1. Introduction
The function of mineral insulating oil in power transformers is to provide insulation and cooling [
1]. Insulation oil in transformers is used in coordination with insulation paper to form an insulation system. The insulation system in oil filled transformers is subjected to variable electric, thermal and chemical constraints that are responsible for its deterioration over time [
2,
3,
4]. The presence of oxygen and humidity in the insulation system are evident due to operation of breather configurations and the degradation of the cellulose fibers in insulation papers. Oxygen and humidity promote early degradation of the transformer oil/paper insulation that leads to an increase in the acidity of the oil and a decrease in the dielectric resistance of the paper. The by-products of the oxidation and hydrolysis may be soluble in oil or settle at the bottom of the tank, thus contributing to oil sedimentation. The soluble by-products are diagnosed by (UV/Vis) spectroscopy, Fourier Transform Infrared Spectroscopy (FTIR), total acid number (TAN), dissipation factor (Tan δ), and interfacial tension (IFT) [
5]. Similarly, insoluble by-products are quantified by the turbidity, particle counter, and color number [
6].
Oxidation and hydrolysis of mineral oil produce acids with different molecular weights. Acids with high molecular weight will have lower affinity to cellulose than those with low molecular weight [
7]. This is because of the hydrophobic nature of alkyl group C
nH
2(
n+1) [
8]. It was reported that the solubility of heavy stearic acids [CH
3(CH
2)
16COOH] and naphtenic acids [R(C
5H
8)(CH
2)
n COOH] is due to mineral oil oxidation. It was noticed that these acids were not adsorbed by the paper rather they are retained in the oil. Later, it was claimed that these acids are not aggressive towards accelerating the age of solid insulation [
9]. Acids with low molecular weight (up to five carbon atoms) are more hydrophilic than high molecular weight acids which have a bigger influence on the degradation rate of the paper through acid hydrolysis. As these acids do not affect the paper as long as they remain dissolved in the oil, it is the accumulation of acids in the oil that leads to formation of the insoluble deposits (muds) and reduces the dielectric rigidity of the oil. It may also reduce the heat transfer capability of the insulation system, if adsorbed on the surface of the solid insulation or accumulate on internal area of the cooling tubes, thus causing a thermal degradation of the insulation paper [
10].
Acids with low molecular weight are more hydrophilic than acids of high molecular weight and have a higher influence on paper degradation [
10]. Acids accumulate in the oil with insoluble deposits and affect the dielectric properties of oil. This may also hamper the heat dissipating nature of the oil by sludge deposition on the insulation paper or in cooling tubes, causing thermal degradation of the oil and paper. It is usual practice to add oxidation inhibitors to oil in order to achieve control of oxidation reactions [
11], however, these inhibitors are only capable of preventing the consequences of oxidation up to a certain extent [
12]. To regain the distinct properties of oil and achieve properties that are close to pristine conditions, regeneration process are adopted [
13].
Hence, there is a need to study oil filtration methods, which act to improve the useful life of mineral oil and will hence be of economic and ecological interest. Two standards were established for the effective maintenance of transformers oil, namely BS EN 60422: 2013 and IEEE Std 637-1985 (R2007). Beside the existing methods of oil treatment based on distillation, adsorption, and ion exchange processes, the membrane technique method could be an interesting alternative for the treatment of the mineral oils. Membrane filtration methods are simple, effective, and economic methods that could be applicable for separation of the suspended and dissolved particles in insulating oils. Membrane methods have the potential to selectively separate the smallest and dissolved decay contents in oil. Membrane technology has been revealed to be a potential candidate for zero discharge of pollutants and the elimination of a wide variety of the compounds present in water while using simple and nonhazardous materials [
14]. In general, regeneration study results depend on several factors including degree of aging, nature of the oil, filtering medium (volume, size, type, and thickness), volume of oil used for filtering, number of passes, temperature, and other experimental conditions. Hence comparative evaluation of the results is not feasible for oil regenerative studies. However, the membrane method used in the present study is investigated in a transformer oil regeneration process for the first time by the authors.
In this paper, the membrane filtration-based treatment and purification of service aged insulating oils has been experimentally studied and reported. The performance of this micro-filtration method has been evaluated by assessing the distinctive characteristics of the membrane, dielectric, and diagnostic characteristics of the oils. Additionally, the chemical characteristics of the membrane and oil r both before and after filtration have been reported by FTIR Studies. Furthermore, the performance of this method is verified by measuring dissolved decay contents, TAN, dissipation factor, relative permittivity, and resistivity of oil before and after filtration. The dielectric properties like dissipation factor, relative permittivity, and resistivity are evaluated for six passes and the percentage improvement of these properties pass by pass are also highlighted.
2. Background and Membrane Separation Technology
Insulation oil is expected to be in effective service for the complete designed life of the transformer (several decades). Due to the various degradation mechanisms of the transformer, the useful life of transformers is reduced and leads to premature aging of oil-paper insulation. It is known that the cost of insulation oils is increasing rapidly due to the reduced availability of the crude petroleum oil feedstocks used in their manufacture. Hence, it is a usual industrial practice to re-use the degraded oil by restoring its dielectric and physiochemical properties through regeneration methods. The process adopted to regain the effectiveness of oil functionalities close to pristine conditions is called oil reclamation or regeneration. This generally involves dehydration, filtration, sedimentation, centrifuging, and adsorption steps. These procedures are attractive because of the increasing price of transformer coolants, cost effectiveness and ecological benefits. Reclamation of oil involves removing moisture, impurities, and oxidation products. In cases where the reclamation process is not economical (i.e., for highly aged oils or ones with excessive chemical contaminants and dissolved contents), the oil is recycled before using it by re-refining. Thus, re-refining is a process of exhaustively removing dissolved chemical contaminants along with other contaminants/particles to make the oil fit for other low performance applications.
The traditional regeneration methods for the purification and treatment of transformer insulating oils include distillation, absorption, and ion exchange processes. These methods involve high equipment costs, noisy procedures and are rather time consuming. Traditional methods also require several pre-testing procedures and are highly energy consuming (required for heating and pumping the oil). Due to the aforementioned reasons, research on regeneration process of used dielectric oils has drawn attention of industry and researchers. Membrane technology used for separation/filtration is an emerging technology due to its continuous operation, low energy consumption, easy adaptability, versatility, and requirement of relatively mild operating conditions in comparison with conventional separation processes.
The membranes used in this technology establish a physical barrier for the passage of the contaminants during cross flow. Pressure-driven membrane methods offer a defined selectivity depending on the size of the pores of the membrane with respect to the size of the contaminants that are to be retained. The efficiency of the membrane is attributable to the intrinsic properties of contaminants in the oil with regard to the chemical nature of the membrane (potential of surface, hydrophilic or hydrophobic, diffusion nature, size and solubility). The membrane filtration is a semi-permeable and selective process under the influence of a driving force. This driving force could be a pressure gradient, a concentration gradient or an electric potential gradient. When it is an action of separation under a pressure gradient, the filtration is attributable to the size of membrane pores and the size of retained particles.
