Investigation on Oxidation Behavior of Super304H and HR3C Steel in High Temperature Steam from a 1000 MW Ultra-Supercritical Coal-Fired Boiler
Abstract
:1. Introduction
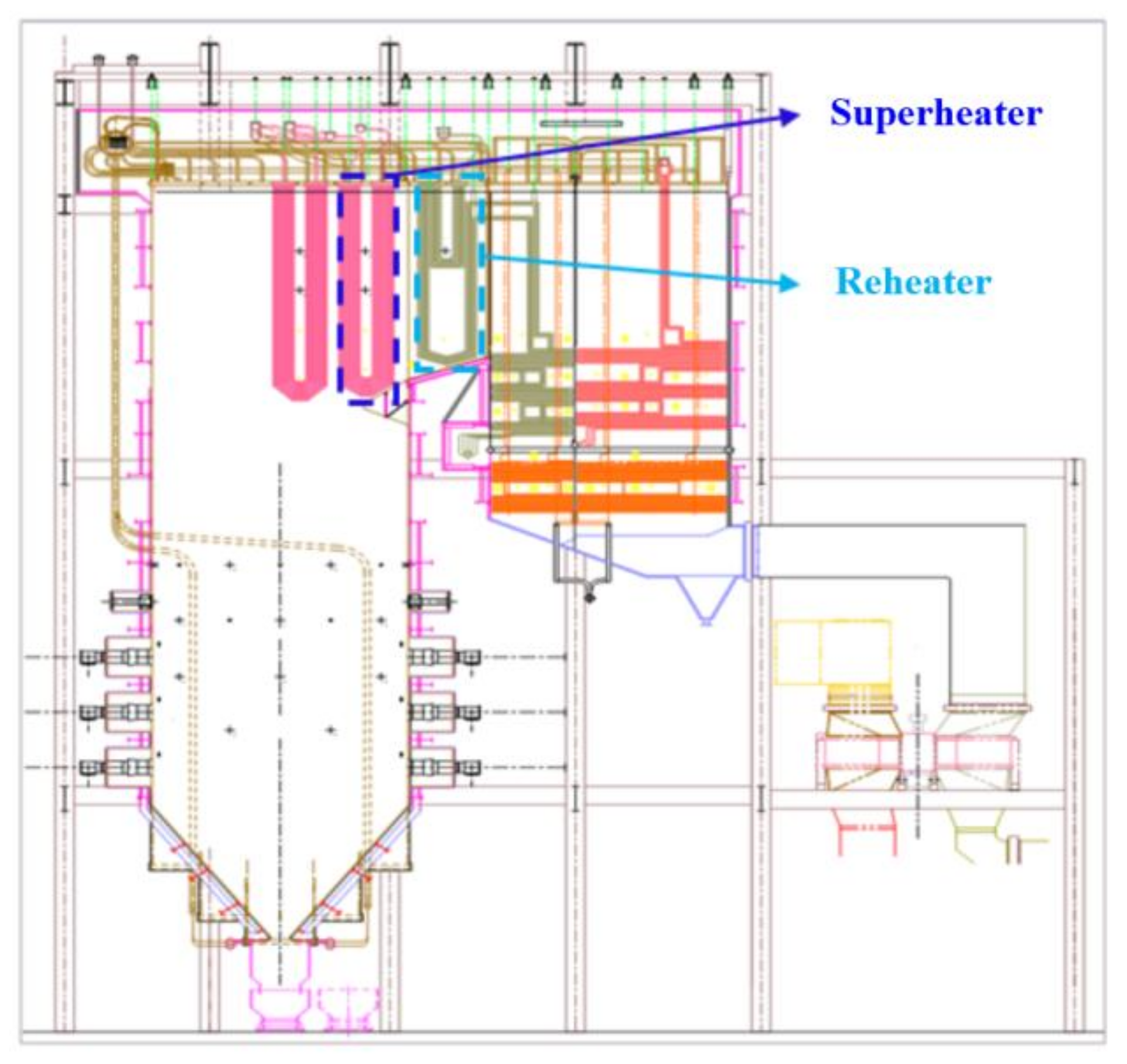
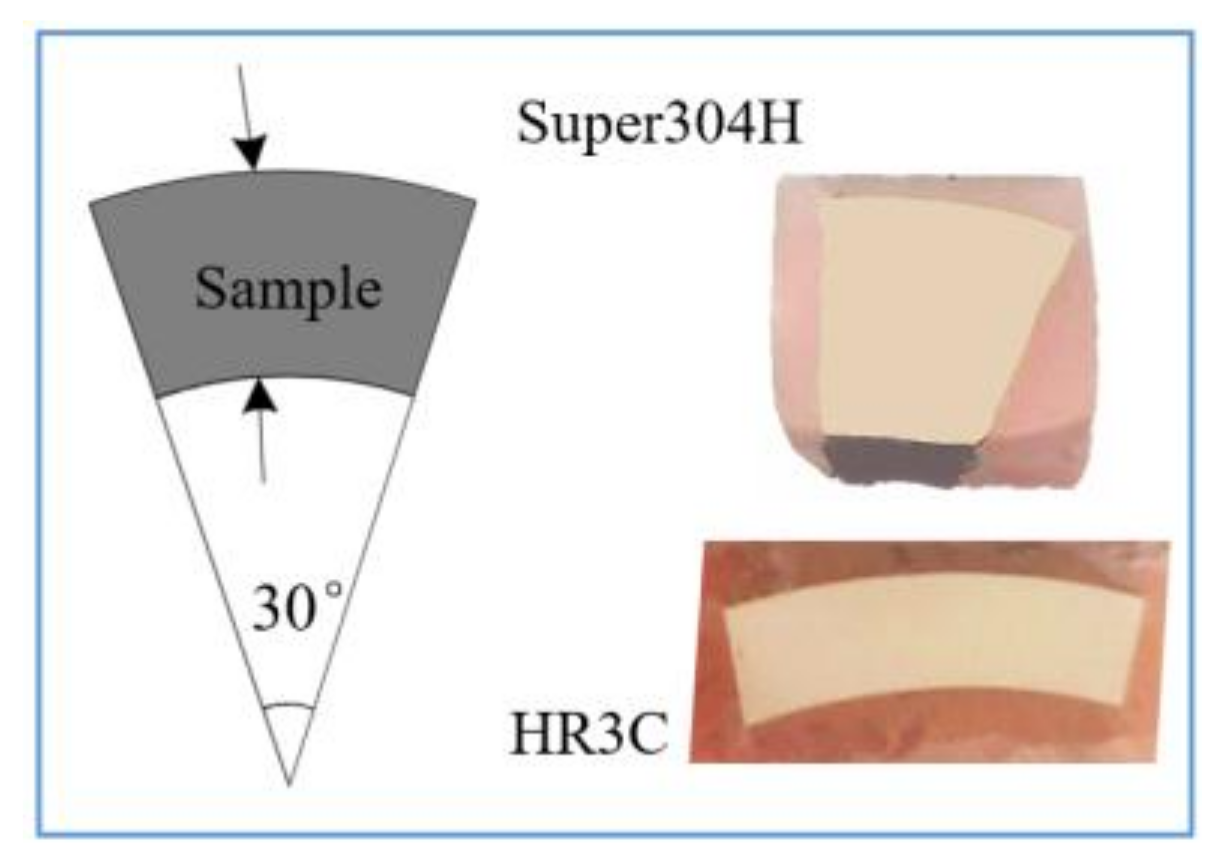
2. Experimental Samples and Methods
3. Experimental Results
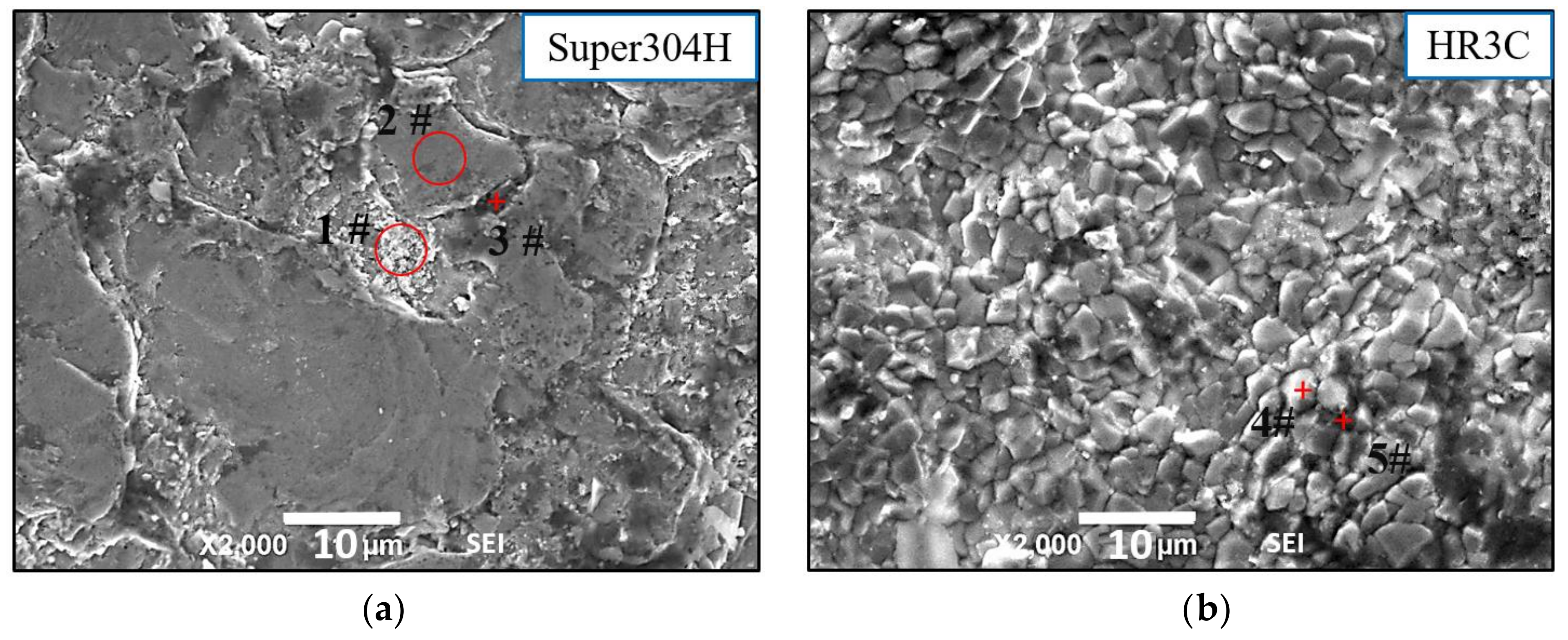
