Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines
Abstract
1. Introduction
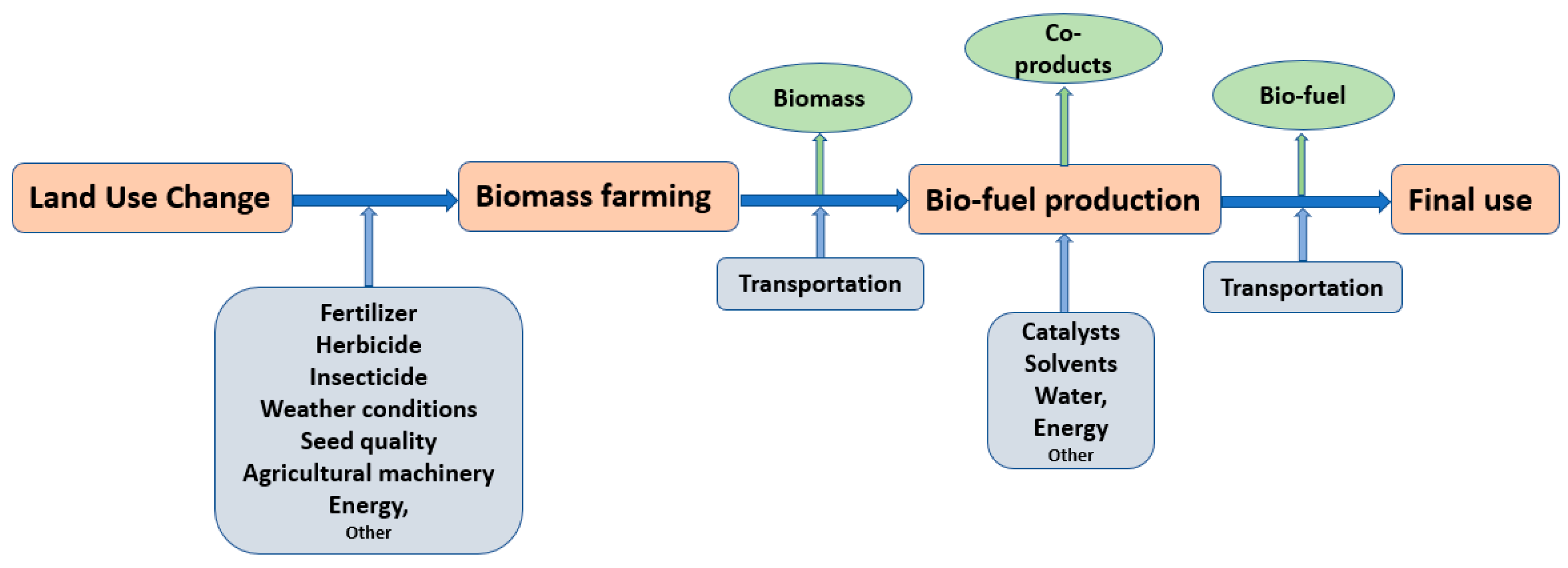
2. Bio-Fuel Production
2.1. Biodiesel Production
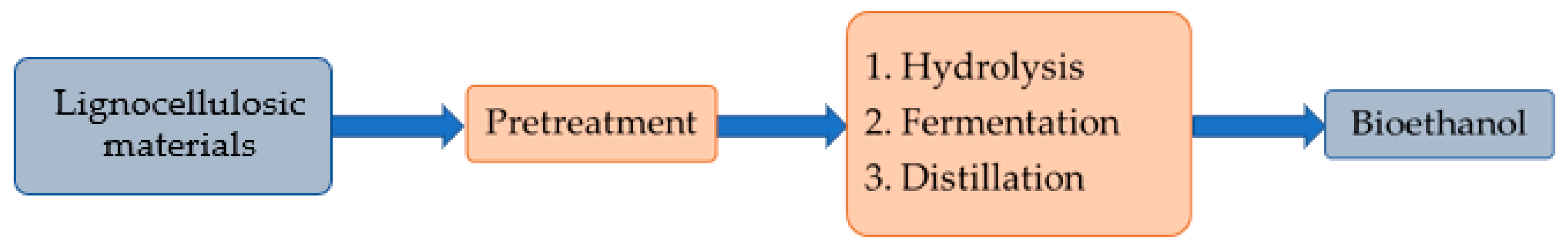
2.2. Bio-Ethanol Production
2.3. Bio-Methanol Production
3. Physico-Chemical Properties of CI Engine Fuels
- -
- The Cloud Point (CP) is the temperature at which solid particles appear in the fuel (microcrystals of ice and crystals of alkanes); consequently, as the name suggests, a clear liquid product becomes cloudy;
- -
- The Cold Filter Plugging Point (CFPP) is the lowest temperature at which a given fuel volume still goes through a standardized filtration device in a specified time;
- -
- Pour Point (PP) is the lowest temperature at which the fuel ceases to flow.
3.1. Biodiesel Physico-Chemical Properties
3.2. Bio-Ethanol/Bio-Methanol Physico-Chemical Properties
4. Use of Diesel–Biodiesel–Alcohols
4.1. Why Are Diesel–Biodiesel–Alcohols Blends Used?
4.2. Influence of Different Diesel–Biodiesel–Alcohol Blends on CI Engine Operation
4.2.1. Combustion Characteristics
4.2.2. Engine Performance
4.2.3. Emissions
5. Summary and Conclusions
- the importance of using alternative biofuels can be attributed to the following aspects:
- the need to pursue energy sustainability by increasing the use of renewable fuels, thus diminishing concerns of limited fossil fuel energy;
- improving the energetic and ecological performance of the IC engine through the better physico-chemical properties of these alternative fuels, compared to those of fossil fuels;
- the effects of using diesel-biodiesel-alcohols blends fuels are:
- regarding combustion characteristics, compared to the case of using fossil diesel fuels: combustion duration decreases in all studied cases; the maximum rate of heat release increases with an increase in the ethanol fraction in the diesel–ethanol blends, and it is slightly lower for biodiesel–diesel–ethanol blends; the ignition delay increases with the increase of the ethanol fraction in diesel–ethanol blends, and it slightly decreases for biodiesel–diesel–ethanol blends or with the addition of a CN improver; cylinder pressure increases with the increase of the ethanol fraction in diesel–ethanol blends, and it slightly decreases or remains unaltered for biodiesel–diesel–ethanol blends;
- concerning engine performance, compared to the case of using fossil diesel fuel: the brake specific fuel consumption is higher in all studied cases; the brake thermal efficiency increases or is comparable; the brake power is very comparable or slightly lower; for exhaust gas temperatures, minor variations were observed; the indicated mean effective pressure has minor variations, or it decreases in cases of more than 35% ethanol in the fuel blends.
- while NOX, smoke, and PM emissions seem to be lower, the improvement of HC and CO emissions depends considerably on the type of fuel blend (ratio and fuels used), and on the engine operating parameters.
