Prospects for Anion-Exchange Membranes in Alkali Metal–Air Batteries
Abstract
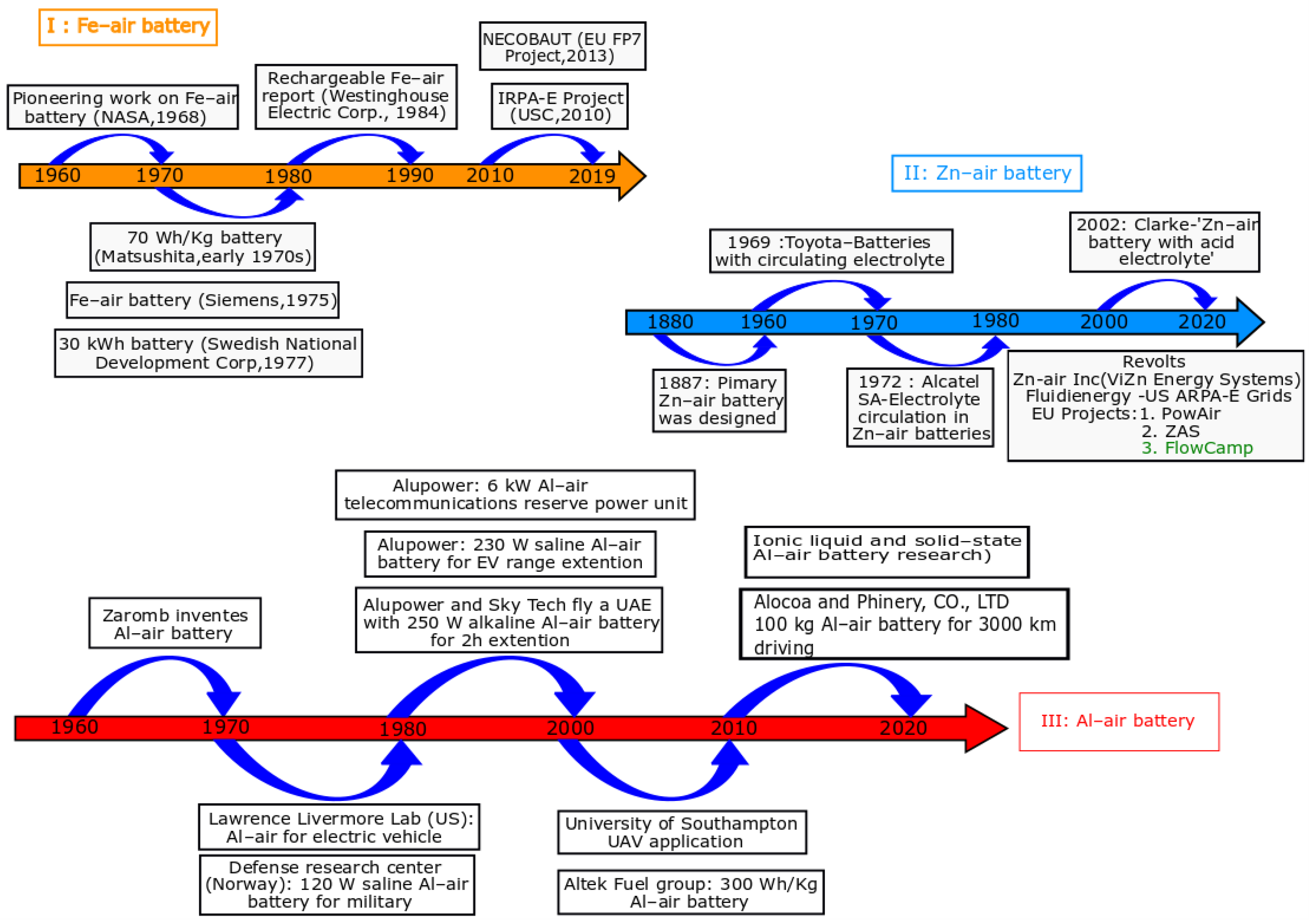
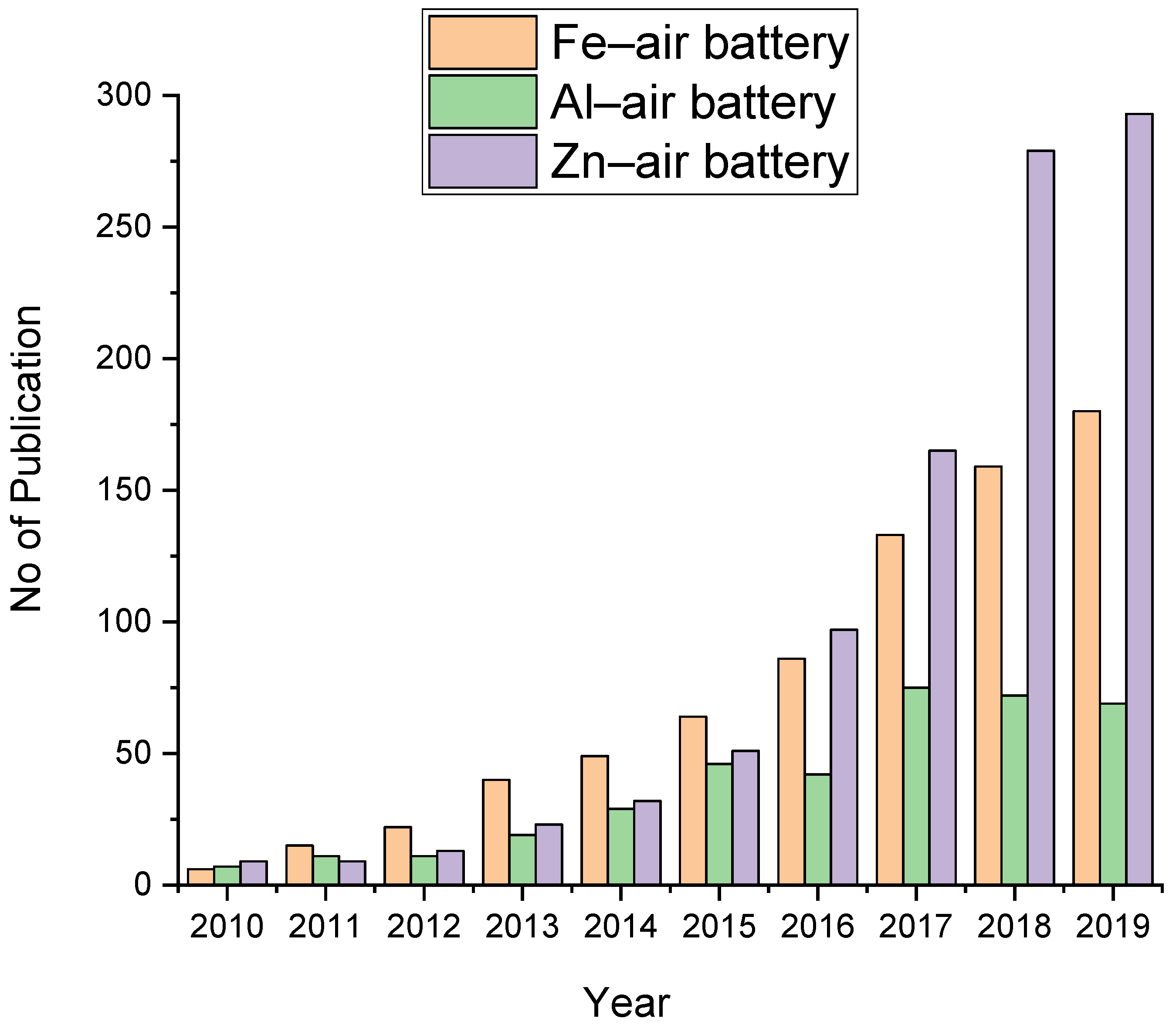
1. Introduction to Alkali Metal–Air Batteries
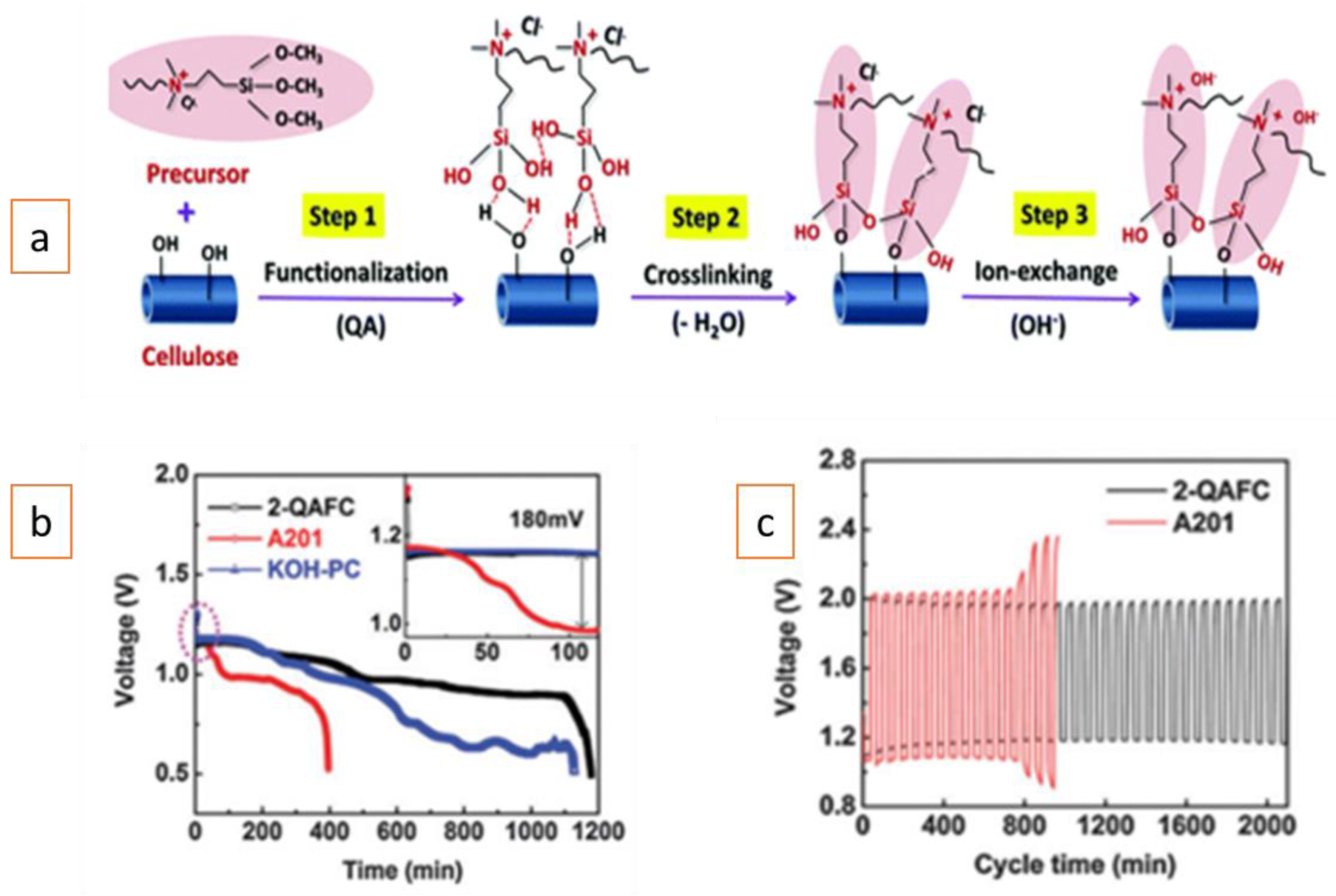
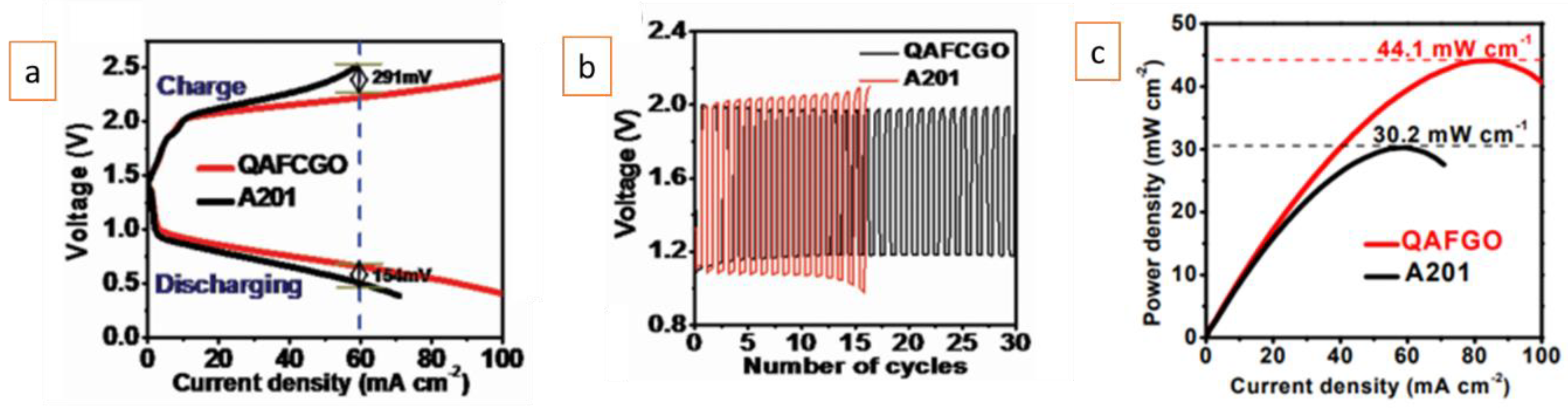
2. Advances in AEMs for Alkali Metal–Air Batteries
3. Overcoming the Remaining Challenges
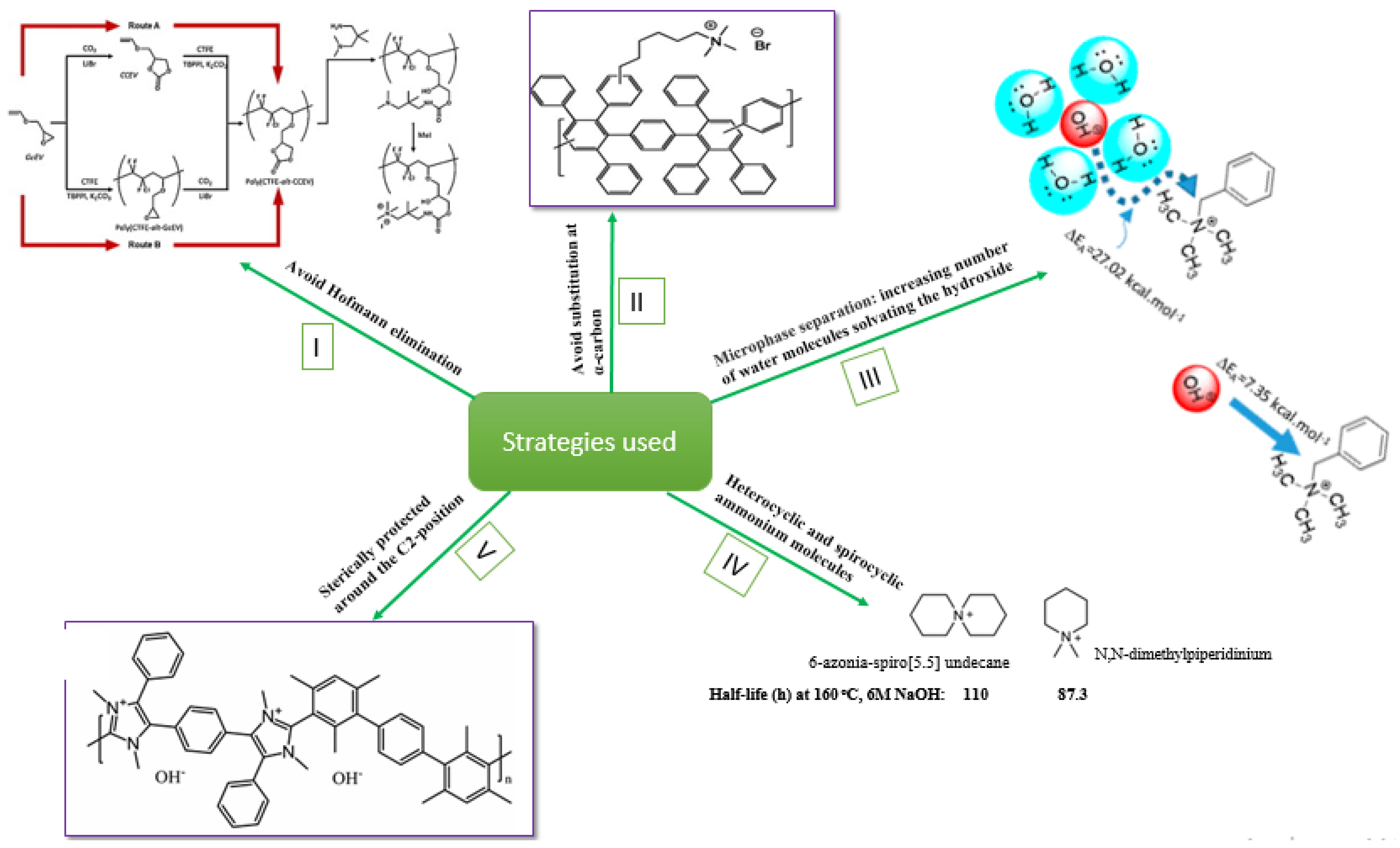
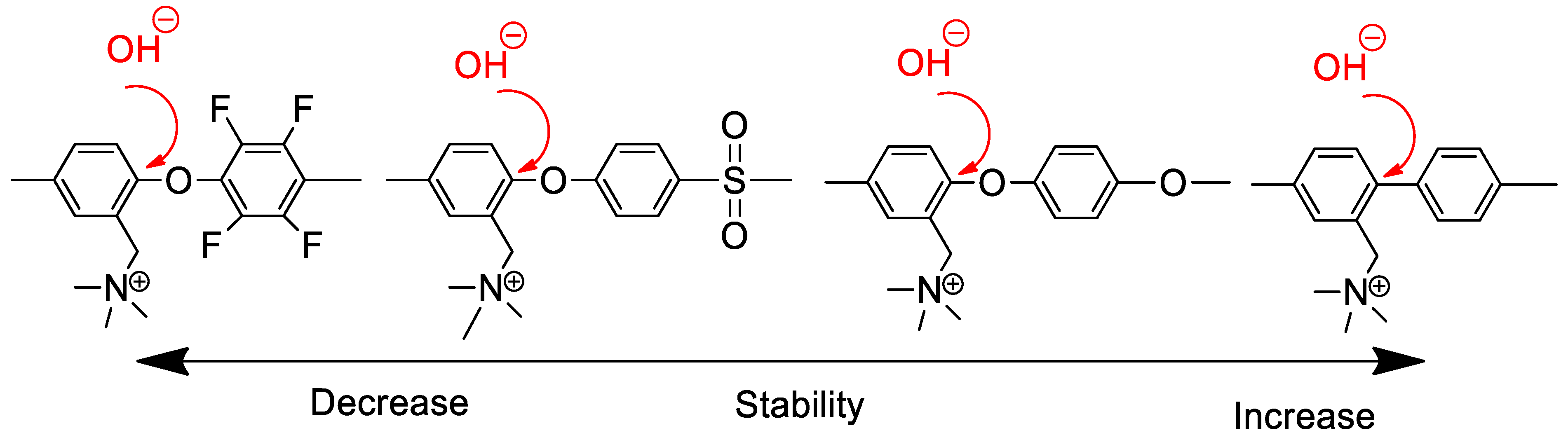
3.1. Low Alkaline Stability of Current AEMs
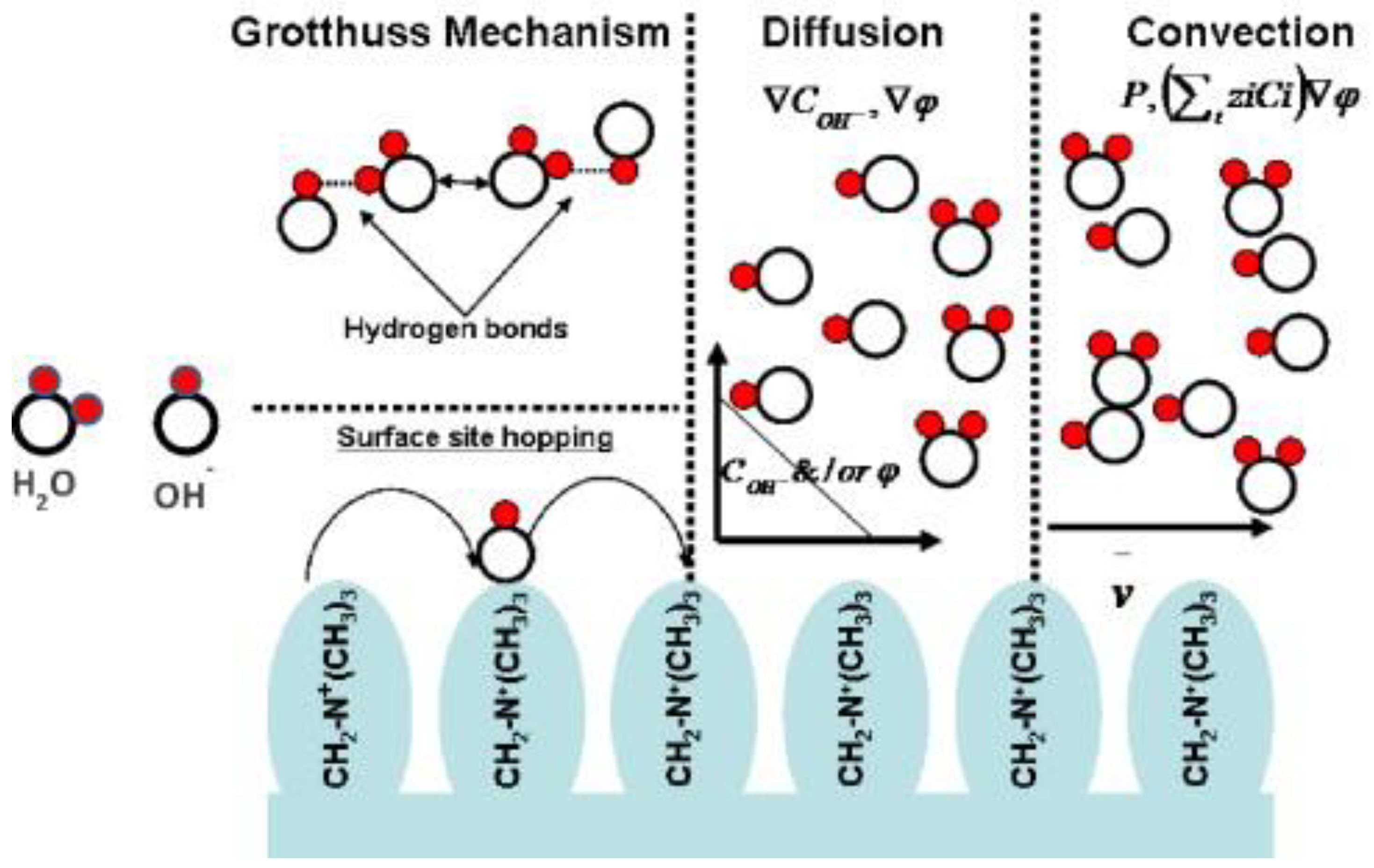
3.2. Hydroxide Conductivity
3.3. Lack of Suitable High-Performing AEMs Designed for Alkali Metal–Air Flow Batteries
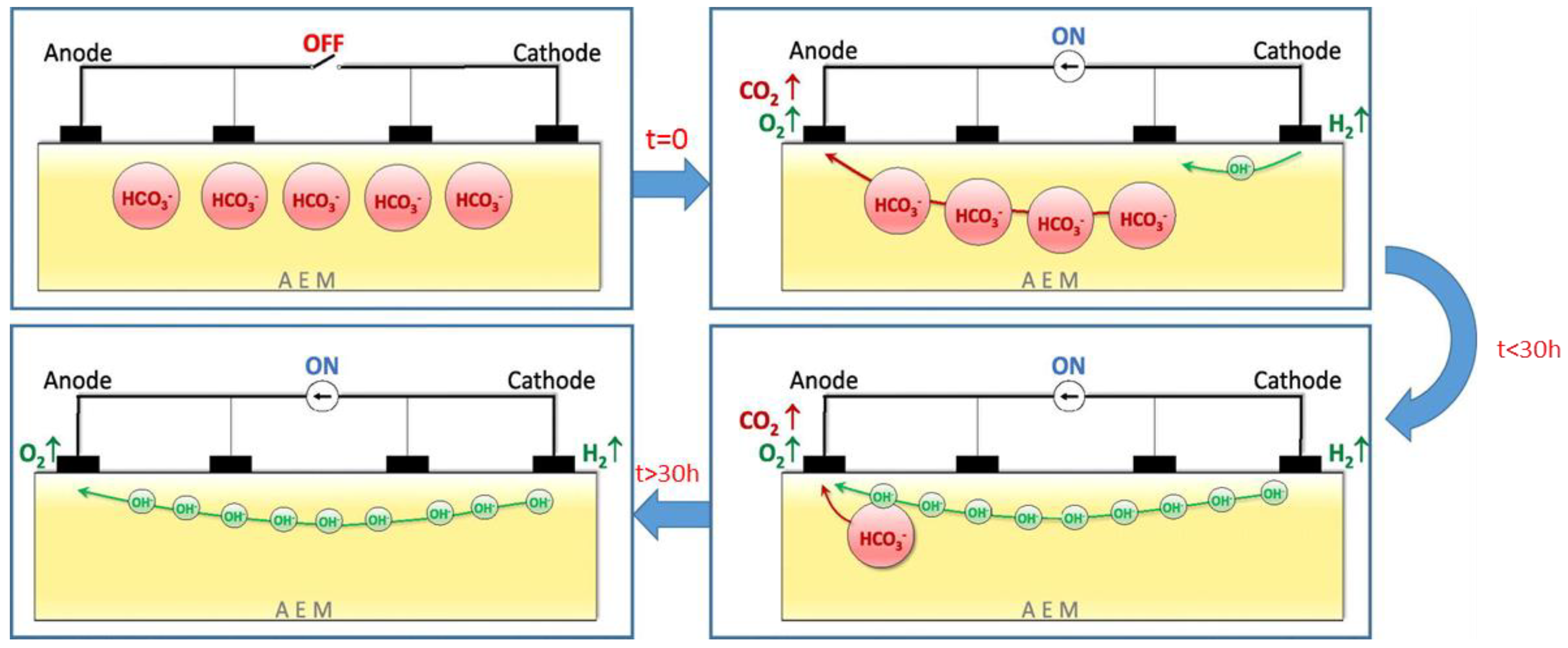
3.4. Lack of Standardization in Membrane Testing Protocol
3.5. Cost
4. Summary and Outlooks
Funding
Conflicts of Interest
References
- Wei, X.; Pan, W.; Duan, W.; Hollas, A.; Yang, Z.; Li, B.; Nie, Z.; Liu, J.; Reed, D.; Wang, W.; et al. Materials and systems for organic redox flow batteries: Status and challenges. ACS Energy Lett. 2017, 2, 2187–2204. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). International Energy Outlook 2016 with Projections to 2040. 2016. Available online: https://www.eia.gov/outlooks/ieo/pdf/0484(2016).pdf (accessed on 10 June 2019).
- Chang, Z.; Zhang, X. Introduction to metal-air batteries: Theory and basic principles. Met.-Air Batter. 2018, 1–9. [Google Scholar] [CrossRef]
- Janoschka, T.; Martin, N.; Hager, M.D.; Schubert, U.S. An aqueous redox-flow battery with high capacity and power: The TEMPTMA/MV system. Angew. Chem. Int. Ed. 2016, 55, 14427–14430. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Chen, J. Metal–air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef] [PubMed]
- May, G.J.; Davidson, A.; Monahov, B. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Pinnangudi, B.; Kuykendal, M.; Bhadra, S. Smart grid energy storage. Power Grid. 2017, 93–135. [Google Scholar] [CrossRef]
