Profitability Analysis and Capital Cost Estimation of a Thermochemical Energy Storage System Utilizing Fluidized Bed Reactors and the Reaction System MgO/Mg(OH)2
Abstract
1. Introduction
2. System Description
3. Economic Analysis
3.1. Capital Cost Estimation
3.2. Component Design
3.3. Expenses
3.4. Income and Economic Performance Evaluation
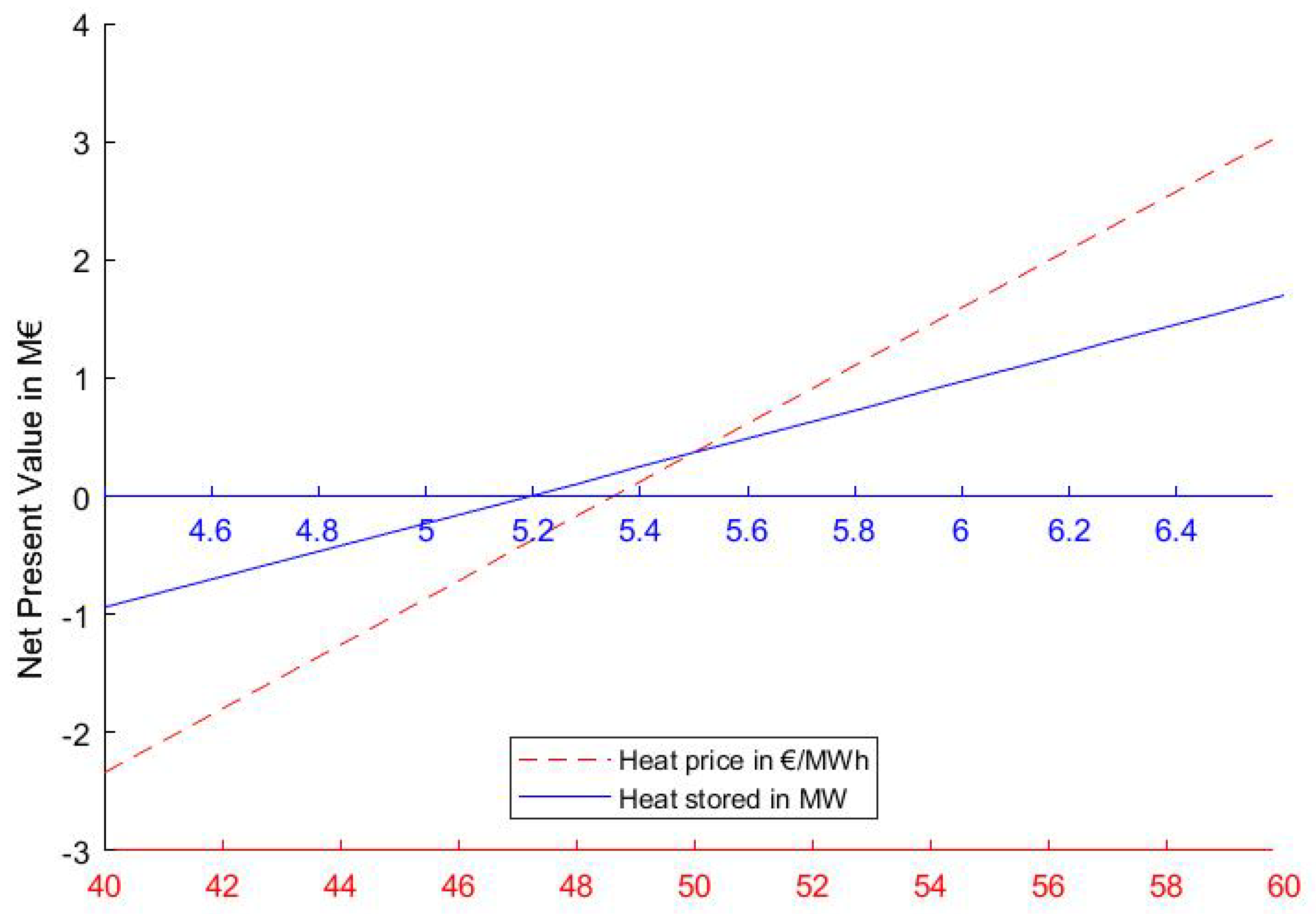
4. Results and Discussion
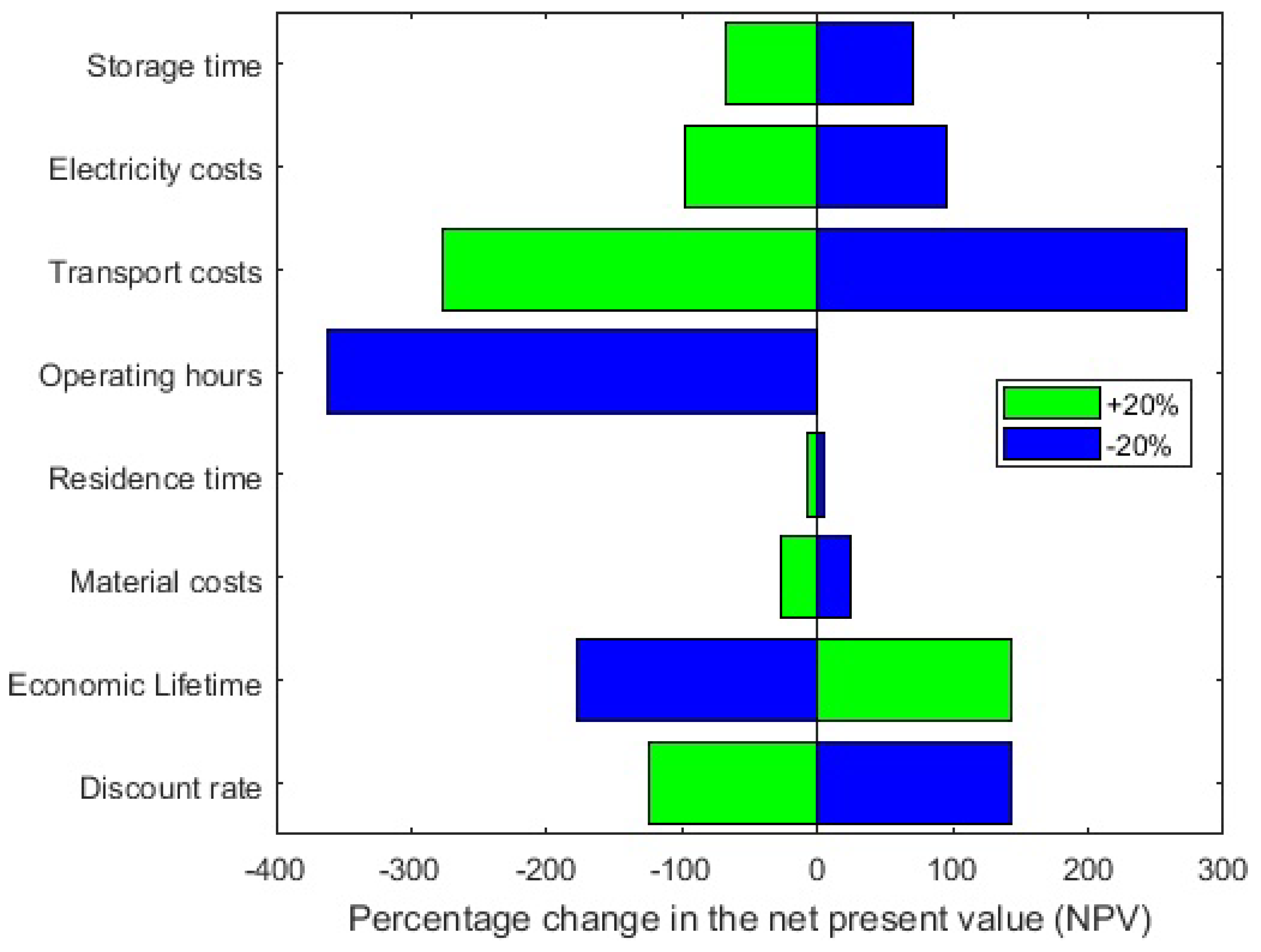
Sensitivity Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Symbols | ||
| A | Heat transfer area | m |
| Reactor cross section | m | |
| Archimedes number | - | |
| Total operating expenses p. a. | € | |
| Fuel costs | ||
| Fix transportation costs | ||
| Charged heat price | ||
| Total maintenance costs | € | |
| Operating labour costs | € | |
| Equipment purchase costs | € | |
| Personal transportation costs | ||
| Total capital investment | € | |
| Initial material costs | € | |
| Transportation costs | € | |
| Variable transportation costs | ||
| Workers wage | ||
| d | Diameter | m |
| Net earnings p. a. | € | |
| Investment site factor | - | |
| Lang factor | - | |
| H | Height | m |
| i | Interest rate | - |
| Base cost index | - | |
| Current cost index | - | |
| k | Heat transfer coefficient | |
| Material stream | ||
| M | Mass | kg |
| Heat flow | kW | |
| S | Wall thickness | m |
| t | Plant service time | years |
| Travel distance | km | |
| Operation time | h | |
| Travel time | h | |
| Minimum fluidization velocity | ||
| V | Volume | m |
| Mean temperature difference | K | |
| Reaction enthalpy | ||
| Mechanical efficiency | - | |
