A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration
Abstract
1. Introduction
AD Process and Main Parameters
2. Anaerobic Co-Digestion (AnCoD)
AnCoD Process and Main Parameters
3. Microbial Diversity in AnCoD
Microbial Community Structure and Synergy
4. Digester Stage
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, H.; Hu, Y. Municipal solid waste (MSW) as a renewable source of energy: Current and future practices in China. Bioresour. Technol. 2010, 101, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Poggio, A.; Grieco, E. Influence of flue gas cleaning system on the energetic efficiency and on the economic performance of a WTE plant. Waste Manag. 2010, 30, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Bujak, J.; Sitarz, P.; Jasiewicz, P. Fuel consumption in the thermal treatment of low-calorific industrial food processing waste. Appl. Energy 2018, 221, 139–147. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrve, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Di Maria, F.; Sisani, F.; Contini, S. Are EU waste-to-energy technologies effective for exploiting the energy in bio-waste? Appl. Energy 2018, 230, 1557–1572. [Google Scholar] [CrossRef]
- Cornelissen, S.; Koper, M.; Deng, Y.Y. The role of bioenergy in a fully sustainable global energy system. Biomass Bioenergy 2012, 41, 21–33. [Google Scholar] [CrossRef]
- Kwietniewska, E.; Tys, J. Process characteristics, inhibition factors and methane yields of anaerobic digestion process, with particular focus on microalgal biomass fermentation. Renew. Sustain. Energy Rev. 2014, 34, 491–500. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, M.; Kumfer, W.; Zhang, W.; Du, Y.; Bai, H. Effect of longitudinal slope of urban underpass tunnels on drivers’ heart rate and speed: A study based on a real vehicle experiment. Tunn. Undergr. Space Technol. 2018, 81, 525–533. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Schuetzle, D.; Schuetzle, R.; Kent Hoekman, S.; Zielinska, B. The effect of oxygen on formation of syngas contaminants during the thermochemical conversion of biomass. Int. J. Energy Environ. Eng. 2015, 6, 405–417. [Google Scholar] [CrossRef]
- Di Maria, F.; Barratta, M.; Bianconi, F.; Placidi, P.; Passeri, D. Solid anaerobic digestion batch with liquid digestate recirculation and wet anaerobic digestion of organic waste: Comparison of system performances and identification of microbial guilds. Waste Manag. 2017, 59, 172–180. [Google Scholar] [CrossRef]
- Mathes, P.G.; Pollard-Durodola, S.D.; Ca´rdenas-Hagan, E.; Linan-Thompson, S.; Vaughn, S. Teaching struggling readers who are native Spanish speakers: What do we know? Lang. Speech Hear. Serv. Sch. 2007, 38, 260. [Google Scholar] [CrossRef]
- European Comission. Best Available Techniques in the Slaughterhouses and Animal By-Products Industries; European Comission: Brussels, Belgium, 2005; 855p. [Google Scholar]
- Martinez, E.; Marcos, A.; Al-Kassir, A.; Jaramillo, M.A.; Mohamad, A.A. Mathematical model of a laboratory-scale plant for slaughterhouse effluents biodigestion for biogas production. Appl. Energy 2012, 95, 210–219. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Cardon, P.V. The Food and Agriculture Organization of the United Nations. Science 1953, 118, 3. [Google Scholar] [CrossRef]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Christopoulou, N.; Goumenaki, M. Optimization of biogas production from olive-oil mill wastewater, by codigesting with diluted poultry-manure. Appl. Energy 2007, 84, 646–663. [Google Scholar] [CrossRef]
- Hilkiah Igoni, A.; Ayotamuno, M.J.; Eze, C.L.; Ogaji, S.O.T.; Probert, S.D. Designs of anaerobic digesters for producing biogas from municipal solid-waste. Appl. Energy 2008, 85, 430–438. [Google Scholar] [CrossRef]
- Karagiannidis, A.; Perkoulidis, G. A multi-criteria ranking of different technologies for the anaerobic digestion for energy recovery of the organic fraction of municipal solid wastes. Bioresour. Technol. 2009, 100, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, R.; Lilly, S.J.; Kacprzak, G.G.; Churchill, C.W. Modeling the distribution of Mg II absorbers around galaxies using background galaxies and quasars. Astrophys. J. 2014, 784, 1–13. [Google Scholar] [CrossRef]
- Bayr, S.; Ojanperä, M.; Kaparaju, P.; Rintala, J. Long-term thermophilic mono-digestion of rendering wastes and co-digestion with potato pulp. Waste Manag. 2014, 34, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Mata-Alvarez, J.; Dosta, J.; Macé, S.; Astals, S. Codigestion of solid wastes: A review of its uses and perspectives including modeling. Crit. Rev. Biotechnol. 2011, 31, 99–111. [Google Scholar] [CrossRef]
- Silvestre, G.; Illa, J.; Fernández, B.; Bonmatí, A. Thermophilic anaerobic co-digestion of sewage sludge with grease waste: Effect of long chain fatty acids in the methane yield and its dewatering properties. Appl. Energy 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Vindis, P.; Stajnko, D.; Lakota, M. Options for Reduction of Maize Silage in Biogas Plant Drazenci. In DAAAM International Scientific Book; 2014; Chapter 9; pp. 121–130. Available online: https://doi.org/10.2507/daaam.scibook.2014.09 (accessed on 20 March 2019).
- Viotti, P.; Di Genova, P.; Falcioli, F. Numerical analysis of the anaerobic co-digestion of the organic fraction from municipal solid waste and wastewater: Prediction of the possible performances at Olmeto plant in Perugia (Italy). Waste Manag. Res. 2004, 22, 115–128. [Google Scholar] [CrossRef]
- Kayhanian, M. Biodegradability of the organic fraction of municipal solid waste in high-solids anaerobic digester. Waste Manag. Res. 1995, 13, 123–136. [Google Scholar] [CrossRef]
- Kangle, K.M.; Kore, V.S.; Kore, S.; Kulkani, G.S. Recent trends in anaerobic codigestion: A review. Univers. J. Environ. Res. 2012, 2, 210–219. [Google Scholar]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Anaerobic Digestion: Decision Support Software. Available online: http://erc.epa.ie/safer/resource?id=48a1566c-c3b5-102a-90c6-0593d266866d (accessed on 21 March 2019).
