Facilely Synthesized NiCo2O4/NiCo2O4 Nanofile Arrays Supported on Nickel Foam by a Hydrothermal Method and Their Excellent Performance for High-Rate Supercapacitance
Abstract
1. Introduction
2. Experimental Methods
2.1. Chemicals and Materials
2.2. Fabrication of the NF@NCO NLAs and NF@NCO/NCO NFAs Materials
2.3. Material Characterizations
2.4. Electrochemical Measurements
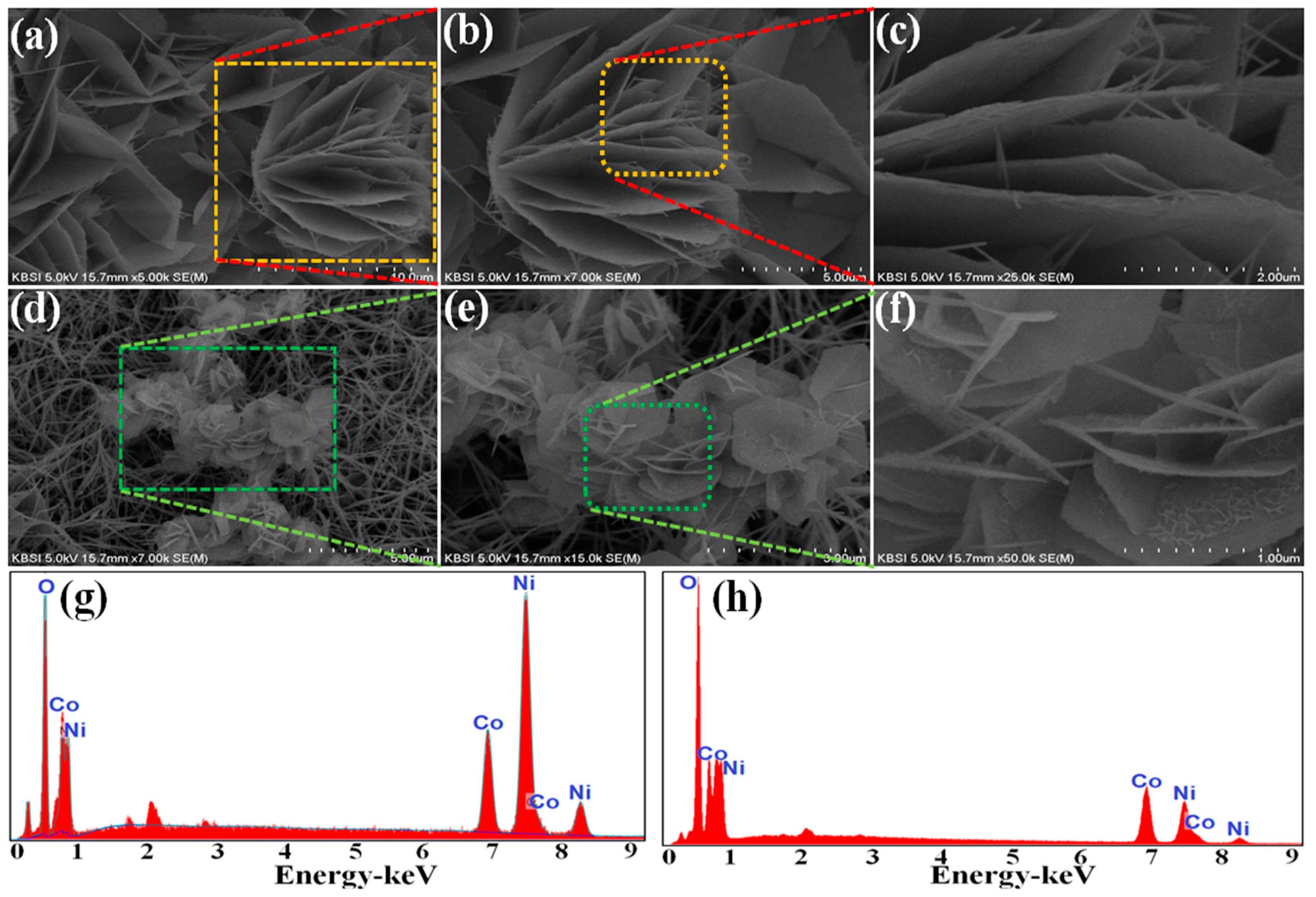
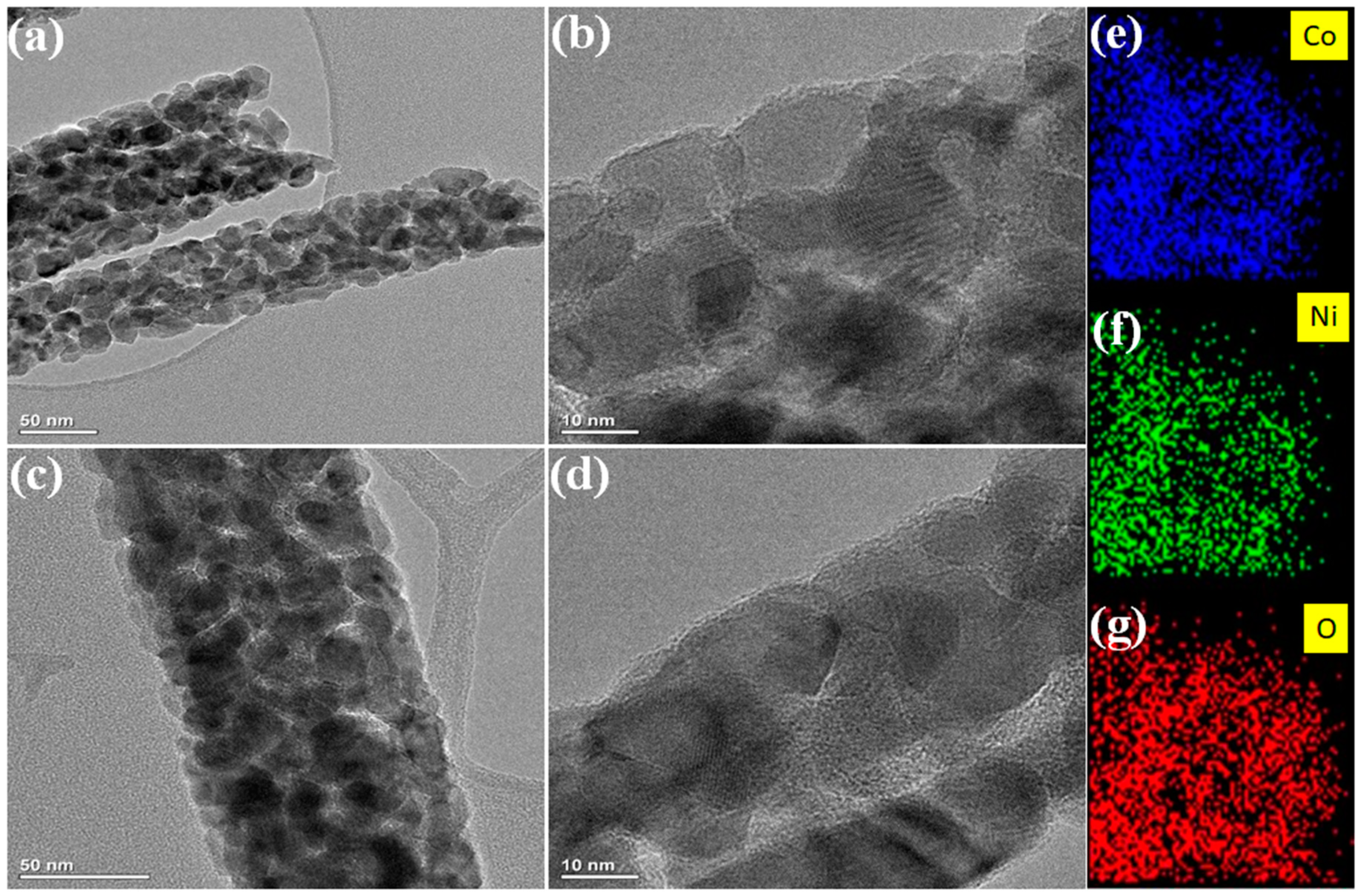
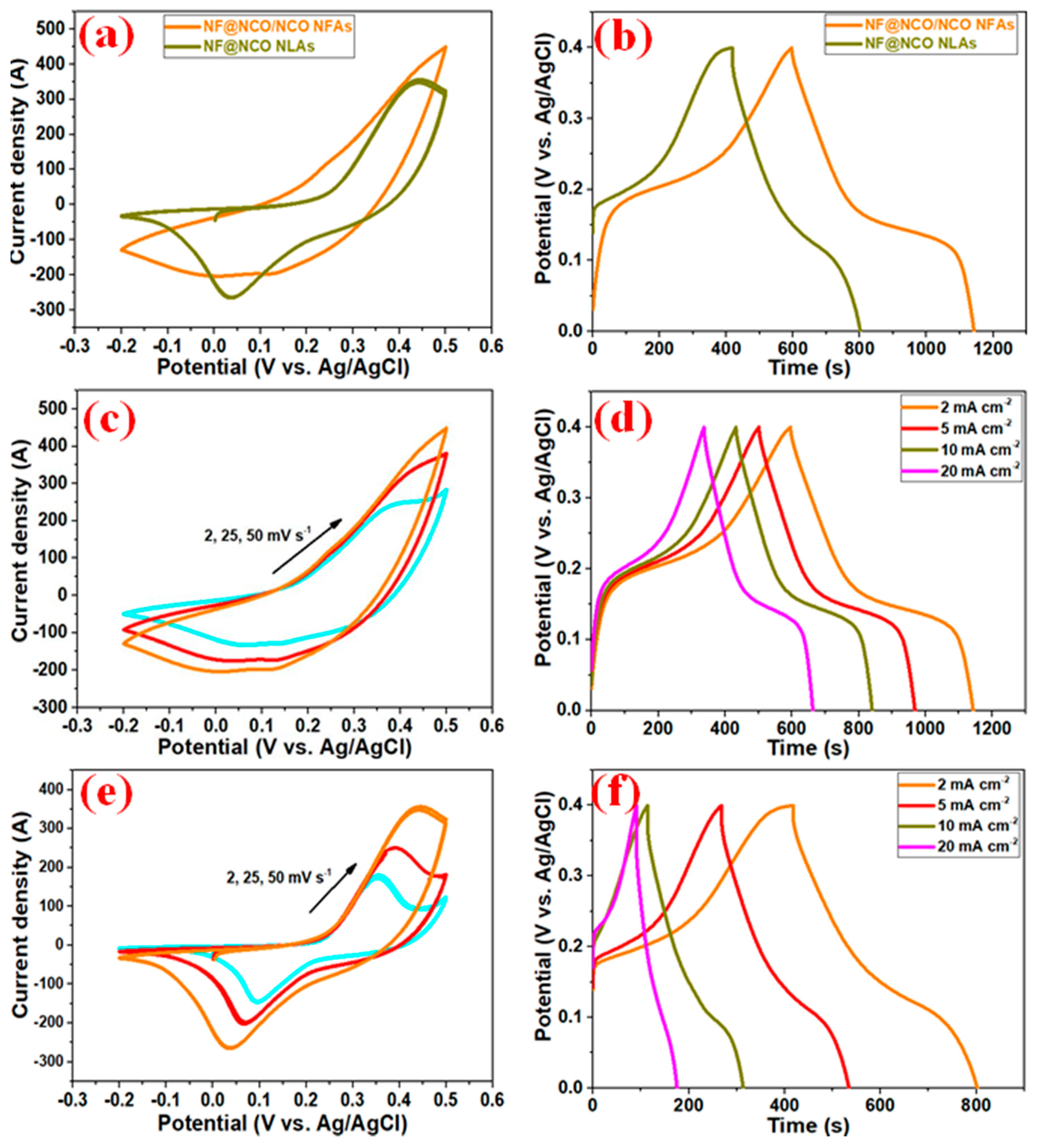
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gobal, F.; Faraji, M. Preparation and electrochemical performances of nanoporous/cracked cobalt oxide layer for supercapacitors. Appl. Phys. A 2014, 117, 2087–2094. [Google Scholar] [CrossRef]
- Yang, M.; Choi, B.G. Preparation of Three-Dimensional Co3O4/Graphene Composite for High-Performance Supercapacitors. Chem. Eng. Commun. 2017, 204, 723–728. [Google Scholar] [CrossRef]
- Le, Z.; Liu, F.; Nie, P.; Li, X.; Liu, X.; Bian, Z.; Chen, G.; Wu, H.B.; Lu, Y. Pseudocapacitive sodium storage in mesoporous single-crystalline-like TiO2-Graphen nanocomposite enables high-performance sodium-ion capacitors. ACS Nano 2017, 11, 2952–2960. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Rao, S.S.; Punnoose, D.; Tulasivarma, C.V.; Gopi, C.V.V.M.; Prabakar, K.; Kim, H.-J. Influence of solvents in the preparation of cobalt sulfide for supercapacitors. R. Soc. Open Sci. 2017, 4, 170427. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kim, H.-J. Effect of Times on a Hierarchical Corn Skeleton-Like Composite of CoO@ZnO as Capacitive Electrode Material for High Specific Performance Supercapacitors. Energies 2018, 11, 3285. [Google Scholar] [CrossRef]