| Round trip efficiency | - | |
| Dynamic viscosity | ||
| Stoichiometric factor | - | |
| Density | ||
| Particle residence time | s |
| Abbreviations | ||
| Carbon dioxide equivalent | ||
| FBR | Fluidized bed reactor15 | |
| Magnesium oxide | ||
| Magnesium hydroxide | ||
| NPV | Net present value | |
| TCES | Thermochemical energy storage | |
| TCM | Thermochemical material | |
| TES | Thermal energy storage | |
| Subscripts | ||
| Reactor bed | ||
| Condenser | ||
| g | Gas | |
| Heat exchanger | ||
| In bed heat exchanger | ||
| p | Particle | |
| R | Reactor | |
| Release | ||
| s | Solid | |
| Storage | ||
| Waste Heat |
References
- Forman, C.; Muritala, I.K.; Pardemann, R.; Meyer, B. Estimating the global waste heat potential. Renew. Sustain. Energy Rev. 2016, 57, 1568–1579. [Google Scholar] [CrossRef]
- Campana, F.; Bianchi, M.; Branchini, L.; de Pascale, A.; Peretto, A.; Baresi, M.; Fermi, A.; Rossetti, N.; Vescovo, R. ORC waste heat recovery in European energy intensive industries: Energy and GHG savings. Energy Convers. Manag. 2013, 76, 244–252. [Google Scholar] [CrossRef]
- Tavakkoli, S.; Lokare, O.R.; Vidic, R.D.; Khanna, V. Systems-Level Analysis of Waste Heat Recovery Opportunities from Natural Gas Compressor Stations in the United States. ACS Sustain. Chem. Eng. 2016, 4, 3618–3626. [Google Scholar] [CrossRef]
- Zhang, H.; Baeyens, J.; Cáceres, G.; Degrève, J.; Lv, Y. Thermal energy storage: Recent developments and practical aspects. Progress Energy Combust. Sci. 2016, 53, 1–40. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.Z.; Li, Y. A review of available technologies for seasonal thermal energy storage. Sol. Energy 2014, 103, 610–638. [Google Scholar] [CrossRef]