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas production from algal biomass: A review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Towey, F. The power of two. Lancet Respir. Med. 2013, 1, 368. [Google Scholar] [CrossRef]
- Heo, N.H.; Park, S.C.; Kang, H. Effects of mixture ratio and hydraulic retention time on single-stage anaerobic co-digestion of food waste and waste activated sludge. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2004, 39, 1739–1756. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Logan, B.E. Environmental Transport Processes, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2012; ISBN 9781118230107. [Google Scholar]
- Kim, S.H.; Han, S.K.; Shin, H.S. Kinetics of LCFA inhibition on acetoclastic methanogenesis, propionate degradation and beta-oxidation. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2004, 39, 1025–1037. [Google Scholar] [CrossRef]
- Pouget, E.R.; Hagan, H.; Des Jarlais, D.C. Meta-analysis of hepatitis c seroconversion in relation to shared syringes and drug preparation equipment. Addiction (Abingt. Engl.) 2012, 107, 1057–1065. [Google Scholar] [CrossRef]
- Azbar, N.; Ursillo, P.; Speece, R.E. Effect of process configuration and substrate complexity on the performance of anaerobic processes. Water Res. 2001, 35, 817–829. [Google Scholar] [CrossRef]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2003; ISBN 0471206938. [Google Scholar]
- Turovskiy, I.S.; Mathai, P.K. Wastewater Sludge Processing; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2005; ISBN 0471700541. [Google Scholar]
- Zahedi, S.; Rivero, M.; Solera, R.; Perez, M. Mesophilic anaerobic co-digestion of sewage sludge with glycerine: Effect of solids retention time. Fuel 2018, 215, 285–289. [Google Scholar] [CrossRef]
- De La Rubia, M.A.; Perez, M.; Romero, L.I.; Sales, D. Effect of solids retention time (SRT) on pilot scale anaerobic thermophilic sludge digestion. Process Biochem. 2006, 41, 79–86. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Divya, D.; Gopinath, L.R.; Merlin Christy, P. A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 2015, 42, 690–699. [Google Scholar] [CrossRef]
- Long, J.H.; Aziz, T.N.; Reyes, F.L.D.L.; Ducoste, J.J. Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Shah, F.A.; Mahmood, Q.; Rashid, N.; Pervez, A.; Raja, I.A.; Shah, M.M. Co-digestion, pretreatment and digester design for enhanced methanogenesis. Renew. Sustain. Energy Rev. 2015, 42, 627–642. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Bayanov, A.; Ivanova, T.; Havrland, B.; Kára, J.; Hanzlíková, I. Effect of different compositions on anaerobic co-digestion of cattle manure and agro-industrial by-products. Agron. Res. 2018, 16, 176–187. [Google Scholar]
- Alqaralleh, R.; Kennedy, K.; Delatolla, R.; Sartaj, M. Biogas recovery from hyper-thermophilic anaerobic co-digestion of thickened waste activated sludge, organic fraction of municipal solid waste and fat, oil and grease. J. Bioremediat. Biodegrad. 2017, 8, 408. [Google Scholar] [CrossRef]
- Panpong, K.; Srisuwan, G.; O-Thong, S.; Kongjan, P. Anaerobic co-digestion of canned seafood wastewater with glycerol waste for enhanced biogas production. Energy Procedia 2014, 52, 328–336. [Google Scholar] [CrossRef]
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Akyol, Ç.; Ozbayram, E.G.; Ince, O.; Kleinsteuber, S.; Ince, B. Anaerobic co-digestion of cow manure and barley: Effect of cow manure to barley ratio on methane production and digestion stability. Environ. Prog. Sustain. Energy 2016, 35, 589–595. [Google Scholar] [CrossRef]