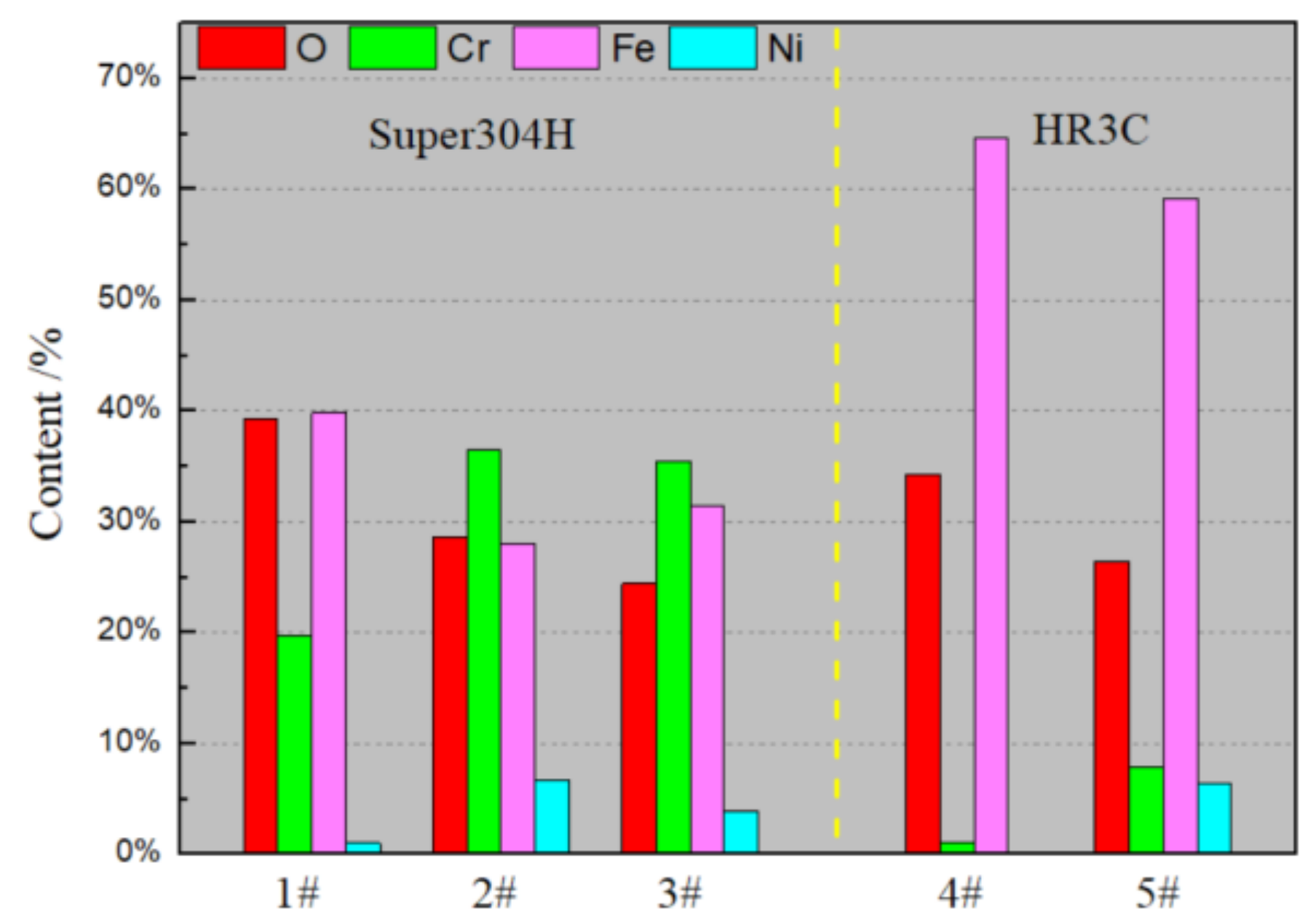
3.1. Surface Analysis
3.2. Cross-Section Analysis
4. Discussion
4.1. Steam Oxidation Mechanism
4.2. Oxidation Layer Spalling Mechanism
5. Conclusions