- Cot-Gores, J.; Castell, A.; Cabeza, L.F. Thermochemical energy storage and conversion: A-state-of-the-art review of the experimental research under practical conditions. Renew. Sustain. Energy Rev. 2012, 16, 5207–5224. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Prieto, C.; Cooper, P.; Fernández, A.I.; Cabeza, L.F. Review of technology: Thermochemical energy storage for concentrated solar power plants. Renew. Sustain. Energy Rev. 2016, 60, 909–929. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Pan, Z.H.; Zhao, C.Y. Gas-solid thermochemical heat storage reactors for high-temperature applications. Energy 2017, 130, 155–173. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Milone, C. Efficiency improvement of heat storage materials for MgO/H2O/Mg(OH)2 chemical heat pumps. Appl. Energy 2016, 162, 31–39. [Google Scholar] [CrossRef]
- Shkatulov, A.; Ryu, J.; Kato, Y.; Aristov, Y. Composite material “Mg(OH)2/vermiculite”: A promising new candidate for storage of middle temperature heat. Energy 2012, 44, 1028–1034. [Google Scholar] [CrossRef]
- Ishitobi, H.; Uruma, K.; Takeuchi, M.; Ryu, J.; Kato, Y. Dehydration and hydration behavior of metal-salt-modified materials for chemical heat pumps. Appl. Therm. Eng. 2013, 50, 1639–1644. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage. Sol. Energy 2016, 135, 800–809. [Google Scholar] [CrossRef]
- Schaube, F.; Koch, L.; Wörner, A.; Müller-Steinhagen, H. A thermodynamic and kinetic study of the de- and rehydration of Ca(OH)2 at high H2O partial pressures for thermo-chemical heat storage. Thermochim. Acta 2012, 538, 9–20. [Google Scholar] [CrossRef]