- Gujer, W.; Zehnder, A.J.B. Conversion processes in anaerobic digestion. Water Sci. Technol. 1983, 15, 127–167. [Google Scholar] [CrossRef]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Moletta, R.; Escoffier, Y.; Ehlinger, F.; Coudert, J.P.; Leyris, J.P. On-line automatic control system. For monitoring an anaerobic fluidized-bed reactor: Response to organic overload. Water Sci. Technol. 1994, 30, 11–20. [Google Scholar] [CrossRef]
- Jenkins, S.R.; Morgan, J.M.; Zhang, X. Measuring the usable carbonate alkalinity of operating anaerobic digesters. Res. J. Water Pollut. Control Fed. 1991, 63, 28–34. [Google Scholar]
- Rozzi, A.; Di Pinto, A.C.; Limoni, N.; Tomei, M.C. Start-up and operation of anaerobic digesters with automatic bicarbonate control. Bioresour. Technol. 1994, 48, 215–219. [Google Scholar] [CrossRef]
- Wilcox, S.J.; Hawkes, D.L.; Hawkes, F.R.; Guwy, A.J. A neural network, based on bicarbonate monitoring, to control anaerobic digestion. Water Res. 1995, 29, 1465–1470. [Google Scholar] [CrossRef]
- Björnsson, L.; Murto, M.; Mattiasson, B. Evaluation of parameters for monitoring an anaerobic co-digestion process. Appl. Microbiol. Biotechnol. 2000, 54, 844–849. [Google Scholar] [CrossRef]
- Frigon, J.C.; Guiot, S.R. Impact of liquid-to-gas hydrogen mass transfer on substrate conversion efficiency of an upflow anaerobic sludge bed and filter reactor. Enzym. Microb. Technol. 1995, 17, 1080–1086. [Google Scholar] [CrossRef]
- Weiland, P.; Rozzi, A. Start-up, operation, monitoring and control of high-rate anaerobic treatment systems: Discusser’s report e anaerobic treatment technology for municipal and wastewater. Water Sci. Technol. 1991, 24, 257–277. [Google Scholar] [CrossRef]
- Pauss, A.; Andre, G.; Perrier, M.; Guiot, S.R. Liquid-to-gas mass transfer in anaerobic processes: Inevitable transfer limitations of methane and hydrogen in the biomethanation process. Appl. Environ. Microbiol. 1990, 56, 1636–1644. [Google Scholar]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef]
- Saha, M.; Eskicioglu, C.; Marin, J. Microwave, ultrasonic and chemo-mechanical pretreatments for enhancing methane potential of pulp mill wastewater treatment sludge. Bioresour. Technol. 2011, 102, 7815–7826. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Giordano, A.; Liotta, F.; Panico, A.; Pirozzi, F. Anaerobic co-digestion of organic wastes. Rev. Environ. Sci. Bio Technol. 2012, 11, 325–341. [Google Scholar] [CrossRef]
- Eastman, J.A.; Ferguson, J.F. Solubilization of particulate organic carbon during the acid phase of anaerobic digestion. Source J. (Water Pollut. Control Fed. Part I 1981, 53, 352–366. [Google Scholar]
- Noike, T.; Endo, G.; Chang, J.-E.; Yaguchi, J.-I.; Matsumoto, J.-I. Characteristics of carbohydrate degradation and the rate-limiting step in anaerobic digestion. Biotechnol. Bioeng. 1985, 27, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; El-Hassan, Z.; Olabi, A.G. Enhanced methane production from waste paper through anaerobic co-digestion with macroalgae. In Proceedings of the 10th International Conference on Sustainable Energy & Environmental Protection: Bioenergy and Biofuels, Madrid, Spain, 26–27 March 2017; pp. 1–9. [Google Scholar]
- Uggetti, E.; Passos, F.; Solé, M.; Garfí, M.; Ferrer, I. Recent achievements in the production of biogas from microalgae. Waste Biomass Valorization 2017, 8, 129–139. [Google Scholar] [CrossRef]
- Martínez-Valdez, F.; Komilis, D.; Saucedo-Castañeda, G.; Barrena, R.; Sanchez, A. The effect of a short term aerobic pretreatment step on the anaerobic co-digestion of the organic fraction of municipal solid wastes: Liquid extract addition versus solid phase addition. Waste Biomass Valorization 2017, 8, 1793–1801. [Google Scholar] [CrossRef]
