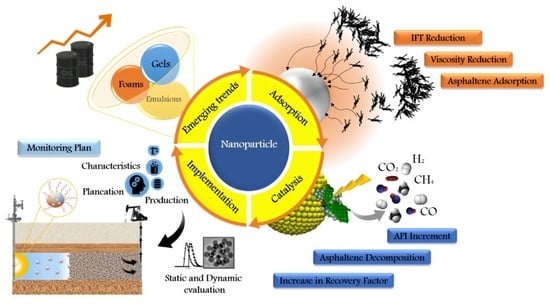
Nanotechnology Applied to Thermal Enhanced Oil Recovery Processes: A Review
Abstract
1. Introduction
2. Physical-Chemical Properties of Heavy (HO), Extra-Heavy Crude Oil (EHO)/Bitumen
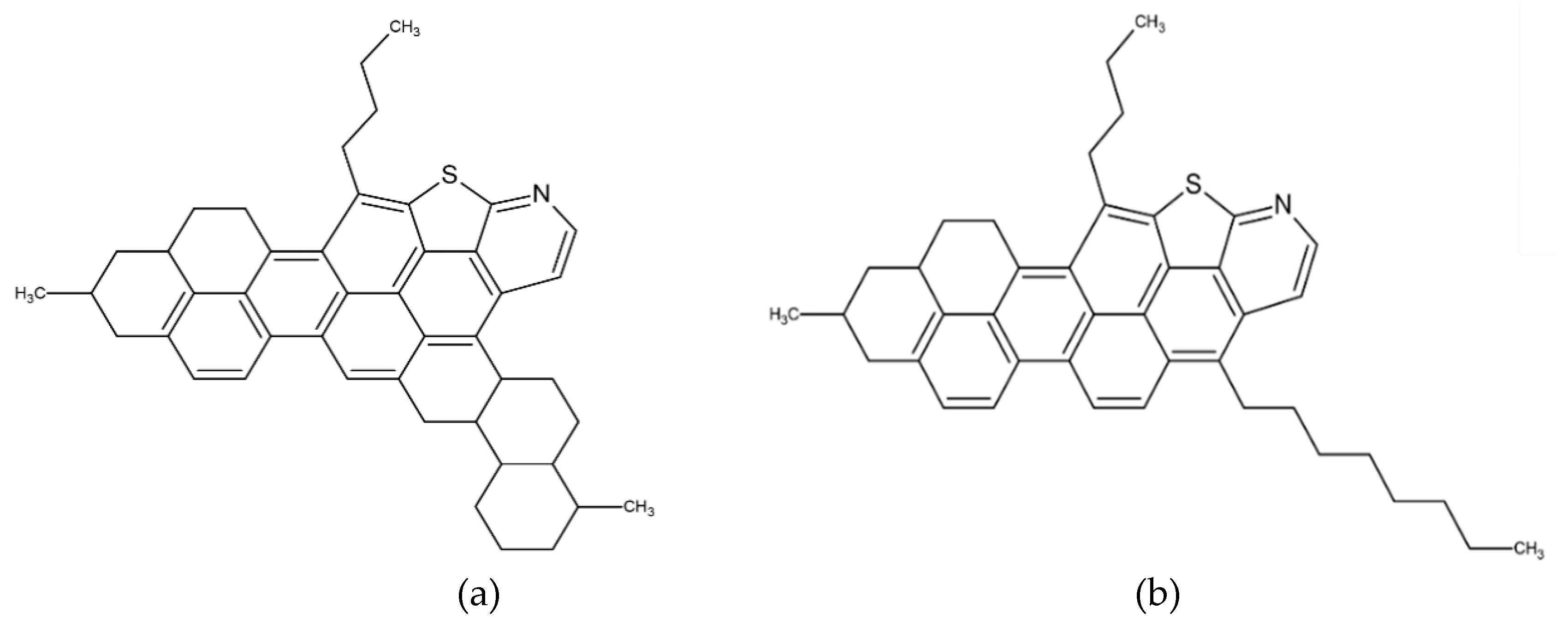
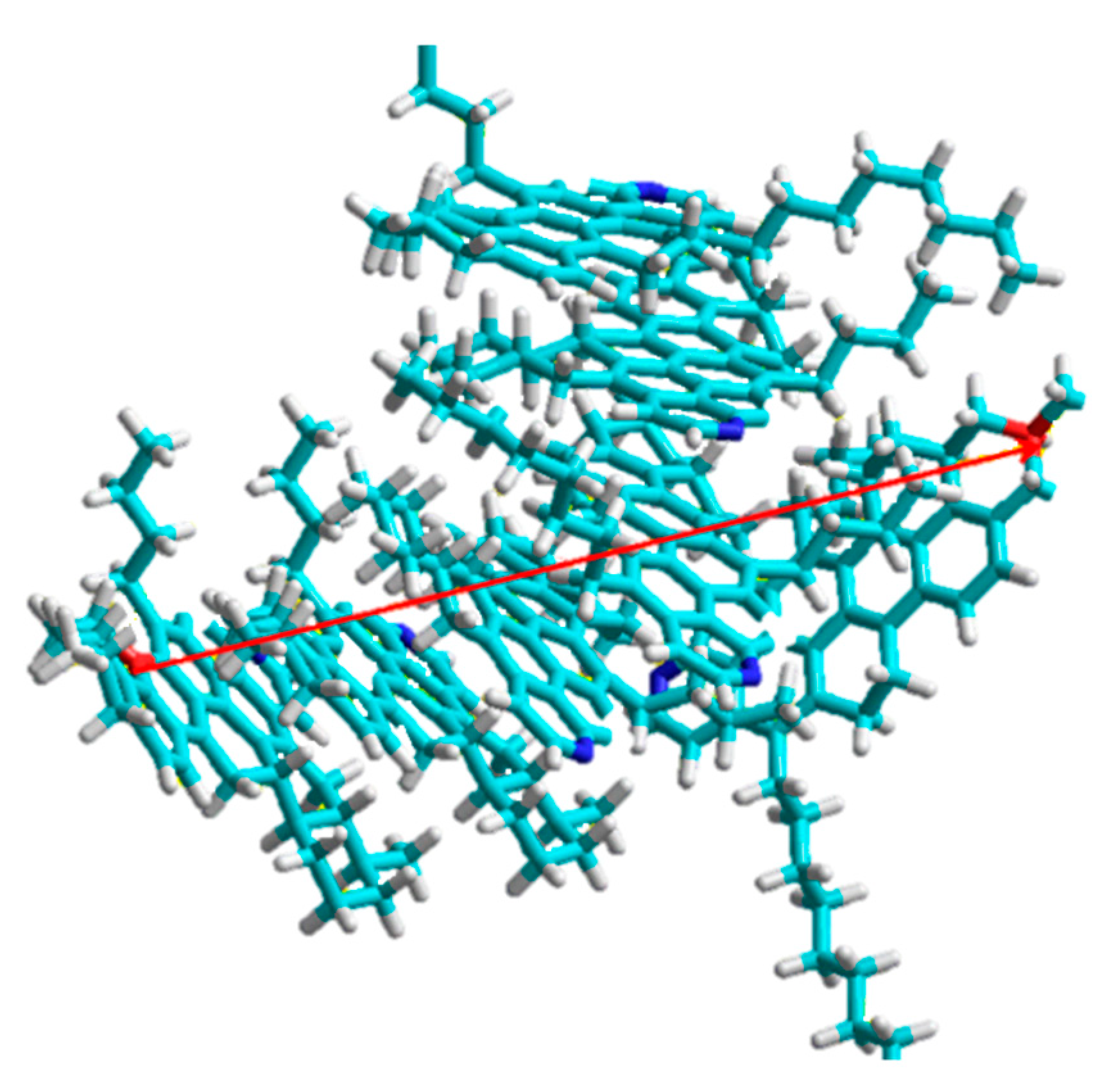
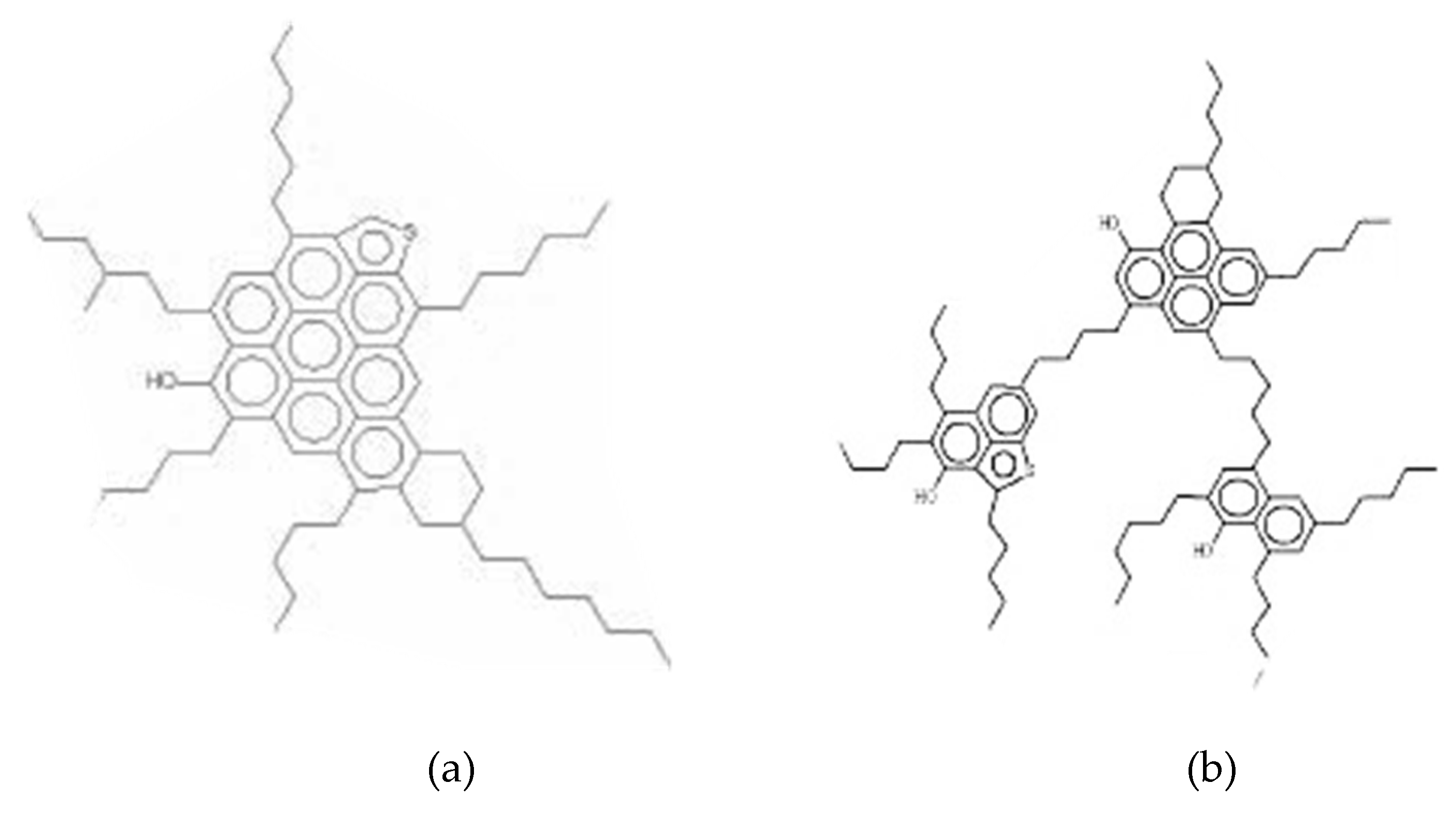
3. Interaction between Heavy Crude Oil Fractions and Nanoparticles
4. Influence of Nanoparticles in the Air Injection Process
4.1. Metal and Metal Oxide Nanoparticles
4.2. SiO2 and SiO2-Based Nanoparticles
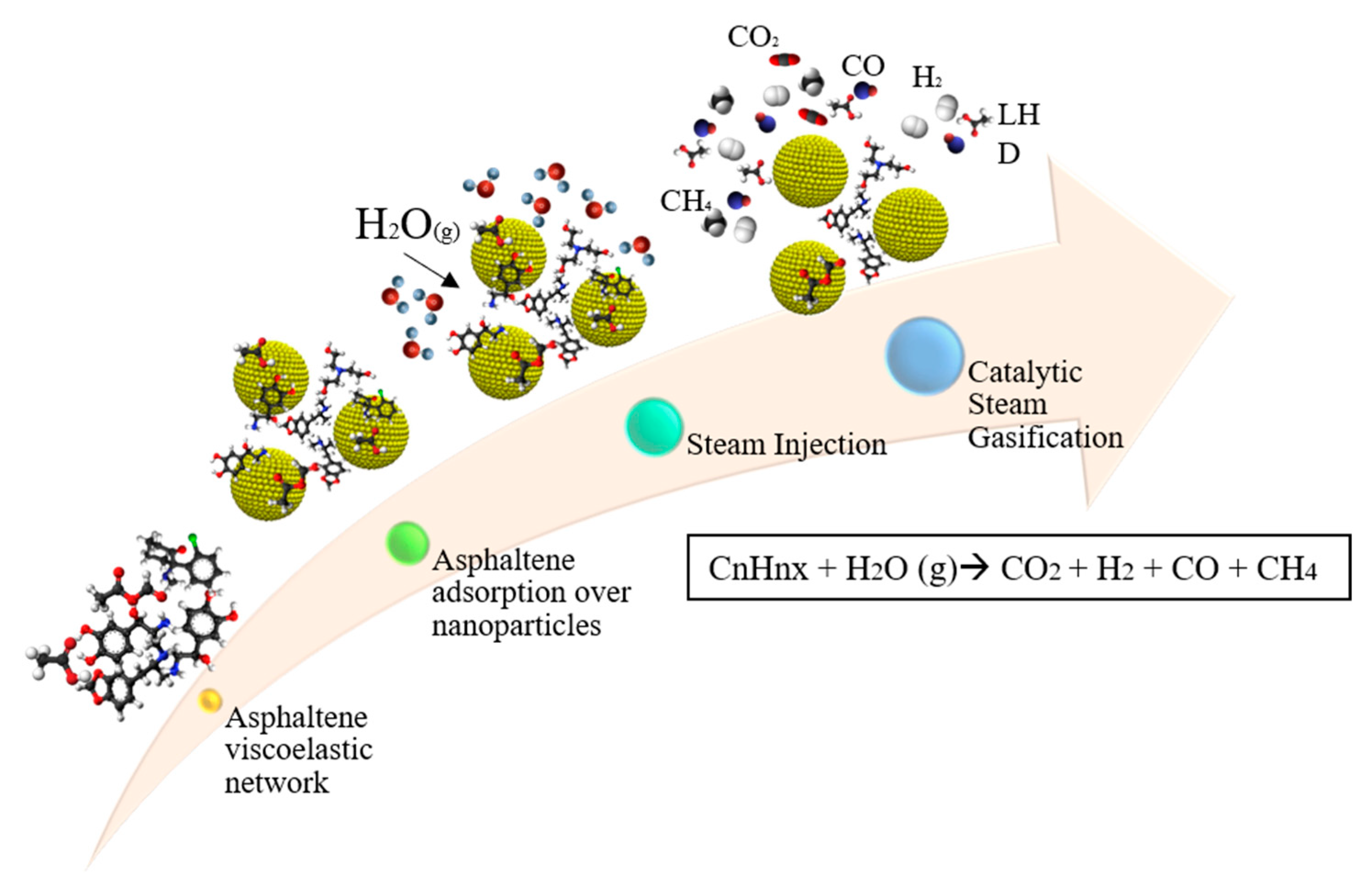
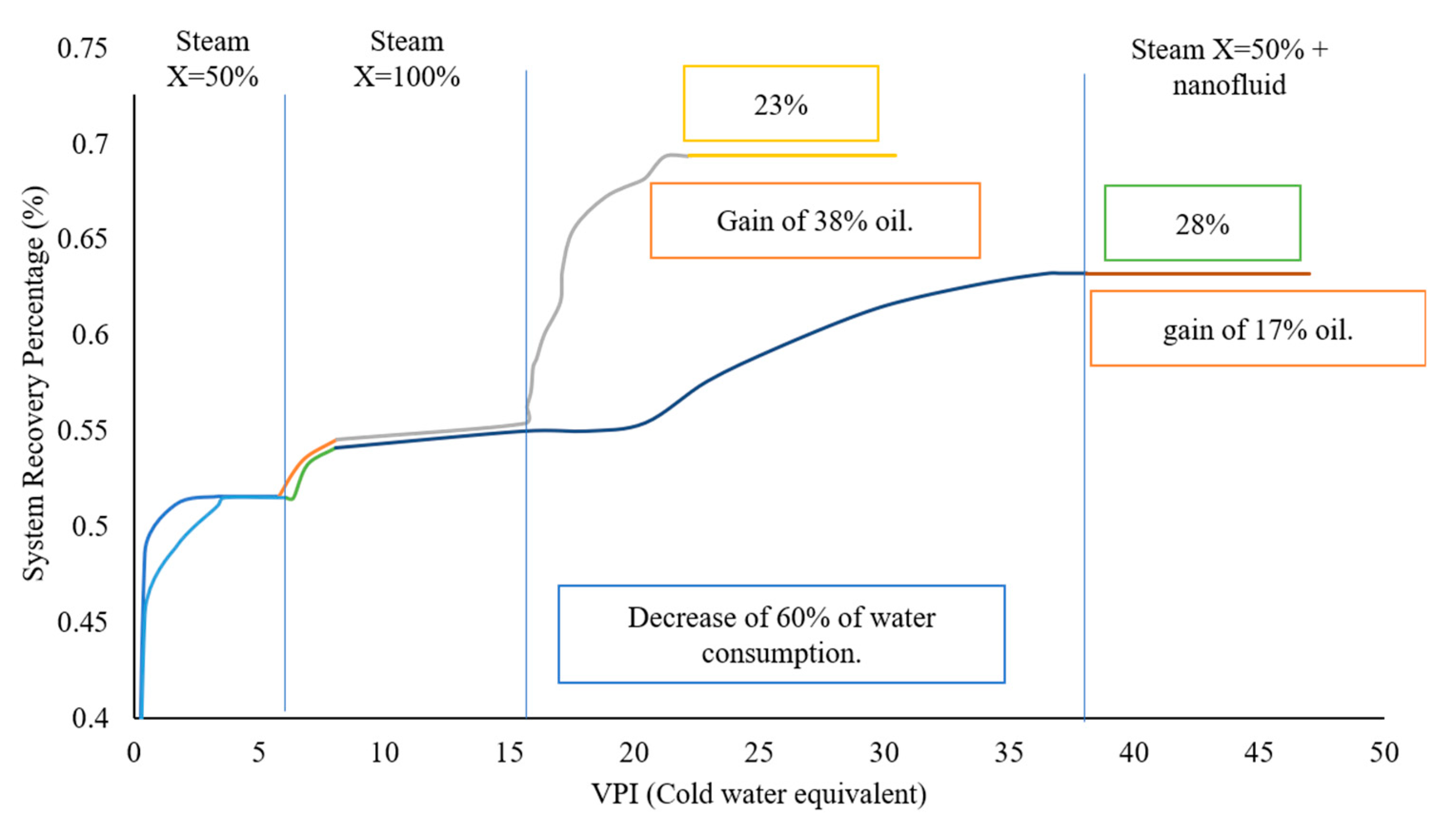
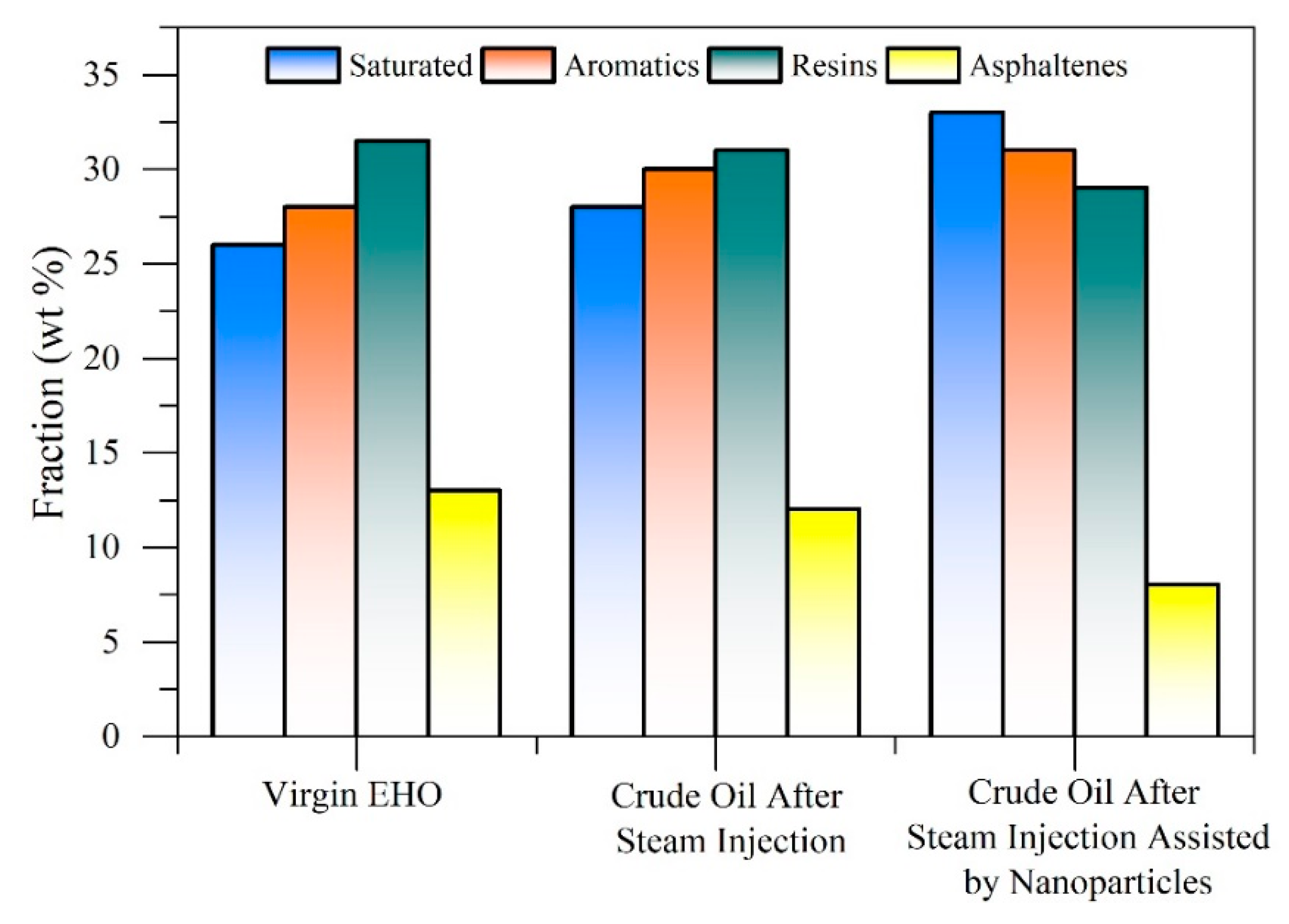
5. Influence of Nanoparticles in Steam Injection Processes
5.1. Metal Oxide Nanoparticles
5.2. Composite Materials
6. Influence of Nanoparticles in Pyrolysis Reactions
