Study on Sensitivity Differences of Critical Spontaneous Ignition Temperature between Alcohol and Hydrocarbon Fuels Based on Reaction Pathway
Abstract
1. Introduction
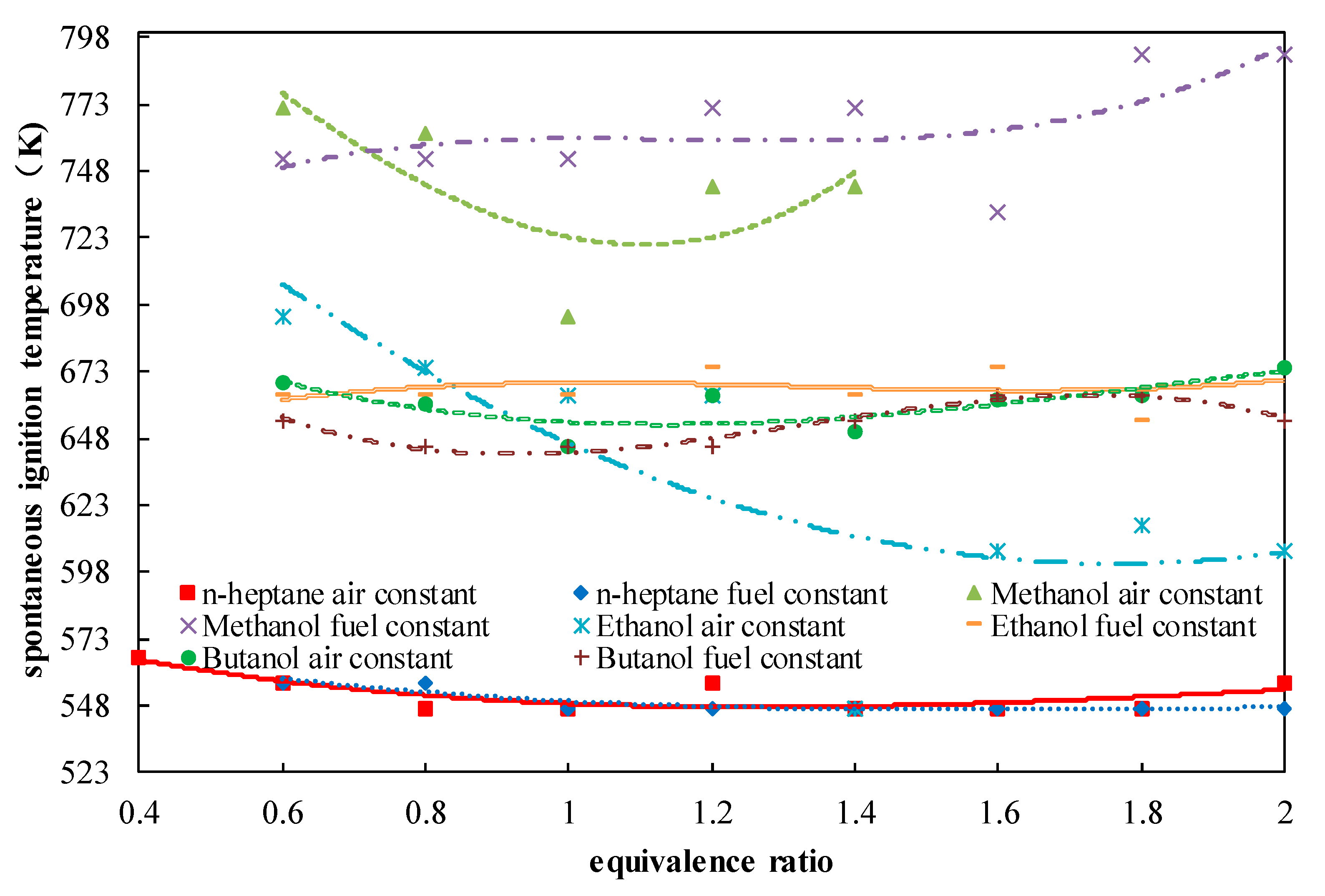
2. Experimental Methods and Results
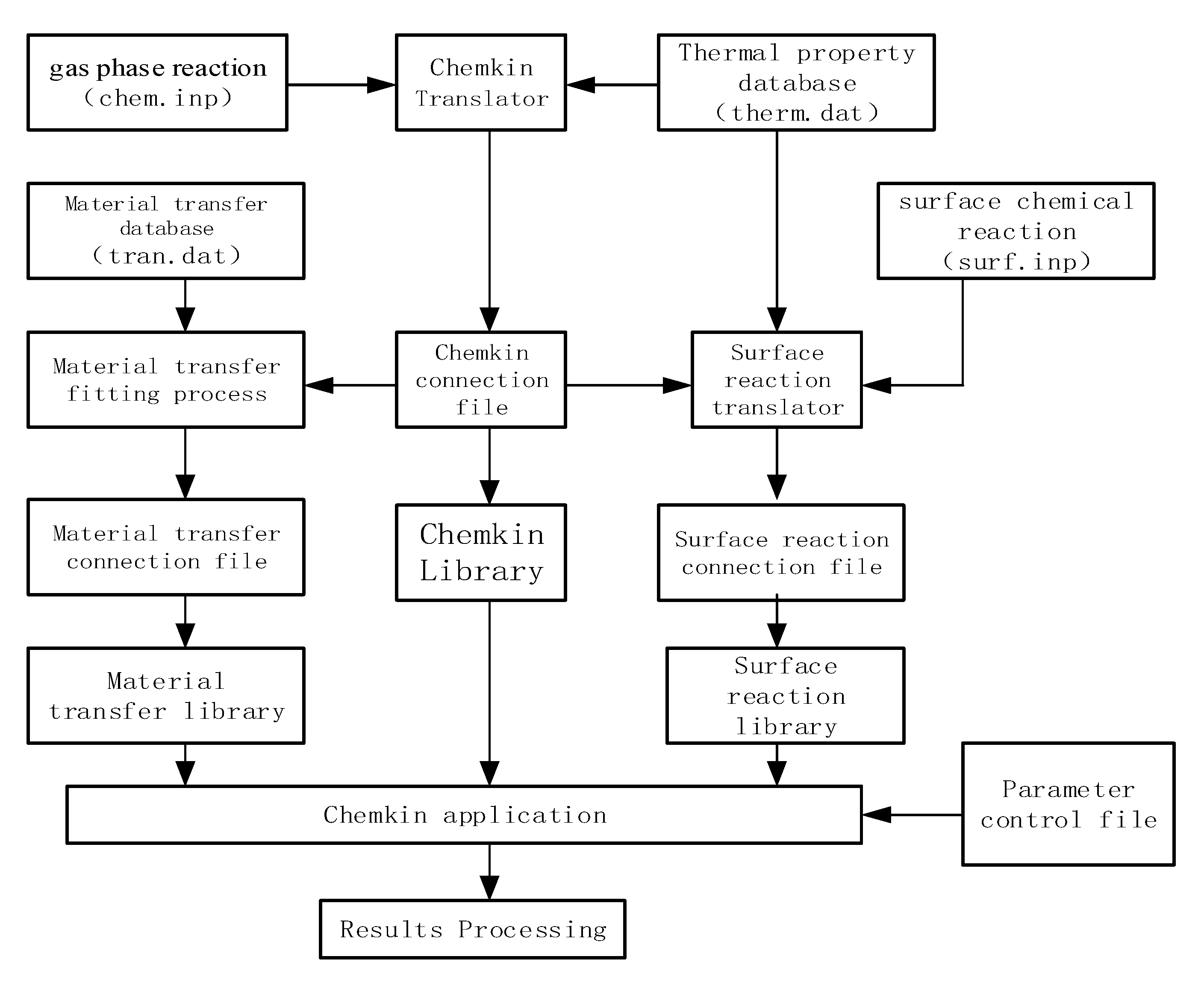
3. Establishment of Simulation Model and Selection of Mechanism
4. Analysis of Dominant Reaction Characteristics of Methanol Spontaneous Ignition
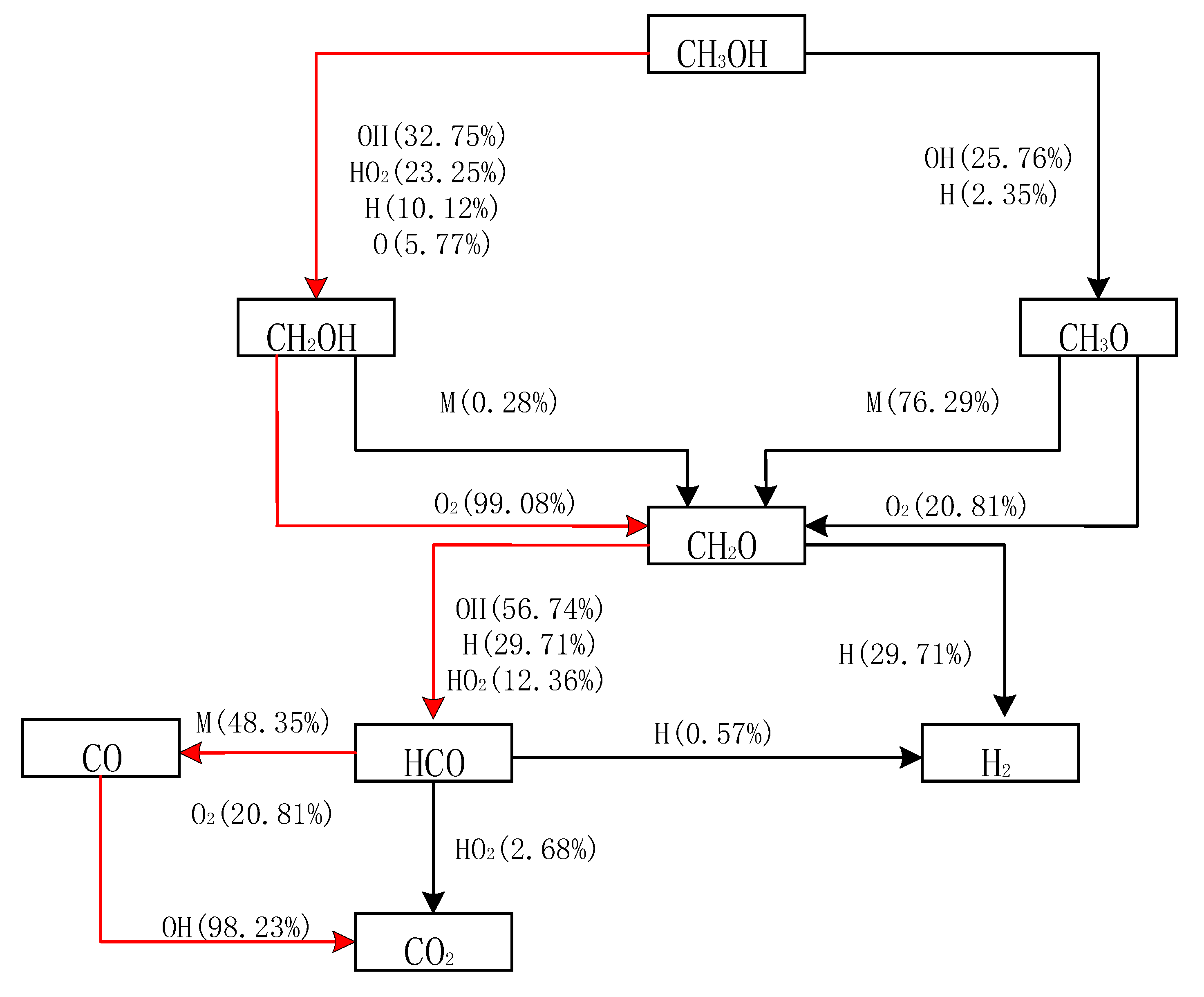
4.1. Determination of Methanol Ignition Markers
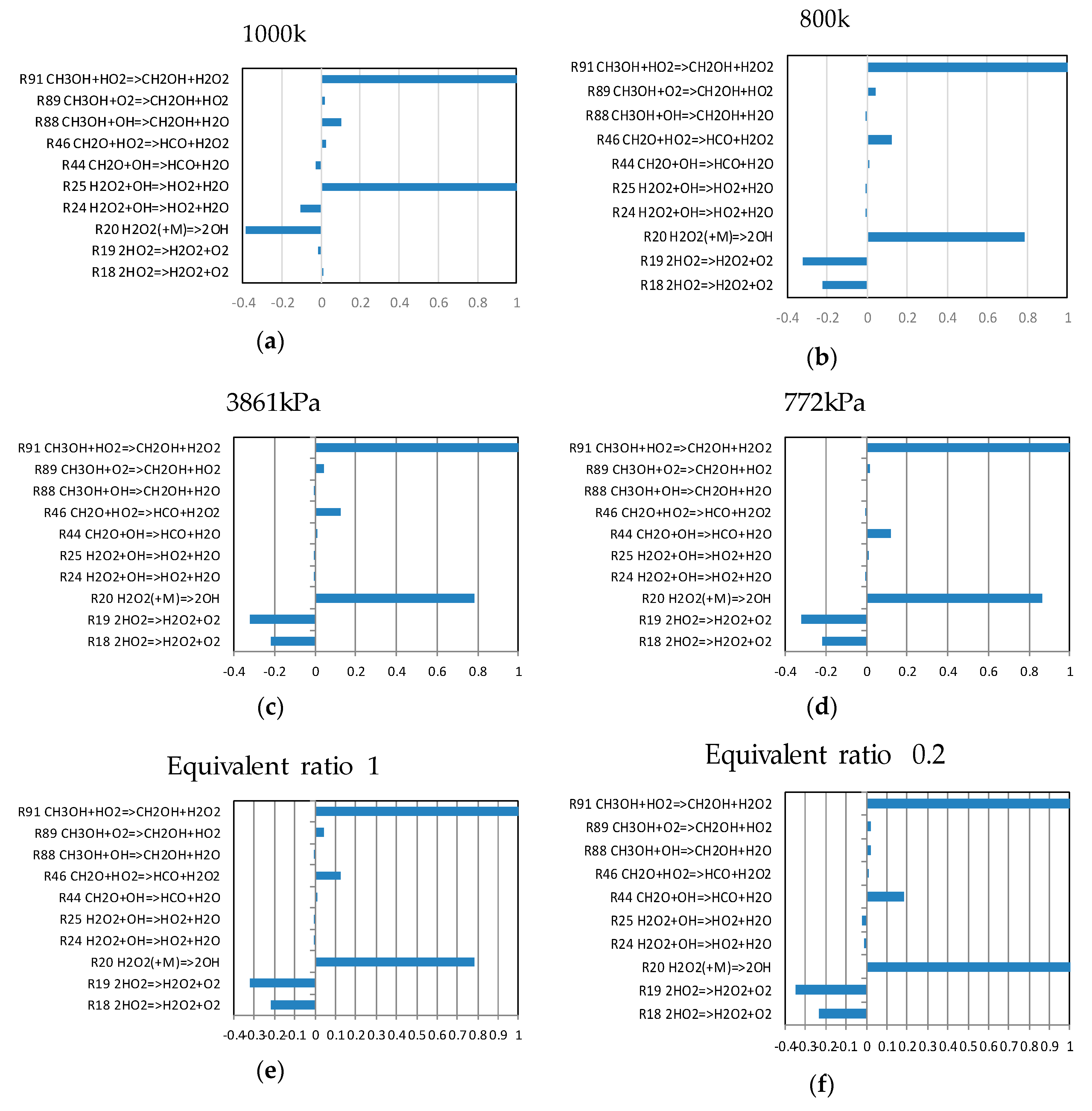
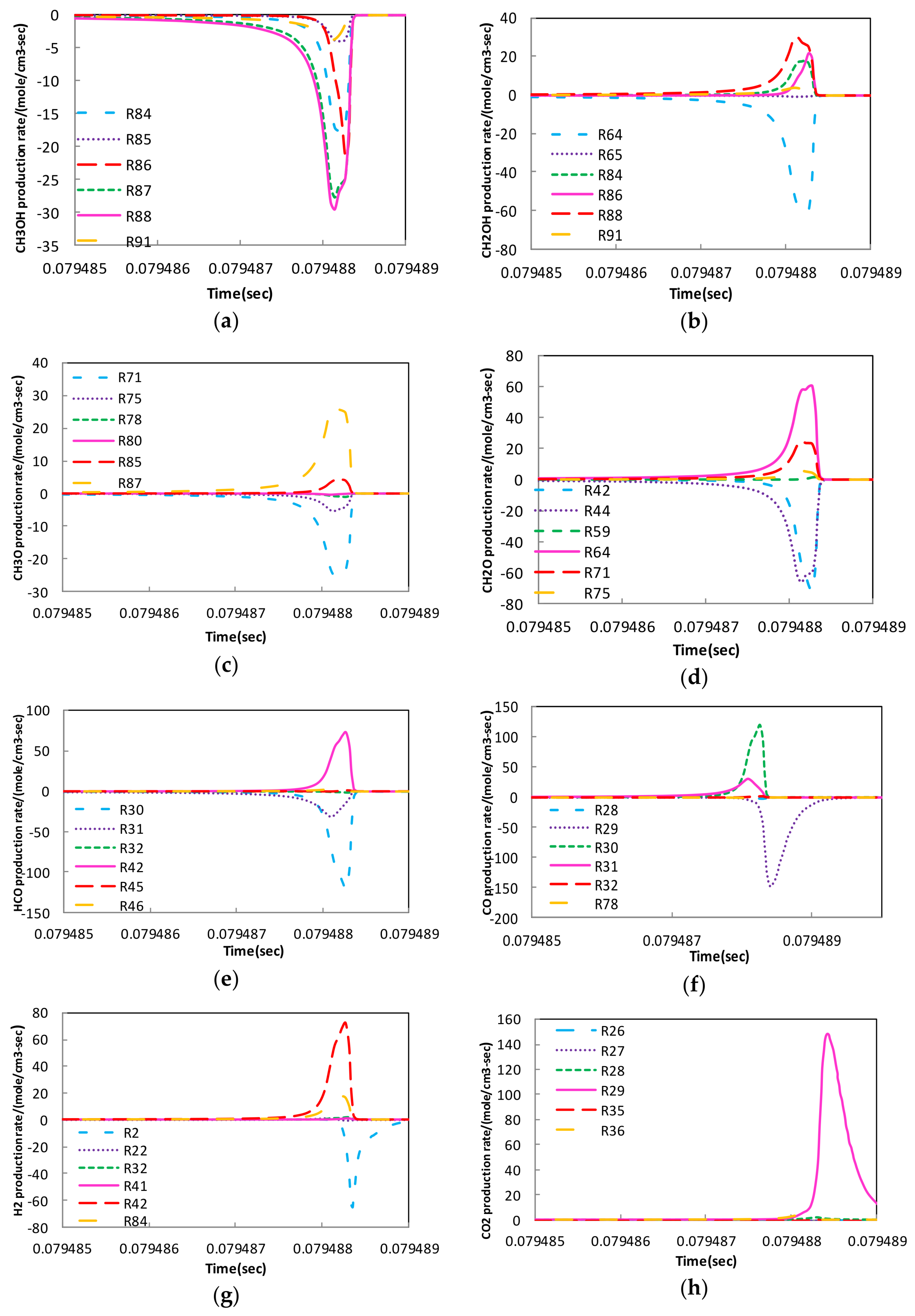
4.2. Sensitivity Analysis Based on OH Group Generation and Consumption
- R91:CH3OH + HO2 = CH2OH+H2O2
- R89:CH3OH + O2 = CH2OH+HO2
- R46:CH2O + HO2 = HCO+H2O2
- R20:H2O2 (+M) = 2OH
- R19:2HO2 = H2O2 + O2
- R18:2HO2 = H2O2 + O2
5. Analysis of the Dominant Reaction Characteristics of N-heptane Spontaneous Combustion
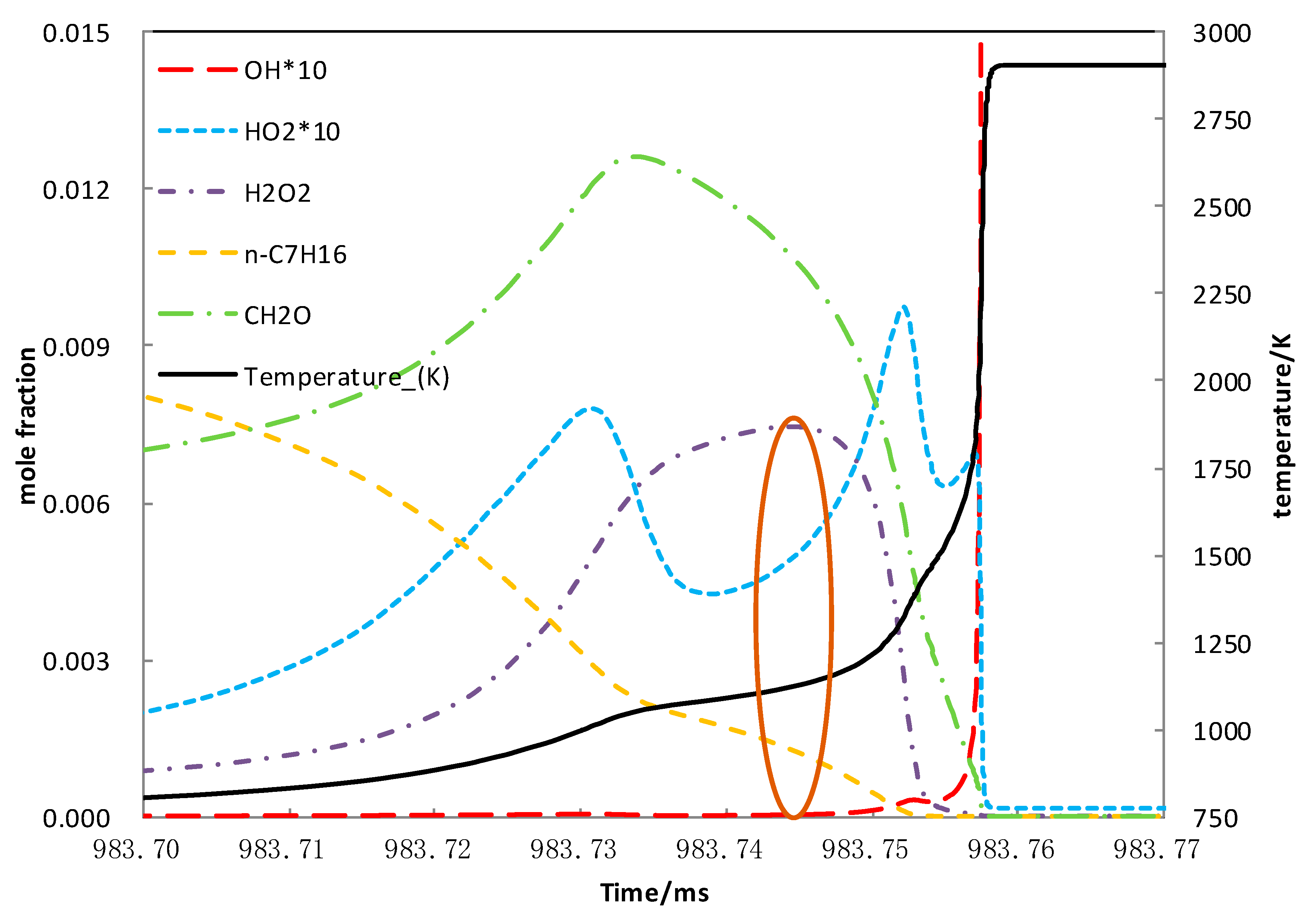
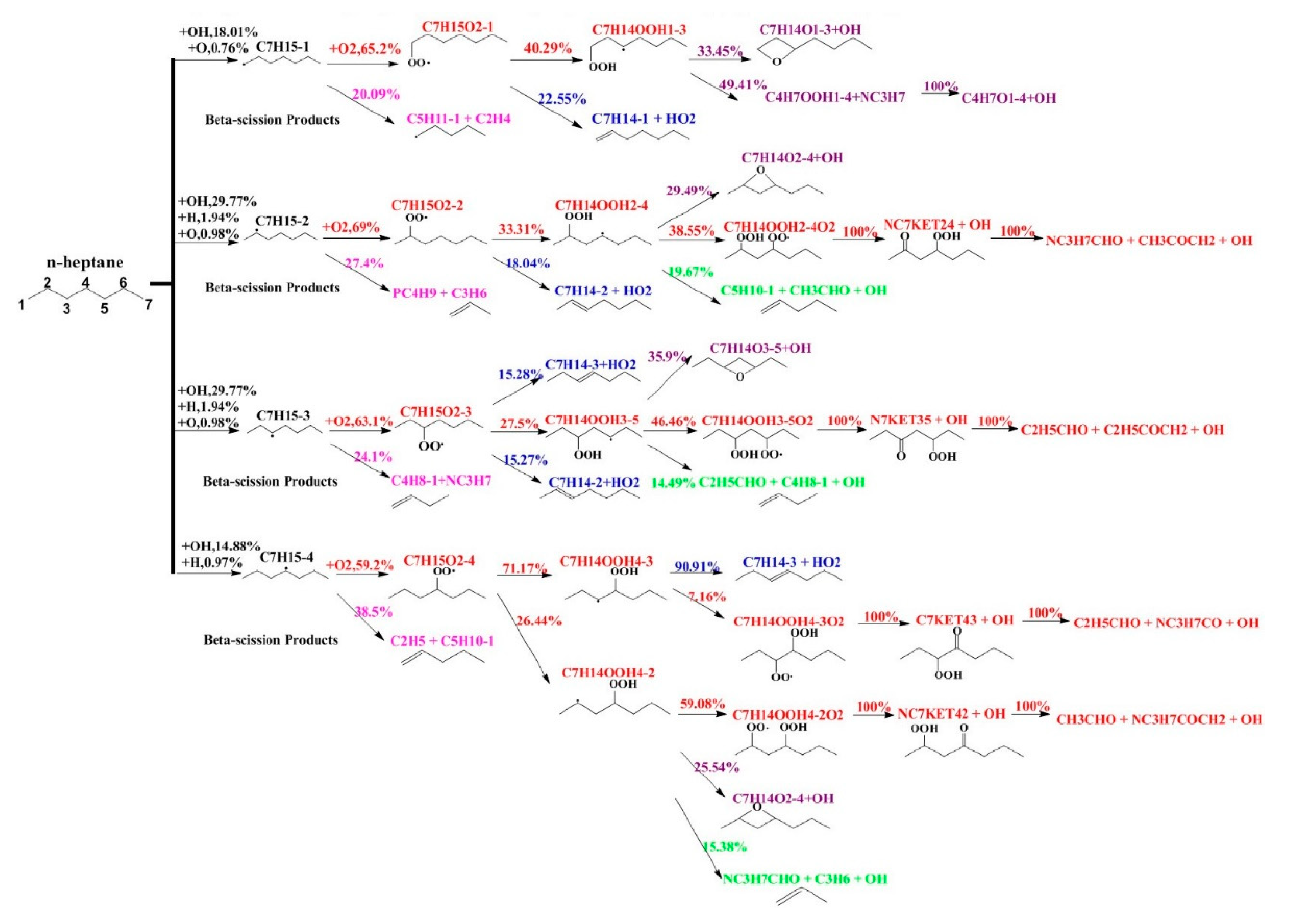
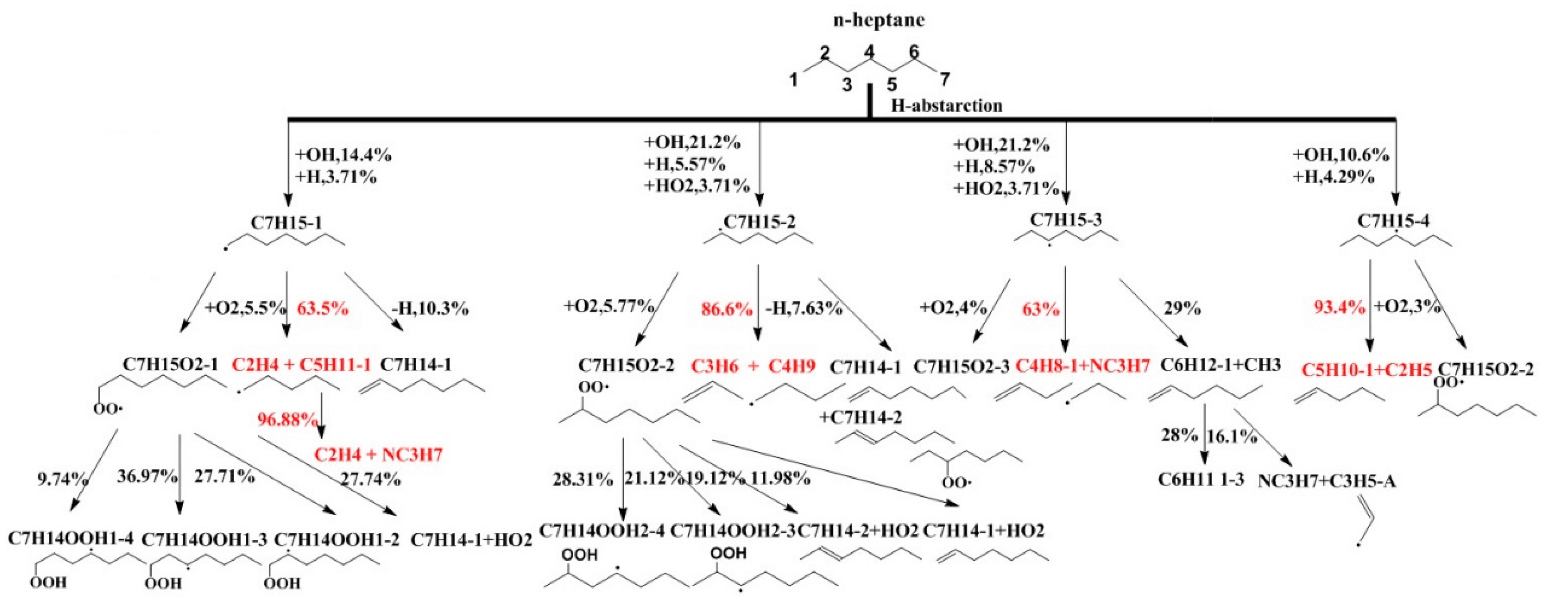
5.1. N-Heptane Ignition Marker
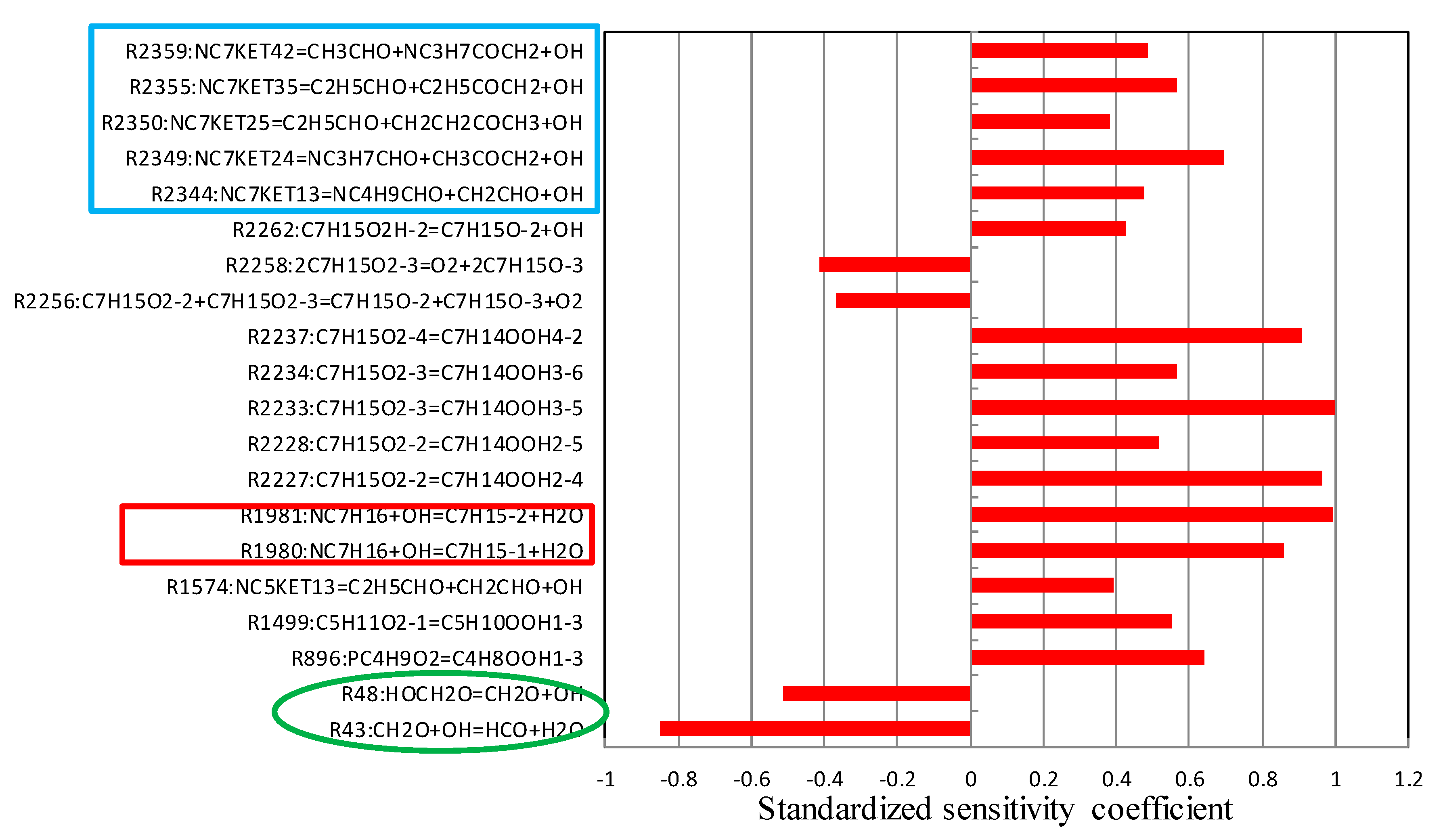
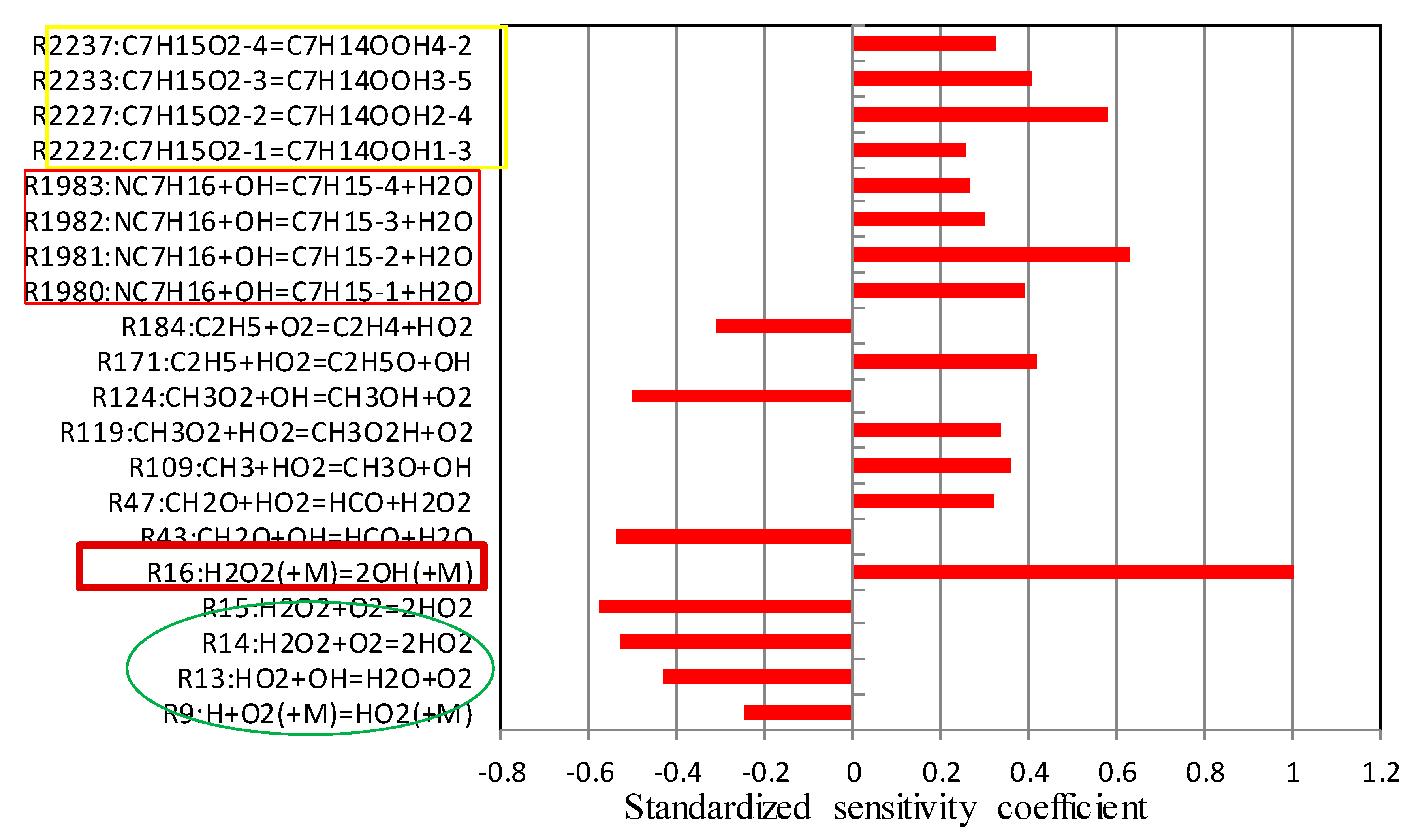
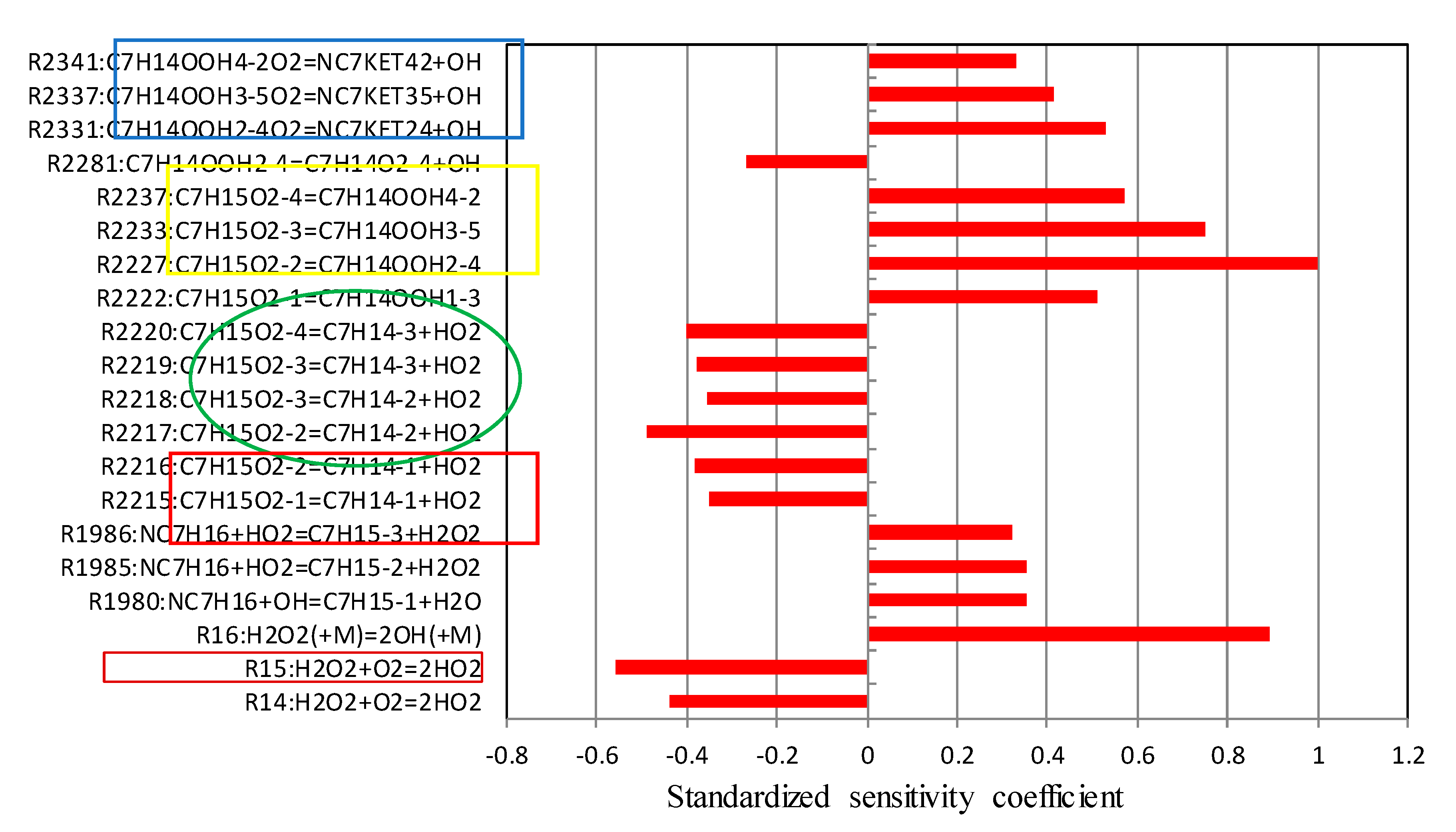
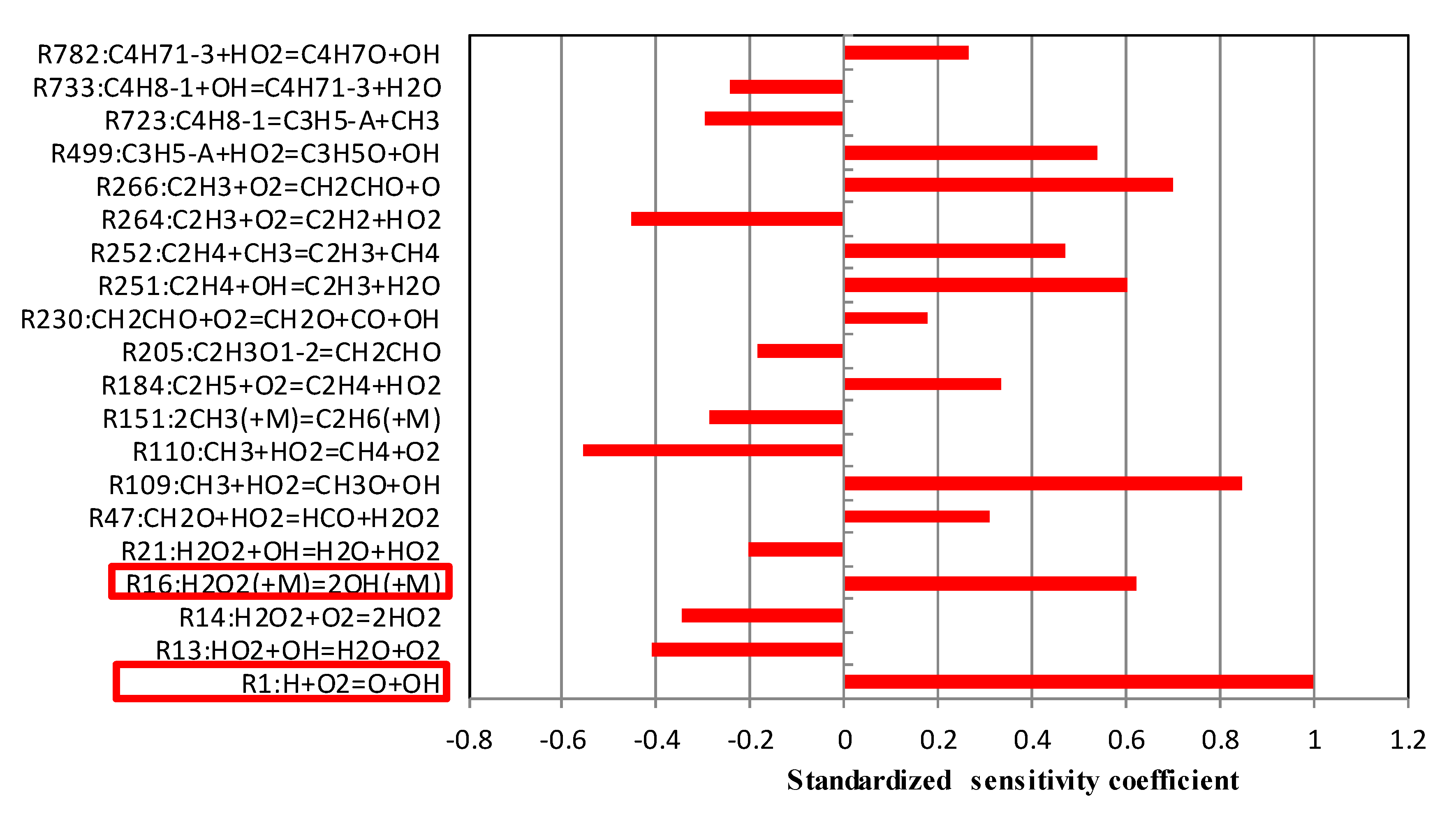
5.2. Sensitivity Analysis Based on OH Group Generation and Consumption