- Du, P.; Lu, N.; Beaudin, M.; Zareipour, H.; Schellenberg, A.; Rosehart, W. Energy storage for mitigating the variability of renewable electricity. Sources Energy Storage Smart Grids 2014, 14, 1–33. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M. Critical review on lithium-ion batteries: Are they safe? sustainable? Ionics 2017, 23, 1933–1947. [Google Scholar] [CrossRef]
- Blomgren, G.E. The development and future of lithium ion batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Sankarasubramanian, S.; Kahky, J.; Ramani, V. Tuning anion solvation energetics enhances potassium-oxygen battery performance. Proc. Natl. Acad. Sci. USA 2019, 116, 14899–14904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.-G.; Xie, Z.; Zhou, Z. Recent progress in rechargeable alkali metal–air batteries. Green Energy Environ. 2016, 1, 4–17. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Metal–air batteries: Will they be the future electrochemical energy storage device of choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; de Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Mori, R. All solid state rechargeable aluminum–air battery with deep eutectic solvent based electrolyte and suppression of byproducts formation. RSC Adv. 2019, 9, 22220–22226. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Niu, J.; Liu, Y.; Bridges, D.; Liu, X.; Pooran, J.; Zhang, Y.; Hu, A. Recent progress of metal-air batteries—A mini review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef]