7. Influence of Nanoparticles on Electromagnetic Heating for Heavy Crude Oil
8. Implementation Plan of Nanotechnology for the Steam Injection Process
9. Environmental Impacts
10. Emerging Trends
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| CNC | Cellulose nanocrystals |
| CNS | Carbon nanospheres |
| CSS | Cyclic Steam Simulation |
| EHO | Extra-heavy crude oil |
| EM | Electromagnetic heating |
| EOR | Enhanced oil recovery |
| HMT | Hexamethylenetetramine |
| HO | Heavy crude oil |
| HPAM | Partially hydrolyzed polyacrylamides |
| HP-DSC | High-pressure differential scan |
| HQ | Hydroquinone |
| HTHP | High temperature and high pressure |
| IEA | International Energy Agency |
| IFT | Interfacial Tension |
| IOR | Improved Oil Recovery |
| ISC | In-Situ Combustion |
| NPs | Nanoparticles |
| PAM | Polyacrylamide |
| SAGD | Steam-Assisted Gravity Drainage |
| SBA-15 | Mesoporous silica |
| SLE | Solid-Liquid Equilibrium |
| RF | Recovery factor |
| THAI | Toe-to-Heel Air Injection |
| THAI/CAPRI | Toe-to-Heel Air Injection (catalytic PRI) |
| TEO | Transition element oxides |
| TEOR | Thermal enhanced oil recovery |
| TGA | Thermal Gravimetric Analysis |
References
- Olsson, G. Water and Energy: Threats and Opportunities; IWA publishing: London, UK, 2015. [Google Scholar]
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010, 3, 700–714. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2017; International Energy Agency: Paris, France, 2017. [Google Scholar]
- Tedeschi, M. RESERVES AND PRODUCTION OF HEAVY CRUDE OIL AND NATURAL BITUMEN. In Proceedings of the 13th World Petroleum Congress, Buenos Aires, Argentina, 1 January 1991; p. 8. [Google Scholar]