- some properties of the alcohol blends, such as stability, lubricity, viscosity, and CFPP, which has a negative influence upon the injection system;
- the low cetane number that affects the combustion process is another important limiting factor;
- the fuel storage and handling problems that are derived from the high volatility of alcohol; in this respect, different technical modifications in the fueling system are necessary; for instance, to avoid vapor locks, special systems for recovering of fuel vapors are necessary, especially when increasing the alcohol amount.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| BEV | Battery electric vehicle |
| BP | Brake power |
| BSFC | Brake-specific fuel consumption |
| BSG | Brewers’ spent grain |
| Bx | Biodiesel blend ratio (i.e., for x = 0, B0, meaning no biodiesel; for x = 100, B100, meaning no diesel fuel) |
| BTE | Brake thermal efficiency |
| CBG | Compressed biogas |
| C&D | Construction and demolition |
| CEN | Commission Européenne de Normalisation/European Committee for Standardization |
| CFPP | Cold filter plugging point, [°C] |
| CI | Compression ignition (engine) |
| CN | Cetane number |
| CNG | Compressed natural gas |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| COV | Coefficient of variance, [%] |
| CP | Cloud Point [°C] |
| Dx | Diesel blend ratio (i.e., for x = 0, D0, meaning no diesel fuel; for x = 100, D100, meaning pure diesel) |
| DxEyBz | x% pure diesel + y% ethanol + z% biodiesel blends |
| DxMyBz | x% pure diesel + y% methanol + z% biodiesel blends |
| Ex | Ethanol blend ratio (i.e., for x = 0, E0, meaning no ethanol; for x = 100, E100, meaning pure ethanol) |
| EC | European Commission |
| EGT | Exhaust gas temperatures |
| EN | European Norms |
| EROEI | Energy return and other energy related issues |
| EU | European Union |
| EV | Electric vehicles |
| FA | Fatty acids |
| FAME | Fatty acids methyl ester |
| FP | Flash point [°C] |
| GHG | Greenhouse gas |
| H2 | Hydrogen |
| HC | (Unburnt) hydrocarbons |
| HDV | Heavy-duty vehicles |
| HFC | Hydrogen fuel cell |
| HFRR | High-frequency reciprocating rig |
| HPSS | High pressure supply system |
| ICE | Internal combustion engine |
| ID | Ignition delay |
| ILUC | Induced land-use change |
| IMEP | Indicated mean effective pressure [bar] |
| ISFC | Indicated specific fuel consumption |
| LCA | Life-cycle assessments |
| LDV | Light-duty vehicles |
| LHR | Low heat rejection |
| LHV | Lower heating value |
| LNG | Liquefied natural gas |
| LPG | Liquid petroleum gas |
| LPSS | Low-pressure supply system |
| LTC | Low temperature combustion |
| LTFT | Low Temperature Flow Test |
| MSW | Municipal solid waste |
| Mx | Methanol blend ratio (i.e., for x = 0, M0, meaning no methanol; for x = 100, M100, meaning pure methanol) |
| NG | Natural gas |
| NOx | Nitric oxides |
| PC | Passenger cars |
| PHEV | Plug-in hybrid electric vehicles |
| PM | Particulate matter |
| PP | Pour point [°C] |
| RON | Research octane number |
| SIE | Spark ignition engine |
| SO2 | Sulfur dioxide |
| SS | Supply systems |
| UHC | Unburned hydrocarbons |
| ULEV | Ultra-low emission vehicles |
| USA | United States of America |
| VHG | Very high gravity |
| ZEV | Zero–low emission vehicles |
References
- Niculescu, R.; Clenci, A.; Iorga-Siman, V.; Zaharia, C. Review on the Use of Bioethanol/Biomethanol—Gasoline Blends in Spark Ignition Engine; Sci. Bul. Automot. Ser. Year XXII, No. 26; University of Pitești: Pitesti, Romania, 2018. [Google Scholar]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- EU Directive 2015-1513; European Commission: Brussels, Belgium, 2014.
- Our Vision for A Clean Planet for All, European Commission; European Commission: Brussels, Belgium, 2018.
- A Clean Planet for All. A European Strategic Long-Term Vision for a Prosperous, Modern, Competitive and Climate Neutral Economy; European Commission: Brussels, Belgium, 2018.
- Storch, M.; Erdenkäufer, S.; Wensing, M.; Will, S.; Zigan, L. The Effect of Ethanol Blending on Combustion and Soot Formation in an Optical DISI Engine Using High-speed Imaging. Phys. Procedia 2015, 66, 77–80. [Google Scholar] [CrossRef]
- Dudley, B. BP Statistical Review of World Energy, 67th ed.; BP: London, UK, 2018. [Google Scholar]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G.; Papagiannakis, R.G.; Kyritsis, D.C. Influence of properties of various common bio-fuels on the combustion and emission characteristics of high-speed DI (direct injection) diesel engine: Vegetable oil, bio-diesel, ethanol, n-butanol, diethyl ether. Energy 2014, 73, 354–366. [Google Scholar] [CrossRef]
- Broatch, A.; Tormos, B.; Olmeda, P.; Novella, R. Impact of biodiesel fuel on cold starting of automotive direct injection diesel engines. Energy 2014, 73, 653–660. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Xu, J.; Ou, X.; Chang, S.; Wu, M. Techno-economic analysis of bioethanol production from lignocellulosic biomass in china: Dilute-acid pretreatment and enzymatic hydrolysis of corn stover. Energies 2015, 8, 4096–4117. [Google Scholar] [CrossRef]
- Iliev, S. A comparison of ethanol and methanol blending with gasoline using a 1-D engine model. Procedia Eng. 2015, 100, 1013–1022. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Rakopoulos, D.C.; Giakoumis, E.G.; Kyritsis, D.C. The combustion of n-butanol/diesel fuel blends and its cyclic variability in a direct injection diesel engine. Proc. Inst. Mech. Eng. Part A J. Power Energy 2011, 225, 289–308. [Google Scholar] [CrossRef]
- Kowalewicz, A.; Wojtyniak, M. Alternative fuels and their application to combustion engines. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2005, 219, 103–125. [Google Scholar] [CrossRef]
- Turner, D.; Xu, H.; Cracknell, R.F.; Natarajan, V.; Chen, X. Combustion performance of bio-ethanol at various blend ratios in a gasoline direct injection engine. Fuel 2011, 90, 1999–2006. [Google Scholar] [CrossRef]
- Caldeira-Pires, A.; da Luz, S.M.; Palma-Rojas, S.; Rodrigues, T.O.; Silverio, V.C.; Vilela, F.; Barbosa, P.C.; Alves, A.M. Sustainability of the biorefinery industry for fuel production. Energies 2013, 6, 329–350. [Google Scholar] [CrossRef]
- O’Connor, D.; Duffield, J.; Tyner, W.; Chen, R.; Wang, M.; Han, J.; Qin, Z.; Taheripour, F. Life cycle energy and greenhouse gas emission effects of biodiesel in the United States with induced land use change impacts. Bioresour. Technol. 2017, 251, 249–258. [Google Scholar] [CrossRef]
- Paredes-Sánchez, J.P.; López-Ochoa, L.M.; López-González, L.M.; Las-Heras-Casas, J.; Xiberta-Bernat, J. Evolution and perspectives of the bioenergy applications in Spain. J. Clean. Prod. 2019, 213, 553–568. [Google Scholar] [CrossRef]
- Alsaleh, M.; Abdul-Rahim, A.S. Determinants of cost efficiency of bioenergy industry: Evidence from EU28 countries. Renew. Energy 2018, 127, 746–762. [Google Scholar] [CrossRef]
- Experts, Report of the European Expert Group on Future Transport Fuels; European Union: Duseldorf, Germany, 2011; pp. 1–81.