- Roßkopf, C.; Haas, M.; Faik, A.; Linder, M.; Wörner, A. Improving powder bed properties for thermochemical storage by adding nanoparticles. Energy Convers. Manag. 2014, 86, 93–98. [Google Scholar] [CrossRef]
- Ishitobi, H.; Ryu, J.; Kato, Y. Combination of Thermochemical Energy Storage and Small Pressurized Water Reactor for Cogeneration System. Energy Procedia 2015, 71, 90–96. [Google Scholar] [CrossRef]
- Kato, Y.; Yamada, M.; Kanie, T.; Yoshizawa, Y. Calcium oxide/carbon dioxide reactivity in a packed bed reactor of a chemical heat pump for high-temperature gas reactors. Nuclear Eng. Des. 2001, 210, 1–8. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C.; Anxionnaz-Minvielle, Z. Conceptual process design of a CaO/Ca(OH)2 thermochemical energy storage system using fluidized bed reactors. Appl. Therm. Eng. 2014, 73, 1087–1094. [Google Scholar] [CrossRef]
- Flegkas, S.; Birkelbach, F.; Werner, A.; Freiberger, N.; Haider, M.; Winter, F.; Weinberger, P. Thermochemical Energy Storage Concept Based on a Reactor Cascade with Different Storage Materials. Int. J. Contemp. Energy 2018, 4, 40–49. [Google Scholar] [CrossRef]
- Schmidt, M.; Szczukowski, C.; Roßkopf, C.; Linder, M.; Wörner, A. Experimental results of a 10 kW high temperature thermochemical storage reactor based on calcium hydroxide. Appl. Therm. Eng. 2014, 62, 553–559. [Google Scholar] [CrossRef]
- Angerer, M.; Djukow, M.; Riedl, K.; Gleis, S.; Spliethoff, H. Simulation of Cogeneration-Combined Cycle Plant Flexibilization by Thermochemical Energy Storage. J. Energy Resour. Technol. 2018, 140, 1–12. [Google Scholar] [CrossRef]