- Miller, R.G.; Sorrell, S.R. The Future of Oil Supply; The Royal Society Publishing: London, UK, 2014. [Google Scholar]
- Santos, R.; Loh, W.; Bannwart, A.; Trevisan, O. An overview of heavy oil properties and its recovery and transportation methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Guarin Arenas, F.; Garcia, C.A.; Diaz Prada, C.A.; Cotes Leon, E.D.J.; Santos, N. A New Inflow Model for Extra-Heavy Crude Oils: Case Study Chichimene Field, Colombia. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Lima, Peru, 1–3 December 2010. [Google Scholar]
- Valbuena, O.H.; Bernal, M.E.; Ramon, J.C.; Xiuxia, T. First Extra-Heavy-Oil Development in Caguan-Putumayo Basin, Colombia, Capella Field. In Proceedings of the SPE Heavy and Extra Heavy Oil Conference: Latin America, Medellín, Colombia, 24–26 September 2014. [Google Scholar]
- Lopez Uribe, J.E.; Chaustre Ruiz, A.J.; Ayala Marin, C.A. Producing Extra-Heavy Oil From Llanos Basin, Colombia, Through Progressive Cavity Pumps and Electric Submersible Pumps: Case Study in the Chichimene Field. In Proceedings of the SPE Heavy and Extra Heavy Oil Conference: Latin America, Medellín, Colombia, 24–26 September 2014. [Google Scholar]
- Muggeridge, A.; Cockin, A.; Webb, K.; Frampton, H.; Collins, I.; Moulds, T.; Salino, P. Recovery rates, enhanced oil recovery and technological limits. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20120320. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Betancur, S.; Franco, C.A.; Cortés, F.B. Magnetite-silica nanoparticles with a core-shell structure for inhibiting the formation damage caused by the precipitation/deposition of asphaltene. J. Magnetohydrodyn. Plasma Res. 2016, 21, 289–322. [Google Scholar]