- Aslan, F.Y.H.A.S.; Altun, Ş. Comparing the lubricity of different biodiesel fuels. In Proceedings of the 6th International Advanced Technologies Symposium, Elazığ, Turkey, 16–18 May 2011; pp. 16–18. [Google Scholar]
- Demirbaş, A. Biodegradability of Biodiesel and Petrodiesel Fuels. Energy Sources Part A Recovery Util. Environ. Effects 2008, 31, 169–174. [Google Scholar] [CrossRef]
- Clenci, A.; Niculescu, R.; Danlos, A.; Iorga-Simăn, V.; Trică, A. Impact of biodiesel blends and Di-Ethyl-Ether on the cold starting performance of a compression ignition engine. Energies 2016, 9, 284. [Google Scholar] [CrossRef]
- Putrasari, Y.; Lim, O. A Review of Gasoline Compression Ignition: A Promising Technology Potentially Fueled with Mixtures of Gasoline and Biodiesel to Meet Future Engine Efficiency and Emission Targets. Energies 2019, 12, 238. [Google Scholar] [CrossRef]
- Clenci, A.; Niculescu, R.; Iorga-Siman, V.; Trica, A.; Danlos, A. On the effect of Di-Ethyl-Ether (DEE) injection upon the cold starting of a biodiesel fuelled compression ignition engine. AIP Conf. Proc. 2017. [Google Scholar] [CrossRef]
- Ghadikolaei, M.A. Effect of alcohol blend and fumigation on regulated and unregulated emissions of IC engines—A review. Renew. Sustain. Energy Rev. 2016, 57, 1440–1495. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; García-Contreras, R. Stability of diesel-bioethanol blends for use in diesel engines. Fuel 2007, 86, 1351–1357. [Google Scholar] [CrossRef]
- Storch, M.; Koegl, M.; Altenhoff, M.; Will, S.; Zigan, L. Investigation of soot formation of spark-ignited ethanol-blended gasoline sprays with single- and multi-component base fuels. Appl. Energy 2016, 181, 278–287. [Google Scholar] [CrossRef]
- Bielaczyc, P.; Woodburn, J.; Klimkiewicz, D.; Pajdowski, P.; Szczotka, A. An examination of the effect of ethanol–gasoline blends’ physicochemical properties on emissions from a light-duty spark ignition engine. Fuel Process. Technol. 2013, 107, 50–63. [Google Scholar] [CrossRef]
- Nakata, K.; Sasaki, N.; Ota, K.; Kawatake, K. The effect of fuel properties on thermal efficiency of advanced spark-ignition engines. Int. J. Engine Res. 2011, 12, 274–281. [Google Scholar] [CrossRef]
- Connolly, D.; Mathiesen, B.V.; Ridjan, I. A comparison between renewable transport fuels that can supplement or replace biofuels in a 100% renewable energy system. Energy 2014, 73, 110–125. [Google Scholar] [CrossRef]
- De Castro, C.; Carpintero, Ó.; Frechoso, F.; Mediavilla, M.; de Miguel, L.J. A top-down approach to assess physical and ecological limits of biofuels. Energy 2014, 64, 506–512. [Google Scholar] [CrossRef]
- Bentivoglio, D.; Finco, A.; Bacchi, M.R.P. Interdependencies between biofuel, fuel and food prices: The case of the Brazilian ethanol market†. Energies 2016, 9, 464. [Google Scholar] [CrossRef]
- Elnashaie, S.S.E.H.; Fateen, S.-E.; El-Ahwany, A.; Moustafa, T.M. Integrated System Approach to Sustainability Bio-Fuels and Bio-Refineries. Bull. Sci. Technol. Soc. 2008, 28, 510–520. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, J.; Lin, G.; Jiang, D.; Yan, X. Switchgrass-based bioethanol productivity and potential environmental impact from marginal lands in China. Energies 2017, 10, 260. [Google Scholar] [CrossRef]
- Eggert, H.; Greaker, M. Promoting second generation biofuels: Does the first generation pave the road? Energies 2014, 7, 4430–4445. [Google Scholar] [CrossRef]
- Baeyens, J.; Kang, Q.; Appels, L.; Dewil, R.; Lv, Y.; Tan, T. Challenges and opportunities in improving the production of bio-ethanol. Prog. Energy Combust. Sci. 2015, 47, 60–88. [Google Scholar] [CrossRef]
- Niculescu, R.; Clenci, A.; Iorga-Siman, V. Diesel Fuels-Physical-Chemical Properties. Development of a Test Method for Distillation of Diesel-Biodiesel-Alcohols Mixtures at Reduced Pressure; Lambert Academic Publishing: Riga, Latvia, 2018. [Google Scholar]
- Murphy, F.; Devlin, G.; Deverell, R.; McDonnell, K. Biofuel production in ireland-an approach to 2020 targets with a focus on algal biomass. Energies 2013, 6, 6391–6412. [Google Scholar] [CrossRef]
- Directive (EU) 2015/1513 of the European Parliament and of The Council of 9 September 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015L1513&from=ro (accessed on 26 March 2019).
