Evaluation of the Nitrous Oxide Emission Reduction Potential of an Aerobic Bioreactor Packed with Carbon Fibres for Swine Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioreactor Construction and Operation
2.2. Analytical Methods for Determining Water Quality and Measuring N2O Emissions
2.3. Characterization of Bacterial Species
3. Results
3.1. Water Quality and Gas Emissions from the Bioreactor
3.2. Bacterial Community Structure of the Biofilms in the Bioreactor
3.3. N2O Generation Reduction Following Introduction of Carbon Fiber into the Actual Plant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eggleston, H.S.B.L.; Miwa, K.; Ngara, T.; Tanabe, K. (Eds.) Chapter 10: Emissions from Livestock and Manure Management in Volume 4 Agriculture, Forestry and Other Land Use. In IPCC (Intergovernmental Panel on Climate Change), 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme; IPCC: Kanagawa, Japan, 2006. [Google Scholar]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Daelman, M.R.J.; van Voorthuizen, E.M.; van Dongen, U.G.J.M.; Volcke, E.I.P.; van Loosdrecht, M.C.M. Seasonal and diurnal variability of N2O emissions from a full-scale municipal wastewater treatment plant. Sci. Total Environ. 2015, 536, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Yamamoto-Ikemoto, R.; Yokoyama, H.; Kawahara, H.; Ogino, A.; Osada, T. Mitigation of nitrous oxide (N2O) emission from swine wastewater treatment in an aerobic bioreactor packed with carbon fibers. Anim. Sci. J. 2015, 86, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Kosonen, H.; Heinonen, M.; Mikola, A.; Haimi, H.; Mulas, M.; Corona, F.; Vahala, R. Nitrous Oxide Production at a Fully Covered Wastewater Treatment Plant: Results of a Long-Term Online Monitoring Campaign. Environ. Sci. Technol. 2016, 50, 5547–5554. [Google Scholar] [CrossRef]
- NIES. National Greenhouse Gas Inventory Report of Japan (NIR); Ministry of the Environment: Tokyo, Japan, 2015. Available online: http://www-gio.nies.go.jp/aboutghg/nir/nir-e.html (accessed on 21 July 2014).