- Angerer, M.; Becker, M.; Härzschel, S.; Kröper, K.; Gleis, S.; Vandersickel, A.; Spliethoff, H. Design of a MW-scale thermo-chemical energy storage reactor. Energy Rep. 2018, 4, 507–519. [Google Scholar] [CrossRef]
- Darkwa, K.; Ianakiev, A.; O’Callaghan, P.W. Modelling and simulation of adsorption process in a fluidised bed thermochemical energy reactor. Appl. Therm. Eng. 2006, 26, 838–845. [Google Scholar] [CrossRef]
- Flegkas, S.; Birkelbach, F.; Winter, F.; Freiberger, N.; Werner, A. Fluidized bed reactors for solid-gas thermochemical energy storage concepts - Modelling and process limitations. Energy 2018, 143, 615–623. [Google Scholar] [CrossRef]
- Kato, Y.; Yamashita, N.; Kobayashi, K.; Yoshizawa, Y. Kinetic Study of the Hydration of Magnesium Oxide for a Chemical Heat Pump. Appl. Therm. Eng. 1996, 16, 853–862. [Google Scholar] [CrossRef]
- Nahdi, K.; Rouquerol, F.; Trabelsi Ayadi, M. Mg(OH)2 dehydroxylation: A kinetic study by controlled rate thermal analysis (CRTA). Solid State Sci. 2009, 11, 1028–1034. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Kinetics of the CaO/Ca(OH) 2 Hydration/Dehydration Reaction for Thermochemical Energy Storage Applications. Ind. Eng. Chem. Res. 2014, 53, 12594–12601. [Google Scholar] [CrossRef]
- Vlaev, L.; Nedelchev, N.; Gyurova, K.; Zagorcheva, M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J. Anal. Appl. Pyrolysis 2008, 81, 253–262. [Google Scholar] [CrossRef]
- Seider, W.D.; Lewin, D.R.; Seader, J.; Widagdo, S.; Gani, R. Product and Process Design Principles: Synthesis, Analysis and Evaluation, 4th ed.; Wiley: New York, NY, USA, 2017. [Google Scholar]