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Ge, J.; Yoon, S.; Choi, N. Using Canola Oil Biodiesel as an Alternative Fuel in Diesel Engines: A Review. Appl. Sci. 2017, 7, 881. [Google Scholar] [CrossRef]
- Zahan, M.K.K.A. Technological Progress in Biodiesel Production: An Overview on Different Types of Reactores. Energy Procedia 2019, 156, 452–457. [Google Scholar] [CrossRef]
- Xuan, S.; Lim, S.; Chyuan, H.; Ling, Y. State of the art review on development of ultrasound-assisted catalytic transesteri fi cation process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Al-muhtaseb, A.H.; Jamil, F.; Al-haj, L.; Tay, M.; Myint, Z.; Mahmoud, E.; Ahmad, M.N.M.; Hasan, A.O.; Ra, S. Biodiesel production over a catalyst prepared from biomass-derived waste date pits. Biotechnol. Rep. 2018, 20, e00284. [Google Scholar] [CrossRef]
- Sulaiman, S.; Fatin, N.; Jamaludin, A.; Kabbashi, N.A. Development of CaO/PVA Catalyst from Fish Bone for Biodiesel Production. Bull. Chem. React. Eng. Catal. 2019, 14, 153–157. [Google Scholar] [CrossRef]
- Kurniati, S.; Soeparman, S.; Yuwono, S.S.; Hakim, L. A Novel Process for Production of Calophyllum Inophyllum Biodiesel with Electromagnetic Induction. Energies 2019, 12, 3–383. [Google Scholar] [CrossRef]
- Strathmann, H. Membrane separation processes: Current relevance and future opportunities. AIChE J. 2001, 47, 1077–1087. [Google Scholar] [CrossRef]
- Thangavelu, S.K.; Ahmed, A.S.; Ani, F.N. Review on bioethanol as alternative fuel for spark ignition engines. Renew. Sustain. Energy Rev. 2016, 56, 820–835. [Google Scholar] [CrossRef]
- Donke, A.; Nogueira, A.; Matai, P.; Kulay, L. Environmental and Energy Performance of Ethanol Production from the Integration of Sugarcane, Corn, and Grain Sorghum in a Multipurpose Plant. Resources 2016, 6, 1. [Google Scholar] [CrossRef]
- Velazquez-Marti, B.; Pérez-Pacheco, S.; Gaibor-Chávez, J.; Wilcaso, P. Modeling of Production and Quality of Bioethanol Obtained from Sugarcane Fermentation Using Direct Dissolved Sugars Measurements. Energies 2016, 9, 319. [Google Scholar] [CrossRef]
- Capecchi, L.; Nissen, L.; Modesto, M.; di Girolamo, G.; Cavani, L.; Barbanti, L. Crop factors influencing ethanol production from sorghum juice and bagasse. Energies 2017, 10, 940. [Google Scholar] [CrossRef]
- Berlowska, J.; Pielech-Przybylska, K.; Balcerek, M.; Cieciura, W.; Borowski, S.; Kregiel, D. Integrated bioethanol fermentation/anaerobic digestion for valorization of sugar beet pulp. Energies 2017, 10, 1255. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Production of ethanol and biomass from thin stillage using food-grade Zygomycetes and Ascomycetes filamentous fungi. Energies 2014, 7, 3872–3885. [Google Scholar] [CrossRef]
- Nuanpeng, S.; Thanonkeo, S.; Yamada, M.; Thanonkeo, P. Ethanol production from sweet sorghum juice at high temperatures using a newly isolated thermotolerant yeast Saccharomyces cerevisiae DBKKU Y-53. Energies 2016, 9, 253. [Google Scholar] [CrossRef]
- Yu, C.Y.; Jiang, B.H.; Duan, K.J. Production of bioethanol from carrot pomace using the thermotolerant yeast kluyveromyces marxianus. Energies 2013, 6, 1794–1801. [Google Scholar] [CrossRef]
- Ariyajaroenwong, P.; Laopaiboon, P.; Jaisil, P.; Laopaiboon, L. Repeated-batch ethanol production from sweet sorghum juice by Saccharomyces cerevisiae immobilized on sweet sorghum stalks. Energies 2012, 5, 1215–1228. [Google Scholar] [CrossRef]
- Hernández, J.P.; Lapuerta, M.; García-Contreras, R.; Agudelo, J.R. Modelling of evaporative losses in n-alcohol/diesel fuel blends. Appl. Therm. Eng. 2016, 102, 302–310. [Google Scholar] [CrossRef]
- Pimentel, D.; Marklein, A.; Toth, M.A.; Karpoff, M.; Paul, G.S.; McCormack, R.; Kyriazis, J.; Krueger, T. Biofuel Impacts on World Food Supply: Use of Fossil Fuel, Land and Water Resources. Energies 2008, 1, 41–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Lohr, L.; Escalante, C.; Wetzstein, M. Ethanol, corn, and soybean price relations in a volatile vehicle-fuels market. Energies 2009, 2, 320–339. [Google Scholar] [CrossRef]
- Zhang, F.; Johnson, D.M.; Wang, J. Life-cycle energy and GHG emissions of forest biomass harvest and transport for biofuel production in Michigan. Energies 2015, 8, 3258–3271. [Google Scholar] [CrossRef]
- Liguori, R.; Soccol, C.R.; Vandenberghe, L.P.D.; Woiciechowski, A.L.; Faraco, V. Second generation ethanol production from brewers’ spent grain. Energies 2015, 8, 2575–2586. [Google Scholar] [CrossRef]
- Ylitervo, P.; Franzén, C.J.; Taherzadeh, M.J. Impact of furfural on rapid ethanol production using a membrane bioreactor. Energies 2013, 6, 1604–1617. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Recent advances in second generation ethanol production by thermophilic bacteria. Energies 2015, 8, 1–30. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Cavalaglio, G.; Gelosia, M.; D’Antonio, S.; Nicolini, A.; Pisello, A.L.; Barbanera, M.; Cotana, F. Lignocellulosic ethanol production from the recovery of stranded driftwood residues. Energies 2016, 9, 634. [Google Scholar] [CrossRef]
- Kandasamy, M.; Hamawand, I.; Bowtell, L.; Seneweera, S.; Chakrabarty, S.; Yusaf, T.; Shakoor, Z.; Algayyim, S.; Eberhard, F. Investigation of ethanol production potential from lignocellulosic material without enzymatic hydrolysis using the ultrasound technique. Energies 2017, 10, 62. [Google Scholar] [CrossRef]
- Gregg, J.S.; Bolwig, S.; Hansen, T.; Solér, O.; Amer-Allam, S.B.; Viladecans, J.P.; Klitkou, A.; Fevolden, A. Value chain structures that define European cellulosic ethanol production. Sustainability 2017, 9, 118. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Pisello, A.L.; Gelosia, M.; Ingles, D.; Pompili, E. Sustainable ethanol production from common reed (Phragmites australis) through simultaneuos saccharification and fermentation. Sustainability 2015, 7, 12149–12163. [Google Scholar] [CrossRef]
- Hansdah, D.; Murugan, S.; Das, L.M. Experimental studies on a DI diesel engine fueled with bioethanol-diesel emulsions. Alexandria Eng. J. 2013, 52, 267–276. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, Y.; Zhang, G.; Fang, Y.; Xiao, Y.; Zhao, H. Improving production of bioethanol from duckweed (Landoltia punctata) by pectinase pretreatment. Energies 2012, 5, 3019–3032. [Google Scholar] [CrossRef]