- Sun, S.; Bao, Z.; Sun, D. Study on emission characteristics and reduction strategy of nitrous oxide during wastewater treatment by different processes. Environ. Sci. Pollut. Res. 2015, 22, 4222–4229. [Google Scholar] [CrossRef] [PubMed]
- Massara, T.M.; Malamis, S.; Guisasola, A.; Baeza, J.A.; Noutsopoulos, C.; Katsou, E. A review on nitrous oxide (N2O) emissions during biological nutrient removal from municipal wastewater and sludge reject water. Sci. Total Environ. 2017, 596–597, 106–123. [Google Scholar] [CrossRef]
- Santín, I.; Barbu, M.; Pedret, C.; Vilanova, R. Control strategies for nitrous oxide emissions reduction on wastewater treatment plants operation. Water Res. 2017, 125, 466–477. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zhang, J.; Huang, X.; Wei, D.; Lan, W.; Hu, Z. Reduction of nitrous oxide emissions from partial nitrification process by using innovative carbon source (mannitol). Bioresour. Technol. 2016, 218, 789–795. [Google Scholar] [CrossRef]
- Osada, T.; Kuroda, K.; Yonaga, M. Reducing nitrous oxide gas emissions from fill-and-draw type activated sludge process. Water Res. 1995, 29, 1607–1608. [Google Scholar] [CrossRef]
- Ikeda-Ohtsubo, W.; Miyahara, M.; Kim, S.W.; Yamada, T.; Matsuoka, M.; Watanabe, A.; Fushinobu, S.; Wakagi, T.; Shoun, H.; Miyauchi, K.; et al. Bioaugmentation of a wastewater bioreactor system with the nitrous oxide-reducing denitrifier Pseudomonas stutzeri strain TR2. J. Biosci. Bioeng. 2013, 115, 37–42. [Google Scholar] [CrossRef]
- Yamashita, T.; Shiraishi, M.; Yamamoto-Ikemoto, R.; Yokoyama, H.; Ogino, A.; Osada, T. Swine wastewater treatment technology to reduce nitrous oxide emission by using an aerobic bioreactor packed with carbon fibres. Anim. Prod. Sci. 2016, 56, 330–336. [Google Scholar] [CrossRef]