- McKerracher, R.D.; Poncedeleon, C.; Wills, R.G.A.; Shah, A.A.; Walsh, F.C. A review of the iron-air secondary battery for energy storage. Chempluschem 2015, 80, 323–335. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. High energy density metal-air batteries: A review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc–air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.T.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal-Air Batteries with High Energy Density: Li-Air versus Zn-Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Sapkota, P.; Kim, H. Zinc–air fuel cell, a potential candidate for alternative energy. J. Ind. Eng. Chem. 2009, 15, 445–450. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically rechargeable zinc-Air batteries: Progress, challenges, and perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Vincent, C.A.; Scrosati, B. Modern Batteries: An Introduction to Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Barsukov, V.; Beck, F. (Eds.) New Promising Electrochemical Systems for Rechargeable Batteries; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Zhang, Z.; Zuo, C.; Liu, Z.; Yu, Y.; Zuo, Y.; Song, Y. All-solid-state Al–air batteries with polymer alkaline gel electrolyte. J. Power Sources 2014, 251, 470–475. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Environ. 2017, 2, 246–277. [Google Scholar] [CrossRef]
- Mokhtar, M.; Talib, M.Z.M.; Majlan, E.H.; Tasirin, S.M.; Ramli, W.M.F.W.; Daud, W.R.W.; Sahari, J. Recent developments in materials for aluminum–air batteries: A review. J. Ind. Eng. Chem. 2015, 32, 1–20. [Google Scholar] [CrossRef]
- Yang, S. Design and analysis of aluminum/air battery system for electric vehicles. J. Power Sources 2002, 112, 162–173. [Google Scholar] [CrossRef]
- Mori, R. A novel aluminium–air rechargeable battery with Al 2 O 3 as the buffer to suppress byproduct accumulation directly onto an aluminium anode and air cathode. RSC Adv. 2014, 4, 30346–30351. [Google Scholar] [CrossRef]
- Heise, G.W. Air-Depolarized Primary Battery. 1925. Available online: https://patents.google.com/patent/US1899615A/en (accessed on 8 October 2019).