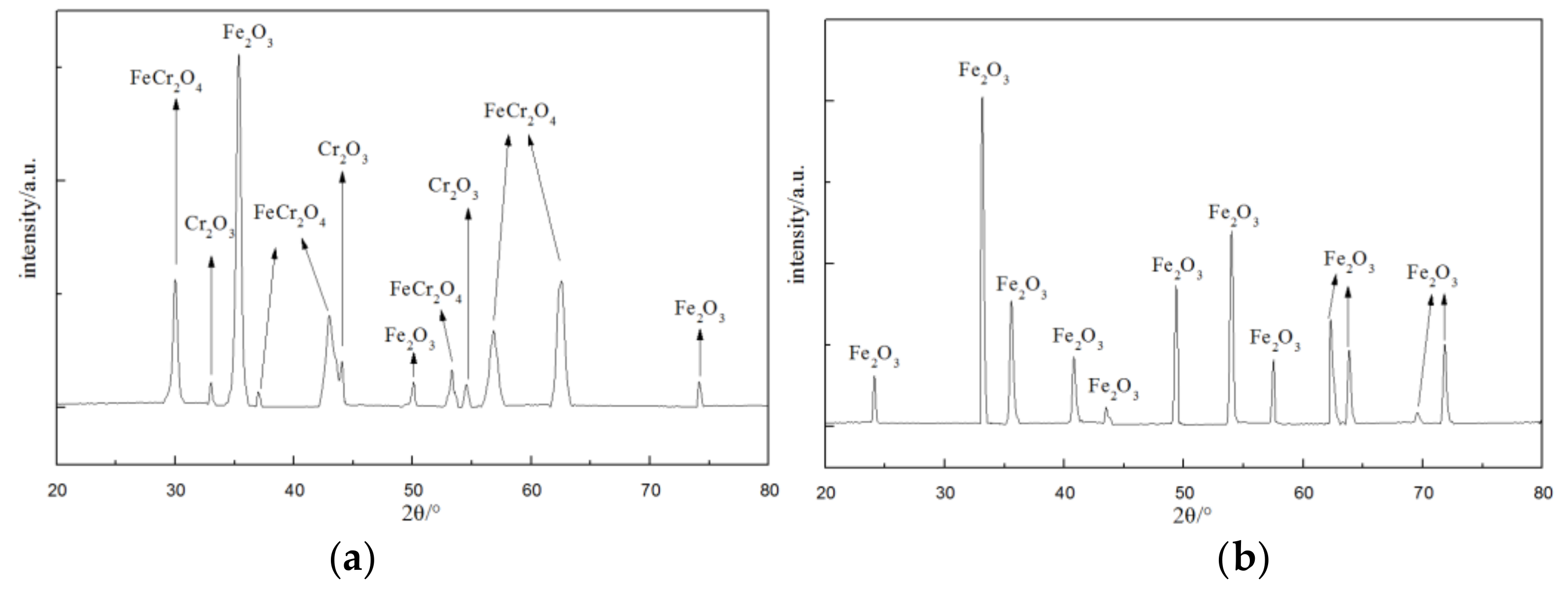
- The steam oxidized surface of Super 304H were mostly Fe2O3, Cr2O3 and FeCr2O4 while that of HR3C was composed of Fe2O3, according XRD analysis.