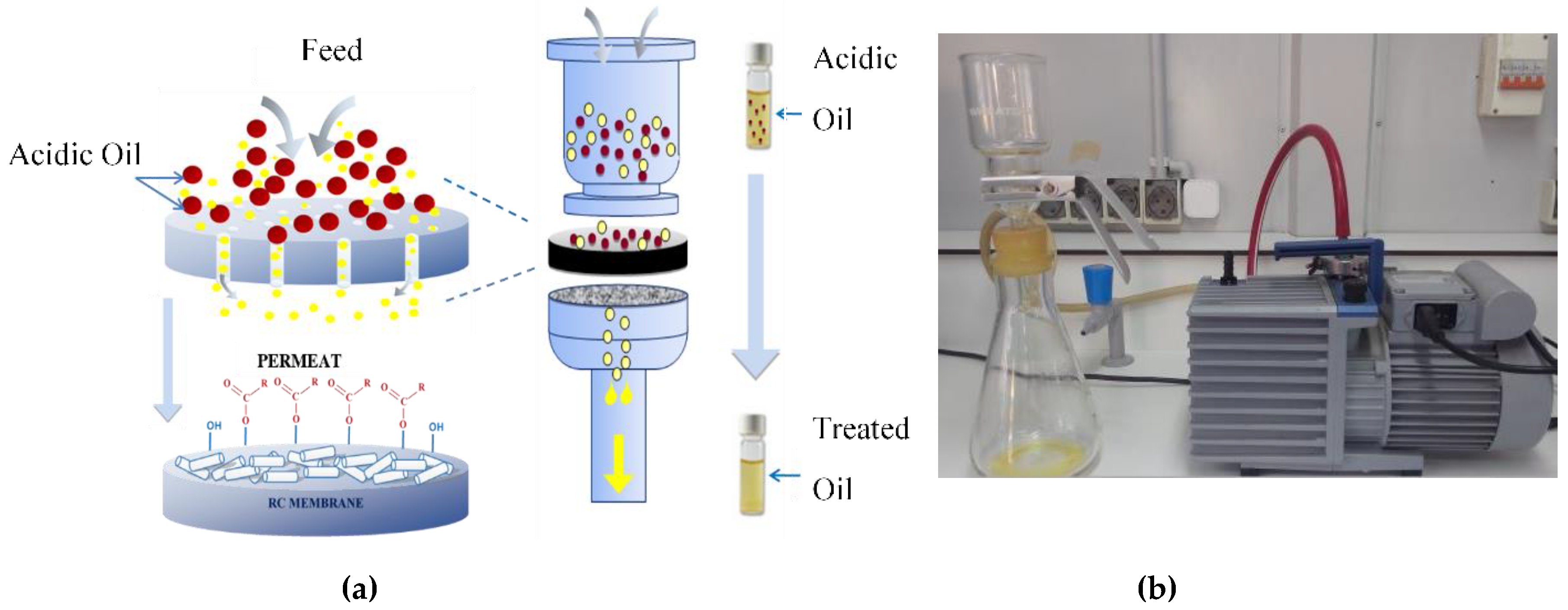
For the present study, a hydrophilic Regenerated Cellulose (RC) type membrane of 47 mm diameter 0.45 µm pores size (Model Porafil Machery-Nagel, Duren, Germany) having a dry mass of 120 mg has been adopted. Regenerated Cellulose membranes are popular for their characteristics, including spontaneous wetting, wet strength, chemically resistant, fit for aqueous and organic media. These membranes are hydrophilic in nature and are mechanically stable. These membranes are made up of cellulose fibers which is generally recommended for filtration studies as per IEEE C57.106 and ASTM D7150. The filtration unit includes an upper bowl and a receiving bowl as well as the fixation of the membrane on a removable plate-support located between the bowls. The oil to be filtered is gently filled in the upper bowl after dehydration. A vacuum pump connected to the receiving bowl with a vacuum pressure applied of the order of 0.5 mm Hg ensures the inhalation of oil and its passage through the membrane filter. The schematic diagram of the microfiltration process is represented in
Figure 1.
Carboxylic acids have an affinity for the cellulose because of their capacity attributed to the hydroxyl (OH-) group. According to Bronsted-Lowry acid-base theory, they react chemically by liberating a proton (H+) and cation RCOO- [
12]. Various mechanisms were proposed in the literature to explain the reticulation of cellulosic polymers. The reaction of carboxylic acids with the cellulose include the fixation of carboxylic acids with hydroxylate cellulosic groups and esterification with another cellulosic hydroxyl group [
15].
3. Experimental Investigation
3.1. Insulating Oil Samples
For the present study, service aged oil samples (approximately 20 years old) have been collected from a high-voltage post situated near the city of Setif, located in eastern Algeria from a 40 MVA power transformer. The physiochemical and dielectric properties of the collected oil sample have been measured according to the International Electrotechnical Commission and the International Organization for Standardization standard specifications. The properties of the service aged oil and properties after dehydration have been summarized in
Table 1 to establish a baseline prior to our membrane filtration study.