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Emissions Management. Heavy Duty Diesel Emissions Guide: Europe, USA and Japan; Johnson Matthey Plc: London, UK, 2012. [Google Scholar]
- Zheng, Y.N. The Critical Spontaneous Combustion Boundary Relevance Research of Single Component Fuel. Ph.D. Thesis, Ji Lin University, Changchun, China, 2017. [Google Scholar]
- Fieweger, M.K.; Blumenthal, R.; Adomeit, G. Self-Ignition of S.I. engine model fuels: A shock tube investigation at high pressure, Combust. Flame 1997, 109, 599–619. [Google Scholar] [CrossRef]
- Burke, U.; Metcalfe, W.K.; Burke, S.M.; Heufer, K.A.; Dagaut, P.; Curran, H.J. A detailed chemical kinetic modeling, ignition delay time and jet-stirred reactor study of methanol oxidation. Combust. Flame 2016, 165, 125–136. [Google Scholar] [CrossRef]
- Noorani, K.E.; Akih-Kumgeh, B.; Bergthorson, J.M. Comparative high temperature shock tube ignition of C1–C4 primary alcohols. Energy Fuels 2010, 24, 5834–5843. [Google Scholar] [CrossRef]
- Aranda, V.; Christensen, J.M.; Alzueta, M.U.; Glarborg, P.; Gersen, S.; Gao, Y.; Mar-shall, P. Experimental and kinetic modeling study of methanol ignition and oxidation at high pressure. Int. J. Chem. Kinet. 2013, 45, 283–294. [Google Scholar] [CrossRef]
- Smith, J.M.; Simmie, J.M.; Curran, H.J. Autoignition of heptanes: Experiments and modeling. Int. J. Chem. Kinet. 2005, 37, 728–736. [Google Scholar] [CrossRef]