- Zaromb, S. The use and behavior of aluminum anodes in alkaline primary batteries. J. Electrochem. Soc. 1962, 109, 1125–1130. [Google Scholar] [CrossRef]
- Öjefors, L.; Carlsson, L. An iron-air vehicle battery. J. Power Sources 1978, 2, 287–296. [Google Scholar] [CrossRef]
- Home-Eos Energy Storage. Available online: https://eosenergystorage.com/#welcome (accessed on 4 October 2019).
- NantEnergy|Progress in Power. Available online: https://nantenergy.com/ (accessed on 4 October 2019).
- Zinc8 Energy Solutions. Available online: https://zinc8energy.com/ (accessed on 4 October 2019).
- Powair. Available online: http://www.powair.eu/ (accessed on 4 October 2019).
- ZAS-Zinc Air Secondary Innovative Nanotech Based Batteries for Efficient Energy Storage. Available online: https://www.sintef.no/zas (accessed on 4 October 2019).
- FlowCamp-the Redox Flow Battery Campus. Available online: https://www.flowcamp-project.eu/ (accessed on 4 October 2019).
- Sun, Y.; Liu, X.; Jiang, Y.; Li, J.; Ding, J.; Hu, W.; Zhong, C. Recent advances and challenges in divalent and multivalent metal electrodes for metal–air batteries. J. Mater. Chem. A 2019, 7, 18183–18208. [Google Scholar] [CrossRef]
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc–air batteries: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Blurton, K.F.; Sammells, A.F. Metal/air batteries: Their status and potential—A review. J. Power Sources 1979, 4, 263–279. [Google Scholar] [CrossRef]
- Liu, P. A Low-Cost Ion Exchange Membrane Separator for Rechargeable Aqueous Batteries. 2018. Available online: https://ecs.confex.com/ecs/aimes2018/webprogram/Paper115710.html (accessed on 4 October 2019).
- Saputra, H.; Othman, R.; Sutjipto, A.G.E.; Muhida, R. MCM-41 as a new separator material for electrochemical cell: Application in zinc–air system. J. Membr. Sci. 2011, 367, 152–157. [Google Scholar] [CrossRef]
- Kiros, Y. Separation and permeability of zincate ions through membranes. J. Power Sources 1996, 62, 117–119. [Google Scholar] [CrossRef]
- Kim, H.W.; Lim, J.M.; Lee, H.J.; Eom, S.W.; Hong, Y.T.; Lee, S.Y. Artificially engineered, bicontinuous anion-conducting/-repelling polymeric phases as a selective ion transport channel for rechargeable zinc-air battery separator membranes. J. Mater. Chem. A 2016, 4, 3711–3720. [Google Scholar] [CrossRef]
- Dewi, E.L.; Oyaizu, K.; Nishide, H.; Tsuchida, E. Cationic polysulfonium membrane as separator in zinc–air cell. J. Power Sources 2003, 115, 149–152. [Google Scholar] [CrossRef]
- Hwang, H.J.; Chi, W.S.; Kwon, O.; Lee, J.G.; Kim, J.H.; Shul, Y.-G. Selective ion transporting polymerized ionic liquid membrane separator for enhancing cycle stability and durability in secondary zinc–air battery systems. ACS Appl. Mater. Interfaces 2016, 8, 26298–26308. [Google Scholar] [CrossRef]
- Zinc-Air Secondary Battery. 2014. Available online: https://patents.google.com/patent/US20140227616 (accessed on 4 October 2019).
- Metal-Air Battery with Ion Exchange Materials. 2010. Available online: https://patents.google.com/patent/US20110027666A1/en (accessed on 4 October 2019).
- Rechargeable Zinc-Air Flow Battery. 2014. Available online: https://patents.google.com/patent/WO2015004069A1/en (accessed on 4 October 2019).
- Sum, E.; Skyllas-Kazacos, M. A study of the V(II)/V(III) redox couple for redox flow cell applications. J. Power Sources 1985, 15, 179–190. [Google Scholar] [CrossRef]
- Sum, E.; Rychcik, M.; Skyllas-kazacos, M. Investigation of the V(V)/V(IV) system for use in the positive half-cell of a redox battery. J. Power Sources 1985, 16, 85–95. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Grossmith, F. Efficient vanadium redox flow cell. J. Electrochem. Soc. 1987, 134, 2950–2953. [Google Scholar] [CrossRef]
- Mai, Z.; Zhang, H.; Li, X.; Xiao, S.; Zhang, H. Nafion/polyvinylidene fluoride blend membranes with improved ion selectivity for vanadium redox flow battery application. J. Power Sources 2011, 196, 5737–5741. [Google Scholar] [CrossRef]
- Zeng, J.; Jiang, C.; Wang, Y.; Chen, J.; Zhu, S.; Zhao, B.; Wang, R. Studies on polypyrrole modified nafion membrane for vanadium redox flow battery. Electrochem. Commun. 2008, 10, 372–375. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 2007, 166, 531–536. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Xu, N.; Peng, L.; Mao, J.; Gong, Q.; Qiao, J. Alkaline exchange polymer membrane electrolyte for high performance of all-solid-state electrochemical devices. ACS Appl. Mater. Interfaces 2018, 10, 29593–29598. [Google Scholar] [CrossRef]
- Fumatech GmbH. Available online: https://www.fumatech.com/Startseite/index.html (accessed on 5 October 2019).
- Carmo, M.; Doubek, G.; Sekol, R.C.; Linardi, M.; Taylor, A.D. Development and electrochemical studies of membrane electrode assemblies for polymer electrolyte alkaline fuel cells using FAA membrane and ionomer. J. Power Sources 2013, 230, 169–175. [Google Scholar] [CrossRef]
- Dekel, D.; Schuster, M.; Ash, U.; Jaouen, F. Critical Raw Materials Elimination by a Top-Down Approach to Hydrogen and Electricity Generation. 2017, pp. 1–13. Available online: https://cordis.europa.eu/project/rcn/206995/factsheet/en (accessed on 9 December 2019).
- Yanagi, H.; Fukuta, K. Anion exchange membrane and ionomer for alkaline membrane fuel cells (AMFCs). ECS Trans. 2008, 257–262. [Google Scholar] [CrossRef]
- Abuin, G.C.; Franceschini, E.A.; Nonjola, P.; Mathe, M.K.; Modibedi, M.; Corti, H.R. A high selectivity quaternized polysulfone membrane for alkaline direct methanol fuel cells. J. Power Sources 2015, 279, 450–459. [Google Scholar] [CrossRef]
- Abbasi, A.; Hosseini, S.; Somwangthanaroj, A.; Mohamad, A.A.; Kheawhom, S. Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-based hydroxide exchange separator membranes for zinc–air battery. Int. J. Mol. Sci. 2019, 20, 3678. [Google Scholar] [CrossRef]
- Willdorf-Cohen, S.; Mondal, A.; Dekel, D.; Diesendruck, C. Chemical stability of poly(phenylene oxide)-based ionomers in an anion exchange-membrane fuel cell environment. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, J.; Song, X.; Zarrin, H.; Tian, X.; Qiao, J.; Rasen, L.; Li, K.; Chen, Z. A flexible solid-state electrolyte for wide-scale integration of rechargeable zinc–air batteries. Energy Environ. Sci. 2016, 9, 663–670. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Song, X.; Jiang, G.; Zarrin, H.; Xu, P.; Li, K.; Yu, A.; Chen, Z. Laminated cross-linked nanocellulose/graphene oxide electrolyte for flexible rechargeable zinc-air batteries. Adv. Energy Mater. 2016, 6, 1600476. [Google Scholar] [CrossRef]
- Zarrin, H.; Sy, S.; Fu, J.; Jiang, G.; Kang, K.; Jun, Y.-S.; Yu, A.; Fowler, M.; Chen, Z. Molecular functionalization of graphene oxide for next-generation wearable electronics. ACS Appl. Mater. Interfaces 2016, 8, 25428–25437. [Google Scholar] [CrossRef] [PubMed]
- Fumasep® Membrane Types Redox-Flow-Batteries. Available online: https://www.fumatech.com (accessed on 5 October 2019).
- Kwon, O.; Hwang, H.J.; Ji, Y.; Jeon, O.S.; Kim, J.P.; Lee, C.; Shul, Y.G. Transparent bendable secondary zinc-air batteries by controlled void ionic separators. Sci. Rep. 2019, 9, 3175. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Bhange, S.N.; Kurungot, S. A 3-D nanoribbon-like Pt-free oxygen reduction reaction electrocatalyst derived from waste leather for anion exchange membrane fuel cells and zinc-air batteries. Nanoscale 2019, 11, 7893–7902. [Google Scholar] [CrossRef]
- Technical Datasheet-Fumapem® FAA-3-50. Available online: https://fuelcellstore.com/spec-sheets/fumapem-faa-3-50-technical-specifications.pdf (accessed on 8 October 2019).