3.2. Proposed Regeneration Process
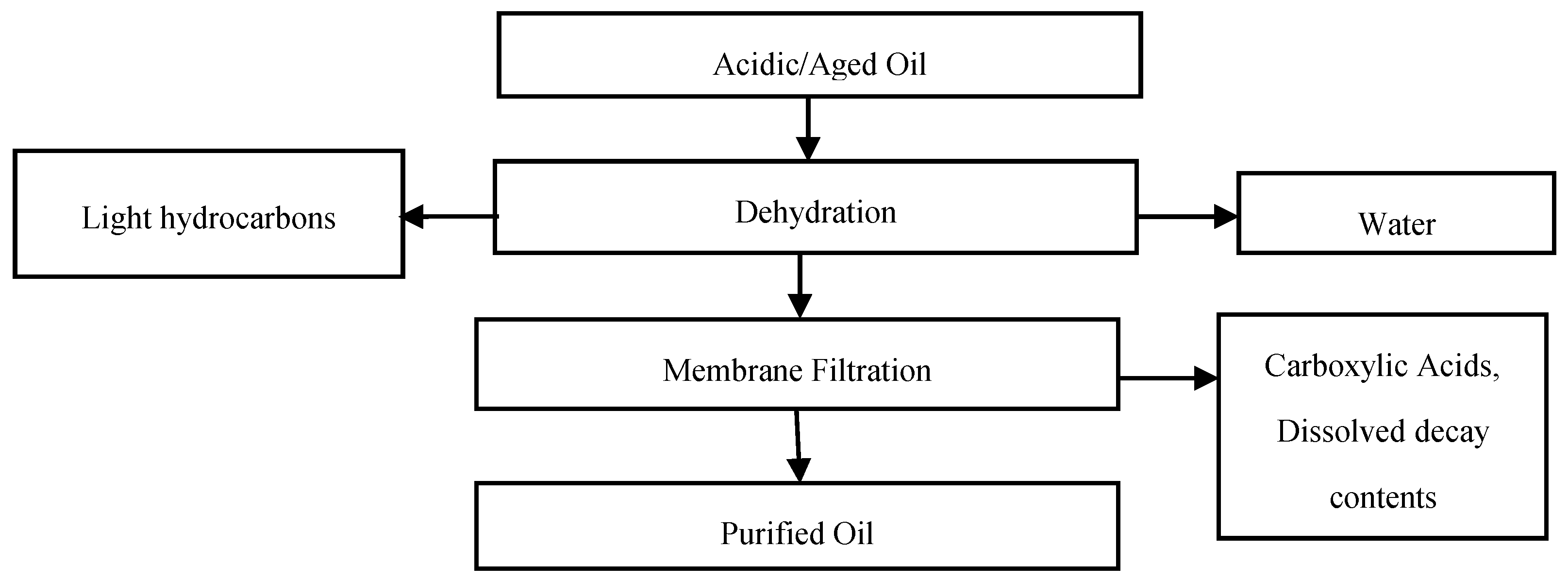
The intent of this work is to filter the aged and acidic oil for a significant improvement in its physiochemical and dielectric properties. The proposed method also aims at regaining the characteristics of the aged insulating oil that are compatible with the requirements mentioned in BS EN 60422: 2013 and IEEE Standard 637-1985 (R2007). The proposed purification method initially involves dehydration and further processing the dehydrated oil through a membrane filtration method. The detailed flow of the present study is illustrated in
Figure 2.
3.3. Dehydration and Filtration
The objective of dehydration is to remove any moisture trapped within the oil before proceeding to the membrane filtration. Dehydration is an effective way to reduce the concentration of dissolved gases and water content in the oil [
16]. In the present work, normal vacuum drying is adopted for oil dehydration at 80 °C and 0.5 mmHg as per the IEEE C57. 637 standard for oil reclamation. In addition to removing water, vacuum dehydration will degas the oil and remove the more volatile acids. The other acids however will be relatively unaffected and the overall acidity of oil will not be improved by the vacuum dehydration method [
17]. The filtration equipment is facilitated with a two stage filtration with 5 microns porosity, for absorbing the water at the first stage of treatment. Later, the dehydrated oil is allowed to flow through a series of dispersal filters of 0.5 micron inside a vacuum chamber. These series of filters form a thin film on which the filtrate is accumulated. The characteristics of the oil before and after dehydration are presented in
Table 1.
4. Performance Analysis of RC Membrane
To understand the mechanism involved in the membrane separation process, characterization of the used membrane is essential. Hence, there is a need to test the membrane retention properties. In this work, membrane retention properties are investigated in continuous mode, by realization of oil cross flow filtration. This study also facilitates in reporting the influence of membrane on the properties of oil. There are two factors that determine the efficiency of a membrane filtration process, selectivity and productivity. The selectivity is expressed by retention coefficient (R). Productivity is expressed by a parameter flow (expressed by L·m−2·h−1). This section emphasizes the membrane characteristics including membrane flux, retention coefficient, sorption process, and membrane mass. The definitions of these parameters have been discussed at the corresponding subsections. The filtrate is dehydrated transformer oil collected from an Algerian utility.
4.1. Membrane Flux (J)
The flow of permeate membrane flux (J) is defined as the time (∆t) taken to collect a volume ∆V of a given filtrate and membrane surface (S) [
18]. The mathematical expression for membrane permeate flux is shown in Equation (1):
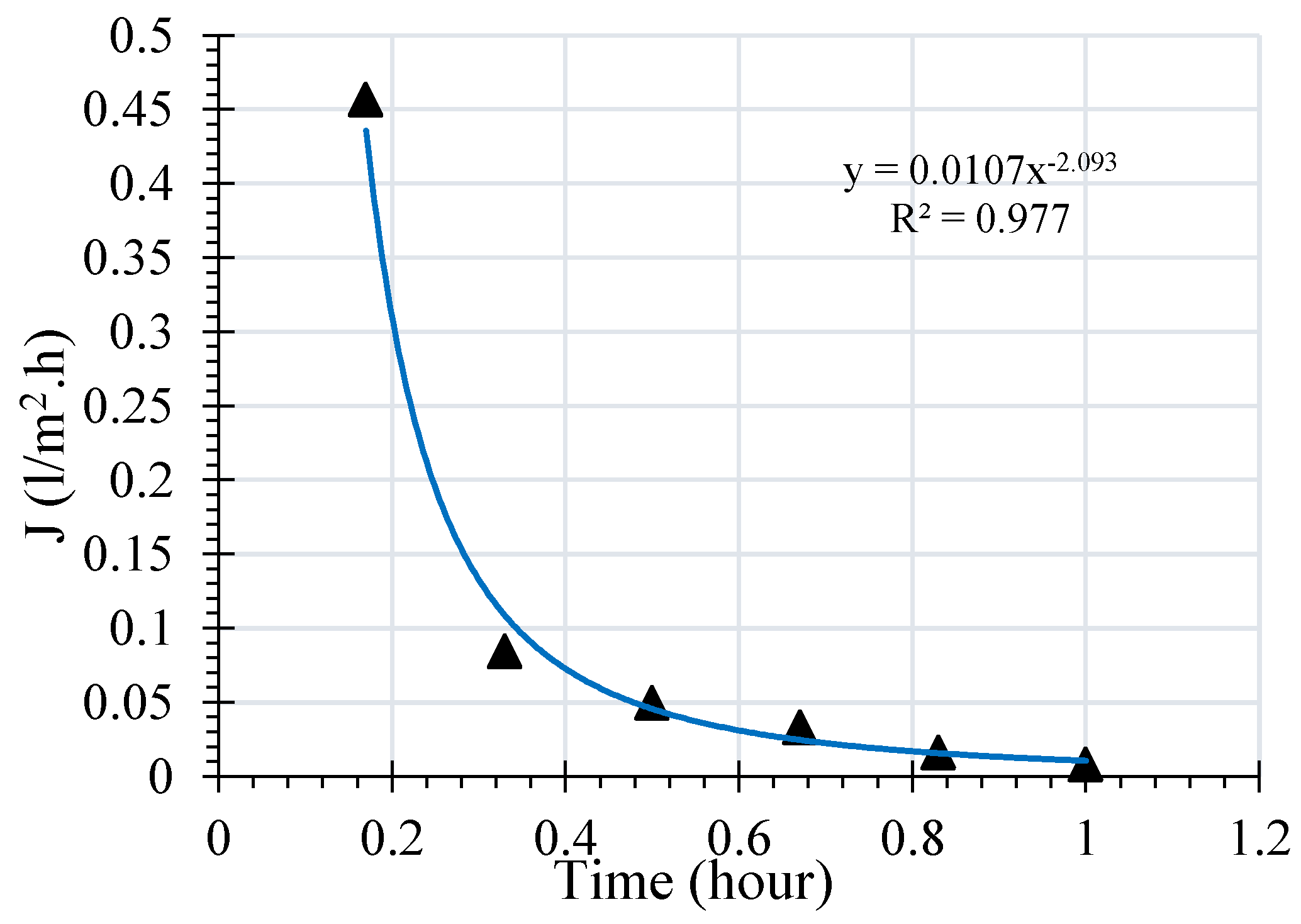
During filtration, accumulation of molecules or macromolecules on the surface of the membrane will reduce the permeation flux. This reduction is due to the adsorption of soluble molecules in the oil. The membrane permeate flux as a function of time is illustrated in
Figure 3. It is observed that the variation is in agreement with the phenomenon of the sorption where a maximal retention is noted at a time of 0.2 h for a maximum flow of 0.46 L/m
2h.