- Rajendran, K.; Rajoli, S.; Taherzadeh, M.J. Techno-economic analysis of integrating first and second-generation ethanol production using filamentous fungi: An industrial case study. Energies 2016, 9, 359. [Google Scholar] [CrossRef]
- Lopez, L.; Velasco, J.; Montes, V.; Marinas, A.; Cabrera, S.; Boutonnet, M.; Järås, S. Synthesis of Ethanol from Syngas over Rh/MCM-41 Catalyst: Effect of Water on Product Selectivity. Catalysts 2015, 5, 1737–1755. [Google Scholar] [CrossRef]
- Oswald, F.; Zwick, M.; Omar, O.; Hotz, E.N.; Neumann, A. Growth and product formation of Clostridium ljungdahlii in presence of cyanide. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Kopke, M.; Held, C.; Hujer, S.; Liesegang, H.; Wiezer, A.; Wollherr, A.; Ehrenreich, A.; Liebl, W.; Gottschalk, G.; Durre, P. Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc. Natl. Acad. Sci. USA 2010, 107, 13087–13092. [Google Scholar] [CrossRef]
- Younesi, H.; Najafpour, G.; Mohamed, A.R. Ethanol and acetate production from synthesis gas via fermentation processes using anaerobic bacterium, Clostridium ljungdahlii. Biochem. Eng. J. 2005, 27, 110–119. [Google Scholar] [CrossRef]
- Richter, H.; Martin, M.E.; Angenent, L.T. A two-stage continuous fermentation system for conversion of syngas into ethanol. Energies 2013, 6, 3987–4000. [Google Scholar] [CrossRef]
- Devarapalli, M.; Lewis, R.; Atiyeh, H. Continuous Ethanol Production from Synthesis Gas by Clostridium ragsdalei in a Trickle-Bed Reactor. Fermentation 2017, 3, 23. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V. Combined biogas and bioethanol production: Opportunities and challenges for industrial application. Energies 2015, 8, 8121–8144. [Google Scholar] [CrossRef]
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- Wang, L.J.; Song, R.Z.; Zou, H.B.; Liu, S.H.; Zhou, L.B. Study on combustion characteristics of a methanol-diesel dual-fuel compression ignition engine. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2008, 222, 619–627. [Google Scholar] [CrossRef]
- Lackner, K.S. Capture of carbon dioxide from ambient air. Eur. Phys. J. Spec. Top. 2009, 176, 93–106. [Google Scholar] [CrossRef]
- Alam, M.; Song, J.; Zello, V.; Boehman, A. Spray and combustion visualization of a direct-injection diesel engine operated with oxygenated fuel blends. Int. J. Engine Res. 2006, 7, 503–521. [Google Scholar] [CrossRef]
- Tesfa, B.; Gu, F.; Mishra, R.; Ball, A.D. LHV predication models and LHV effect on the performance of CI engine running with biodiesel blends. Energy Convers. Manag. 2013, 71, 217–226. [Google Scholar] [CrossRef]
- García, M.; Gonzalo, A.; Sánchez, J.L.; Arauzo, J.; Peña, J.Á. Prediction of normalized biodiesel properties by simulation of multiple feedstock blends. Bioresour. Technol. 2010, 101, 4431–4439. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Subbaiah, G.V.; Gopal, K.R.; Hussain, S.A.; Prasad, B.D.; Reddy, K.T.; Pradesh, A. Rice Bran Oil Biodiesel As an Additive in Diesel-Ethanol Blends for Diesel Engines. Int. J. Recent Res. Appl. Stud. 2010, 3, 334–342. [Google Scholar]
- Shudo, T.; Nakajima, T.; Hiraga, K. Simultaneous reduction in cloud point, smoke, and NOxemissions by blending bioethanol into biodiesel fuels and exhaust gas recirculation. Int. J. Engine Res. 2009, 10, 15–26. [Google Scholar] [CrossRef]
- Ali, O.M.; Yusaf, T.; Mamat, R.; Abdullah, N.R.; Abdullah, A.A. Influence of chemical blends on palm oil methyl esters’ cold flow properties and fuel characteristics. Energies 2014, 7, 4364–4380. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M.P. Impact of cold flow properties of biodiesel on engine performance. Renew. Sustain. Energy Rev. 2014, 31, 650–656. [Google Scholar] [CrossRef]
- Yasin, M.H.M.; Mamat, R.; Yusop, A.F.; Rahim, R.; Aziz, A.; Shah, L.A. Fuel physical characteristics of biodiesel blend fuels with alcohol as additives. Procedia Eng. 2013, 53, 701–706. [Google Scholar] [CrossRef]
- Beatrice, C.; Napolitano, P.; Guido, C. Injection parameter optimization by DoE of a light-duty diesel engine fed by Bio-ethanol/RME/diesel blend. Appl. Energy 2014, 113, 373–384. [Google Scholar] [CrossRef]
- Li, W.; Ren, Y.; Wang, X.B.; Miao, H.; Jiang, D.M.; Huang, Z.H. Combustion characteristics of a compression ignition engine fuelled with diesel-ethanol blends. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2008, 222, 265–274. [Google Scholar] [CrossRef]
- Sastry, G.R.K.; Deb, M.; Panda, J.K. Effect of Fuel Injection Pressure, Isobutanol and Ethanol Addition on Performance of Diesel-biodiesel Fuelled D.I. Diesel Engine. Phys. Procedia 2015, 66, 81–84. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Pyrc, M.; Sobiepański, M. Investigation on combustion process and emissions characteristic in direct injection diesel engine powered by wet ethanol using blend mode. Fuel Process. Technol. 2016, 149, 86–95. [Google Scholar] [CrossRef]
- Bharadwaz, Y.D.; Rao, B.G.; Rao, V.D.; Anusha, C. Improvement of biodiesel methanol blends performance in a variable compression ratio engine using response surface methodology. Alexandria Eng. J. 2016, 55, 1201–1209. [Google Scholar] [CrossRef]
- Soni, D.K.; Gupta, R. Numerical investigation of emission reduction techniques applied on methanol blended diesel engine. Alexandria Eng. J. 2016, 55, 1867–1879. [Google Scholar] [CrossRef]
- Hasimoglu, C. Exhaust emission characteristics of a low-heat-rejection diesel engine fuelled with 10 per cent ethanol and 90 per cent diesel fuel mixture. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2008, 222, 93–100. [Google Scholar] [CrossRef]
- Mofijur, M.; Rasul, M.G.; Hyde, J. Recent developments on internal combustion engine performance and emissions fuelled with biodiesel-diesel-ethanol blends. Procedia Eng. 2015, 105, 658–664. [Google Scholar] [CrossRef]
- Niculescu, R.; Iosub, I.; Clenci, A.; Zaharia, C.; Iorga-Simǎn, V. Development of a test method for distillation of diesel-biodiesel-alcohols mixtures at reduced pressure. IOP Conf. Ser. Mater. Sci. Eng. 2017. [Google Scholar] [CrossRef]
- How, H.G.; Masjuki, H.H.; Kalam, M.A.; Teoh, Y.H. Engine performance, emission and combustion characteristics of a common-rail diesel engine fuelled with bioethanol as a fuel additive in coconut oil biodiesel blends. Energy Procedia 2014, 61, 1655–1659. [Google Scholar] [CrossRef]