- Osada, T.; Shiraishi, M.; Hasegawa, T.; Kawahara, H. Methane, nitrous oxide and ammonia generation in full-scale swine wastewater purification facilities. Front. Environ. Sci. Eng. 2017, 11, 10. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; Association, A.P.H.; Association, A.W.W.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- IPCC. IPCC (Intergovernmental Panel on Climate Change), Climate Change 2013: The Physical Science Basis. Intergovernmental Panel on Climate Change, Stockholm; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Sabba, F.; Picioreanu, C.; Nerenberg, R. Mechanisms of nitrous oxide (N2O) formation and reduction in denitrifying biofilms. Biotechnol. Bioeng. 2017, 114, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sekiguchi, Y.; Hanada, S.; Imachi, H.; Ohashi, A.; Harada, H.; Kamagata, Y. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2006, 56, 1331–1340. [Google Scholar]
- Sekiguchi, Y.; Yamada, T.; Hanada, S.; Ohashi, A.; Harada, H.; Kamagata, Y. Anaerolinea thermophila gen. nov., sp. nov. and Caldilinea aerophila gen. nov., sp. nov., novel filamentous thermophiles that represent a previously uncultured lineage of the domain Bacteria at the subphylum level. Int. J. Syst. Evol. Microbiol. 2003, 53, 1843–1851. [Google Scholar] [CrossRef]
- Kragelund, C.; Thomsen, T.R.; Mielczarek, A.T.; Nielsen, P.H. Eikelboom’s morphotype 0803 in activated sludge belongs to the genus Caldilinea in the phylum Chloroflexi. FEMS Microbiol. Ecol. 2011, 76, 451–462. [Google Scholar] [CrossRef]
- Eikelboom, D.H. Filamentous organisms observed in activated sludge. Water Res. 1975, 9, 365–388. [Google Scholar] [CrossRef]
- Martins, A.M.P.; Pagilla, K.; Heijnen, J.J.; van Loosdrecht, M.C.M. Filamentous bulking sludge—A critical review. Water Res. 2004, 38, 793–817. [Google Scholar] [CrossRef] [PubMed]
- Sanford, R.A.; Wagner, D.D.; Wu, Q.; Chee-Sanford, J.C.; Thomas, S.H.; Cruz-García, C.; Rodríguez, G.; Massol-Deyá, A.; Krishnani, K.K.; Ritalahti, K.M.; et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc. Natl. Acad. Sci. USA 2012, 109, 19709–19714. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Xiong, X.; Xu, Z.; Lu, C.; Cheng, H.; Lyu, X.; Zhang, J.; He, W.; Deng, W.; Lyu, Y.; et al. Bacterial Communities in the Rhizospheres of Three Mangrove Tree Species from Beilun Estuary, China. PLoS ONE 2016, 11, e0164082. [Google Scholar] [CrossRef]