The evidence of irreversible deposit on the surface of the membrane is generally due to the equilibrium between the repulsive forces (particle-particle or particle-membrane) and the trail forces of the permeate. For a given flow, repulsive forces are overcome by the permeate trail forces, which allows the formation of a deposit on the membrane surface which creates an additional resistance to the flow of permeate.
4.2. Retention Coefficient (R)
It is the ratio of concentration of the species in the filtrate (C
F) to the concentration of the species in the upstream oil sample (C
0) [
19]. The expression of retention coefficient is shown in Equation (2):
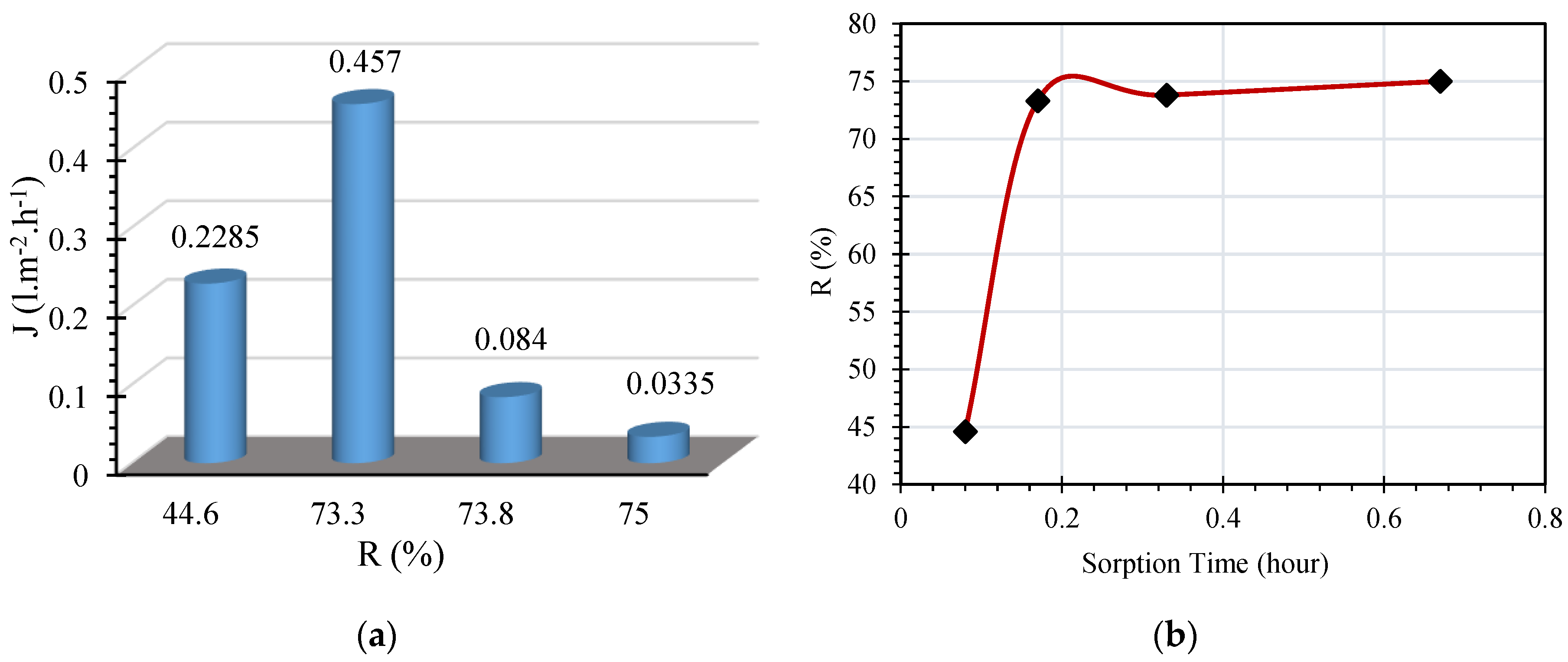
The sorption of carboxylic acids/colloidal particles accumulated in the insulating liquid is due to the hydrophilic interactions that occur when the membrane is loaded with oil. Permeate flux as a function of retention coefficient is illustrated in
Figure 4a.
It is noticed that a retention coefficient of 75% is recorded by the RC membrane after one pass. This highlighted the possibility for operation of an interesting sorption of the acids and colloidal particles by the RC membrane. During the operations of cross flow microfiltration, the retention coefficient exhibited a tendency to increase rapidly up to a certain value. With further increase in sorption time, further rises of R were not seen and is observed to be almost constant. The same is presented in
Figure 4b. There is a chance for the filtration process to cause a progressive sealing of the membrane pores, which is observed by a reduction in the permeation flow. Such a sealing could occur externally due to adsorption of colloidal particles formed in oil due to oil-paper insulation degradation. It could also be internally sealed due to dissolved decay contents formed due to oxidation of oil-paper insulation because of the absorption nature of RC membrane. Hence, the reduction in permeation flow is a resultant of adsorption and absorption phenomena, formation of the filtration cake by retained elements and dissolved decay contents settled in the membrane pores. The same is expressed as sealing and saturation of the membrane. Also, the resistance to filtration may be related to the effective size of the membrane pores.