- Xu, S.; Lin, M.C. Theoretical study on the kinetics for OH reactions with CH3OH and C2H5OH. Proc. Combust. Inst. 2007, 31, 159–166. [Google Scholar] [CrossRef]
- Lu, X.; Ji, L.; Zu, L.; Hou, Y.; Huang, C.; Huang, Z. Experimental study and chemical analysis of n-heptane homogeneous charge compression ignition combustion with port injection of reaction inhibitors. Combust. Flame 2007, 149, 261–270. [Google Scholar] [CrossRef]
- Li, R.; Liu, Z.; Han, Y.; Tan, M.; Xu, Y.; Tian, J.; Yan, J.; Chai, J.; Liu, J.; Yu, X. Experimental and Kinetic Modeling Study of Autoignition Characteristics of n-Heptane/Ethanol by Constant Volume Bomb and Detail Reaction Mechanism. Energy Fuels 2017, 31, 13610–13626. [Google Scholar] [CrossRef]
- Li, R.; Liu, Z.; Han, Y.; Tan, M.; Xu, Y.; Tian, J.; Chong, D.; Chai, J.; Liu, J.; Li, Z. Experimental and Numerical Investigation into the Effect of Fuel Type and Fuel/Air Molar Concentration on Autoignition Temperature of n-Heptane, Methanol, Ethanol, and Butanol. Energy Fuels 2017, 31, 2572–2584. [Google Scholar] [CrossRef]
- ASTM D7668-14a Standard Test Method for Determination of Derived Cetane Number (DCN) of Diesel Fuel Oils—Ignition Delay and Combustion Delay Using a Constant Volume Combustion Chamber Method; ASTM International: West Conshohocken, PA, USA, 2014.
- Liu, J. The Characteristic and Parameter Sensitivity Analysis of Alcohol Fuel in Auto-Ignition Combustion; Jilin University: Changchun, China, 2018. [Google Scholar]