- Singh, A.K.; Sarkar, D.; Karmakar, K.; Mandal, K.; Khan, G.G. High-Performance Supercapacitor Electrode based on Cobalt Oxide—Manganese Dioxide-Nickel Oxide Ternary 1D Hybrid Nanotubes. ACS Appl. Mater. Interfaces 2016, 8, 20786–20792. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Chu, D.; Li, S. Ethanol directed morphological evolution of hierarchical CeOx architectures as advance electrochemical capacitors. J. Mater. Chem. A 2015, 3, 13970–13977. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kim, H.-J. Preparation and electrochemical performance of NiCo2O4@NiCo2O4 composite nanoplates for high performance supercapacitor applications. New J. Chem. 2018, 42, 19971–19978. [Google Scholar] [CrossRef]
- Liu, S.; Mao, C.; Niu, Y.; Yi, F.; Hou, J.; Lu, S.; Jiang, J.; Xu, M.; Li, C.M. Facile Synthesis of Novel Networked Ultralong Cobalt Sulfide Nanotubes and Its Application in Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 25568–25573. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S. Co3O4/ZnO nanoheterostructure derived from core–shell ZIF-8@ZIF-67 for supercapacitors. RSC Adv. 2016, 6, 52137–52142. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.-J. Wearable super-high specific performance supercapacitors using a honeycomb with folded silk-like composite of NiCo2O4 nanoplates decorated with NiMoO4 honeycombs on nickel foam. Dalton Trans. 2018, 47, 15545–15554. [Google Scholar] [CrossRef]
- Bai, J.; Wang, K.; Feng, J.; Xiong, S. ZnO/CoO and ZnCo2O4 Hierarchical Bipyramid Nanoframes: Morphology Control, Formation Mechanism, and Their Lithium Storage Properties. ACS Appl. Mater. Interfaces 2015, 7, 22848–22857. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Zhang, J.Z.; Shi, G.W.; Liu, Y.