- Mandal, M.; Huang, G.; Kohl, P.A. Highly conductive anion-exchange membranes based on cross-linked poly(norbornene): Vinyl addition polymerization. ACS Appl. Energy Mater. 2019, 2, 2447–2457. [Google Scholar] [CrossRef]
- Wang, L.; Bellini, M.; Miller, H.A.; Varcoe, J.R. A high conductivity ultrathin anion-exchange membrane with 500+ h alkali stability for use in alkaline membrane fuel cells that can achieve 2 W cm−2 at 80 °C. J. Mater. Chem. A 2018, 6, 15404–15412. [Google Scholar] [CrossRef]
- Fu, J.; Qiao, J.; Lv, H.; Ma, J.; Yuan, X.-Z.; Wang, H. Alkali doped poly(vinyl alcohol) (PVA) for anion-exchange membrane fuel cells: Ionic conductivity, chemical stability and FT-IR characterizations. ECS Trans. 2010, 25, 15–23. [Google Scholar] [CrossRef]
- Yokota, N.; Shimada, M.; Ono, H.; Akiyama, R.; Nishino, E.; Asazawa, K.; Miyake, J.; Watanabe, M.; Miyatake, K. Aromatic copolymers containing ammonium-functionalized oligophenylene moieties as highly anion conductive membranes. Macromolecules 2014, 47, 8238–8246. [Google Scholar] [CrossRef]
- Sun, L.; Guo, J.; Zhou, J.; Xu, Q.; Chu, D.; Chen, R. Novel nanostructured high-performance anion exchange ionomers for anion exchange membrane fuel cells. J. Power Sources 2012, 202, 70–77. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Xie, X.; Zhang, F. Novel cross-linked anion exchange membranes with diamines as ionic exchange functional groups and crosslinking groups. Int. J. Hydrogen Energy 2014, 39, 13718–13724. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; Varcoe, J.R.; Ong, A.L.; Poynton, S.D.; Xu, T. Development of imidazolium-type alkaline anion exchange membranes for fuel cell application. J. Membr. Sci. 2012, 415–416, 242–249. [Google Scholar] [CrossRef]
- Yoshimura, K.; Koshikawa, H.; Yamaki, T.; Shishitani, H.; Yamamoto, K.; Yamaguchi, S.; Tanaka, H.; Maekawa, Y. Imidazolium cation based anion-conducting electrolyte membranes prepared by radiation induced grafting for direct hydrazine hydrate fuel cells. J. Electrochem. Soc. 2014, 161, F889–F893. [Google Scholar] [CrossRef]
- Cotanda, P.; Sudre, G.; Modestino, M.A.; Chen, X.C.; Balsara, N.P. High anion conductivity and low water uptake of phosphonium containing diblock copolymer membranes. Macromolecules 2014, 47, 7540–7547. [Google Scholar] [CrossRef]
- Noonan, K.J.T.; Hugar, K.M.; Kostalik, H.A.; Lobkovsky, E.B.; Abruña, H.D.; Coates, G.W. Phosphonium-functionalized polyethylene: A new class of base-stable alkaline anion exchange membranes. J. Am. Chem. Soc. 2012, 134, 18161–18164. [Google Scholar] [CrossRef]
- Sajjad, S.D.; Hong, Y.; Liu, F. Synthesis of guanidinium-based anion exchange membranes and their stability assessment. Polym. Adv. Technol. 2014, 25, 108–116. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Zhang, X.; Wang, W.; Xie, X.; Pei, P. Degradation of guanidinium-functionalized anion exchange membrane during alkaline environment. Int. J. Hydrogen Energy 2014, 39, 13710–13717. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Couture, G.; Ladmiral, V.; Améduri, B. Comparison of epoxy- and cyclocarbonate-functionalised vinyl ethers in radical copolymerisation with chlorotrifluoroethylene. J. Fluor. Chem. 2015, 171, 124–132. [Google Scholar] [CrossRef]
- Hibbs, M.R. Alkaline stability of poly(phenylene)-based anion exchange membranes with various cations. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1736–1742. [Google Scholar] [CrossRef]
- Jannasch, P. Tethering cations to aromatic polymers via flexible spacers to enhance the performance of alkaline fuel cell membranes. ECS Trans. 2015, 69, 369–375. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline stability of quaternary ammonium cations for alkaline fuel cell membranes and ionic liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yan, T.; Li, Z.; Thurn-Albrecht, T.; Binder, W.H. Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes. Energy Environ. Sci. 2012, 5, 7888–7892. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-based polymer electrolyte membranes: Importance of morphology on ion transport and membrane stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef]