- Chai, J. Analysis of Main Reaction Characteristics of Alkane Fuel and Its Sensitive Parameters; Jilin University: Changchun, China, 2018. [Google Scholar]
- Hatim, M.; Simeon, C. An experimental and numerical analysis of the influence of the inlet temperature, equivalence ratio and compression ratio on the HCCI auto-ignition process of Primary Reference Fuels in an engine. Fuel Process Technol. 2008, 89, 1218–1226. [Google Scholar] [CrossRef]
- Lv, H. Application of Chemkin Softwear in Numerical Simulation of Homogeneous Charge Compression Ignition and Fuel Modification. Ph.D. Thesis, Chang An University, Changan, China, June 2010. [Google Scholar]
- Ilbas, M.; Crayford, A.; Yilmaz, I.; Bowen, P.; Syred, N. Laminar-burning velocities of hydrogen–air and hydrogen–methane–air mixtures: An experimental study. Int. J. Hydrogen Energy 2006, 31, 1768–1779. [Google Scholar] [CrossRef]
- Cashdollar, K.L.; Zlochower, I.A.; Green, G.M.; Thomas, R.A.; Hertzberg, M. Flammability of methane, propane, and hydrogen gases. J. Loss Prev. Process Ind. 2000, 13, 327–340. [Google Scholar] [CrossRef]
- Thomas, W.R.; Callahan, T.J. Homogeneous Charge Compression Ignition of Diesel Fuel; SAE Technical Paper 961160; SAE International: Warrendale, PN, USA, 1996. [Google Scholar] [CrossRef]