- Solé, A.; Martorell, I.; Cabeza, L.F. State of the art on gas-solid thermochemical energy storage systems and reactors for building applications. Renew. Sustain. Energy Rev. 2015, 47, 386–398. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O. Fluidization Engineering, 2nd ed.; Butterworth-Heinemann: Boston, MA, USA, 1991. [Google Scholar]
- Towler, G.P.; Sinnott, R.K. Chemical Engineering Design: Principles, Practice, and Economics of Plant and Process Design, 2nd ed.; Butterworth-Heinemann: Boston, MA, USA, 2013. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraq-Hill Higher Education: New York, NY, USA, 2003. [Google Scholar]
- Chemical Engineering Plant Cost Index: (17.10.2017). Available online: www.chemengonline.com (accessed on 16 December 2019).
- Wen, C.Y.; Yu, Y.H. A generalized method for predicting the minimum fluidization velocity. AIChE J. 1966, 12, 610–612. [Google Scholar] [CrossRef]
- VDI-Gesellschaft (Ed.) VDI Heat Atlas, 2nd ed.; VDI Buch, Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Bundesministeriums für Verkehr und Digitale Information (Ed.) Entwicklung eines Modells zur Berechnung von modalen Verlagerungen im Güterverkehr für die Ableitung konsistenter Bewertungsansätze für die Bundesverkehrswegeplanung: Endbericht; Bundesministeriums für Verkehr und digitale Information: Berlin, Germany, 2016. [Google Scholar]
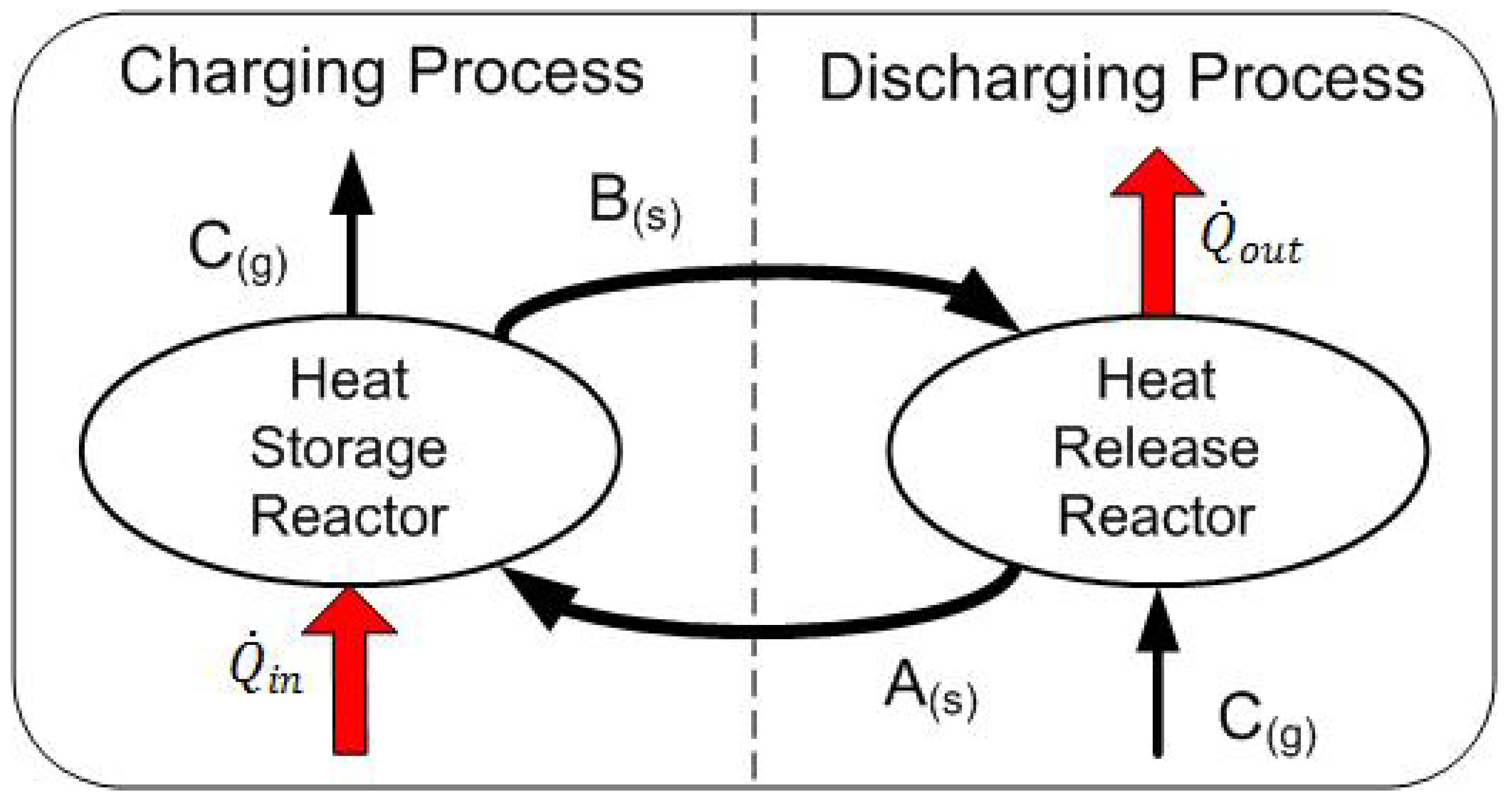
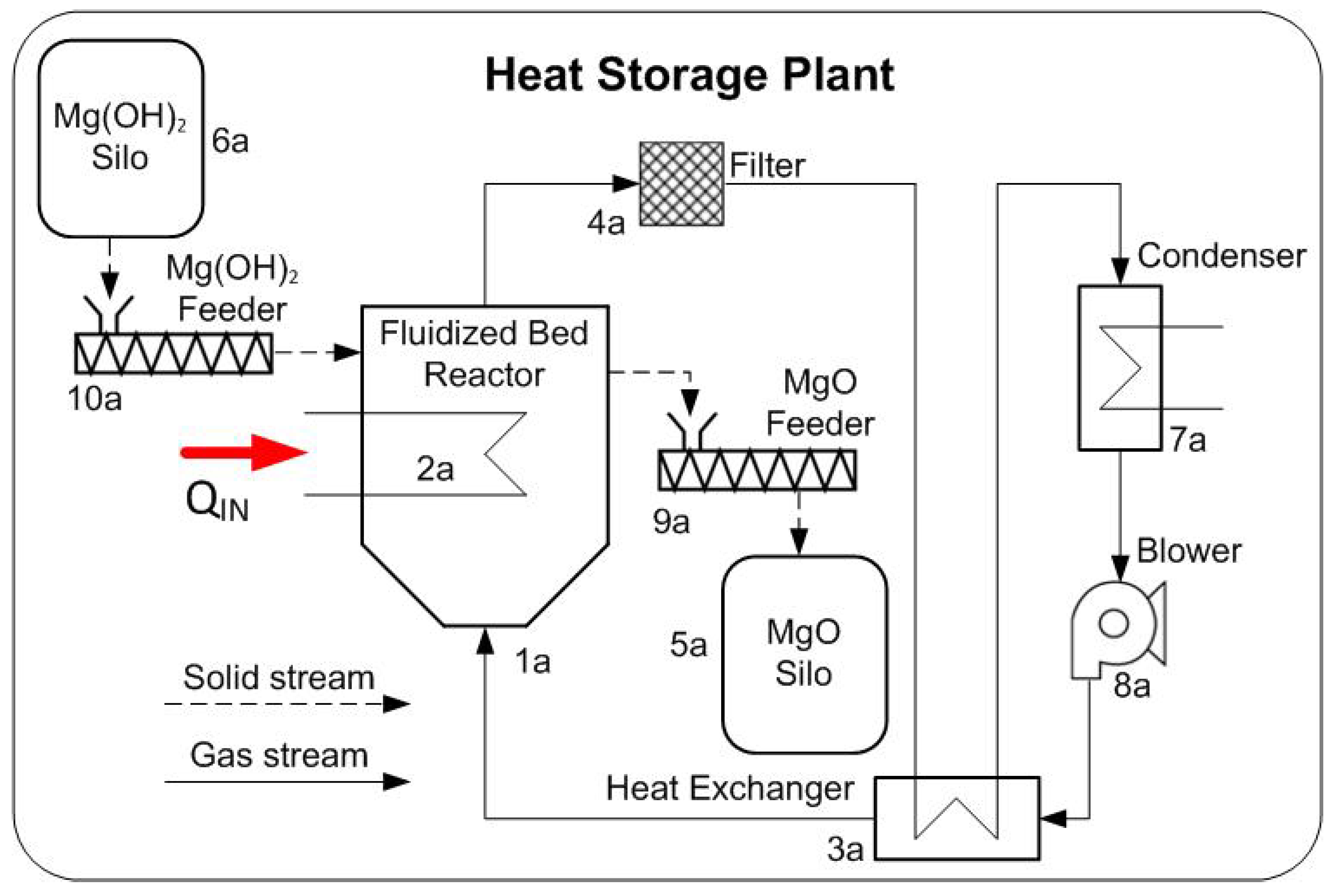



| Heat Storage Plant | Heat Release Plant |
|---|---|
| Storage process | Release Process |
| = 5.5 MW | - |