- As the results of SEM and EDS, the oxidation layer of the Super 304H sample was thick and continuous while the oxidation layer of the HR3C was thin and unevenly distributed. Furthermore, the oxidation products of the two materials could be divided into two layers, the outer layer enriched in O element and Fe element, and the inner layer enriched in O element and Cr element.
- The surface of the Super 304H sample was found to be spalling, and the spalling position located at the interface between the inner and outer oxidation layers. To the opposite, the oxide scale of HR3C was not found to have signs of spalling. The main reasons causing this situation were the low Cr and Ni content and the existence of thermal coefficients of expansion discrepancy between the outer and inner layer under fluctuated periodically operating conditions of the boiler.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wright, I.G.; Dooley, R.B. A Review of the Oxidation Behaviour of Structural Alloys in Steam. Int. Mater. Rev. 2010, 55, 129–167. [Google Scholar] [CrossRef]
- Choi, S.M.; Park, J.S.; Sohn, H.S.; Kim, S.H.; Cho, H.H. Thermal Characteristics of Tube Bundles in Ultra-Supercritical Boilers. Energies 2016, 9, 779. [Google Scholar] [CrossRef]
- Viswanathan, R.; Sarver, J.; Tanzosh, J.M. Boiler Materials for Ultra-supercritical Coal Power Plants-stearmside Oxidation. J. Mater. Eng. Perform. 2006, 15, 255–274. [Google Scholar] [CrossRef]
- Valicek, J.; Palkova, Z.; Harnicarova, M.; Kusnerova, M.; Lukac, O. Thermal and Performance Analysis of a Gasification Boiler and Its Energy Efficiency Optimization. Energies 2017, 10, 1066. [Google Scholar] [CrossRef]
- Abe, F.; Kutsumi, H.; Haruyama, H.; Okubo, H. Improvement of Oxidation Resistance of 9 Mass% Chromium Steel for Advanced-ultra Supercritical Power Plant Boilers by Pre-oxidation treatment. Corros. Sci. 2017, 114, 1–9. [Google Scholar] [CrossRef]
- Gomez, M.A.; Comesana, R.; Alvarez Feijoo, H.P.; Eguia, P. Simulation of the Effect of Water Temperature on Domestic Biomass Boiler Performance. Energies 2012, 5, 1044–1061. [Google Scholar] [CrossRef]
- Wang, C.N.; Chou, M.T.; Hsu, H.P.; Wang, J.W.; Selvaraj, S. The Efficiency Improvement by Combining HHO Gas, Coal and Oil in Boiler for Electricity Generation. Energies 2017, 10, 251. [Google Scholar] [CrossRef]
- Chen, G.H.; Zhang, Q.; Liu, J.J.; Wang, J.Q.; Yu, X.H.; Hua, J.; Bai, X.L.; Zhang, T.; Zhang, J.H.; Tang, W.M. Microstructures and Mechanical Properties of T92/Super304H Dissimilar Steel Weld Joints After High-temperature Ageing. Mater. Design 2017, 44, 469–475. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Z.F.; Tu, H.Y.; Schmauder, S.; Wu, G.X. Microstructure Evolution in HR3C Austenitic Steel During Long-term Creep at 650 degrees C. Mater. Sci. Eng., A 2017, 681, 74–84. [Google Scholar] [CrossRef]