4.3. Sorption Process
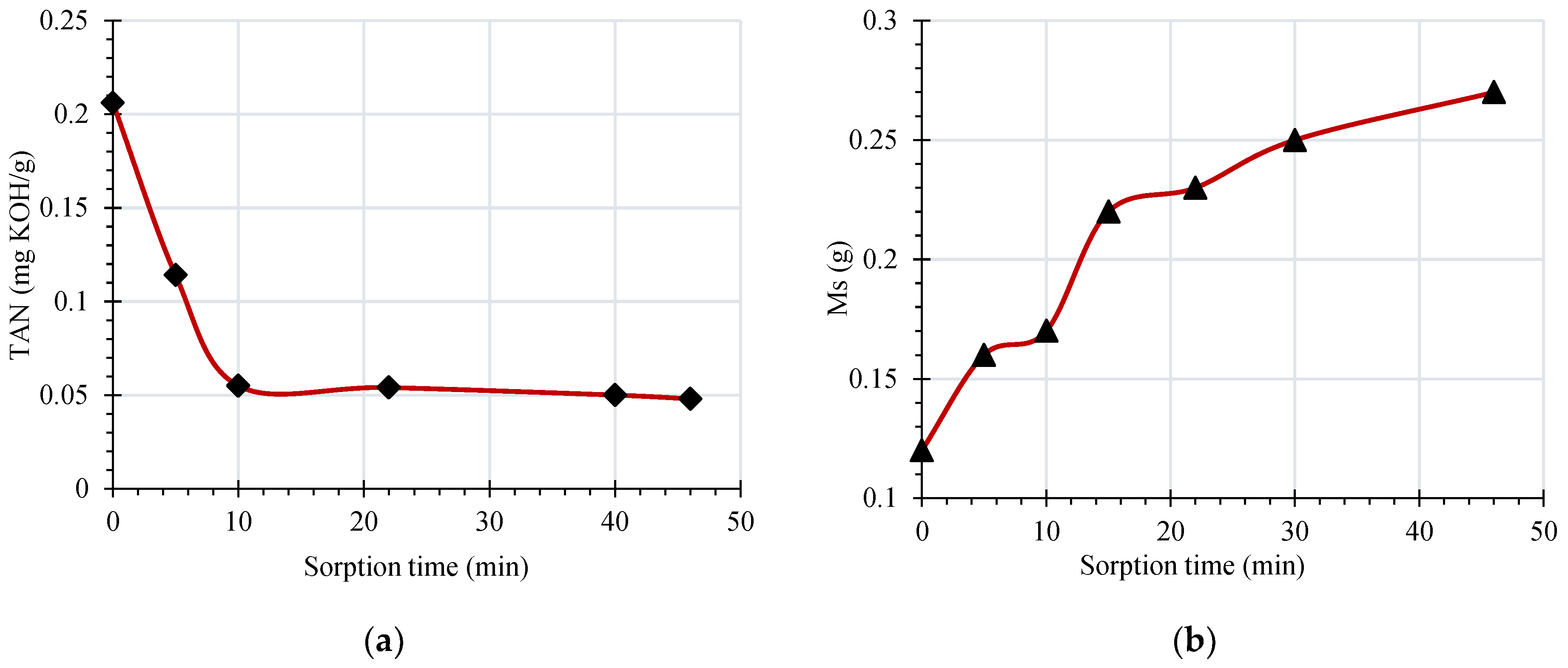
The sorption kinetics of the membrane have been measured in order to determine the effectiveness of the membrane. Change in TAN of oil and mass of the membrane are reported to reflect membrane sorption phenomena. Changes in TAN address to how much extent the membrane is reducing the acidity of oil with sorption time. Similarly, the membrane mass address the effectiveness of the membrane is retaining the unwanted colloidal particles and decay contents in the oil. The acidity of the aged insulating oil is measured after dehydration as per IEC 62021 and is found to be 0.206 mg KOH/g and is later subjected to filtration. The filtration proses and tests are conducted at ambient temperature and the dry mass of RC membrane is 120 mg. Later, a 73 mL volume of oil was filtered and several samplings of the filtrate have been made at various times. At the sorption equilibrium, the mass of retained acids by the membrane as well as the value of the index of acidity of the filtered oil have been measured. The variation of TAN and the mass of the membrane as a function of sorption time are presented in
Figure 5. In
Figure 5a, reduction of acidity is noticed with sorption time, this is because of filtration process. The acidic particles in oil are retained by the membrane, thus reducing the neutralization number of oil with time. In support of this, mass of membrane is observed to increase as a function of sorption time which is presented in
Figure 5b.
4.4. Variation of the Membrane Mass
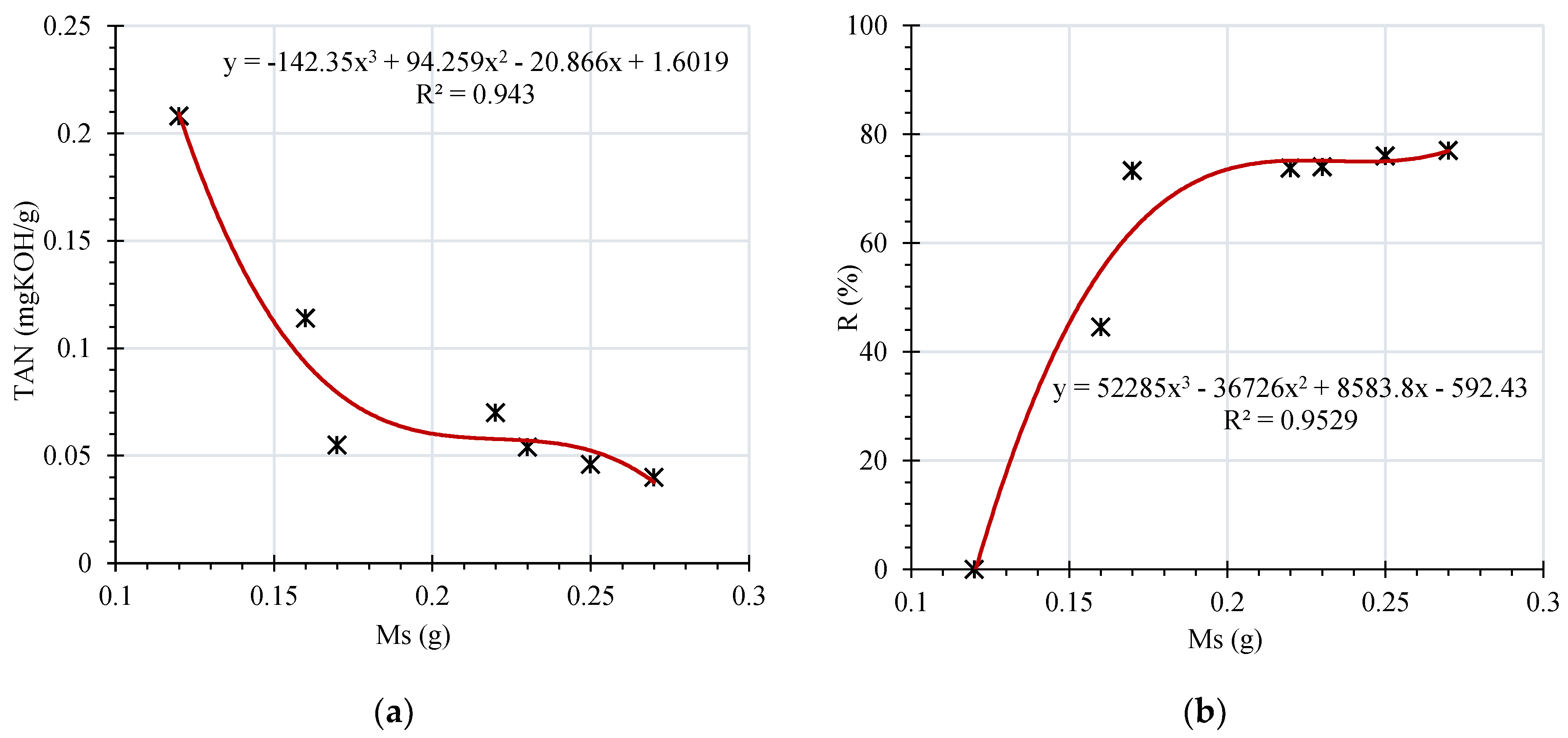
The high retention coefficients observed during sorption evaluation are dependent on the mass of the membrane. The effect of the membrane mass (Ms) on the retention of carboxylic acids has been studied on a series of dry RC membrane with a surface of 69.4 cm
2 and a mass of 120 mg (dry). A specific volume of oil has been added to the dry membrane. Measurements of TAN and R as a function of membrane masse are presented in
Figure 6.