| = 1.5 h | = 2.5 h |
| Heat Storage Reactor | Heat Release Reactor |
| Heat Exchanger | - |
| - | |
| - | |
| Condenser | - |
| - | |
| - | |
| Economic Parameters | Transport Parameters |
| Operating hours p. a.: 8000 h | Distance between sites: 30 km |
| Heat price: 50 €/MWh | Transport time: 1 h |
| Real interest rate: % | Material Parameters |
| Economic lifetime: 15 years | Material costs: 200 €/t |
| Required Material: 2360 t |
| Capital investment | 4.95 M€ |
| Operating costs p. a. | 0.93 M€ |
| Earnings p. a. | 1.53 M€ |
| NPV | 0.37 M€ |
| Component (No.) | Size Factor | Size Factor Value (S) | Purchase Costs () |
|---|---|---|---|
| Reactor (1a) | Mass, kg Diameter, m Height, m | 6872 kg 5 m m | 67.3 k € |
| Reactor heat exchanger (2a) | Surface area, m2 | m2 | 25.6 k € |
| Heat exchanger (3a) | Surface area, m2 | m2 | 63 k € |
| Bag filter (4a) | Gas flow rate, m3/h | 3 m3/h | 42.8 k € |
| Bin | |||
| Bin (5a) | Volume, m3 | 695 m3 | 58 k € |
| Bin (6a) | Volume, m3 | 1253 m3 | 76.1 k € |
| Condenser (7a) | Surface area, m2 | m2 | 22 k € |
| Blower (8a) | Drive power, kW | kW | 37.3 k € |
| Screw conveyor | |||
| feeder (9a) | Volumetric flow rate, m3/h | m3/h | 3.7 k € |
| feeder (10a) | Volumetric flow rate, m3/h | m3/h | 4.2 k € |
| Total heat storage plant equipment costs: | 400 k € | ||
| Component (No.) | Size Factor | Size Factor Value (S) | Purchase Costs () |
|---|---|---|---|
| Reactor (1b) | Mass, kg Diameter, m Height, m | 4061 kg 5 m m | 52.5 k € |
| Reactor heat exchanger (2b) | Surface area, m2 | 438 m2 | 36.9 k € |
| Bag filter (3b) | Gas flow rate, m3/h | m3/h | 70.2 k € |
| Bin | |||