- Dekel, D.R.; Willdorf, S.; Ash, U.; Amar, M.; Pusara, S.; Dhara, S.; Srebnik, S.; Diesendruck, C.E. The critical relation between chemical stability of cations and water in anion exchange membrane fuel cells environment. J. Power Sources 2018, 375, 351–360. [Google Scholar] [CrossRef]
- Dekel, D.R.; Amar, M.; Willdorf, S.; Kosa, M.; Dhara, S.; Diesendruck, C.E. Effect of water on the stability of quaternary ammonium groups for anion exchange membrane fuel cell applications. Chem. Mater. 2017, 29, 4425–4431. [Google Scholar] [CrossRef]
- Strasser, D.J.; Graziano, B.J.; Knauss, D.M. Base stable poly(diallylpiperidinium hydroxide) multiblock copolymers for anion exchange membranes. J. Mater. Chem. A. 2017, 5, 9627–9640. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Poly(N,N-diallylazacycloalkane)s for anion-exchange membranes functionalized with N-spirocyclic quaternary ammonium cations. Macromolecules 2017, 50, 2784–2793. [Google Scholar] [CrossRef]
- Pham, T.H.; Jannasch, P. Aromatic polymers incorporating bis-N-spirocyclic quaternary ammonium moieties for anion-exchange membranes. ACS Macro Lett. 2015, 4, 1370–1375. [Google Scholar] [CrossRef]
- Vogel, C.; Komber, H.; Meier-Haack, J. Temperature-stable anion-exchange materials from cyclopolymerization of quaternary ammonium halides. React. Funct. Polym. 2017, 117, 34–42. [Google Scholar] [CrossRef]
- Thomas, O.D.; Soo, K.J.W.Y.; Peckham, T.J.; Kulkarni, M.P.; Holdcroft, S. A stable hydroxide-conducting polymer. J. Am. Chem. Soc. 2012, 134, 10753–10756. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wright, A.G.; Britton, B.; Weissbach, T.; Skalski, T.J.G.; Ward, J.; Peckham, T.J.; Holdcroft, S. Cationic polyelectrolytes, stable in 10 M KOH aq at 100 °C. ACS Macro Lett. 2017, 6, 1089–1093. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C.T. Prospects for alkaline anion-exchange membranes in low Ttemperature fuel cells. Fuel Cells 2005, 5, 187–200. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Kinsinger, C.L.; Yang, Y.; Seifert, S.; Yan, Y.; Maupin, C.M.; Liberatore, M.W.; Herring, A.M. Anion exchange membranes composed of a poly(2,6-dimethyl-1,4-phenylene oxide) random copolymer functionalized with a bulky phosphonium cation. J. Membr. Sci. 2016, 506, 50–59. [Google Scholar] [CrossRef]
- Dang, H.-S.; Jannasch, P. Alkali-stable and highly anion conducting poly(phenylene oxide)s carrying quaternary piperidinium cations. J. Mater. Chem. A 2016, 4, 11924–11938. [Google Scholar] [CrossRef]
- Lin, J.; Yan, X.; He, G.; Chen, W.; Zhen, D.; Li, T.; Ma, L.; Wu, X. Thermoplastic interpenetrating polymer networks based on polybenzimidazole and poly (1, 2-dimethy-3-allylimidazolium) for anion exchange membranes. Electrochim. Acta 2017, 257, 9–19. [Google Scholar] [CrossRef]
- Lin, C.X.; Huang, X.L.; Guo, D.; Zhang, Q.G.; Zhu, A.M.; Ye, M.L.; Liu, Q.L. Side-chain-type anion exchange membranes bearing pendant quaternary ammonium groups via flexible spacers for fuel cells. J. Mater. Chem. A 2016, 4, 13938–13948. [Google Scholar] [CrossRef]
- Arges, C.G.; Wang, L.; Parrondo, J.; Ramani, V. Best practices for investigating anion exchange membrane suitability for alkaline electrochemical devices: Case study using quaternary ammonium poly(2,6-dimethyl 1,4-phenylene)oxide anion exchange membranes. J. Electrochem. Soc. 2013, 160, F1258–F1274. [Google Scholar] [CrossRef]
- Nuñez, S.A.; Hickner, M.A. Quantitative 1H NMR analysis of chemical stabilities in anion-exchange membranes. ACS Macro Lett. 2013, 2, 49–52. [Google Scholar] [CrossRef]