- Berntsson, W.A.; Denbratt, I. HCCI Combustion Using Charge Stratification for Combustion Control; SAE Paper 2007-01-0210; SAE International: Warrendale, PN, USA, 2007. [Google Scholar] [CrossRef]
- Korakianitis, T.; Namasivayam, A.M.; Crookes, R.J. Natu-ral gas fueled spark-ignition (SI) and compression-ignition(CI) engine performance and emissions. Prog. Energy Combust. Sci. 2011, 37, 89–112. [Google Scholar] [CrossRef]
- Guang, H.; Zheng, Y.; Zhen, H.; Lu, X. Experimental Study of n-Heptane Ignition Delay with Carbon dioxide addition in a Rapid Compression Machine under Low Temperature Conditions. Chin. Sci. Bull. 2012, 57, 3953–3960. [Google Scholar] [CrossRef]
- Dahnz, C.; Han, K.; Spicher, U.; Magar, M.; Schiessl, R.; Maas, U. Investigations on pre-ignition in highly supercharged SI engines. SAE Int. J. Engines 2010, 3, 214–224. [Google Scholar] [CrossRef]
- Amann, M.; Alger, T.; Mehta, D. The effect of EGR on low-speed pre-ignition in boosted SI engines. SAE Int. J. Engines 2011, 4, 235–245. [Google Scholar] [CrossRef]
- Khan, M.Y.; Johnson, K.C.; Durbin, T.D.; Jung, H.; Cocker, D.R., III; Bishnu, D.; Giannelli, R. Characterization of PM-PEMS for in-use measurements conducted during validation testing for the PM-PEMS measurement allowance program. Atmos. Environ. 2012, 55, 311–318. [Google Scholar] [CrossRef]
- Onishi, S.; Jo, S.H.; Shoda, K.; Jo, P.D.; Kato, S. Active Thermo-Atmosphere Combustion (ATAC)—A new Combustion Process for Internal Combustion Engines; SAE Paper 790501; SAE International: Warrendale, PN, USA, 1979. [Google Scholar] [CrossRef]