-M. One step hydrothermal synthesis of two-dimentional cobalt sulfide for high performance supercapacitors. Mater. Lett. 2014, 131, 45–48. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, W.; Song, S.; Hao, Q.; Fan, W.; Xia, X.; Zhang, X.; Wang, Q.; Liu, C.; Yan, D. Solvothermal synthesis and electrochemical performance in super-capacitors of Co3O4/C flower-like nanostructures. J. Power Sources 2014, 248, 1281–1289. [Google Scholar] [CrossRef]
- Peng, Y.; Le, Z.; Wen, M.; Zhang, D.; Chen, Z.; Bin Wu, H.; Li, H.; Lu, Y. Mesoporous single-crystal-like TiO2 mesocages threaded with carbon nanotubes for high-performance electrochemical energy storage. Nano Energy 2017, 35, 44–51. [Google Scholar] [CrossRef]
- Lei, C.; Chen, Z.; Sohn, H.; Wang, X.; Le, Z.; Weng, D.; Shen, M.; Wang, G.; Lu, Y. Better lithium-ion storage materials made through hierarchical assemblies of active nanorods and nanocrystals. J. Mater. Chem. A 2014, 2, 17536–17544. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Xia, D.; Zhao, Y.; Guo, D.; Qi, T.; Wan, H. In situ growth of NiCo2O4 nanotube arrays on Ni foam for supercapacitors: Maximizing utilization efficiency at high mass loading to achieve ultrahigh areal pseudocapacitance. J. Power Sources 2014, 254, 249–257. [Google Scholar] [CrossRef]
- Zou, R.; Xu, K.; Wang, T.; He, G.; Liu, Q.; Liu, X.; Zhang, Z.; Hu, J. Chain-like NiCo2O4 nanowires with different exposed reactive planes for high-performance supercapacitors. J. Mater. Chem. A 2013, 1, 8560. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, M.; Yang, J.; Su, H.; Huang, W.; Dong, X. Selective synthesis of hierarchical mesoporous spinal NiCo2O4 for high-performance supercapacitors. Nanoscale 2014, 6, 4303–4308. [Google Scholar] [CrossRef]