- Parrondo, J.; Wang, Z.; Jung, M.-S.J.; Ramani, V. Reactive oxygen species accelerate degradation of anion exchange membranes based on polyphenylene oxide in alkaline environments. Phys. Chem. Chem. Phys. 2016, 18, 19705–19712. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Elabd, Y.A. Chemical stability of anion exchange membranes for alkaline fuel cells. In Polymers for Energy Storage and Delivery: Polyelectrolytes for Batteries and Fuel Cells; American Chemical Society: Washington, DC, USA, 2012; pp. 233–251. [Google Scholar] [CrossRef]
- Danks, T.N.; Slade, R.C.T.; Varcoe, J.R. Alkaline anion-exchange radiation-grafted membranes for possible electrochemical application in fuel cells. J. Mater. Chem. 2003, 13, 712–721. [Google Scholar] [CrossRef]
- Sata, T.; Yamane, Y.; Matsusaki, K. Preparation and properties of anion exchange membranes having pyridinium or pyridinium derivatives as anion exchange groups. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 49–58. [Google Scholar] [CrossRef]
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends †. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [PubMed]
- Neagu, V.; Bunia, I.; Plesca, I. Ionic polymers VI. Chemical stability of strong base anion exchangers in aggressive media. Polym. Degrad. Stab. 2000, 70, 463–468. [Google Scholar] [CrossRef]
- Ross, G.; Watts, J.; Hill, M.; Morrissey, P. Surface modification of poly(vinylidene fluoride) by alkaline treatment part 2. process modification by the use of phase transfer catalysts. Polymer 2001, 42, 403–413. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef]
- Fujimoto, C.; Kim, D.-S.; Hibbs, M.; Wrobleski, D.; Kim, Y.S. Backbone stability of quaternized polyaromatics for alkaline membrane fuel cells. J. Membr. Sci. 2012, 423–424, 438–449. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Tignor, S.E.; Krause, J.A.; Choe, Y.-K.; Bae, C. Systematic alkaline stability study of polymer backbones for anion exchange membrane applications. Macromolecules 2016, 49, 3361–3372. [Google Scholar] [CrossRef]
- Hickner, M.A. Strategies for developing new anion exchange membranes and electrode ionomers. Electrochem. Soc. Interface 2017, 26, 69–73. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.S. Quaternized aryl ether-free polyaromatics for alkaline membrane fuel cells: Synthesis, properties, and performance—A topical review. J. Mater. Chem. A 2018, 6, 15456–15477. [Google Scholar] [CrossRef]
- Amel, A.; Zhu, L.; Hickner, M.; Ein-Eli, Y. Influence of sulfone linkage on the stability of aromatic quaternary ammonium polymers for alkaline fuel cells. J. Electrochem. Soc. 2014, 161, F615–F621. [Google Scholar] [CrossRef]
- Choe, Y.-K.; Fujimoto, C.; Lee, K.-S.; Dalton, L.T.; Ayers, K.; Henson, N.J.; Kim, Y.S. Alkaline stability of benzyl trimethyl ammonium functionalized polyaromatics: A computational and experimental study. Chem. Mater. 2014, 26, 5675–5682. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells: Theory and Practice; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Eriksson, B.; Grimler, H.; Carlson, A.; Ekström, H.; Lindström, R.W.; Lindbergh, G.; Lagergren, C. Quantifying water transport in anion exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 4930–4939. [Google Scholar] [CrossRef]
- Grew, K.N.; Ren, X.; Chu, D. Effects of temperature and carbon dioxide on anion exchange membrane conductivity. Electrochem. Solid-State Lett. 2011, 14, B127–B131. [Google Scholar] [CrossRef]
- Grew, K.N.; Chiu, W.K.S. A dusty fluid model for predicting hydroxyl anion conductivity in alkaline anion exchange membranes. J. Electrochem. Soc. 2010, 157, B327–B337. [Google Scholar] [CrossRef]
- Takaba, H.; Hisabe, T.; Shimizu, T.; Alam, M.K. Molecular modeling of OH− transport in poly(arylene ether sulfone ketone)s containing quaternized ammonio-substituted fluorenyl groups as anion exchange membranes. J. Membr. Sci. 2017, 522, 237–244. [Google Scholar] [CrossRef]