- Han, Y.; Li, R.; Liu, Z.; Tian, J.; Wang, X.; Kang, J. Feasibility analysis and performance characteristics investigation of spatial recuperative expander based on organic Rankine cycle for waste heat recovery. Energy Convers. Manag. 2016, 121, 335–348. [Google Scholar] [CrossRef]
- Morteza, F.; Rahim, K.S.; Mohsen, P. EGR and Intake Charge Temperature Effects on Dual-Fuel HCCI Combustion and Emissions Characteristics; SAE Paper 2011-24-0050; Capri: Napoli, Italy, 2011. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.C.; Han, Y.; Tian, J.; Wang, J.; Fang, J. Experimental Investigation of the Loading Strategy of an Automotive Diesel Engine under Transient Operation Conditions. Energies 2018, 11, 1293. [Google Scholar] [CrossRef]
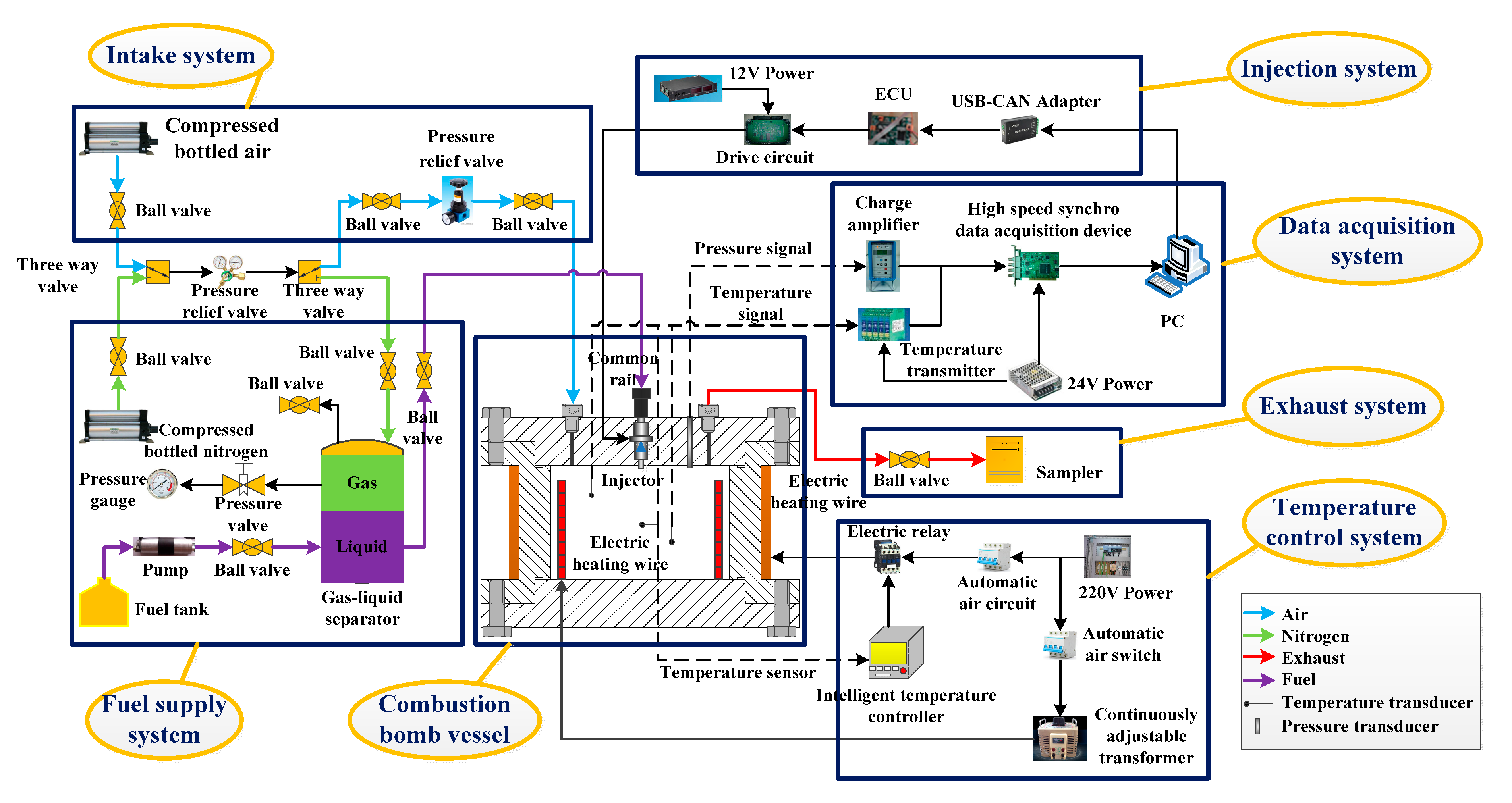


| Apparatus | Manufacturer | Type |
|---|---|---|
| Temperature transducer | MingYang (Tianjin, China ) | WRNK-191 |
| Pressure transducer | Kistler (Winterthur, Switzerland) | KISTLER6125B |
| Temperature transmitter | PARAGON (Forestville, CA, USA) | PA-15-4-1 (K) |
| Charge amplifier | Kistler (Winterthur, Switzerland) | 5051A |
| Electrical control unit | Freescale (Austin, TX, USA) | MC9S12XEP100MAL |
| USB | DAM-C3110 (Beijing, China) | USB-CAN |
| Multi-function data USB | HengRuiFeng (Suzhou, China) | USB2.0 |