It is to be noticed that the mass reported in
Figure 6 is the wet mass of the membrane which is increased with filtration. This increase may be attributable to absorption and adsorption of colloidal particles, dissolved decay contents, and acid particles of the oil on/in the membrane. The active number of the sites of the membrane are increased, while acidity of oil is decreased with treatment. Hence, it is inferred that, retention coefficient of the membrane increases and acidity of oil reduces with increase in Ms. One may assume this observation until active filtration is seen through the membrane. It is to be informed that, rate of filtration reduces with filtration time due to sealing of the membrane pores due to settlement of the colloidal particles, dissolved decay contents, and acid particles of the oil on/in the membrane. This time is depended on the deterioration rate of oil/paper insulation and thickness of membrane. However, further investigations are to be carried out to comment on the correlation between degree of aging and optimal thickness of membrane. Future investigations are also to be emphasized on absorption coefficient, effect of transmembrane pressure difference on the flux.
5. Discussion
The performance of the membrane is verified by adopting appropriate diagnostic and dielectric measurements. For analyzing the performance of the proposed method explicitly, comparative measurements of the oil and membrane for both before and after the oil treatment have been evaluated as discussed.
5.1. Acidity
In order to check the influence of RC membrane on reducing the acidity of the oil, efforts have been made to evaluate the membrane performance by testing acidic oil in a cyclic process. Dehydrated acidic oil is passed through the RC membrane module for six cycles and a new membrane has been adopted for every cycle at the ambient temperature. For each cycle the TAN values of the filtered oil has been measured. The cycle wise TAN measurement details have been presented in
Table 2. It is observed that, 83.98% of acidity has been reduced after six cycles of filtration.
5.2. Ultraviolet Visible Infrared Spectrometry
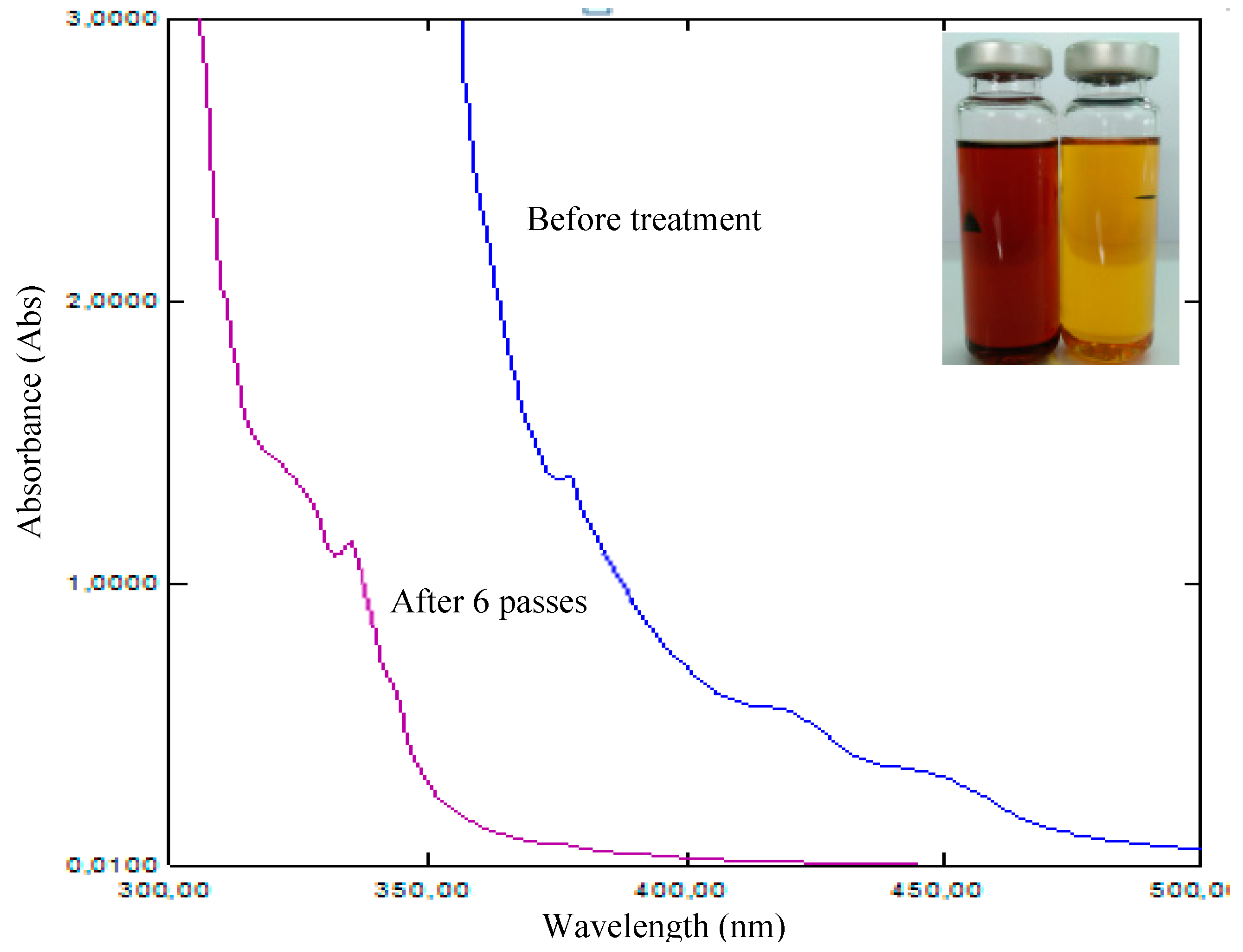
Further, to demonstrate the rigidity of the proposed RC membrane oil purification process, UV/Vis spectroscopy measurements have been carried for acidic oil and treated oil as per ASTM D6802. UV/Vis is a potential method to characterize the oil for estimating the concentration of dissolved decay contests in oil [
20,
21,
22]. The UV/Vis spectral curves for acidic and treated/purified oils are presented in
Figure 7.