- Yasin, M.H.M.; Mamat, R.; Yusop, A.F.; Aziz, A.; Najafi, G. Comparative Study on Biodiesel-methanol-diesel Low Proportion Blends Operating with a Diesel Engine. Energy Procedia 2015, 75, 10–16. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Saray, R.K.; Poorghasemi, K.; Maghbouli, A. A numerical investigation on combustion and emission characteristics of a dual fuel engine at part load condition. Fuel 2016, 166, 309–319. [Google Scholar] [CrossRef]
- Al-lwayzy, S.H.; Yusaf, T. Combustion of microalgae oil and ethanol blended with diesel fuel. Energies 2015, 8, 13985–13995. [Google Scholar] [CrossRef]
- Ali, O.M.; Abdullah, N.R.; Mamat, R.; Abdullah, A.A. Comparison of the Effect of Different Alcohol Additives with Blended Fuel on Cyclic Variation in Diesel Engine. Energy Procedia 2015, 75, 2357–2362. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Zheng, Z.; Gu, J.; Wang, H.; Yao, M. Experimental and simulation investigation of the combustion characteristics and emissions using n-butanol/biodiesel dual-fuel injection on a diesel engine. Energy 2014, 74, 741–752. [Google Scholar] [CrossRef]
- Ileri, E.; Atmanli, A.; Yilmaz, N. Comparative analyses of n-butanol–rapeseed oil–diesel blend with biodiesel, diesel and biodiesel–diesel fuels in a turbocharged direct injection diesel engine. J. Energy Inst. 2016, 89, 586–593. [Google Scholar] [CrossRef]
- Kumar, M.S.; Bellettre, J.; Tazerout, M. The use of biofuel emulsions as fuel for diesel engines: A review. Proc. Inst. Mech. Eng. Part A J. Power Energy 2009, 223, 729–742. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lee, W.J.; Wu, T.S.; Wu, C.Y.; Chen, S.J. Use of water containing acetone-butanol-ethanol for NOx-PM (nitrogen oxide-particulate matter) trade-off in the diesel engine fueled with biodiesel. Energy 2014, 64, 678–687. [Google Scholar] [CrossRef]

| Name of FA | Structure (xx:y) 1 | Type 2 | Formula |
|---|---|---|---|
| Lauric | 12:0 | S | C12H24O2 |
| Myristic | 14:0 | S | C14H28O2 |
| Palmitic | 16:0 | S | C16H32O2 |
| Stearic | 18:0 | S | C18H36O2 |
| Oleic cis-9- | 18:1 | US | C18H34O2 |
| Linoleic cis-9, cis-12- | 18:2 | US | C18H32O2 |
| Linoleic | 18:3 | US | C18H30O2 |
| Arachidic | 20:0 | S | C20H40O2 |
| Behenic | 22:0 | S | C22H44O2 |
| Erucle | 22:1 | US | C22H42O2 |
| Lignoceric | 24:0 | S | C24H48O2 |
| Vegetable Oil | FA Composition (wt %) | |||||
|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 | |
| Cottonseed | 28.7 | – | 0.9 | 13.0 | 57.4 | – |
| Rapeseed | 3.5 | – | 0.9 | 64.1 | 22.3 | 8.2 |
| Rapeseed | 3 | – | 1 | 64 | 22 | 8 |
| Safflower-seed | 7.3 | – | 1.9 | 13.6 | 77.2 | – |
| Safflower | 9 | – | 2 | 12 | 78 | – |
| High oleic Safflower oil | 5 | – | 2 | 79 | 13 | – |
| Sunflower | 6.4 | 0.1 | 2.9 | 17.7 | 72.9 | – |
| Sunflower | 6 | – | 3 | 17 | 74 | – |
| Sesame | 13.1 | – | 3.9 | 52.8 | 30.2 | – |
| Sesame | 13 | – | 4 | 53 | 30 | – |
| Linseed | 5.1 | 0.3 | 2.5 | 18.9 | 18.1 | 55.1 |
| Linseed | 5 | – | 2 | 20 | 18 | 55 |
| Palm | 42.6 | 0.3 | 4.4 | 40.5 | 10.1 | 0.2 |
| Palm tree | 35 | – | 7 | 44 | 14 | – |
| Corn marrow | 11.8 | – | 2.0 | 24.8 | 61.3 | – |
| Corn | 12 | – | 2 | 25 | 6 | Tr |
| Soybean | 13.9 | 0.3 | 2.1 | 23.2 | 56.2 | 4.3 |
| Soya bean | 14 | – | 4 | 24 | 52 | – |
| Soya bean | 12 | – | 3 | 23 | 55 | 6 |
| Property | SAF-Flower | Soy | Sun-Flower | Tallow | Yellowgrease | Coco-Nut | Corn | Jotro-Pha | Palm | Rape-Seed | Cottonseed [8] | Rice Bran [86] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfur content [ppm] | - | 2 | 2 | 7 | 5 | 3 | 4 | 5 | 2 | 4 | - | - |
| Kinematic viscosity [@40 °C, mm2/s] | 4.14 | 4.26 | 4.42 | 4.69 | 4.80 | 2.75 | 4.19 | 4.75 | 4.61 | 4.50 | 4.00 | 4.63 |
| Cloud point, CP [°C] | -4 | 0 | 2 | 13 | 8 | -3 | -3 | 5 | 14 | -3 | - | - |
| Cloud Filter Plugging Point (CFPP) [°C] | −6 | −4 | −2 | 13 | 1 | −5 | −8 | - | 9 | −12 | - | - |
| Pour point, PP [°C] | −7 | −4 | −2 | 10 | 3 | −9 | −2 | 0 | 13 | −10 | - | 3 |
| Flash point, FP [°C] | 174 | 159 | 175 | 124 | 161 | 113 | 171 | 152 | 163 | 169 | - | 165 |
| Cetane number, CN | 51.1 | 51.3 | 51.1 | 58.9 | 56.9 | 59.3 | 55.7 | 55.7 | 61.9 65.8 [87] | 53.7 | 52.0 | 56.2 |
| Iodine value | 141.0 | 125.5 | 128.7 | 65.9 | 89.9 | 18.5 | 101.0 | 109.5 | 54.0 | 116.1 | - | 102.0 |
| Specific gravity | 0.879 | 0.882 | 0.878 | 0.878 | 0.879 | 0.874 | 0.883 | 0.876 | 0.873 | 0.879 | 0.885 | - |
| Lower heating value [MJ/kg] | - | 37.0 | 35.3 | 37.2 | 37.6 | 35.2 | 39.9 | 37.7 | 37.3 | 37.6 | 37.5 | 38.725 |
| Higher heating value [MJ/kg] | 42.2 | 39.7 | 40.6 | 37.0 | 39.4 | 38.1 | 43.1 | 40.7 | 40.6 | 41.1 | - | - |
| Average chain length | 17.8 | 17.9 | 18.1 | 17.3 | 18.5 | 13.4 | 17.6 | 18.3 | 17.2 | 17.9 | - | - |
| Average Unsaturation | 1.63 | 1.50 | 1.59 | 0.59 | 1.06 | 0.12 | 1.46 | 1.15 | 0.62 0.494 [88] | 1.31 | - | - |
| Boiling point [°C] | - | - | -- | - | - | - | - | - | - | - | 280–400 | - |
| Stoichiometricair-fuel ratio | - | - | - | - | - | - | - | - | - | - | 12.5 | - |
| Number of references | 4 | 59 | 20 | 12 | 37 | 7 | 6 | 23 | 44 | 39 | 1 | 1 |
| Properties Fuel | Specifications | |
|---|---|---|
| (Fuel) Standard/Test Method | Limits | |
| Density at 15 °C (kg/m3) | (DIESEL) EN590/EN 3675 | 820–845 |
| (B100) EN 14214/EN 3675 | 860–900 | |
| Kinematic viscosity at 40 °C (mm2/s) | (DIESEL) EN 590/EN 3104 | 2.0–4.5 |
| (B100) EN 14214/EN 3104-3105 | 3.5–5.0 | |
| (B100) ASTM D 6751-08/D 445 | 1.9–6.0 | |
| Cetane number | (DIESEL) EN590/EN5165 | Min. 51 |
| (DIESEL) ASTM D975-07/D613 | Min. 40 | |
| (B6–20) ASTM D7467-08/D613 | Min. 40 | |
| (B100) EN14214/EN5165 | Min. 51 | |
| (B100) ASTM D6751-08/D613 | Min. 47 | |
| Water content (% v/v) | (DIESEL) EN590/EN12937 | Max. 0.02 |
| (B6–20) ASTM D7467-08/D2709 | Max. 0.05 | |
| (B100) EN14214/EN12937 | Max. 0.05 | |
| (B100) ASTM D6751-08/D2709 | Max. 0.05 | |
| Distillation characteristics, T90 (°C) | (DIESEL) ASTM D975/D86 | Max. 338 |
| (B6-20) ASTM D7467-08/D86 | Max. 343 | |
| (B100) ASTM D6751-12/D7501; D1160 | Max. 360 | |
| Lubricity, HFRR@60 °C Micrometer | (DIESEL) ASTM590/ISO12156-1 | Max. 460 |
| (B6-20) ASTM D7467-08/D6079 | Max. 460 | |
| (B100) ASTM D6751-08/ | - | |
| Flash Point (°C) | (DIESEL) EN590/EN2719 | Min. 55 |
| (B6-20) ASTM D7467-08/ | Min. 38 | |
| (B100) EN14214/EN2719; 3679 | Min. 101 | |
| (B100) ASTM D6751-12/D93 | Min. 93 | |
| Sulfur content (mg/kg) | (DIESEL) EN590/EN20846; 20884 | Max. 50 |
| (B6-20) ASTM D7467-08/ | - | |
| (B100) EN14214/EN20846; 20,884 | Max. 10 | |
| Type of Fuel | Standard Specifications | |