- Arelli, V.; Begum, S.; Anupoju, G.R.; Kuruti, K.; Shailaja, S. Dry anaerobic co-digestion of food waste and cattle manure: Impact of total solids, substrate ratio and thermal pre treatment on methane yield and quality of biomanure. Bioresour. Technol. 2018, 253, 273–280. [Google Scholar] [CrossRef]
- Tedesco, S.; Benyounis, K.Y.; Olabi, A.G. Mechanical pretreatment effects on macroalgae-derived biogas production in co-digestion with sludge in Ireland. Energy 2013, 61, 27–33. [Google Scholar] [CrossRef]
- Lin, J.; Zuo, J.; Ji, R.; Chen, X.; Liu, F.; Wang, K.; Yang, Y. Methanogenic community dynamics in anaerobic co-digestion of fruit and vegetable waste and food waste. J. Environ. Sci. (China) 2012, 24, 1288–1294. [Google Scholar] [CrossRef]
- Supaphol, S.; Jenkins, S.N.; Intomo, P.; Waite, I.S.; Donnell, A.G.O.; O’Donnell, A.G. Microbial community dynamics in mesophilic anaerobic co-digestion of mixed waste. Bioresour. Technol. 2011, 102, 4021–4027. [Google Scholar] [CrossRef]
- Bedoya, K.; Coltell-Simon, O.; Cabarcas, F.; Alzate, J.F. Metagenomic assessment of the microbial community and methanogenic pathways in biosolids from a municipal wastewater treatment plant in Medellín, Colombia. Sci. Total Environ. 2019, 648, 572–581. [Google Scholar] [CrossRef]
- Ike, M.; Inoue, D.; Miyano, T.; Liu, T.T.; Sei, K.; Soda, S.; Kadoshin, S. Microbial population dynamics during startup of a full-scale anaerobic digester treating industrial food waste in Kyoto eco-energy project. Bioresour. Technol. 2010, 101, 3952–3957. [Google Scholar] [CrossRef]
- Kim, E.; Lee, J.; Han, G.; Hwang, S. Comprehensive analysis of microbial communities in full-scale mesophilic and thermophilic anaerobic digesters treating food waste-recycling wastewater. Bioresour. Technol. 2018, 259, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Rivière, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 2009, 3, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, Y.; Xiao, W.; Zhou, Z.; Yan, X. Performance and spatial community succession of an anaerobic baffled reactor treating acetone-butanol-ethanol fermentation wastewater. Bioresour. Technol. 2011, 102, 7407–7414. [Google Scholar] [CrossRef]
- Jihen, T.; Baligh, M.; Amel, F.; Said, N.; Moktar, H.; Maher, G.; Hassib, B. Microbial ecology overview during anaerobic codigestion of dairy wastewater and cattle manure and use in agriculture of obtained bio-fertilisers. Bioresour. Technol. 2015, 198, 141–149. [Google Scholar]
- Wang, H.; Lehtomäki, A.; Tolvanen, K.; Puhakka, J.; Rintala, J. Impact of crop species on bacterial community structure during anaerobic co-digestion of crops and cow manure. Bioresour. Technol. 2009, 100, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, R.; El-Mashad, H.M.; Dong, R. Effect of feed to inoculum ratios on biogas yields of food and green wastes. Bioresour. Technol. 2009, 100, 5103–5108. [Google Scholar] [CrossRef]
- Martín-González, L.; Castro, R.; Pereira, M.A.; Alves, M.M.; Font, X.; Vicent, T. Thermophilic co-digestion of organic fraction of municipal solid wastes with FOG wastes from a sewage treatment plant: Reactor performance and microbial community monitoring. Bioresour. Technol. 2011, 102, 4734–4741. [Google Scholar] [CrossRef]
- Yang, Z.H.; Xu, R.; Zheng, Y.; Chen, T.; Zhao, L.J.; Li, M. Characterization of extracellular polymeric substances and microbial diversity in anaerobic co-digestion reactor treated sewage sludge with fat, oil, grease. Bioresour. Technol. 2016, 212, 164–173. [Google Scholar] [CrossRef]