- Lei, Y.; Li, J.; Wang, Y.; Gu, L.; Chang, Y.; Yuan, H.; Xiao, D. Rapid Microwave-Assisted Green Synthesis of 3D Hierarchical Flower-Shaped NiCo2O4 Microsphere for High-Performance Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Yedluri, A.K.; Kim, H.-J. Enhanced electrochemical performance of nanaoplate nickel cobaltite (NiCo2O4) supercapacitor applications. RSC Adv. 2019, 9, 1115–1122. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Zhang, X.; Shen, L.; Lou, X.W. Ultrathin Mesoporous NiCo2O4 Nanosheets Supported on Ni Foam as Advanced Electrodes for Supercapacitors. Adv. Funct. Mater. 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, X.; Wu, Z.; Song, W.; Hou, H.; Wu, Z.; He, X.; Chen, Q.; Banks, C.E. Spinal NiCo2O4 for use as a high-performance supercapacitor electrode material: Understanding of its electrochemical properties. J. Power Sources 2014, 267, 888–900. [Google Scholar] [CrossRef]
- Liu, R.; Ma, L.; Huang, S.; Mei, J.; Li, E.; Yuan, G. Large areal mass and high scalable and flexible cobalt Oxide/Graphene/Bacterial cellulose electrode for supercapacitors. J. Phys. Chem. C 2016, 120, 28480–28488. [Google Scholar] [CrossRef]
- Cui, C.; Xu, J.; Wang, L.; Guo, D.; Mao, M.; Ma, J.; Wang, T. Growth of NiCo2O4@MnMoO4 Nanocolumn Arrays with Superior Pseudocapacitor Properties. ACS Appl. Mater. Interfaces 2016, 8, 8568–8575. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.Q.; Yang, F.; Zhao, Y.L.; Xu, X.; Xu, L.; Luo, Y.Z. Hierarchical MnMoO(4)/CoMoO(4) heterostructured nanowires with enhanced supercapacitor performance. Nat. Commun. 2011, 2, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, W.; Yang, J.; Tang, H.; Ye, Z.; Zeng, Y.; Lu, J. Three-Dimensional NiCo2O4@MnMoO4 Core–Shell Nanoarrays for High-Performance Asymmetric Supercapacitors. Langmuir 2017, 33, 10446–10454. [Google Scholar] [CrossRef]