- Pereira, A.D.; Leal, C.D.; Dias, M.F.; Etchebehere, C.; Chernicharo, C.A.; de Araujo, J.C. Effect of phenol on the nitrogen removal performance and microbial community structure and composition of an anammox reactor. Bioresour. Technol. 2014, 166, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zehr, J.P.; Ward, B.B. Nitrogen Cycling in the Ocean: New Perspectives on Processes and Paradigms. Appl. Environ. Microbiol. 2002, 68, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Jetten, M.S.M.; Wagner, M.; Fuerst, J.; van Loosdrecht, M.; Kuenen, G.; Strous, M. Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Curr. Opin. Biotechnol. 2001, 12, 283–288. [Google Scholar] [CrossRef]
- Lee, K.C.-Y.; Dunfield, P.F.; Morgan, X.C.; Crowe, M.A.; Houghton, K.M.; Vyssotski, M.; Ryan, J.L.J.; Lagutin, K.; McDonald, I.R.; Stott, M.B. Chthonomonas calidirosea gen. nov., sp. nov., an aerobic, pigmented, thermophilic micro-organism of a novel bacterial class, Chthonomonadetes classis nov., of the newly described phylum Armatimonadetes originally designated candidate division OP10. Int. J. Syst. Evol. Microbiol. 2011, 61, 2482–2490. [Google Scholar] [CrossRef]
- McBride, M.J.; Liu, W.; Lu, X.; Zhu, Y.; Zhang, W. The Family Cytophagaceae. In The Prokaryotes: Other Major Lineages of Bacteria and the Archaea; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 577–593. [Google Scholar]
- Kovaleva, O.L.; Merkel, A.Y.; Novikov, A.A.; Baslerov, R.V.; Toshchakov, S.V.; Bonch-Osmolovskaya, E.A. Tepidisphaera mucosa gen. nov., sp. nov., a moderately thermophilic member of the class Phycisphaerae in the phylum Planctomycetes, and proposal of a new family, Tepidisphaeraceae fam. nov., and a new order, Tepidisphaerales ord. nov. Int. J. Syst. Evol. Microbiol. 2015, 65 Pt 2, 549–555. [Google Scholar] [CrossRef]