- Razaviarani, V.; Buchanan, I.D. Anaerobic co-digestion of biodiesel waste glycerin with municipal wastewater sludge: Microbial community structure dynamics and reactor performance. Bioresour. Technol. 2015, 182, 8–17. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.C.; Lee, J.; Wang, C.H.; Dai, Y.; Wah Tong, Y. Three-stage anaerobic co-digestion of food waste and horse manure. Sci. Rep. 2017, 7, 1269. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Biogas production from food waste via co-digestion and digestion—Effects on performance and microbial ecology. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Guo, X.; Zuo, J.; Wang, Y.; Zhang, M. A comparative study of thermophilic and mesophilic anaerobic co-digestion of food waste and wheat straw: Process stability and microbial community structure shifts. Waste Manag. 2018, 75, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Khura, T.K. Production of methane from anaerobic digestion of jatropha and pongamia oil cakes. Appl. Energy 2012, 93, 148–159. [Google Scholar] [CrossRef]
- Liew, L.N.; Shi, J.; Li, Y. Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 2012, 46, 125–132. [Google Scholar] [CrossRef]
- Cárdenas-Cleves, L.M.; Marmolejo-Rebellón, L.F.; Torres-Lozada, P. Anaerobic codigestion of sugarcane press mud with food waste: Effects on Hydrolysis stage, methane yield, and synergistic effects. Int. J. Chem. Eng. 2018, 2018, 9351848. [Google Scholar] [CrossRef]
- Osman, M.M.M.; Shao, X.; Zhao, D.; Basheer, A.K.; Jin, H.; Zhang, Y. Methane Production from alginate-extracted and non-extracted waste of Laminaria japonica: Anaerobic mono- and synergetic co-digestion effects on yield. Sustainability 2019, 11, 1269. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wen, X.; Xu, M.; Huang, X. Characteristics of extracellular polymeric substances and bacterial communities in an anaerobic membrane bioreactor coupled with online ultrasound equipment. Bioresour. Technol. 2012, 117, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Kim, S.; Bae, J.; Choi, O.; Ju, D.; Lee, J.; Sung, H.; Park, S.; Sang, B.I.; Um, Y. A pilot scale two-stage anaerobic digester treating food waste leachate (FWL): Performance and microbial structure analysis using pyrosequencing. Process Biochem. 2014, 49, 301–308. [Google Scholar] [CrossRef]
- Nopharatana, A.; Clarke, W.P.; Pullammanappallil, P.C.; Silvey, P.; Chynoweth, D.P. Evaluation of methanogenic activities during anaerobic digestion of municipal solid waste. Bioresour. Technol. 1998, 64, 169–174. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef] [PubMed]
- Veeken, A.; Hamelers, B. Effect of temperature on hydrolysis rates of selected biowaste components. Bioresour. Technol. 1999, 69, 249–254. [Google Scholar] [CrossRef]
- Leclerc, M.; Delgènes, J.P.; Godon, J.J. Diversity of the archaeal community in 44 anaerobic digesters as determined by single strand conformation polymorphism analysis and 16S rDNA sequencing. Environ. Microbiol. 2004, 6, 809–819. [Google Scholar] [CrossRef]
- Osaka, T.; Yoshie, S.; Tsuneda, S.; Hirata, A.; Iwami, N.; Inamori, Y. Identification of acetate- or methanol-assimilating bacteria under nitrate-reducing conditions by stable-isotope probing. Microb. Ecol. 2006, 52, 253–266. [Google Scholar] [CrossRef]
- Lorenzen, J.M.; Schauerte, C.; Hübner, A.; Kölling, M.; Martino, F.; Scherf, K.; Batkai, S.; Zimmer, K.; Foinquinos, A.; Kaucsar, T.; et al. Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur. Heart J. 2015, 36, 2184–2196. [Google Scholar] [CrossRef]
- Weiss, A.; Jérôme, V.; Freitag, R.; Mayer, H.K. Diversity of the resident microbiota in a thermophilic municipal biogas plant. Appl. Microbiol. Biotechnol. 2008, 81, 163–173. [Google Scholar] [CrossRef]
- Lalman, J.; Bagley, D.M. Effects of C18 long chain fatty acids on glucose, butyrate and hydrogen degradation. Water Res. 2002, 36, 3307–3313. [Google Scholar] [CrossRef]