It is observed that the concentration of dissolved decay contents has been remarkably reduced after purification through the RC membrane. The absorbance of the oil is reduced from 382 to 335 nm after subjecting the aged oil to the membrane treatment process. Color is one of the important characteristics of the oil to reveal its quality [
23]. In the present study, the color of the oil indicates the degree of purification employed by the membrane filtration method. The change in color of the oil sample before and after filtration with six passes is highlighted in the inset of
Figure 7. The change in color of the oil with filtration aids to the spectral curves and supports the degree of filtration that is achieved by the membrane filtration.
5.3. Fourier Transform Infrared Spectrometry
To further investigate the changes in chemical properties caused by the proposed method, FTIR spectroscopy measurements have been also carried out. This measurement also ensures the elimination of carboxylic acids from the aged oil after filtration. FTIR spectral curves of oils and membranes are presented in
Figure 8. In
Figure 8a, a strong band and wide asymmetric absorption between 3200 cm
−1 and 3400 cm
-1 is due to the vibration of stretching O-H attributed to the hydrogen bonds in new membrane [
24]. On the other hand, the molecular interactions between the acidic oil and the cellulosic polymers are evident by an absorption band at 1729.55 cm
−1 (C=O stretching of the vibrations of a group ester) and 1207.95 cm
−1 (stretching of the vibrations of the bridge C-O-C) [
20]. Generally, all the acidic oils represent characteristics bands situated in the spectral region between 1700 cm
−1 and 1730 cm
−1 [
25]. This is due to the vibrations that are attributable to the functional group C=O which confirms the presence of carboxylic acids.
Further, increase in thermal oxidation with aging of oil is generally accompanied by nitration which introduces nitrogen oxides (NO, NO
2 and N
2O) to the oil [
26]. The effect of the nitration on the quality of the oil is similar to that of oxidation, which either increases the viscosity of the oil or involves in increasing insoluble sediments. The function of the nitration is identified in the spectral region between 1600 cm
−1 and 1650 cm
−1 which is not seen in the spectral curve of purified oil, thus ensuring the disappearance of carboxylic acids and nitrated compounds in the purified oil.
5.4. Dissipation factor, Resistivity and Relative Permittivity
After filtration, it is desired that the oil regain all the dielectric parameters to a range that is acceptable for use in oil-filled transformers [
27,
28]. To further comment on the quality of the oil after filtration Dissipation factor (Tan δ), Resistivity, and Relative Permittivity (ε
r) measurements have been carried out for the filtered oil. The details of the measurements are summarized in
Table 3.
It is noticed that the dissipation factor varied from 0.194 to 0.131 for oil before and after filtration after six passes. Similarly, improvement of resistivity is noticed to be from 1.45 × 109 to 2.17 × 109, which represents a 49.7% improvement of of the dissipation factor with filtration after six passes. This variation reflects a decrease of the concentration of the conductive contaminants after filtration. Albeit relative permittivity changed to 2.13 with filtration, so a significant change is not seen. The details of breakdown voltage and density have not been presented in view of the high oil requirements for measurements. The effect the membrane technology on micro and nanoparticles dissolved in the oil is also to be foreseen. The optimal number of passes and its relation to the aging factor of oil are also to be investigated.
6. Conclusions
Insulating oil is expected to serve effectively for several decades of expected or designed life of a transformer. Generally, oil is reclaimed after a certain period based on its dielectric characterization and its performance. In this work, a membrane separation technology method is demonstrated and analyzed for the transformer oil treatment process. The effectiveness of the proposed method is evaluated by analyzing service-aged oil samples. It is found that the oil properties have been regained significantly with application of membrane technology. Analyses using UV spectroscopy, FTIR spectroscopy, acidity, dissipation factor, color, resistivity, and relative permittivity proved the potential of the proposed regenerative method. Dissipation factor, resistivity, and relative permittivity are improved by 32.5%, 49.7%, and 0.5%, respectively, after six passes. Results are also highlighted by studying the oil and RC membrane chemical interactions between the functional groups of carboxylic acids and cellulosic fiber before and after treatment. RC membrane treatment process proved a significant retention of oil properties.
Future Scope
Further investigations are to be carried out to comment on the correlation between degree of aging and optimal thickness of membrane. The effect the membrane technology on micro and nanoparticles dissolved in the oil is also to be foreseen. Future investigations are also to be emphasized on absorption coefficient and effect of transmembrane pressure difference on the flux. The optimal number of passes and its relation to the aging factor of oil is also to be investigated.
Author Contributions
This work was done under the supervision of H-Z.Z., director of the Laboratoire de Génie Chimique at the Université Saad Dahlab (Blida, Algeria) and I.F., holder of the Research Chair on the Aging of Power Network Infrastructure (ViAHT), L.S. designed this research and gave the whole guidance. L.S. and U.M.R. collected all the data, carried out calculations, result display and analysis and wrote the manuscript. The final draft of paper was thoroughly reviewed by I.F. and H-Z.Z. All authors read and approved the final manuscript.