- Cheng, J.; Lu, Y.; Qiu, K.; Yan, H.; Xu, J.; Han, L.; Liu, X.; Luo, J.; Kim, J.-K.; Luo, Y. Hierarchical Core/Shell NiCo2O4@NiCo2O4 Nanocactus Arrays with Dual-functionalities for High Performance Supercapacitors and Li-ion Batteries. Sci. Rep. 2015, 5, 12099. [Google Scholar] [CrossRef]
- Gu, Z.; Zhang, X. NiCo2O4@MnMoO4 core–shell flowers for high performance supercapacitors. J. Mater. Chem. A 2016, 4, 8249–8254. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Huang, Z.; Ren, L.; Qi, X.; Wei, X.; Zhong, J. Facile hydrothermal synthesis of NiMoO4@CoMoO4 hierarchical nanospheres for supercapacitor applications. Phys. Chem. Chem. Phys. 2015, 17, 20795–20804. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, F.; Hu, Z.; Liu, Y. Template synthesis of C@NiCo2O4 hollow microsphere as electrode material for supercapacitor. J. Alloys Compd. 2018, 749, 305–312. [Google Scholar] [CrossRef]
- Karmakar, S.; Varma, S.; Behera, D.; Varma, S. Investigation of structural and electrical transport properties of nano-flower shaped NiCo2O4 supercapacitor electrode materials. J. Alloys Compd. 2018, 757, 49–59. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Yang, L.; Shen, L.; Zhang, X. Facile template-free synthesis of ultralayered mesoporous nickel cobaltite nanowires towards high-performance electrochemical capacitors. J. Mater. Chem. 2012, 22, 16084–16090. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Sun, X. NiCo2O4@NiO hybrid arrays with improved electrochemical performance for pseudocapacitors. J. Mater. Chem. A 2015, 3, 13900–13905. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Liu, Y.; Ju, Z.; Qian, Y. High Electrochemical Performance of Monodisperse NiCo2O4 Mesoporous Microspheres as an Anode Material for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, T.; Saied, S.O.; Abbot, A.M.; Sullivan, J.L. Reduction of oxides of iron, cobalt, titanium and niobium by low-energy ion bombardment. J. Phys. D Appl. Phys. 1989, 22, 1185–1195. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Li, Q.; Zheng, S.; Xu, Y.; Du, P.; Chen, C.; Zhao, J.; Xue, H.; Xu, Q.; et al. Nitrogen-Doped Cobalt Oxide Nanostructures Derived from Cobalt-Alanine Complexes for High-Performance Oxygen Evolution Reactions. Adv. Funct. Mater. 2018, 28, 1800886. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yedluri, A.K.; Araveeti, E.R.; Kim, H.-J. Facilely Synthesized NiCo2O4/NiCo2O4 Nanofile Arrays Supported on Nickel Foam by a Hydrothermal Method and Their Excellent Performance for High-Rate Supercapacitance. Energies 2019, 12, 1308. https://doi.org/10.3390/en12071308
Yedluri AK, Araveeti ER, Kim H-J. Facilely Synthesized NiCo2O4/NiCo2O4 Nanofile Arrays Supported on Nickel Foam by a Hydrothermal Method and Their Excellent Performance for High-Rate Supercapacitance. Energies. 2019; 12(7):1308. https://doi.org/10.3390/en12071308
Chicago/Turabian StyleYedluri, Anil Kumar, Eswar Reddy Araveeti, and Hee-Je Kim. 2019. "Facilely Synthesized NiCo2O4/NiCo2O4 Nanofile Arrays Supported on Nickel Foam by a Hydrothermal Method and Their Excellent Performance for High-Rate Supercapacitance" Energies 12, no. 7: 1308. https://doi.org/10.3390/en12071308
APA StyleYedluri, A. K., Araveeti, E. R., & Kim, H.-J. (2019). Facilely Synthesized NiCo2O4/NiCo2O4 Nanofile Arrays Supported on Nickel Foam by a Hydrothermal Method and Their Excellent Performance for High-Rate Supercapacitance. Energies, 12(7), 1308. https://doi.org/10.3390/en12071308