- Nathao, C.; Sirisukpoka, U.; Pisutpaisal, N. Production of hydrogen and methane by one and two stage fermentation of food waste. Int. J. Hydrogen Energy 2013, 38, 15764–15769. [Google Scholar] [CrossRef]
- Azbar, N.; Speece, R.E. Two-phase, two-stage, and single-stage anaerobic process comparison. J. Environ. Eng. 2001, 127, 240–248. [Google Scholar] [CrossRef]
- Bekkering, J.; Broekhuis, A.A.; Van Gemert, W.J.T. Optimisation of a green gas supply chain—A review. Bioresour. Technol. 2010, 101, 450–456. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Gómez, X.; Escapa, A.; Morán, A. Evaluation and simultaneous optimization of bio-hydrogen production using 32factorial design and the desirability function. J. Power Sour. 2007, 169, 131–139. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Anaerobic mesophilic co-digestion of ensiled sorghum, cheese whey and liquid cow manure in a two-stage CSTR system: Effect of hydraulic retention time. Bioresour. Technol. 2015, 175, 553–562. [Google Scholar] [CrossRef]
- Demeyer, D.I.; Henderickx, H.K. The effect of C18 unsaturated fatty acids on methane production in vitro by mixed rumen bacteria. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1967, 137, 484–497. [Google Scholar] [CrossRef]
- Koster, I.W.; Cramer, A. Inhibition of methanogenesis from acetate in granular sludge by long-chain fatty acids. Appl. Environ. Microbiol. 1987, 53, 403–409. [Google Scholar] [PubMed]
- Thies, E.; Jenkins, T.; Stutzenberger, F. Effects of the detergent Tween 80 on Thermomonospora curvata. World J. Microbiol. Biotechnol. 1994, 10, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, K.; Matsuo, T.; Nagase, M. Mechanism of inhibition caused by long-chain fatty acids in anaerobic digestion process. Biotechnol. Bioeng. 1981, 23, 1591–1610. [Google Scholar] [CrossRef]
- Beccari, M.; Majone, M.; Torrisi, L. Two-reactor system with partial phase separation for anaerobic treatment of olive oil mill effluents. Water Sci. Technol. 1998, 38, 53–60. [Google Scholar] [CrossRef]
- Dinsdale, R.M.; Premier, G.C.; Hawkes, F.R.; Hawkes, D.L. Two-stage anaerobic co-digestion of waste activated sludge and fruit/vegetable waste using inclined tubular digesters. Bioresour. Technol. 2000, 72, 159–168. [Google Scholar] [CrossRef]
- Kinnunen, M.; Hilderbrandt, D.; Grimberg, S.; Rogers, S.; Mondal, S. Comparative study of methanogens in one- and two-stage anaerobic digester treating food waste. Renew. Agric. Food Syst. 2015, 30, 515–523. [Google Scholar] [CrossRef]
- Klocke, M.; Nettmann, E.; Bergmann, I.; Mundt, K.; Souidi, K.; Mumme, J.; Linke, B. Characterization of the methanogenic Archaea within two-phase biogas reactor systems operated with plant biomass. Syst. Appl. Microbiol. 2008, 31, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Shen, F.; Yuan, H.; Pang, Y.; Chen, S.; Zhu, B.; Zou, D.; Liu, Y.; Ma, J.; Yu, L.; Li, X. Performances of anaerobic co-digestion of fruit & vegetable waste (FVW) and food waste (FW): Single-phase vs. two-phase. Bioresour. Technol. 2013, 144, 80–85. [Google Scholar] [PubMed]
- Shin, S.G.; Han, G.; Lim, J.; Lee, C.; Hwang, S. A comprehensive microbial insight into two-stage anaerobic digestion of food waste-recycling wastewater. Water Res. 2010, 44, 4838–4849. [Google Scholar] [CrossRef]
- Lafitte-Trouqué, S.; Forster, C.F. Dual anaerobic co-digestion of sewage sludge and confectionery waste. Bioresour. Technol. 2000, 71, 77–82. [Google Scholar] [CrossRef]
- Ratanatamskul, C.; Wattanayommanaporn, O.; Yamamoto, K. An on-site prototype two-stage anaerobic digester for co-digestion of food waste and sewage sludge for biogas production from high-rise building. Int. Biodeterior. Biodegrad. 2015, 102, 143–148. [Google Scholar] [CrossRef]