| Intake Pressure (psi) | Intake Pressure (kPa) | Temperature (K) | Air Amount (mol) | Equivalent Ratio | Fuel (mol) |
|---|---|---|---|---|---|
| 240 | 1655 | 473 | 0.427 | 1 | 0.0596 |
| 220 | 1517 | 473 | 0.391 | 1 | 0.0546 |
| 200 | 1379 | 473 | 0.356 | 1 | 0.0497 |
| 180 | 1241 | 473 | 0.320 | 1 | 0.0447 |
| 160 | 1103 | 473 | 0.284 | 1 | 0.0397 |
| 140 | 965 | 473 | 0.249 | 1 | 0.0348 |
| 120 | 827 | 473 | 0.213 | 1 | 0.0298 |
| 100 | 689 | 473 | 0.178 | 1 | 0.0248 |
| 80 | 552 | 473 | 0.142 | 1 | 0.0199 |
| Intake Pressure (psi) | Intake Pressure (kPa) | Temperature (K) | Air Amount (mol) | Equivalent Ratio | Fuel (mol) |
|---|---|---|---|---|---|
| 200 | 1379 | 473 | 0.356 | 2.0 | 0.0993 |
| 200 | 1379 | 473 | 0.356 | 1.8 | 0.0894 |
| 200 | 1379 | 473 | 0.356 | 1.6 | 0.0795 |
| 200 | 1379 | 473 | 0.356 | 1.4 | 0.0695 |
| 200 | 1379 | 473 | 0.356 | 1.2 | 0.0596 |
| 200 | 1379 | 473 | 0.356 | 1.0 | 0.0497 |
| 200 | 1379 | 473 | 0.356 | 0.8 | 0.0397 |
| 200 | 1379 | 473 | 0.356 | 0.6 | 0.0298 |
| 200 | 1379 | 473 | 0.356 | 0.4 | 0.0199 |
| Intake Pressure (psi) | Intake Pressure (kPa) | Temperature (K) | Air Amount (mol) | Equivalent Ratio | Fuel (mol) |
|---|---|---|---|---|---|
| 100.00 | 689.48 | 473 | 0.178 | 2.0 | 0.0497 |
| 111.1.1 | 766.08 | 473 | 0.198 | 1.8 | 0.0497 |
| 125.00 | 861.84 | 473 | 0.222 | 1.6 | 0.0497 |
| 142.86 | 984.97 | 473 | 0.254 | 1.4 | 0.0497 |