- Wunderlin, P.; Mohn, J.; Joss, A.; Emmenegger, L.; Siegrist, H. Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res. 2012, 46, 1027–1037. [Google Scholar] [CrossRef]
- Zheng, H.; Hanaki, K.; Matsuo, T. Production of nitrous oxide gas during nitrification of wastewater. Water Sci. Technol. 1994, 30, 133–141. [Google Scholar] [CrossRef]
- Tallec, G.; Garnier, J.; Billen, G.; Gousailles, M. Nitrous oxide emissions from secondary activated sludge in nitrifying conditions of urban wastewater treatment plants: Effect of oxygenation level. Water Res. 2006, 40, 2972–2980. [Google Scholar] [CrossRef]
- Otte, S.; Grobben, N.G.; Robertson, L.A.; Jetten, M.S.; Kuenen, J.G. Nitrous oxide production by Alcaligenes faecalis under transient and dynamic aerobic and anaerobic conditions. Appl. Environ. Microbiol. 1996, 62, 2421–2426. [Google Scholar]
- Schulthess, R.V.; Gujer, W. Release of nitrous oxide (N2O) from denitrifying activated sludge: Verification and application of a mathematical model. Water Res. 1996, 30, 521–530. [Google Scholar] [CrossRef]
- Hanaki, K.; Hong, Z.; Matsuo, T. Production of Nitrous Oxide Gas during Denitrification of Wastewater. Water Sci. Technol. 1992, 26, 1027–1036. [Google Scholar] [CrossRef]
- Park, K.Y.; Inamori, Y.; Mizuochi, M.; Ahn, K.H. Emission and control of nitrous oxide from a biological wastewater treatment system with intermittent aeration. J. Biosci. Bioeng. 2000, 90, 247–252. [Google Scholar] [CrossRef]
- Thörn, M.; Sörensson, F. Variation of nitrous oxide formation in the denitrification basin in a wastewater treatment plant with nitrogen removal. Water Res. 1996, 30, 1543–1547. [Google Scholar] [CrossRef]
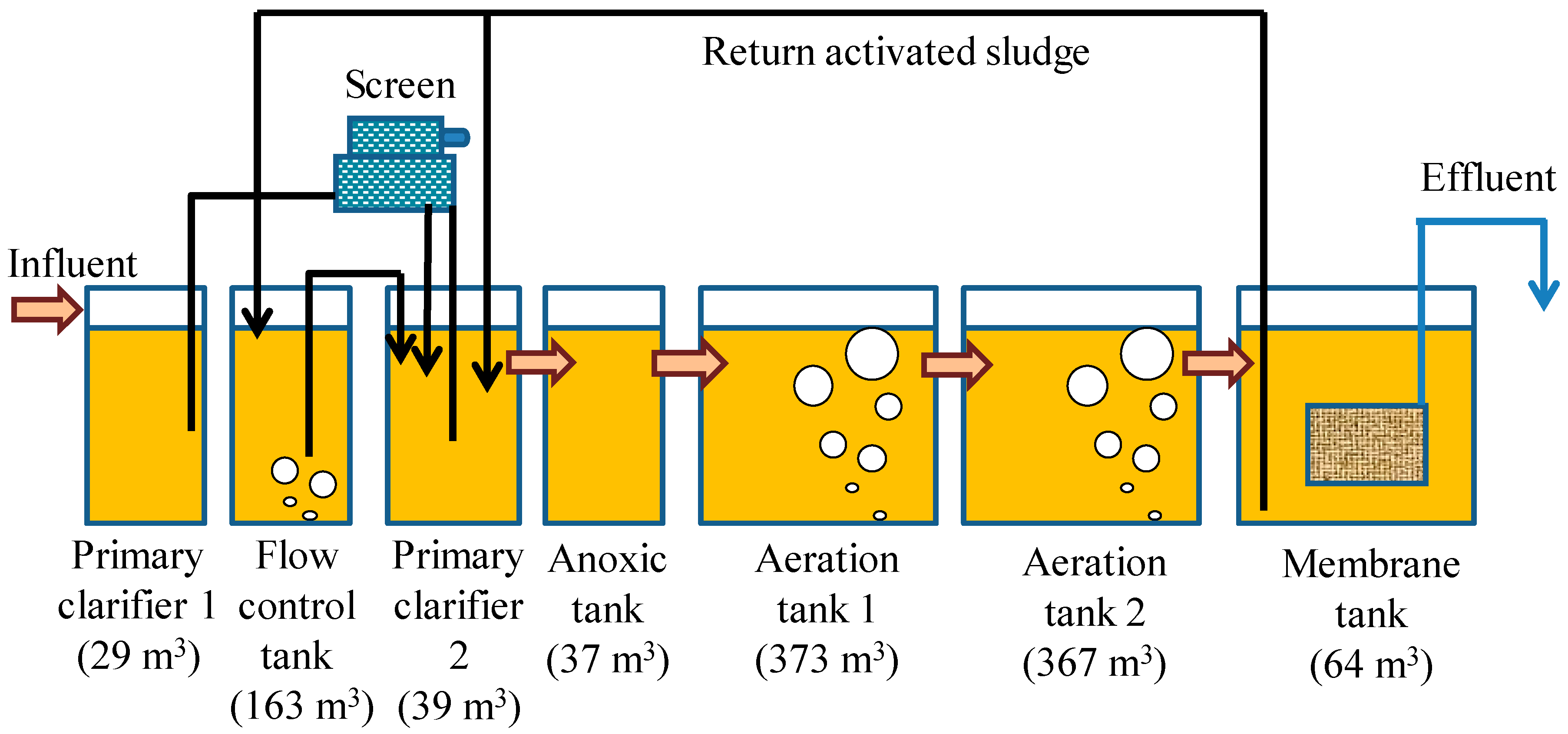


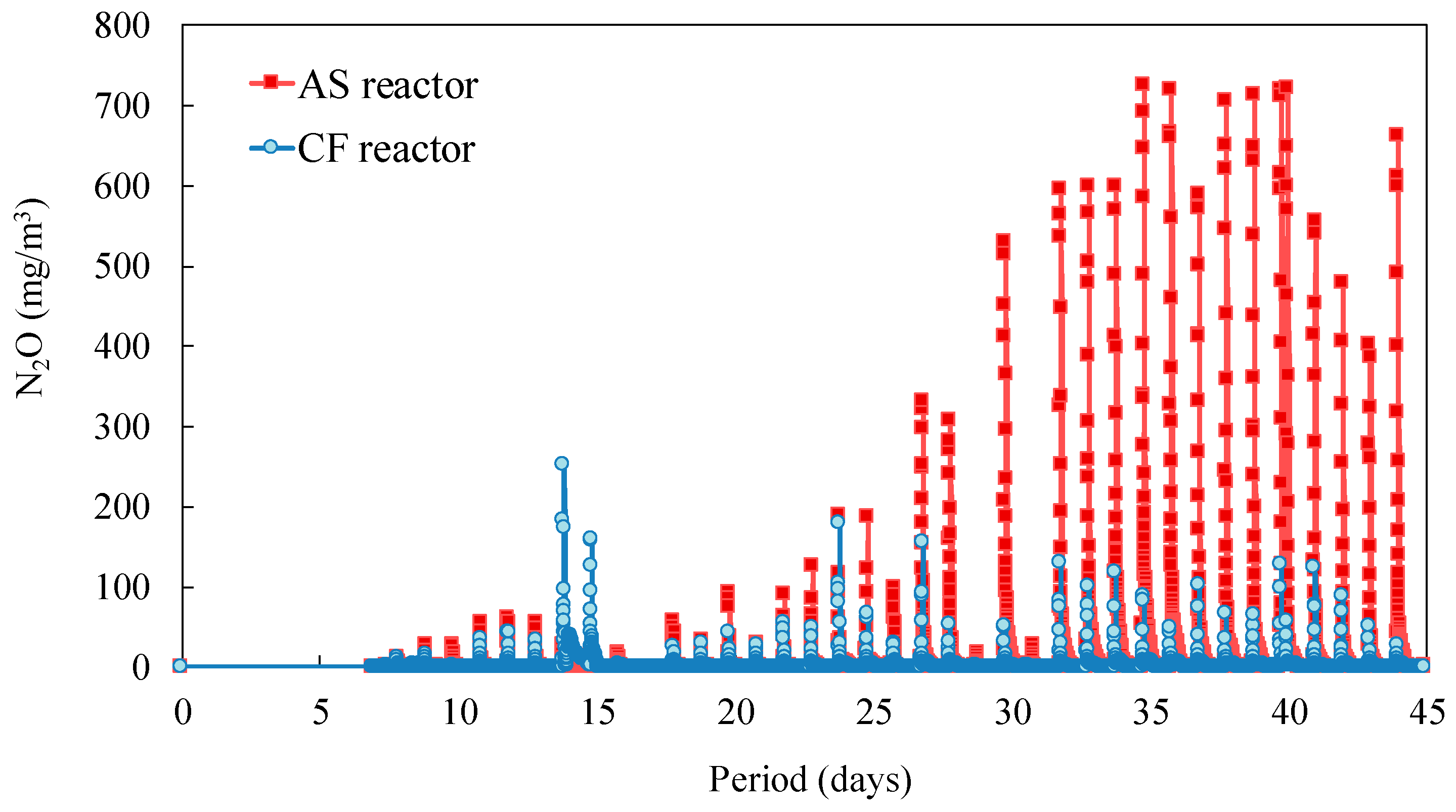


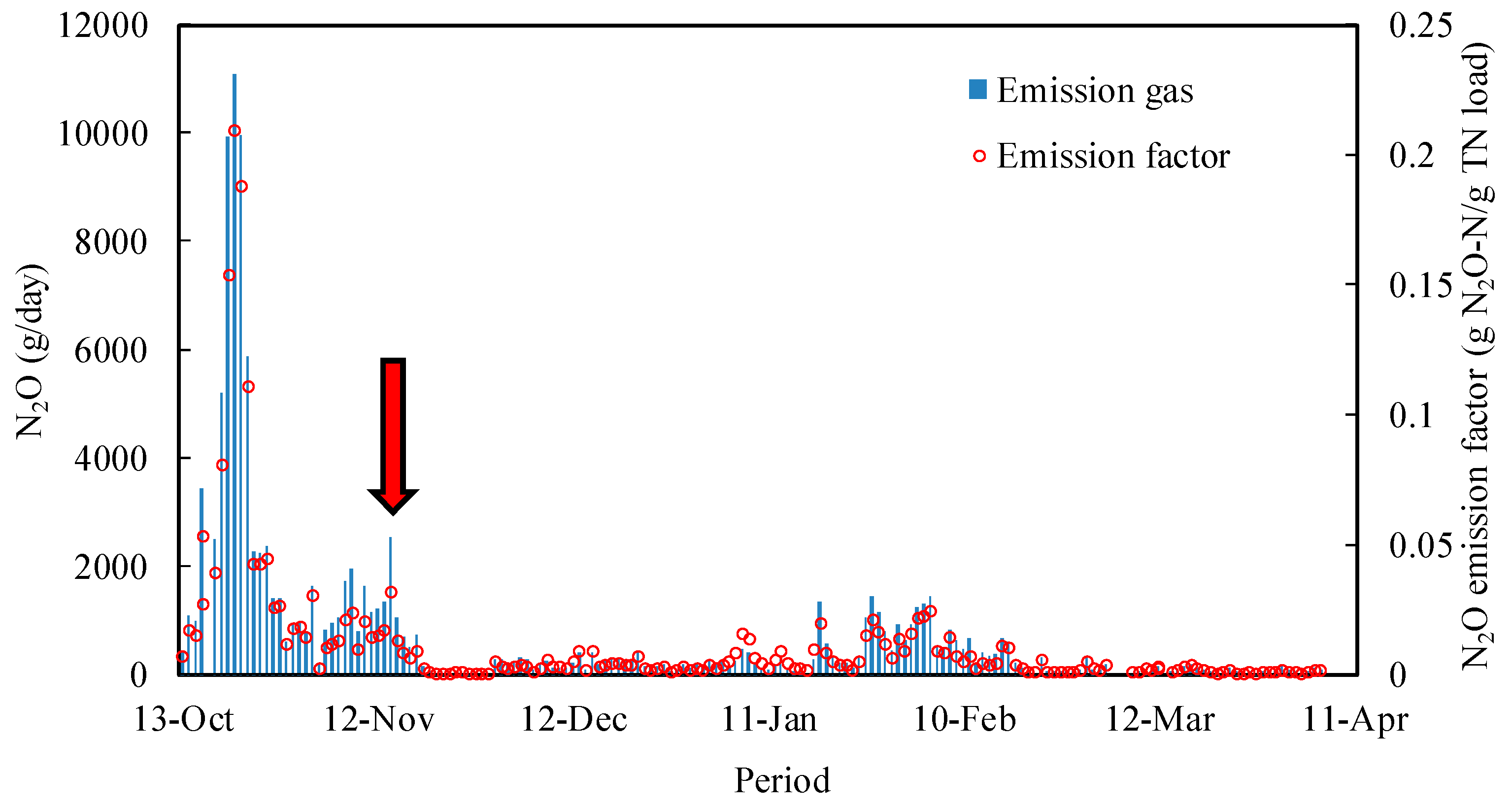
| AS Reactor | CF Reactor | |
|---|---|---|
| Bioreactor | ||
| Height (cm) | 110 | 110 |
| Width (cm) | 90 | 90 |
| Depth (cm) | 90 | 90 |
| Water phase (L) | 700 | 700 |
| Carbon Fiber Carrier | ||
| Diameter (µm) | - | 7 |
| Number of fibers | - | 24,000 |