- Viitala, H.; Galfi, I.; Taskinen, P. Initial Oxidation Behaviour of Niobium Stabilized TP347H Austenitic Stainless Steel–Effect of Grain Size and Temperature. Mater. Corros. 2017, 66, 851–862. [Google Scholar] [CrossRef]
- Zurek, J.; Wessel, E.; Niewolak, L.; Schmitz, F.; Kern, T.U.; Singheiser, L.; Quadakkers, W.J. Anomalous Temperature Dependence of Oxidation Kinetics During Steam Oxidation of Ferritic Steels in the Temperature Range 550-650 degrees C. Corros. Sci. 2004, 46, 2301–2317. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Ennis, P.J.; Zurek, J.; Michalik, M. Steam Oxidation of Ferritic Steels-laboratory Test Kinetic Data. Mater. High Temp. 2005, 22, 47–60. [Google Scholar] [CrossRef]
- Wright, I.G.; Dooley, R.V. Morphologies of Oxide Growth and Exfoliation in Superheater and Reheater Yubing of Steam Boiler. Mater. High Temp. 2011, 28, 40–57. [Google Scholar] [CrossRef]
- Hansson, A.N.; Korcakova, L.; Hald, J.; Montgomery, M. Long Term Steam Oxidation of TP347HFG in Power Plants. Mater. High Temp. 2005, 22, 263–267. [Google Scholar] [CrossRef]
- Liang, Z.Y.; Singh, P.M.; Zhao, Q.X.; Wang, Y.S. High Temperature Oxidation of Newly Developed Alloy 282 in the Flowing-Air and Steam Condition at 900-1100 degrees C. Oxid. Met. 2015, 84, 291–305. [Google Scholar] [CrossRef]
- Rosser, J.C.; Bass, M.I.; Cooper, C.; Lant, T.; Brown, P.D.; Connolly, B.J. Steam Oxidation of Super 304H and Shot-peened Super304H. Mater. High Temp. 2012, 29, 95–106. [Google Scholar] [CrossRef]
- Yuan, J.Y.; Wu, X.M.; Wang, W.; Zhu, S.L.; Wang, F.H. The Effect of Surface Finish on the Scaling Behavior of Stainless Steel in Steam and Supercritical Water. Oxid. Met. 2013, 79, 541–551. [Google Scholar] [CrossRef]
- Yue, Z.W.; Fu, M.; Wang, X.G.; Li, X.G. Effect of Shot Peening on the Oxidation Resistance of TP304H and HR3C Steels in Water Vapor. Oxid. Met. 2012, 77, 17–26. [Google Scholar] [CrossRef]
- Iseda, A.; Okada, H.; Semba, H.; Igarashi, M. Long Term Creep Properties and Microstructure of SUPER304H, TP347HFG and HR3C for A-USC Boilers. Energy Met. 2007, 2, 199–206. [Google Scholar] [CrossRef]
- Dudziak, M.; Lukaszewicz, M.; Simms, N.J.; Nicholls, J.R. Steam Oxidation of TP347HFG, Super 304H and HR3C-analysis of Significance of Steam Flowrate and Specimen Surface Finish. Corros. Eng. Sci. Techn. 2015, 50, 272–282. [Google Scholar] [CrossRef]
- Lukaszewicz, M.; Simms, N.J.; Dudziak, T.; Nicholls, J.R. Effect of Steam Flow Rate and Sample Orientation on Steam Oxidation of Ferritic and Austenitic Steels at 650 and 700 degrees C. Oxid. Met. 2013, 79, 473–483. [Google Scholar] [CrossRef]
- Dudziak, T.; Lukaszewicz, M.; Simms, N.J.; Nicholls, J.R. Impact Specimen Geometry on T23 and TP347HFG Steels Behaviour During Steam Oxidation at Harsh Condition. Corros. Eng. Sci. Techn. 2017, 52, 46–53. [Google Scholar] [CrossRef]
- Liu, L.L.; Liu, S.; Guo, Q.Q.; Shen, J.; Li, W.B.; Niu, Y. Effects of Cr Addition and a Magnetron Sputtered Film on the Oxidation-Sulfidation Behavior of Fe-xCr-5Si Alloys in H2-CO2-H2S Mixture at 800 degrees C. J. Electrochem. Soc. 2017, 164, C269–C275. [Google Scholar] [CrossRef]