Acknowledgments
Ms Safiddine is grateful to Société de Maintenance des Equipements Industriels, Spa (Blida, Algeria) for assistance with needful materials and equipment.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| UV/Vis | Ultra Violet Visible Infrared Spectroscopy. |
| FTIR | Fourier Transform Infrared Spectroscopy |
| TAN | Total Acid Number |
| Tan δ | dissipation factor |
| IFT | Interfacial Tension |
| RC | Regenerated Cellulose |
| J | Membrane Flux |
| R | Retention Coefficient |
References
- Fofana, I. 50 years in the development of insulating liquids. IEEE Electr. Insul. Mag. 2013, 29, 13–25. [Google Scholar] [CrossRef]
- N’Cho, J.S.; Fofana, I.; Beroual, A. Review of Modern Physicochemical-based Diagnostic Techniques for Assessing Insulation condition in Aged Transformers. Energies 2016, 9, 367. [Google Scholar] [CrossRef]
- Dombek, G.; Gielniak, J. Fire Safety and Electrical Properties of Mixtures of Synthetic Ester/Mineral Oil and Synthetic Ester/Natural Esterm. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1846–1852. [Google Scholar] [CrossRef]
- Fofana, I.; Hadjadj, Y. Electrical-based Diagnostic Techniques for Assessing Insulation condition in Aged Transformers. Energies 2016, 9, 679. [Google Scholar] [CrossRef]
- Rao, U.M.; Sood, Y.R.; Jarial, R.K. Physiometric and Fourier transform infrared spectroscopy analysis of cellulose insulation in blend of mineral and synthetic ester oils for transformers. IET Sci. Meas. Technol. 2017, 11, 297–304. [Google Scholar] [CrossRef]
- Ncho, J.S.; Fofana, I.; Beroual, A.; Aka-Ngnui, T.; Sabau, J. Verification of Insulation Oil Reclamation by Turbidity and Spetrophotometry Measurements. J. Energy Power Eng. 2012, 6, 703–712. [Google Scholar]
- Kouassi, K.; Fofana, I.; Cisse, L.; Hadjadj, Y.; Yapi, K.; Diby, K. Impact of Low Molecular Weight Acids on Oil Impregnated Paper Insulation Degradation. Energies 2018, 11, 1465. [Google Scholar] [CrossRef]
- Ingebrigtsen, S.; Dahlund, M.; Hansen, W.; Linhjell, D.; Lundgaard, L. Solubility of carboxylic acids in paper (Kraft)-oil insulation systems. In Proceedings of the IEEE International Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Boulder, CO, USA, 17–20 October 2004; pp. 253–257. [Google Scholar]
- Lundgaard, L.; Hansen, W.; Ingebrigtsen, S.; Linhjell, D.; Dahlund, M. Aging of Kraft paper by acid catalyzed hydrolysis. In Proceedings of the IEEE International Conference on Dielectric Liquids (ICDL), Coimbra, Portugal, 26 June–1 July 2005; pp. 381–384. [Google Scholar]
- Ageing of Cellulose in Mineral-Oil Insulated Transformers; CIGRE Task Force D1.01.10; CIGRE Brochure; CIGRE: Paris, France, 2007; No. 323.
- Martin, D.; Saha, T.K. A Review of the Techniques Used by Utilities to Measure the Water Content of Transformer Insulation Paper. IEEE Electr. Insul. Mag. 2017, 33, 8–16. [Google Scholar] [CrossRef]
- Lelekakis, N.; Wijaya, J.; Martin, D.; Susa, D. The effect of acid accumulation in power-transformer oil on the aging rate of paper insulation. IEEE Electr. Insul. Mag. 2014, 30, 19–26. [Google Scholar] [CrossRef]
- Ward, B. Application of Filtration System for On-Line Oil Reclamation, Degassing, and Dehydration; EPRI Report 1002046; EPRI: Palo Alto, CA, USA, 2003. [Google Scholar]
- Jepsen, K.; Hansen, L.; Mai, C.; Yang, Z. Challenges of membrane filtration for produced water treatment in offshore oil & gas production. In Proceedings of the IEEE International Conference on Oceans, Monterey, CA, USA, 19–23 September 2016; pp. 1–8. [Google Scholar]
- Ravanchi, M.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Oumert, L.; Zafour, A.H.Z.; Fofana, I.; Abdelhak, S.; Guerbas, F.; Boucherit, A. Transformer Oil Regeneration by Combining Several Strategies Enhanced by the Use of Four Adsorbents. IET Gener. Transm. Distrib. 2017, 11, 2912–2920. [Google Scholar]
- IEEE Guide for the Reclamation of Insulating Oil and Criteria for Its Use; ANSI/IEEE Std. 637-1985; IEEE Standards Association: Piscataway, NJ, USA, 1985; pp. 1–125.
- Farahani, S.K.; Halek, F.S.; Hosseini, S.M. Physico-chemical characterization, morphology and performance of polyethersulfone based membrane for glycerol removal from biodiesel produced through trans-esterification of waste cooking oils. Korean J. Chem. Eng. 2015, 32, 2097–2102. [Google Scholar] [CrossRef]
- Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. Comparison of membrane fouling at constant flux and constant transmembrane pressure conditions. J. Membr. Sci. 2014, 454, 505–515. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, L.; Wang, A.; Li, H.; Liao, W.; Guo, L. Effects of Thermal Aging on Moisture Diffusion in Insulation Paper Immersed with Mineral Oil. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1888–1896. [Google Scholar] [CrossRef]
- Sarathi, R.; Soumya, T.; Suraj, K.P.; Mishra, A.K. Understanding the influence of ambience on thermal ageing of natural ester oil. IET Sci. Meas. Technol. 2018. [Google Scholar] [CrossRef]
- Rao, U.M.; Sood, Y.R.; Jarial, R.K. Oxidation stability enhancement of a blend of mineral and synthetic ester oils. IEEE Electr. Insul. Mag. 2016, 32, 43–47. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Feipeng, W.; Zhengyong, H.; Quan, Z. A Novel Aging Indicator of Transformer Paper Insulation Based on Dispersion Staining Colors of Cellulose Fibers in Oil. IEEE Electr. Insul. Mag. 2018, 34, 8–16. [Google Scholar] [CrossRef]
- Rao, U.M.; Harish, P.; Goutham, N. Performance analysis of transformer oil/paper insulation with ester and mixed dielectric fluids. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1853–1862. [Google Scholar] [CrossRef]
- Munajad, A.; Subroto, C.; Suwarno, S. Fourier Transform Infrared (FTIR) Spectroscopy Analysis of Transformer Paper in Mineral Oil-Paper Composite Insulation under Accelerated Thermal Aging. Energies 2018, 11, 364. [Google Scholar] [CrossRef]
- Munajad, A.; Subroto, C.; Suwarno, S. Study on the Effects of Thermal Aging on Insulating Paper for High Voltage Transformer Composite with Natural Ester from Palm Oil Using Fourier Transform Infrared Spectroscopy (FTIR) and Energy Dispersive X-ray Spectroscopy (EDS). Energies 2017, 10, 1857. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Huang, B.; Hao, J.; Chen, G. Review of Research Progress on the Electrical Properties and Modification of Mineral Insulating Oils Used in Power Transformers. Energies 2018, 11, 487. [Google Scholar] [CrossRef]
- Su, L.; Contreras, J.M.; Velez, P.; Fernandez, A.; Martin, F. Analytical Method to Estimate the Complex Permittivity of Oil Samples. Sensors 2018, 18, 984. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).