| Length (cm) | - | 25 |
| Fixed carbon fiber units | - | 104 |
| Feed cycle (h) | 24 | 24 |
| Average feed volume (L/day) | 30 | 30 |
| Aeration rate (m3/h) | 6 | 6 |
| Influent | Effluent from AS Reactor | Effluent from CF Reactor | |
|---|---|---|---|
| MLSS (mg/L) | - | 8301 ± 455 | 7542 ± 835 |
| BOD (mg/L) | 9936 ± 2179 | 20 ± 25 | 19 ± 23 |
| COD (mg/L) | 5898 ± 2654 | 181 ± 80 | 170 ± 69 |
| SS (mg/L) | 12,972 ± 7870 | 90 ± 39 | 139 ± 86 |
| TN (mg/L) | 2658 ± 1002 | 311 ± 116 | 300 ± 103 |
| NH4+-N (mg/L) | 1392 ± 321 | 4 ± 5 | 4 ± 6 |
| NO2−-N (mg/L) | 5 ± 8 | 48 ± 68 | 26 ± 44 |
| NO3−-N (mg/L) | 5 ± 4 | 234 ± 137 | 169 ± 73 |
| TP (mg/L) | 412 ± 152 | 90 ± 34 | 69 ± 29 |
| pH | 6.7 to 7.8 | 5.9 to 8.0 | 6.9 to 7.9 |
| (mean ± SD) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, T.; Shiraishi, M.; Yokoyama, H.; Ogino, A.; Yamamoto-Ikemoto, R.; Osada, T. Evaluation of the Nitrous Oxide Emission Reduction Potential of an Aerobic Bioreactor Packed with Carbon Fibres for Swine Wastewater Treatment. Energies 2019, 12, 1013. https://doi.org/10.3390/en12061013
Yamashita T, Shiraishi M, Yokoyama H, Ogino A, Yamamoto-Ikemoto R, Osada T. Evaluation of the Nitrous Oxide Emission Reduction Potential of an Aerobic Bioreactor Packed with Carbon Fibres for Swine Wastewater Treatment. Energies. 2019; 12(6):1013. https://doi.org/10.3390/en12061013
Chicago/Turabian StyleYamashita, Takahiro, Makoto Shiraishi, Hiroshi Yokoyama, Akifumi Ogino, Ryoko Yamamoto-Ikemoto, and Takashi Osada. 2019. "Evaluation of the Nitrous Oxide Emission Reduction Potential of an Aerobic Bioreactor Packed with Carbon Fibres for Swine Wastewater Treatment" Energies 12, no. 6: 1013. https://doi.org/10.3390/en12061013
APA StyleYamashita, T., Shiraishi, M., Yokoyama, H., Ogino, A., Yamamoto-Ikemoto, R., & Osada, T. (2019). Evaluation of the Nitrous Oxide Emission Reduction Potential of an Aerobic Bioreactor Packed with Carbon Fibres for Swine Wastewater Treatment. Energies, 12(6), 1013. https://doi.org/10.3390/en12061013