|---|---|---|
| US | EN | |
| Ethanol | ASTM4806 ASTM5798 | EN 15376 1 |
| Methanol | ASTM D5797 2 | EN 228-2008 (E) 3 |
| Property | Methanol | Ethanol |
|---|---|---|
| Formula | CH3OH | CH3CH2OH |
| Molecular weight [kg/kmol] | 32 | 46 |
| Oxygen (wt %) | 50.0 | 34.8 |
| Density@20 °C [kg/m3] | (787–792) | (780–820) 450 [93] |
| Lower heating value (LHV) [MJ/kg] | (19.9–22.7) | (26.4–26.9) 28,959 [93]; 29.38 [69] |
| Octane number, RON | (109–114) | (107–111) |
| Cetane number, CN | 3 | (5–8) 10 [86]; 11 [1,94] |
| Stoichiometric air–fuel ratio, A/F [kg/kg] | 6.5 | (8.9–9.0) |
| Latent heat of vaporization [kJ/kg] | (1101–1168) | (837–840) 880 [92]; 879 [13] |
| Boiling point [°C] | (64–65) | (78–78.3) |
| Flame speed [m/s] | - | 0.39 |
| Specific heat [kJ/kg K] | 1.44 | 2.4 |
| Vapor pressure@20 °C, [KPa] | 12.9 52 [13] | 5.9 21 [13] |
| Kinematic viscosity@20 °C [mm2/s] | (0.759–1.01) | 1.16–1.87 |
| Flash point [°C] | (11–12) | 9–17.2 22 [86]; 24 [69] |
| Auto-ignition temperature, [°C] | (470–500) | (365–450) 636 [92] |
| Fuels/Experimental Details | Results | Reference Fuel | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Duration of Combustion DoC | Rate of Heat Release RoHR | Injection Delay ID | In-cylinder pressure pcyl | In-cylinder temperature Tcyl | |||
| B10D90; B20D80; B50D50; E5D95; E10D90; E15D85
| NA | Higher at the beginning of combustion and lower later on into the expansion stroke for BxDy blends | Slightly decreased for BxDy blends | Higher at the beginning of combustion and lower later on into the expansion stroke for BxDy blends | Higher at the beginning of combustion and lower later on into the expansion stroke for BxDy blends | Fossil diesel, D100 | [8] |
| The cumulative heat release values are lower at the beginning and catch up later on during the expansion stroke for ExDy blends | Slightly increased for ExDy blends | Unaltered for ExDy blends | Slightly reduced for ExDy blends | ||||
E5D95; E10D90; E15D85
| Decreases when increasing the bioethanol percentage | Higher. The maxi- mum value is found to be the highest for D85E15 followed by D95E5, D90E10 | Higher at all loads | Higher at full load. The maximum cylinder pressure increases when increasing the amount of alcohol | NA | Fossil diesel, D100 | [69] |
B10E20D70
| NA | NA | NA | A slight decrease | NA | B10D90 | [91] |
E5D95; E10D90; E15D85; E20D80
| The total combustion duration decreases when increasing the ethanol amount or the oxygen mass fraction with and without a CN improver. | The maximum value increases with increasing the ethanol fraction in the blends | Increases with the increase of the ethanol fraction | Without the CN improver, it increases with an increase in the ethanol fraction | NA | Fossil diesel, D100 | [92] |
| NA | Decreases with the addition of a small amount of a CN improver to diesel–ethanol blends | With CN improver, it decreases, but it remains bigger than the case of neat diesel fuel | |||||
E5D95; E10D90; … E45D55
| Decreases in both cases with the increase of the ethanol fraction. LPSS—the decrease is linear. HPPS—combustion duration decreases to 15% ethanol in the diesel blend; after this, it remains near-constant of up to 35% ethanol | NA | Increases with the increase of the ethanol fraction. With the increase of pressure in the supply system (HPSS), it grows less | Higher with an increasing ethanol amount and supply pressure | NA | Fossil diesel, D100 | [94] |
B20E5D75
| NA | Slightly lower | NA | Slightly lower | NA | Fossil diesel, D100 | [100] |
B10E10D80
| NA | NA | NA | Very comparable | NA | Fossil diesel, D100 | [103] |
| Diesel–Methanol dual-fuel
| Decreases with an increase in methanol mass fraction | Increases with an increase in methanol mass fraction | Increases with an increase in methanol mass fraction | Higher with increasing in methanol mass fraction | NA | Fossil diesel, D100 | [80] |
| Fuels/Experimental Details | Results | Reference Fuel | Ref. | ||||
|---|---|---|---|---|---|---|---|
| BSFC | BTE | BP | EGT | IMEP | |||
| B10D90; B20D80; B50D50; E5D95; E10D90; E15D85
| Increased with all bio-fuel blends | Slightly higher | NA | Slightly lower | NA | Fossil diesel, D100 | [8] |
| Review on the effect of ethanol in the biodiesel-diesel blend | Higher | NA | NA | NA | NA | Fossil diesel, D100 | [98] |
B95M5, B90M10, B85M15
| Increases with methanol content in the blend | Increases with methanol content in the blend | NA | NA | NA | Fossil diesel, D100 | [95] |
| B20M5D75, B20M10D70, B20D80, D100
| Increases with methanol amount, especially at low engine loads | NA | NA | Increases with methanol amounts, especially at high engine loads | NA | Fossil diesel, D100 | [101] |
E5D95; E10D90; … E45D55
| NB. The authors did not specify the kind of SFC that was being reported (BSFC or ISFC?) When using HPSS: almost constant up to 35% ethanol fuel fraction in diesel blends; further increasing the ethanol percentage generates an increase in BSFC | Increases, the maximum increase is at 35% ethanol when using HPSS | NA | NA | Almost constant, until 35% ethanol. Cycle variation, expressed as COVIMEP, increases with increasing ethanol amount | Fossil diesel, D100 | [94] |
| When using LPSS: it increases with the increase of ethanol fraction | It decreases when using LPSS | It slightly decreases. Cycle variation, expressed as leftIMEP, increases with increasing ethanol amount | |||||
| Isobutanol/ethanol as additives in diesel biodiesel blends B20D70Alcohol10 B10D80Alcohol10 B30D70, D100, B100
| For some blends, it increases, for others it decreases; however, there is a decreasing trend for all blends with the increase in injection pressure (200 to 275 bar) | Higher when the injection pressure is between 225–250 bar | NA | NA | NA | Fossil diesel, D100 | [93] |
| Smaller or almost equal at an injection pressure below 225 and above 250 bar. | |||||||
B20E5D75
| (2.0–2.7)% higher | (3.0–5.4)% higher | NA | NA | NA | Fossil diesel, D100 | [100] |
B10E20D70
| Higher | NA | NA | NA | NA | B10 | [91] |
| B100; B10E5D85; B10E10D80; B10E15D75
| Higher | A maximum BTE of 28.2% was observed with blend B10E15 | NA | At B10E15 it was slightly lower | NA | Fossil diesel, D100 | [86] |
B95E5
| Slightly lower | NA | Slightly higher | NA | NA | B100 | [88] |
| Higher | NA | Lower | NA | NA | Fossil diesel, D100 | ||
B10E10D80