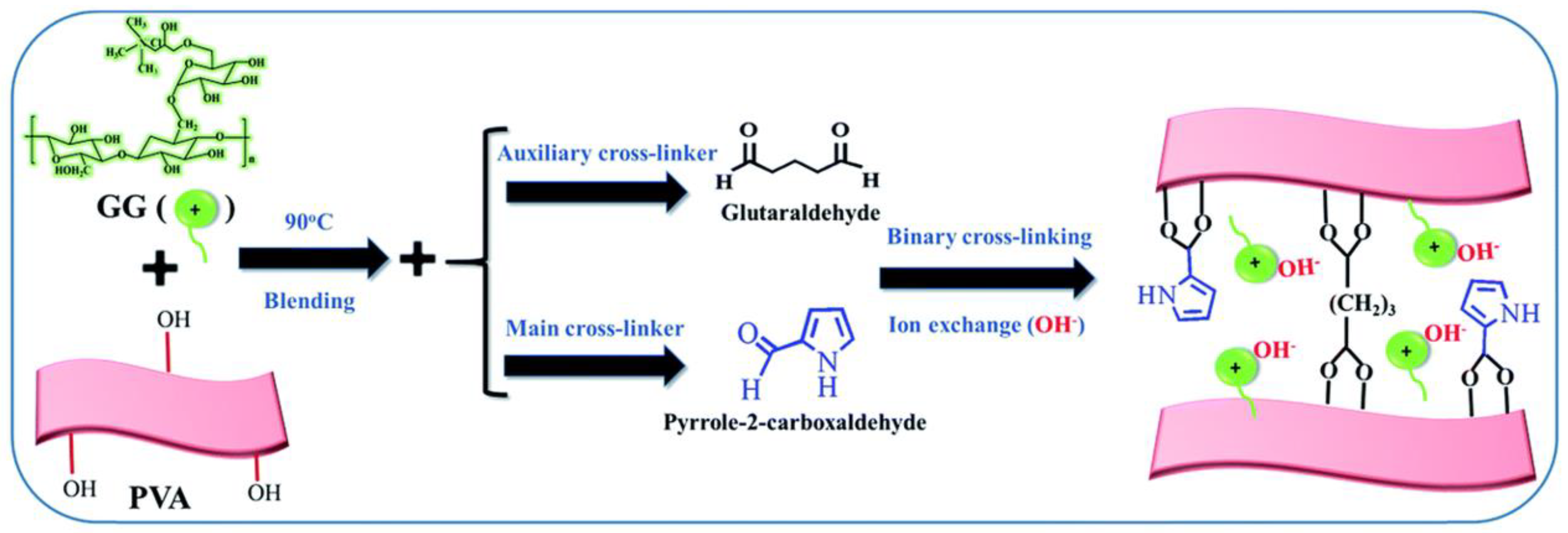
- Wang, M.; Xu, N.; Fu, J.; Liu, Y.; Qiao, J. High-performance binary cross-linked alkaline anion polymer electrolyte membranes for all-solid-state supercapacitors and flexible rechargeable zinc–air batteries. J. Mater. Chem. A 2019, 7, 11257–11264. [Google Scholar] [CrossRef]
- Kreuer, K.-D.; Jannasch, P. A practical method for measuring the ion exchange capacity decrease of hydroxide exchange membranes during intrinsic degradation. J. Power Sources 2018, 375, 361–366. [Google Scholar] [CrossRef]
- Li, N.; Leng, Y.; Hickner, M.A.; Wang, C.-Y. Highly stable, anion conductive, comb-shaped copolymers for alkaline fuel cells. J. Am. Chem. Soc. 2013, 135, 10124–10133. [Google Scholar] [CrossRef] [PubMed]
- Ponce-González, J.; Whelligan, D.K.; Wang, L.; Bance-Soualhi, R.; Wang, Y.; Peng, Y.; Peng, H.; Apperley, D.C.; Sarode, H.N.; Pandey, T.P.; et al. High performance aliphatic-heterocyclic benzyl-quaternary ammonium radiation-grafted anion-exchange membranes. Energy Environ. Sci. 2016, 9, 3724–3735. [Google Scholar] [CrossRef]
- Ziv, N.; Dekel, D.R. A practical method for measuring the true hydroxide conductivity of anion exchange membranes. Electrochem. Commun. 2018, 88, 109–113. [Google Scholar] [CrossRef]
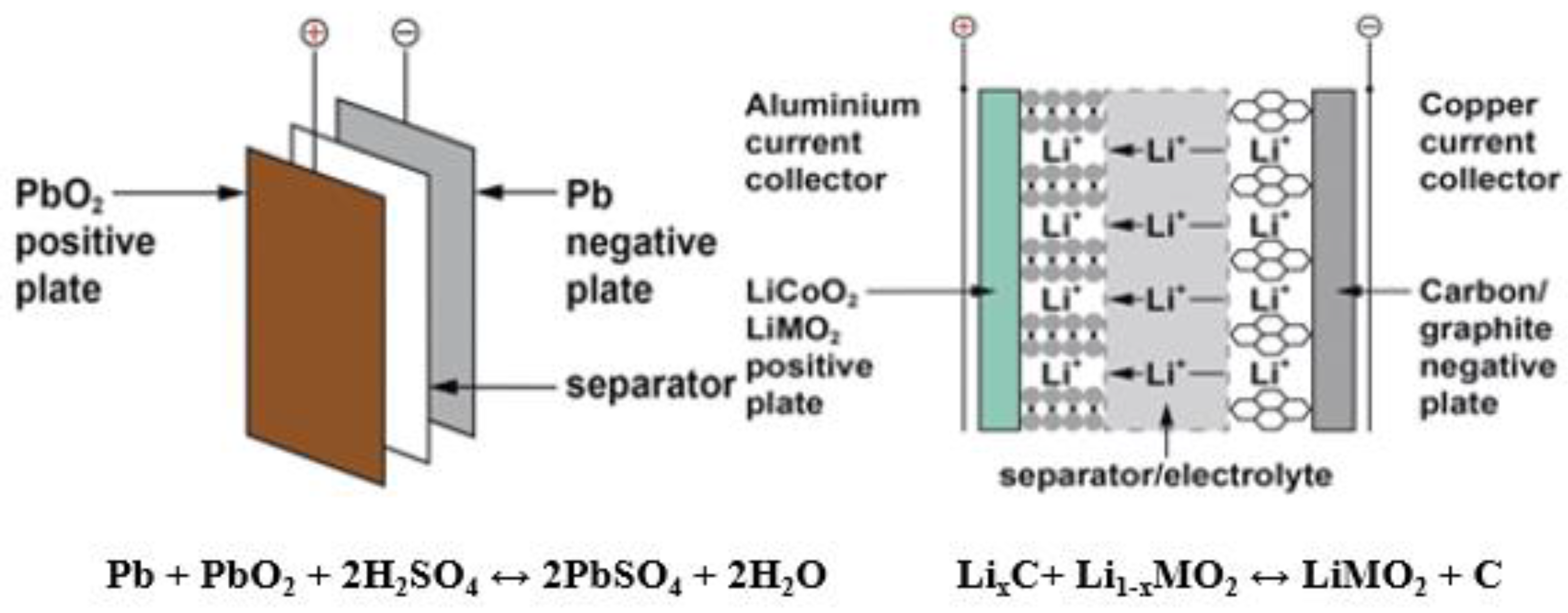
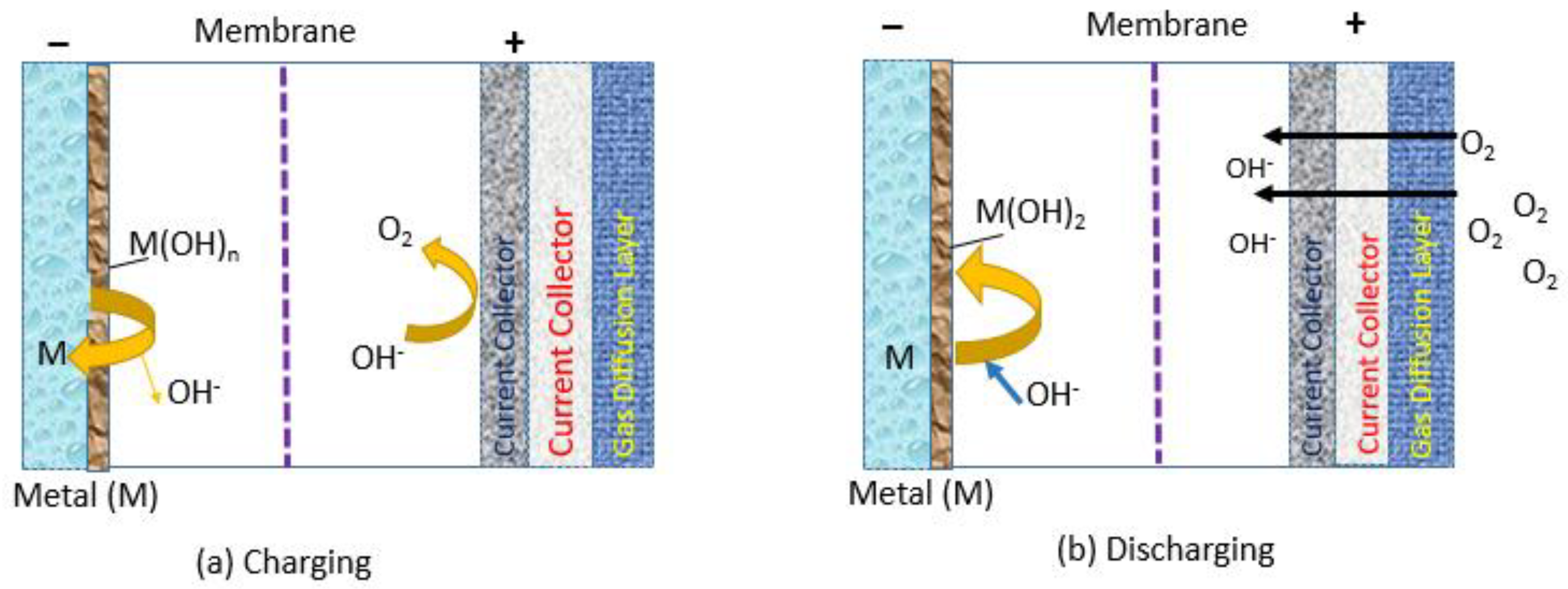
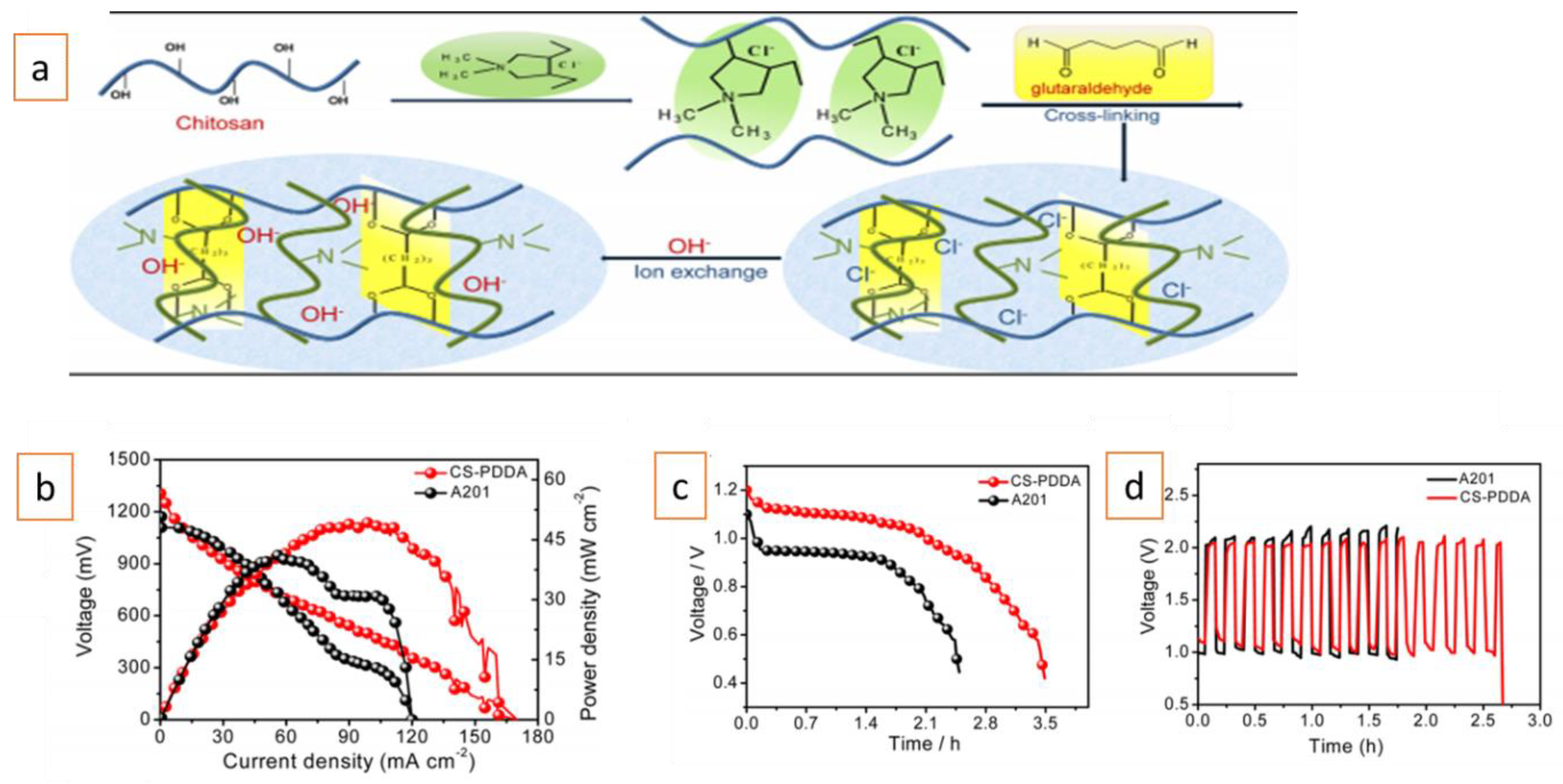
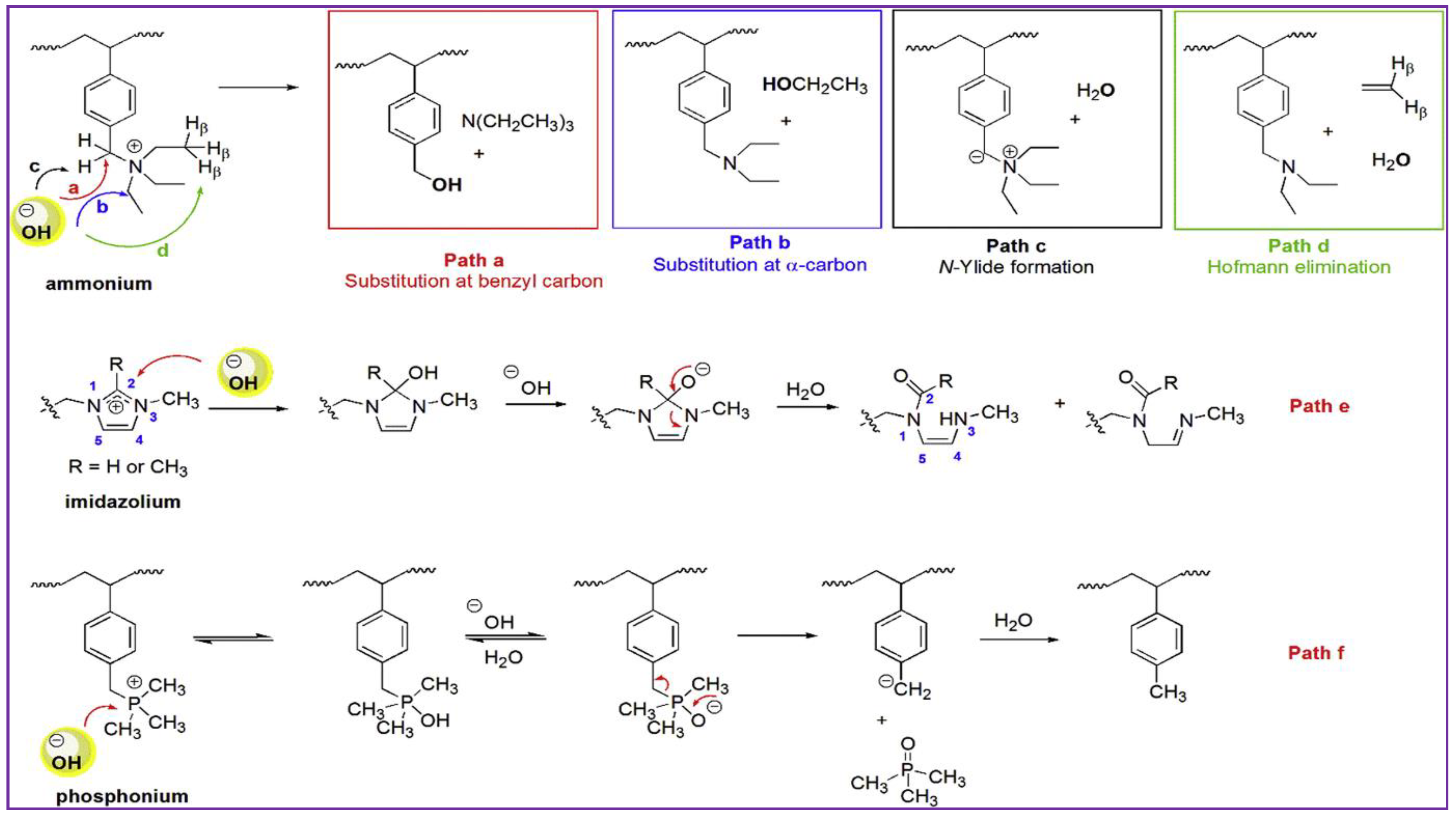
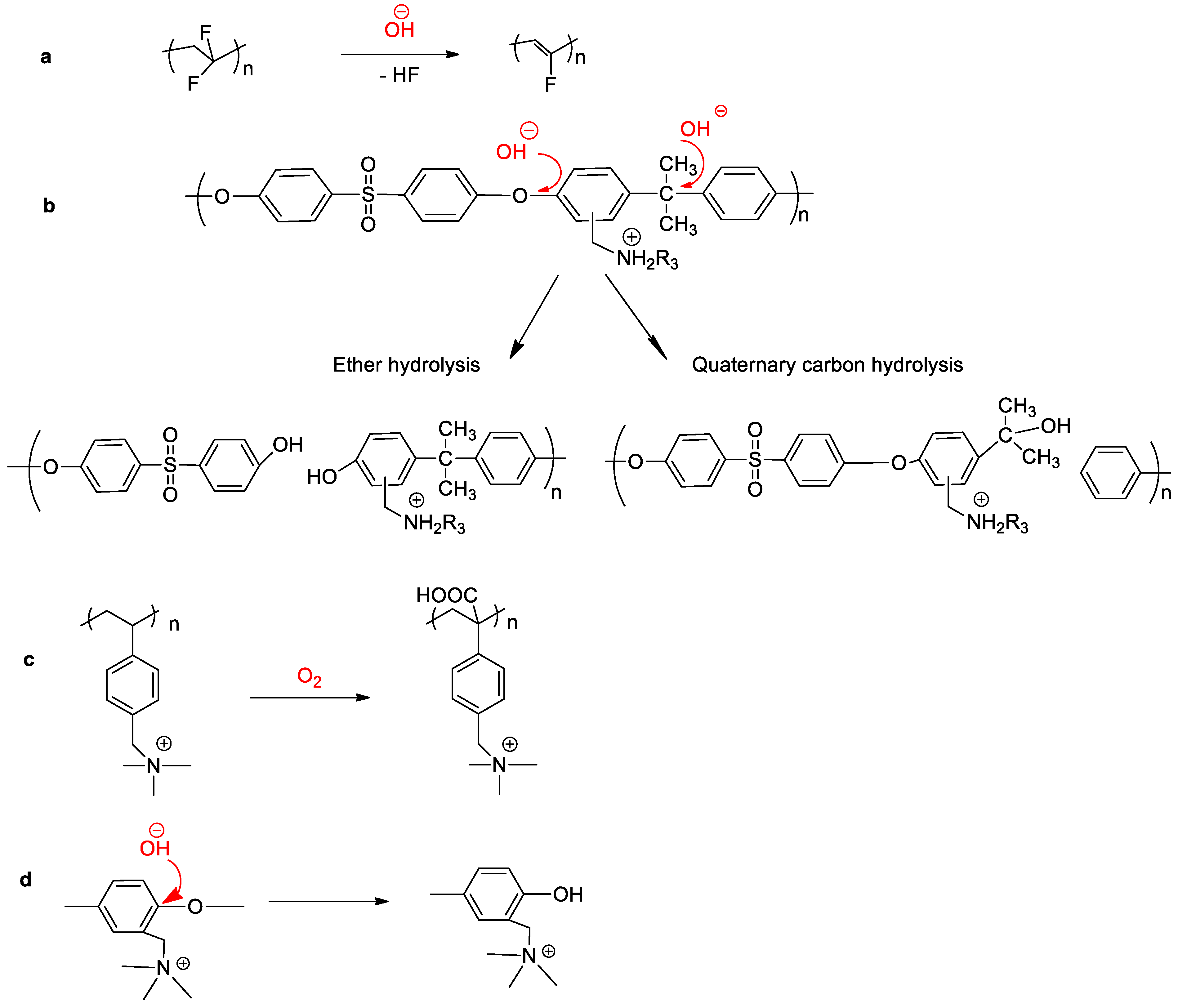
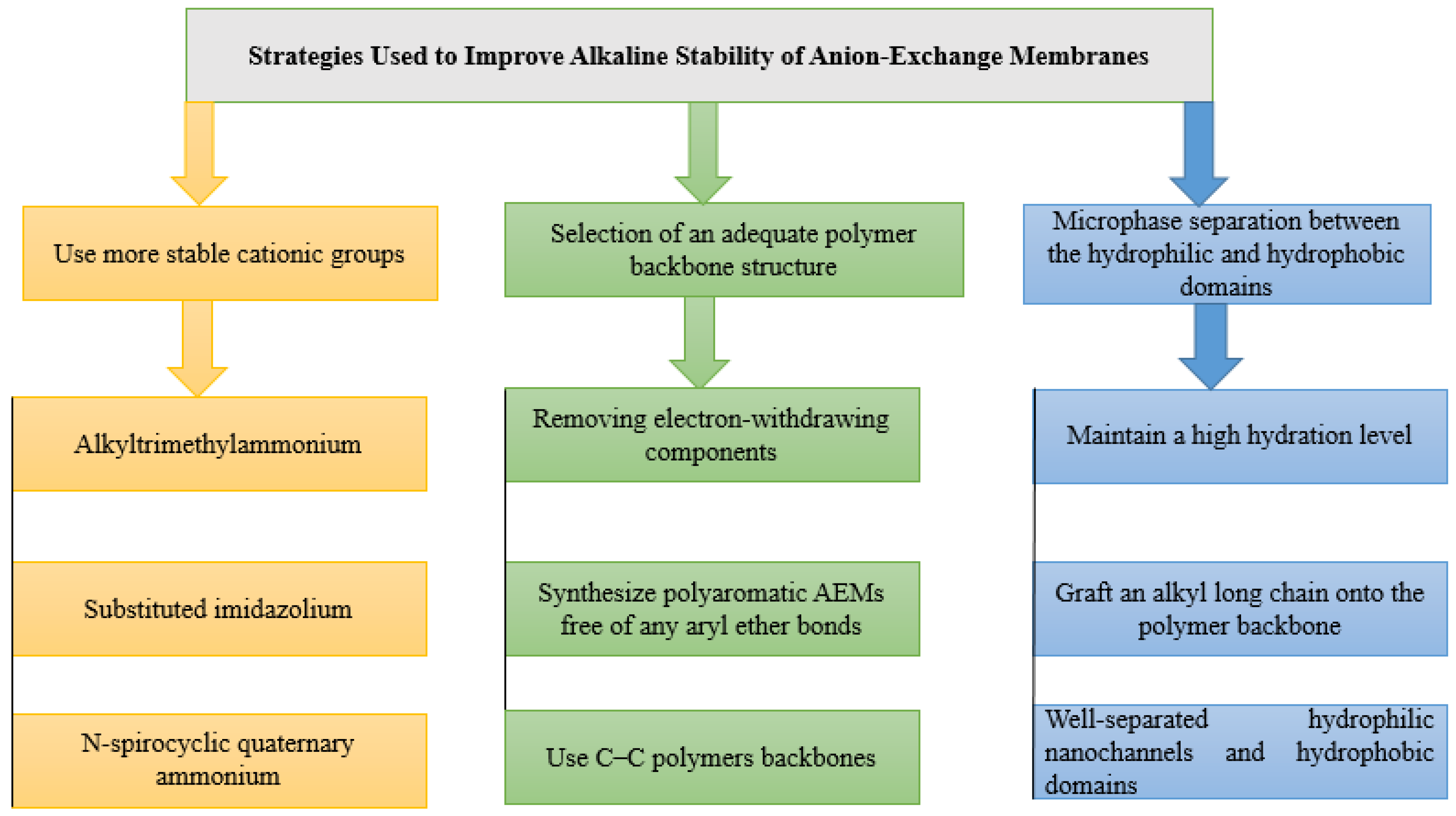
| Battery Systems | Zn–Air Battery | Fe–Air Battery | Al–Air Battery |
|---|---|---|---|
| Cathode reaction | O2 + 2H2O + 4e− ↔ 4OH− | ||
| Anode reaction | Zn + 4OH− ↔ Zn(OH)42− + 2e− Zn(OH)42− ↔ ZnO + H2O + 2OH− | Fe + 2OH− ↔ Fe(OH)2 + 2e− 3Fe(OH)2 + 2OH− ↔ Fe3O4 + 4H2O + 2e− | Al + 4OH− ↔ Al(OH)4−+ 3e− |
| Overall reaction: | 2Zn + O2 ↔ 2ZnO | 3Fe + 2O2 ↔ Fe3O4 | 2Al + 3O2 ↔ Al2O3 |
| Theoretical voltage (V) | 1.65 | 1.28 | 2.71 |
| Year invented | 1878 | 1968 | 1962 |
| Cost of metals ($US/Kg)* | 2.6 | 0.5 | 1.9 |
| Theoretical energy density (Wh/kg) | 1086 | 764 | 2796 |
| Specific Capacity (mA h/g) | 820 | 2974 | 2980 |
| Major strengths | High energy density, low cost, and environmental friendliness | -Do not form dendrites -Fourth most abundant element on the earth | -Inexpensive, safe, and is the third most abundant element in the Earth’s crust. -Significant cost savings and safety |
| Potential applications | Suitable for use in larger passenger vehicles | A range of applications, including automotive | Electric vehicles, military for aircraft and underwater vehicles |
| Remaining challenges | -Dendrite formation/growth -Bifunctional catalyst -Carbonation -Suitable membrane | Efficient and moderate-cost bifunctional -oxygen electrodes -Low-cost iron electrodes able to decrease corrosion and hydrogen evolution -New cell designs using additive manufacturing technologies | High rate of Al self-corrosion in alkaline solutions (under both open-circuit and discharge conditions) |
| Refs. | [5,21,22,23,24,25] | [19,25,26,27] | [14,25,28,29,30,31,32] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsehaye, M.T.; Alloin, F.; Iojoiu, C. Prospects for Anion-Exchange Membranes in Alkali Metal–Air Batteries. Energies 2019, 12, 4702. https://doi.org/10.3390/en12244702
Tsehaye MT, Alloin F, Iojoiu C. Prospects for Anion-Exchange Membranes in Alkali Metal–Air Batteries. Energies. 2019; 12(24):4702. https://doi.org/10.3390/en12244702
Chicago/Turabian StyleTsehaye, Misgina Tilahun, Fannie Alloin, and Cristina Iojoiu. 2019. "Prospects for Anion-Exchange Membranes in Alkali Metal–Air Batteries" Energies 12, no. 24: 4702. https://doi.org/10.3390/en12244702
APA StyleTsehaye, M. T., Alloin, F., & Iojoiu, C. (2019). Prospects for Anion-Exchange Membranes in Alkali Metal–Air Batteries. Energies, 12(24), 4702. https://doi.org/10.3390/en12244702