- Bertin, L.; Grilli, S.; Spagni, A.; Fava, F. Innovative two-stage anaerobic process for effective codigestion of cheese whey and cattle manure. Bioresour. Technol. 2013, 128, 779–783. [Google Scholar] [CrossRef]
- Nasr, N.; Elbeshbishy, E.; Hafez, H.; Nakhla, G.; Hesham El Naggar, M. Comparative assessment of single-stage and two-stage anaerobic digestion for the treatment of thin stillage. Bioresour. Technol. 2012, 111, 122–126. [Google Scholar] [CrossRef]
- Park, Y.; Hong, F.; Cheon, J.; Hidaka, T.; Tsuno, H. Comparison of thermophilic anaerobic digestion characteristics between single-phase and two-phase systems for kitchen garbage treatment. J. Biosci. Bioeng. 2008, 105, 48–54. [Google Scholar] [CrossRef]
- Rao, P.; Arcury, T.A.; Quandt, S.A. Student participation in community-based participatory research to improve migrant and seasonal farmworker environmental health: Issues for success. J. Environ. Educ. 2004, 35, 3–15. [Google Scholar] [CrossRef]
- Yu, H.W.; Samani, Z.; Hanson, A.; Smith, G. Energy recovery from grass using two-phase anaerobic digestion. Waste Manag. 2002, 22, 1–5. [Google Scholar] [CrossRef]
- Kim, S.; Shin, H. Enhanced Lipid degradation in an upflow anaerobic sludge blanket reactor by integration with an acidogenic reactor. Water Environ. Res. 2010, 82, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Schievano, A.; Tenca, A.; Scaglia, B.; Merlino, G.; Rizzi, A.; Daffonchio, D.; Oberti, R.; Adani, F. Two-stage vs single-stage thermophilic anaerobic digestion: Comparison of energy production and biodegradation efficiencies. Environ. Sci. Technol. 2012, 46, 8502–8510. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.; Martín-Marroquín, J.M.; Sastre, E. Single-phase and two-phase anaerobic co-digestion of residues from the treatment process of waste vegetable oil and pig manure. Bioenergy Res. 2014, 7, 670–680. [Google Scholar] [CrossRef]
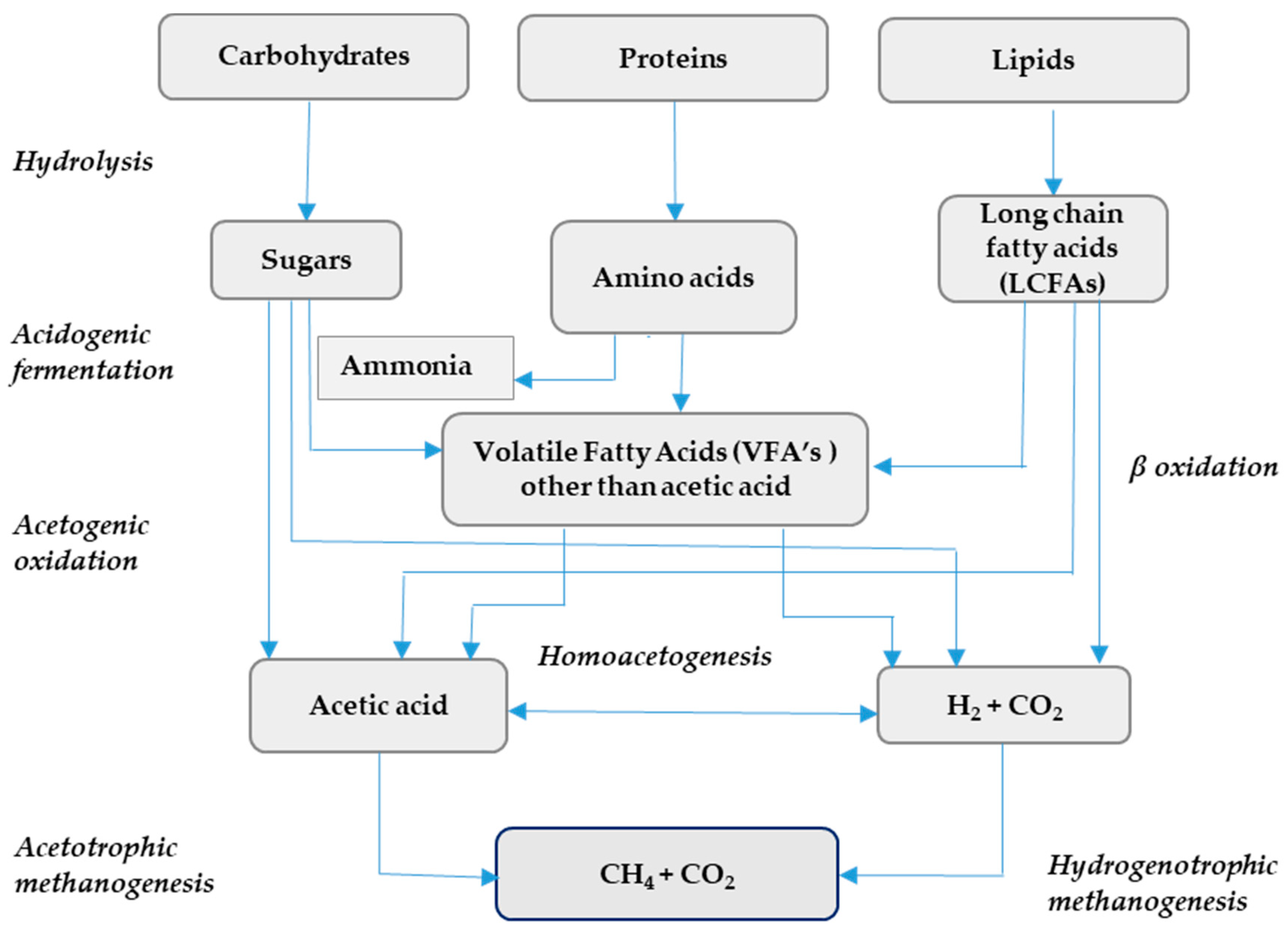
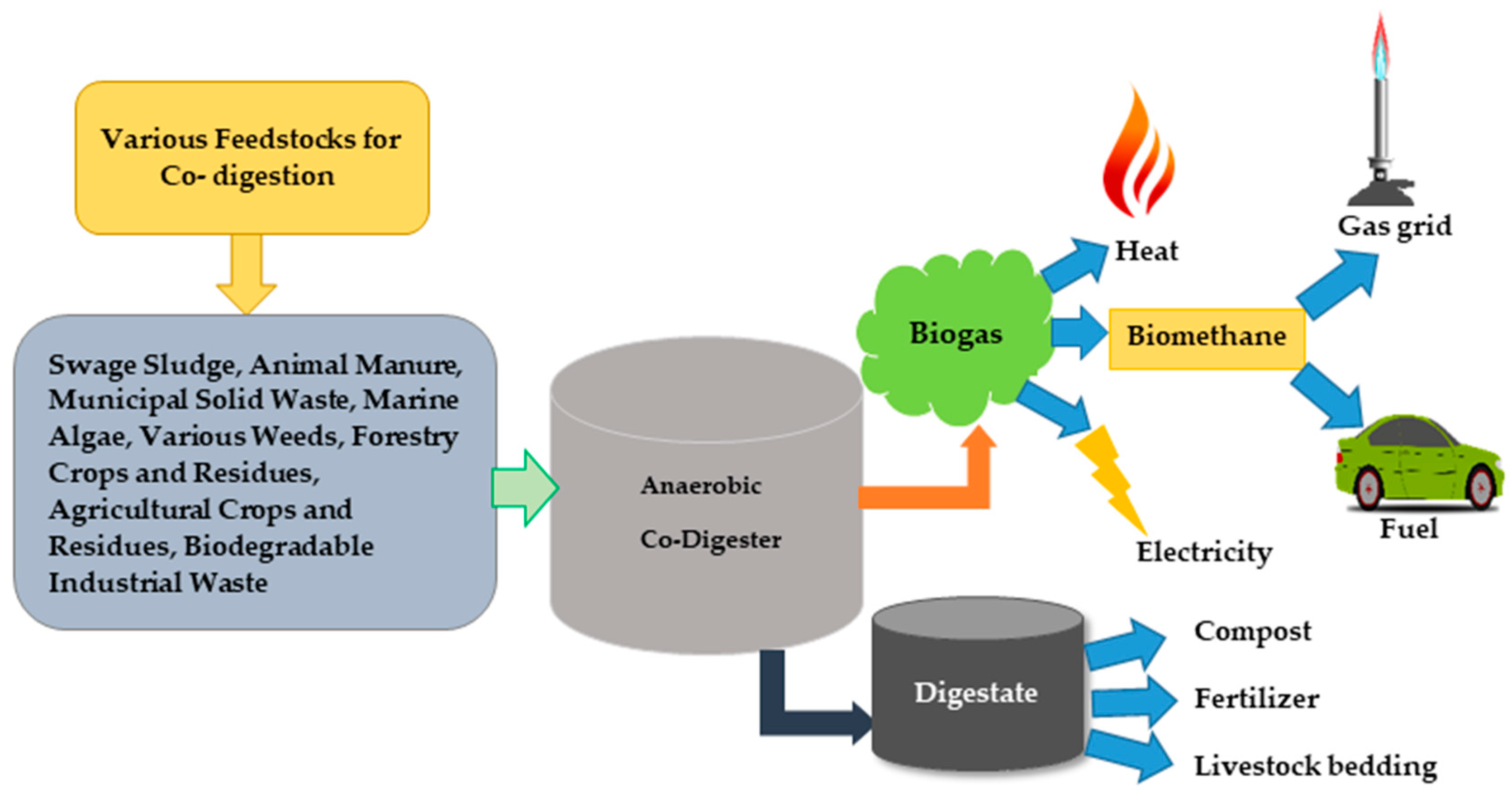
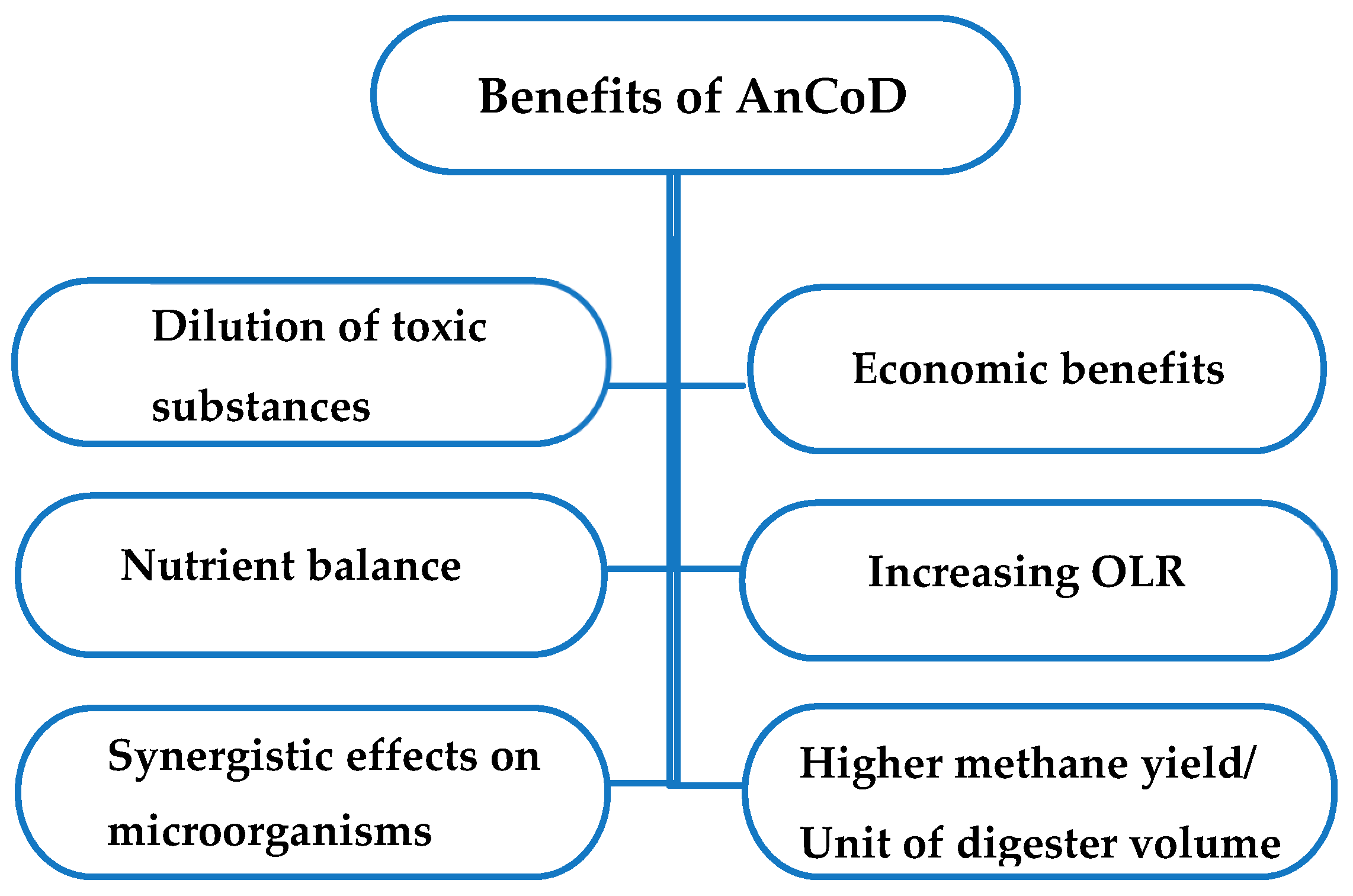
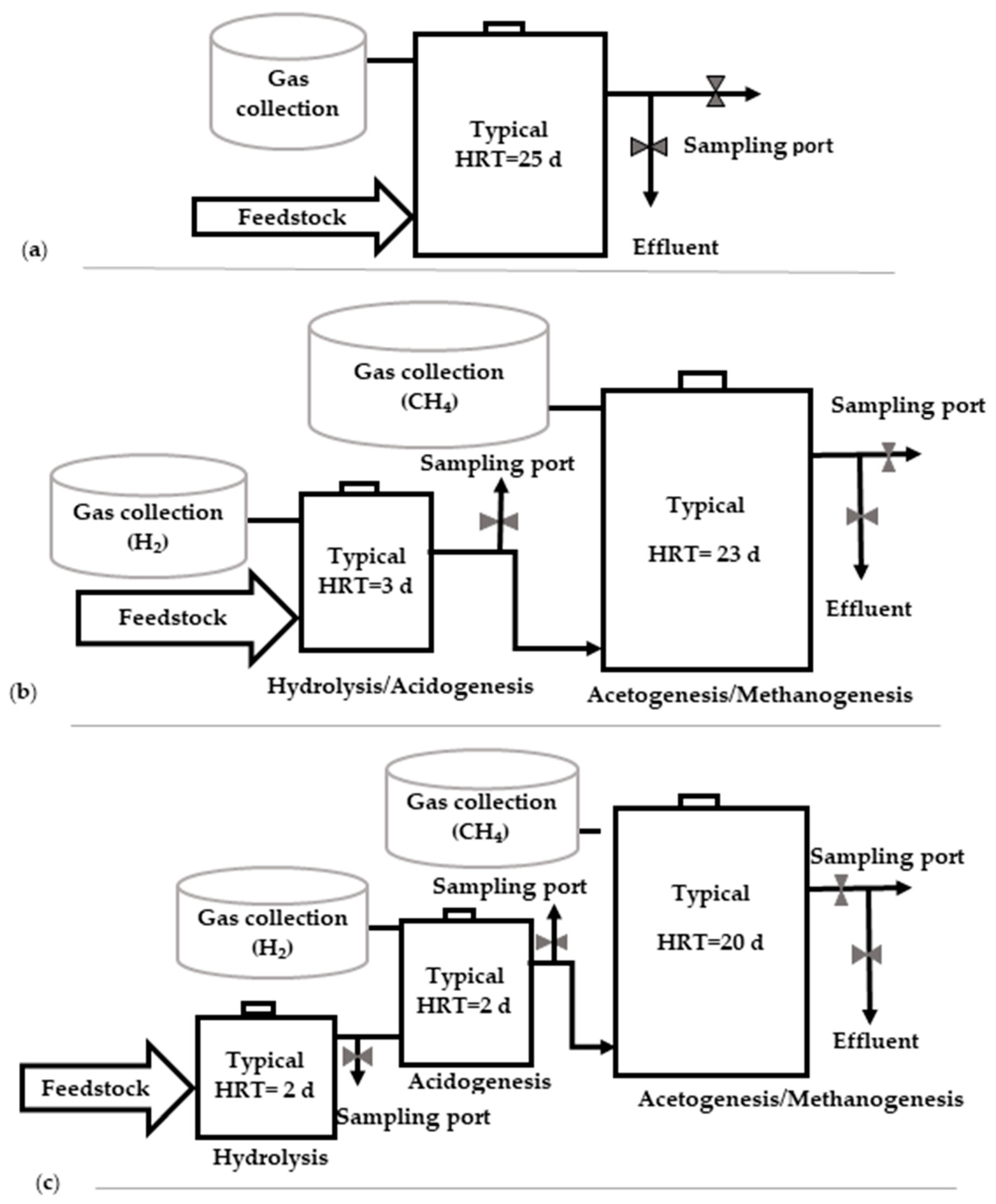
| Feedstocks with Max C/N Ratio <20 | C/N Ratio | Feedstocks with Max C/N Ratio ≤40 | C/N Ratio | Feedstocks with C/N Ratio Around or >50 | C/N Ratio |
|---|---|---|---|---|---|
| TWAS 1 | 6–9 | OFMSW 3 | 24 | Potatoes | 35–60 |
| CSW 2 | 11 | Cow dung | 16–25 | Oat straw | 48–50 |
| Poultry manure | 5–15 | Horse manure | 20–25 | Corn stalks/straw | 50–56 |
| Pig manure | 6–14 | Kitchen Waste | 25–29 | Fallen leaves | 50–53 |
| Goat manure | 10–17 | Peanut shoots/hulls | 20–31 | Rice straw | 51–67 |
| Grass/Grass trimmings | 12–16 | Slaughterhouse waste | 22–37 | Seaweed | 70–79 |
| Alfalfa | 12–17 | Mixed food waste | 15–32 | Algae | 75–100 |
| Food Waste | 3–17 | Waste cereal | 16–40 | Sugar cane/bagasse | 140–150 |
| - | - | Sugar beet/Sugar foliage | 35–40 | Sawdust | 200–500 |
| - | - | Waste cereals | 16–40 | - | - |
| Feedstocks | Microbial Consortia | Digester Mode | HRT | Methane Yield/Biogas Increase % | Ref # |
|---|---|---|---|---|---|
| Food, fruit, vegetable + night soil waste | Methanosaeta (predominant methanogen) + hydrogenotrophs | Full scale wet fed-batch | 18–20 d | NA | [75] |
| C/N 8.6 | |||||
| Fruit vegetable waste + Food waste | Methanoculleus, Methanosaeta, Methanosarcina | CSTR (mesophilic) | NA 1 | 0.49 m3 CH4/kg VS | [77] |
| Dairy wastewater + Cattle manure | Uncultured Bacteroidetes, Firmicutes, Synergistetes, Syntrophomonas strains Methanosarcina species | ASBR (mesophilic) | 20 d | biogas produced: 0.87 L/g VS removed | [81] |
| C/N 24.7 | |||||