| 166.67 | 1149.13 | 473 | 0.296 | 1.2 | 0.0497 |
| 200.00 | 1378.95 | 473 | 0.356 | 1.0 | 0.0497 |
| 250 | 1723.69 | 473 | 0.444 | 0.8 | 0.0497 |
| 333.33 | 2298.25 | 473 | 0.593 | 0.6 | 0.0497 |
| 500.00 | 3447.38 | 473 | 0.889 | 0.4 | 0.0497 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, Z.; Ren, X.; Han, Y.; Wang, J.; Fang, J. Study on Sensitivity Differences of Critical Spontaneous Ignition Temperature between Alcohol and Hydrocarbon Fuels Based on Reaction Pathway. Energies 2019, 12, 475. https://doi.org/10.3390/en12030475
Liu Q, Liu Z, Ren X, Han Y, Wang J, Fang J. Study on Sensitivity Differences of Critical Spontaneous Ignition Temperature between Alcohol and Hydrocarbon Fuels Based on Reaction Pathway. Energies. 2019; 12(3):475. https://doi.org/10.3390/en12030475
Chicago/Turabian StyleLiu, Qiang, Zhongchang Liu, Xiaoming Ren, Yongqiang Han, Jun Wang, and Jian Fang. 2019. "Study on Sensitivity Differences of Critical Spontaneous Ignition Temperature between Alcohol and Hydrocarbon Fuels Based on Reaction Pathway" Energies 12, no. 3: 475. https://doi.org/10.3390/en12030475
APA StyleLiu, Q., Liu, Z., Ren, X., Han, Y., Wang, J., & Fang, J. (2019). Study on Sensitivity Differences of Critical Spontaneous Ignition Temperature between Alcohol and Hydrocarbon Fuels Based on Reaction Pathway. Energies, 12(3), 475. https://doi.org/10.3390/en12030475