| Very comparable | NA | Very comparable | Minor variations | NA | Fossil diesel, D100 | [103] |
B30D70; B50D50
| Higher | NA | Lower | NA | NA | Fossil diesel, D100 | [13] |
D95E5; D90E10; D85E15
| Higher for all loads (only at 25% load until 5% ethanol, it decreases) | NA | NA | Slightly increases with the increase of ethanol for all loads | NA | Fossil diesel, D100 | [69] |
| Fuels/Experimental Details | Results | Reference Fuel | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| NOx | Smoke | CO | UHC | PM | CO2 | |||
| B10D90; B20D80; B50D50; E5D95; E10D90; E15D85
| Decreases for all blends Increases with the percentage of biofuel in the blend | Decreases with an increase of the percentage of biofuel in the blend | Decreases for all blends Increases with the percentage of biofuel in the blend | Increases | NA | NA | Fossil diesel, D100 | [8] |
| Review on the effects of ethanol in the biodiesel–diesel blend | Decreases significantly | Decreases with the increase of the percentage of ethanol | NA | Decreases significantly | Decreases | NA | Fossil diesel, D100 | [98] |
B95M5, B90M10, B85M15
| Decreases while adding methanol in biodiesel fuel | Decreases while adding methanol in biodiesel fuel | Increases while adding methanol in biodiesel fuel | Increases | NA | NA | Fossil diesel, D100 | [95] |
| B20M5D75, B20M10D70, B20D80, D100
| Decreases significantly Decreases while adding methanol | NA | Slightly increases while adding methanol | NA | NA | NA | Fossil diesel, D100 | [101] |
E5D95; E10D90; … E45D55
| Increases with the increase of the ethanol fraction in the blend until the combustion process starts to deteriorate | NA | Eight-times decrease (35, 45% ethanol), LPSS | Increases with a higher ethanol fraction, or it was almost at a constant level. It improves for 20–35% ethanol in case of HPSS-1bar | NA | Decreases with the increase of the ethanol amount It is higher at HPSS | Fossil diesel, D100 | [94] |
| Five-times Decrease (35, 45% ethanol), HPSS | ||||||||
| Isobutanol/ethanol as an additive in diesel–biodiesel blends B20-D70-alcohol10 B10-D80-alcohol10 B30-D70 D100 B100
| NOx emissions were decreased slightly; increase with an increase of the injection pressure (200 to 275 bar) | NA | NA | NA | NA | NA | Fossil diesel, D100 | [93] |
B20E5D75
| Decreases | Decreases | Decreases | NA | NA | NA | Fossil diesel, D100 | [100] |
B10E20D70
| Decreases | NA | Increases at low loads | Increases at low loads | Decreases | NA | B10 | [91] |
| B100; B10E5D85; B10E10D80; B10E15D75
| Low emissions for biodiesel and for all of the other fuel blends at lower loads High emissions at higher loads | Decreases; the lowest value was obtained for the blend B10E15 | Lower; minimum CO emissions for B10E15 | Increases with the increase of the ethanol percentage, but lower than those of the diesel at higher loads | NA | Higher | Fossil diesel, D100 | [86] |
B80E20; B60E40
| Decreases with the increase of ethanol amount | Decreases with the increase of ethanol (oxygen) amount | Slightly increases for B60E40These emissions are not very high and could be after-treated by oxidation catalysts | Slightly increases. These emissions are not very high and could be after-treated by oxidation catalysts | NA | NA | - | [87] |
B10E10D80
| Substantially lower | NA | Lower | Considerably lower | NA | Almost the same. Negligible reduction at low engine speeds | Fossil diesel, D100 | [103] |
E10D90
| Lower | Lower | NA | NA | NA | NA | Engine standard | [97] |
B30D70; B50D50; B100
| Increases | NA | Decreases | Decreases | Decreases | Fossil diesel, D100 | [13] | |
Different blends of diesel–ethanol
| NA | Decreases | Decreases | NA | NA | Drastically decreases with the fraction of ethanol | Fossil diesel, D100 | |
Different blends of diesel–ethanol
| Decreases when using alcohol fuels in fumigation mode | Decreases in both modes (fumigation and blended modes). | Increases in fumigation mode | Increases in fumigation mode | Decreases in both modes (fumigation and blended modes). | Decreases with using alcohol fuels in fumigation mode | Fossil diesel, D100 | [25] |
| Increases when using alcohol fuels in blended mode | Decreases with the use of alcohol fuels in blended mode | Decreases in blended mode | Increases with using alcohol fuels in blended mode | |||||
| E5D95; E10D90; E15D85
| Lower Decreases with bioethanol amount increase | Decreases substantially with an increase in the amount of bioethanol (E5 decrease is 2.31%; E15 decrease is 20.8%) | NA | Lower for E5D95 Higher for E10D90 | NA | NA | Fossil diesel, D100 | [69] |
| D90M10; D80M20; D70M30 | Decreases with an increase in the percentage of methanol in diesel blends | Increases with an increase in the percentage of methanol in diesel blends | Decreases with an increasing percent of methanol | Decreases with the increasing percent of methanol | NA | NA | Fossil diesel, D100 | [96] |
| 15 vol % water addition reduces NO emissions by up to 95% | 15 vol % water addition, the soot mass fraction could decrease by up to 14 % | 15 vol % water addition, reduces CO emission up to 29% | 15 vol % water addition in D70M30 results in a 36% reduction in HC emission | NA | D70M30 | |||
| 20% increase in the amount of EGR reduces NO by 36% | NA | NA | NA | NA | ||||
| Diesel–methanol dual-fuel
| Decreases with an increase of the percent of the injected mass of methanol | Decreases with an increase of the percent of the injected mass of methanol | Increases with an increase in the percent of the injected mass of methanol | Increases with increase of the percent of the injected mass of methanol | NA | NA | Fossil diesel, D100 | [80] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculescu, R.; Clenci, A.; Iorga-Siman, V. Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines. Energies 2019, 12, 1194. https://doi.org/10.3390/en12071194
Niculescu R, Clenci A, Iorga-Siman V. Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines. Energies. 2019; 12(7):1194. https://doi.org/10.3390/en12071194
Chicago/Turabian StyleNiculescu, Rodica, Adrian Clenci, and Victor Iorga-Siman. 2019. "Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines" Energies 12, no. 7: 1194. https://doi.org/10.3390/en12071194
APA StyleNiculescu, R., Clenci, A., & Iorga-Siman, V. (2019). Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines. Energies, 12(7), 1194. https://doi.org/10.3390/en12071194