| Cow manure + grass silage | Clostridia, unclassified Bacteria, Bacteroidets | CSTR mesophilic | 20 d | NA | [82] |
| Cow manure + oat straw | Clostridia, unclassified Bacteria, Bacteroidets, Deltaproteobacteria | CSTR (mesophilic) | 20 d | NA | |
| Cow manure + sugar beet tops | unclassified Bacteria,Clostridia, Bacteroidets, Bacilli | CSTR (mesophilic) | 20 d | NA | |
| Food wastewater + WAS | Dominated by Methanothermobacter and Methanosarcina | CSTR (thermophilic) | 20 d | Max biogas: 316.11 mL CH4/g COD removed | [83] |
| STP-OGW + SC-OFMSW | Methanobacterium, Methanoculleus, Methanothermobacter uncultured archaea | Batch (thermophilic) | 14.4 d | 52% biogas and 36% methane increase | [84] |
| Sewage sludge + FOG | Dominantly Methanosaeta, and N09 | Semi-continuous (mesophilic) | 15 d | 35% biogas increase | [85] |
| biodiesel waste glycerin + municipal waste sludge | Dominated by Methanosaeta and Methanomicrobium | Two-stage CSTR (mesophilic) | 20 d | 100% biogas and 120% methane increase | [86] |
| Food waste + horse manure | Dominated by Aminobacterium, Clostridium, Proteiniphilum, and Saccharofermentans | compact three-stage | NA | 11–23% methane increase | [87] |
| Food waste + cow manure | Firmicutes, Methanobacterium and Methanosaeta | CSTR mesophilic | 20 d | 26% methane increase | [88] |
| Thermotogae, Firmicutes, Synergistetes and Methanothermobacter | CSTR thermophilic | 20 d | methane did not increase by co-digestion | ||
| Food waste + Wheat straw | Dominated by Bacteroidetes and Methanothrix | CSTR mesophilic | NA | 30% biogas increase | [89] |
| Dominated by Thermotogae and Methanosarcina | CSTR thermophilic | NA | 45% biogas increase |
| Digester Mode | Feedstocks | Mixing Ratio | HRT | Biogas/Methane Content | Ref # |
|---|---|---|---|---|---|
| Single-stage (CSTR, mesophilic) | Sewage sludge + confectionery waste | NA 1 | 20 d | Methane yield: 0.36-0.28 m3/kg VS applied (76–82% methane) 2 | [113] |
| Two-stage (CSTR, thermophilic/mesophilic) | 12 d | Methane yield: 0.3–0.34 m3/kg VS applied (66–76% methane) 2 | |||
| Single stage (plug flow) | Food waste + sewage sludge | 7:1 (weight) | 24 d | Biogas production: 1704.59 ± 52.12 L/d | [114] |
| 16 d | 1209.17 ± 48.44 L/d | ||||
| Single-stage (UASB) | Slaughter house + milk wastewater | NA | 2.14 d | 40% Methane increase by two-stage reactor | [120] |
| Two-stage (CSTR/UASB) | 2.9 d | ||||
| Single-stage (CSTR, thermophilic) | Market biowaste + swine manure | 1:4 (weight) | 25 d | 0.55 dm3/L digester d | [121] |
| Two-stage (CSTR, thermophilic) | 3/22 d | 0.54 dm3/L digester d | |||
| Single-stage (Batch) | Oil processing wastewater + pig manure | 1:3 (weight) | 20 d | Average biogas: 0.33 m3/kgVS removed, (0.66% methane) | [122] |
| Two-stage (Batch) | 2/18 d | Average biogas: 0.4 m3/kgVS removed, (0.67% methane) | |||
| Single-stage | Food waste/horse manure | NA | 20 d | 45.4 L cumulative methane production | [123] |
| Two-stage | 4/16 d | 50.7 L cumulative methane | |||
| Three-stage | 2/2/16 | 55.7 L cumulative methane production |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. https://doi.org/10.3390/en12061106
Rabii A, Aldin S, Dahman Y, Elbeshbishy E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies. 2019; 12(6):1106. https://doi.org/10.3390/en12061106
Chicago/Turabian StyleRabii, Anahita, Saad Aldin, Yaser Dahman, and Elsayed Elbeshbishy. 2019. "A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration" Energies 12, no. 6: 1106. https://doi.org/10.3390/en12061106
APA StyleRabii, A., Aldin, S., Dahman, Y., & Elbeshbishy, E. (2019). A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies, 12(6), 1106. https://doi.org/10.3390/en12061106





