; Han, C.H.; Kim, K. Effects of ammonia concentration on the thermodynamic performances of ammonia-water based power cycles. Thermochim. Acta 2012, 530, 7–16. [Google Scholar] [CrossRef]
- Kim, K.H.; Ko, H.J.; Kim, K. Assessment of pinch point characteristics in heat exchangers and condensers of ammonia-water based power cycles. Appl. Energy 2014, 113, 970–981. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Klein, S.A. Absorption power cycles. Energy 1996, 21, 21–27. [Google Scholar] [CrossRef]
- Spiecker, S.; Weber, C. The future of the European electricity system and the impact of fluctuating renewable energy—A scenario analysis. Energy Policy 2014, 65, 185–197. [Google Scholar] [CrossRef]
- Zhang, X.X.; He, M.G.; Zhang, Y. A review of research on the Kalina cycle. Renew. Sustain. Energy Rev. 2012, 16, 5309–5318. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Thermodynamic analysis of a novel ammonia-water trilateral Rankine cycle. Acta 2008, 477, 7–15. [Google Scholar] [CrossRef]
- Guzović, Z.; Lončar, D.; Ferdelji, N. Possibilities of electricity generation in the Republic of Croatia by means of geothermal energy. Energy 2010, 35, 3429–3440. [Google Scholar] [CrossRef]
- Singh, O.K.; Kaushik, S.C. Energy and exergy analysis and optimization of Kalina cycle with a coal fired steam power plant. Appl. Eng. 2013, 51, 787–800. [Google Scholar] [CrossRef]
- Li, R.; Wang, H.; Yao, E.; Zhang, S. Thermo-Economic Comparison and Parametric Optimizations among Two Compressed Air Energy Storage System Based on Kalina Cycle and ORC. Energies 2017, 10, 15. [Google Scholar] [CrossRef]
- Guo, Z.W.; Zhang, Z.; Chen, Y.P.; Wu, J.F.; Dong, C. Dual-pressure vaporization Kalina cycle for cascade reclaiming heat resource for power generation. Energy Convers. Manag. 2015, 106, 557–565. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Zhang, Z.; Chen, Y.P.; Wu, J.F. Parameter optimization of dual-pressure vaporization Kalina cycle with second evaporator parallel to economizer. Energy 2016, 112, 420–429. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Chen, L.; Dai, Y. Comprehensive analysis and optimization of Kalina-Flash cycles for low-grade heat source. Appl. Eng. 2018, 131, 540–552. [Google Scholar] [CrossRef]
- Kim, K.H.; Han, C.H.; Ko, H.J. Comparative Thermodynamic Analysis of Kalina and Kalina Flash Cycles for Utilizing Low-Grade Heat Sources. Energies 2018, 11, 3311. [Google Scholar] [CrossRef]
- Ogriseck, S. Integration of Kalina cycle in a combined heat and power plant, a case study. Appl. Eng. 2009, 29, 2843–2848. [Google Scholar] [CrossRef]
- Shankar, R.; Srinivas, T. Cooling cogeneration cycles. Appl. Sol. Energy 2017, 53, 61–71. [Google Scholar] [CrossRef]
- Kim, K.H.; Perez-Blanco, H. Performance analysis of a combined organic Rankine cycle and vapor compression cycle for power and refrigeration cogeneration. Appl. Eng. 2015, 91, 964–974. [Google Scholar] [CrossRef]
- Goswami, D.Y. Solar thermal power technology: Present status and ideas for the future. Energy Sources 1998, 20, 137–145. [Google Scholar] [CrossRef]
- Xu, F.; Goswami, D.Y.; Bhagwat, S.S. A combined power/cooling cycle. Energy 2000, 25, 233–246. [Google Scholar] [CrossRef]
- Sadrameli, S.M.; Goswami, D.Y. Optimum operating conditions for a combined power and cooling thermodynamic cycle. Appl. Energy 2007, 84, 254–265. [Google Scholar] [CrossRef]
- Zhang, N.; Lior, N. Development of a novel combined absorption cycle for power generation and refrigeration. J. Energy Res. Technol. 2007, 129, 254–265. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, N. Proposal and analysis of a novel ammonia–water cycle for power and refrigeration cogeneration. Energy 2007, 32, 961–970. [Google Scholar] [CrossRef]
- Jawahar, C.P.; Saravanan, R.; Bruno Coronas, J.C. Simulation studies on gaxbasedKalina cycle for both power and cooling applications. Appl. Eng. 2013, 50, 1522–1529. [Google Scholar]
- Zheng, D.X.; Chen, B.; Qi, Y.; Jin, H.G. Thermodynamic analysis of a novel absorption power/cooling combined cycle. Appl. Energy 2006, 83, 311–323. [Google Scholar] [CrossRef]