| Bin (4b) | Volume, m3 | 1253 m3 | 76.1 k € |
| Bin (5b) | Volume, m3 | 695 m3 | 58 k € |
| Blower (6b) | Drive power, kW | 337 kW | 73 k € |
| Screw conveyor | |||
| feeder (7b) | Volumetric flow rate, m3/h | m3/h | 4.2 k € |
| feeder (8b) | Volumetric flow rate, m3/h | m3/h | 3.7 k € |
| Total heat release plant equipment costs: | 374.6 k € | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flegkas, S.; Birkelbach, F.; Winter, F.; Groenewold, H.; Werner, A. Profitability Analysis and Capital Cost Estimation of a Thermochemical Energy Storage System Utilizing Fluidized Bed Reactors and the Reaction System MgO/Mg(OH)2. Energies 2019, 12, 4788. https://doi.org/10.3390/en12244788
Flegkas S, Birkelbach F, Winter F, Groenewold H, Werner A. Profitability Analysis and Capital Cost Estimation of a Thermochemical Energy Storage System Utilizing Fluidized Bed Reactors and the Reaction System MgO/Mg(OH)2. Energies. 2019; 12(24):4788. https://doi.org/10.3390/en12244788
Chicago/Turabian StyleFlegkas, Stylianos, Felix Birkelbach, Franz Winter, Hans Groenewold, and Andreas Werner. 2019. "Profitability Analysis and Capital Cost Estimation of a Thermochemical Energy Storage System Utilizing Fluidized Bed Reactors and the Reaction System MgO/Mg(OH)2" Energies 12, no. 24: 4788. https://doi.org/10.3390/en12244788
APA StyleFlegkas, S., Birkelbach, F., Winter, F., Groenewold, H., & Werner, A. (2019). Profitability Analysis and Capital Cost Estimation of a Thermochemical Energy Storage System Utilizing Fluidized Bed Reactors and the Reaction System MgO/Mg(OH)2. Energies, 12(24), 4788. https://doi.org/10.3390/en12244788