- Ericsson, T. A Study of the Cr-depleted Surface Layers Formed on Four Cr-Ni Steels During Oxidation in Steam at 600 and 800 degree C. Oxid. Met. 1970, 2, 401–417. [Google Scholar] [CrossRef]
- Young, D.J.; Pint, B.A. Chromium Volatilization Rates from Cr2O3 Scales into Flowing Gases Containing Water Vapor. Oxid. Met. 2006, 66, 137–153. [Google Scholar] [CrossRef]
- Yan, W.J.; Ya, Y.Q.; Du, F.; Shao, H.; Zhao, P.T. Spectrometer-Based Line-of-Sight Temperature Measurements during Alkali-Pulverized Coal Combustion in a Power Station Boiler. Energies 2017, 10, 1375. [Google Scholar] [CrossRef]
- Zhao, Y.; Men, Y.H.; Han, P. Modeling and Simulation of Complex Fluid Networks in the Flue Gas System of a Boiler. Energies 2017, 10, 1432. [Google Scholar] [CrossRef]

| Alloy | Fe | C | Si | Mn | P | S | Ni | Cr | V | B | N | Cu | Nb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Super304H | Bal. | 0.08 | 0.22 | 0.75 | 0.025 | 0.002 | 8.68 | 18.71 | <0.1 | <0.0005 | 0.09 | 2.97 | 0.48 |
| HR3C | Bal. | 0.06 | 0.42 | 1.13 | 0.012 | 0.002 | 19.56 | 25.61 | <0.1 | <0.0005 | 0.28 | - | 0.39 |
| Material | Size (mm) | Component | Location | Elevation | Temperature (°C) | Pressure (MPa) |
|---|---|---|---|---|---|---|
| Super304H | φ51 × 9 | rear panel superheater | 30–12 | outlet section, four meters from elbow | 580–607 | 25.98–26.17 |
| HR3C | φ60 × 4 | final-stage reheater (rear panel) | 20–1 | outlet section, six meters from elbow | 590–617 | 7.05–7.25 |
| Material | Compound | 2θ |
|---|---|---|
| Super304H | Fe2O3 | 35.592, 50.045, 74.406 |
| Cr2O3 | 33.307, 43.945, 53.553 | |
| FeCr2O4 | 30.112, 37.093, 43.088, 53.516, 57.980, 62.612 | |
| HR3C | Fe2O3 | 24.133, 33.169, 35.624, 40.806, 43.518, 49.468, 54.030, 57.533, 62.407, 63.938, 69.625, 71.922 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ma, H.; Wang, Y.; Xue, M.; Zhao, Q. Investigation on Oxidation Behavior of Super304H and HR3C Steel in High Temperature Steam from a 1000 MW Ultra-Supercritical Coal-Fired Boiler. Energies 2019, 12, 521. https://doi.org/10.3390/en12030521
Li J, Ma H, Wang Y, Xue M, Zhao Q. Investigation on Oxidation Behavior of Super304H and HR3C Steel in High Temperature Steam from a 1000 MW Ultra-Supercritical Coal-Fired Boiler. Energies. 2019; 12(3):521. https://doi.org/10.3390/en12030521
Chicago/Turabian StyleLi, Jingjing, Haidong Ma, Yungang Wang, Min Xue, and Qinxin Zhao. 2019. "Investigation on Oxidation Behavior of Super304H and HR3C Steel in High Temperature Steam from a 1000 MW Ultra-Supercritical Coal-Fired Boiler" Energies 12, no. 3: 521. https://doi.org/10.3390/en12030521
APA StyleLi, J., Ma, H., Wang, Y., Xue, M., & Zhao, Q. (2019). Investigation on Oxidation Behavior of Super304H and HR3C Steel in High Temperature Steam from a 1000 MW Ultra-Supercritical Coal-Fired Boiler. Energies, 12(3), 521. https://doi.org/10.3390/en12030521