- Yu, Z.; Han, J.; Liu, H.; Zhao, H. Theoretical study on a novel ammonia-water cogeneration system with adjustable cooling to power ratios. Appl. Energy 2014, 122, 53–61. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Wu, J.; Zhu, Z. Thermodynamic analysis on a modified Kalina cycle with parallel cogeneration of power and refrigeration. Energy Convers. Manag. 2018, 163, 1–12. [Google Scholar] [CrossRef]
- Hua, J.Y.; Chen, Y.P.; Wang, Y.D.; Roskilly, A.P. Thermodynamic analysis of ammonia–water power/chilling cogeneration cycle with low-grade waste heat. Appl. Eng. 2014, 64, 483–490. [Google Scholar] [CrossRef]
- Ghaebi, H.; Parikhani, T.; Rostamzadeh, H.; Farhang, B. Thermodynamic and thermo-economic analysis and optimization of a novel combined cooling and power(CCP) cycle by integrating of ejector refrigeration and Kalina cycles. Energy 2017, 139, 262–276. [Google Scholar] [CrossRef]
- Rashidi, J.; Yoo, C.K. A novel Kalina power-cooling cycle with an ejector absorption refrigeration cycle: Thermodynamic modelling and pinch analysis. Energy Convers. Manag. 2018, 162, 225–238. [Google Scholar] [CrossRef]
- Shokati, N.; Ranjbar, F.; Yari, M. A comprehensive exergoeconomic analysis of absorption power and cooling cogeneration cycles based on Kalina, part 1: Simulation. Energy Convers. Manag. 2018, 158, 437–459. [Google Scholar] [CrossRef]
- Shankar, R.; Srinivas, T. Performance investigation of Kalina cooling cogeneration cycles. Int. J. Refrig. 2018, 86, 163–185. [Google Scholar] [CrossRef]
- Xu, F.; Goswami, D.Y. Thermodynamic properties of ammonia-water mixtures for power cycle application. Energy 1999, 24, 525–536. [Google Scholar] [CrossRef]





| Symbol | Parameter | Data | Unit |
|---|---|---|---|
| Ts | source temperature | 180 | °C |
| T4 | separator temperature | Ts-15 | °C |
| PH | separator pressure | 32 | bar |
| T1, T7 | condensation temperature | 35 | °C |
| T16, T18 | water inlet temperature | 30 | °C |
| T20 | temperature of chilled water in | 20 | °C |
| T21 | temperature of chilled water out | 15 | °C |
| ΔTpp | pinch temperature difference | 5 | °C |
| ηp | isentropic efficiency of pump | 75 | % |
| ηt | isentropic efficiency of turbine | 75 | % |
| xb | basic ammonia fraction | 45 | % |
| State | x(%) | T | P(bar) | h (kJ/kg) | s (kJ/kgK) | m (kg/s) |
|---|---|---|---|---|---|---|
| 1 | 45.0 | 35.0 | 3.23 | 0.0 | 0.000 | 0.343 |
| 2 | 45.0 | 35.5 | 32.00 | 4.5 | 0.004 | 0.343 |
| 3 | 45.0 | 120.1 | 32.00 | 395.9 | 1.122 | 0.343 |
| 4 | 45.0 | 165.0 | 32.00 | 1027.0 | 2.657 | 0.343 |
| 5 | 80.7 | 165.0 | 32.00 | 1887.4 | 4.869 | 0.106 |
| 6 | 80.7 | 118.8 | 10.92 | 1740.3 | 4.996 | 0.106 |
| 7 | 80.7 | 35.0 | 10.92 | 108.9 | 0.109 | 0.106 |
| 8 | 80.7 | −0.6 | 3.23 | 108.9 | 0.163 | 0.106 |
| 9 | 80.7 | 15.0 | 3.23 | 700.3 | 2.327 | 0.106 |
| 10 | 29.0 | 165.0 | 32.00 | 640.2 | 1.662 | 0.237 |
| 11 | 29.0 | 40.5 | 32.00 | 72.8 | 0.146 | 0.237 |
| 12 | 29.0 | 41.0 | 3.23 | 72.8 | 0.155 | 0.237 |
| 13 | 45.0 | 49.1 | 3.23 | 267.4 | 0.861 | 0.343 |
| 14 | 180.0 | 671.5 | 1.814 | 1.000 | ||
| 15 | 130.1 | 455.1 | 1.308 | 1.000 | ||
| 16 | 30.0 | 20.9 | 0.070 | 1.778 | ||
| 17 | 42.3 | 72.5 | 0.236 | 1.778 | ||
| 18 | 30.0 | 20.8 | 0.069 | 3.071 | ||
| 19 | 43.6 | 77.3 | 0.252 | 3.071 | ||
| 20 | 20.0 | −21.0 | −0.071 | 2.992 | ||
| 21 | 15.0 | −42.0 | −0.143 | 2.992 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.H. Thermodynamic Analysis of Kalina Based Power and Cooling Cogeneration Cycle Employed Once Through Configuration. Energies 2019, 12, 1536. https://doi.org/10.3390/en12081536
Kim KH. Thermodynamic Analysis of Kalina Based Power and Cooling Cogeneration Cycle Employed Once Through Configuration. Energies. 2019; 12(8):1536. https://doi.org/10.3390/en12081536
Chicago/Turabian StyleKim, Kyoung Hoon. 2019. "Thermodynamic Analysis of Kalina Based Power and Cooling Cogeneration Cycle Employed Once Through Configuration" Energies 12, no. 8: 1536. https://doi.org/10.3390/en12081536
APA StyleKim, K. H. (2019). Thermodynamic Analysis of Kalina Based Power and Cooling Cogeneration Cycle Employed Once Through Configuration. Energies, 12(8), 1536. https://doi.org/10.3390/en12081536