- Betancur, S.; Giraldo, L.J.; Carrasco-Marín, F.; Riazi, M.; Manrique, E.J.; Quintero, H.; García, H.A.; Franco-Ariza, C.A.; Cortés, F.B. Importance of the Nanofluid Preparation for Ultra-Low Interfacial Tension in Enhanced Oil Recovery Based on Surfactant–Nanoparticle–Brine System Interaction. ACS Omega 2019, 4, 16171–16180. [Google Scholar] [CrossRef]
- Yarveicy, H.; Habibi, A.; Pegov, S.; Zolfaghari, A.; Dehghanpour, H. Enhancing oil recovery by adding surfactants in fracturing water: A Montney case study. In Proceedings of the SPE Canada Unconventional Resources Conference, Calgary, AB, Canada, 13–14 March 2018. [Google Scholar]
- Sheng, J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Oxford, UK, 2010. [Google Scholar]
- Assef, Y.; Pereira Almao, P. Evaluation of Cyclic Gas Injection in Enhanced Recovery from Unconventional Light Oil Reservoirs: Effect of Gas Type and Fracture Spacing. Energies 2019, 12, 1370. [Google Scholar] [CrossRef]
- Alagorni, A.H.; Yaacob, Z.B.; Nour, A.H. An overview of oil production stages: Enhanced oil recovery techniques and nitrogen injection. Int. J. Environ. Sci. Dev. 2015, 6, 693. [Google Scholar] [CrossRef]
- Caudle, B.; Dyes, A. Improving Miscible Displacement by Gas-Water Injection. In Proceedings of the 32nd Annual Fall Meeting of Society of Petroleum Engineers, Dallas, TX, USA, 6–9 October 1957. [Google Scholar]
- Jia, B.; Tsau, J.-S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, S.S.; Luo, P. Coupling immiscible CO2 technology and polymer injection to maximize EOR performance for heavy oils. J. Can. Pet. Technol. 2010, 49, 25–33. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of nanoparticles in enhanced oil recovery: A critical review of recent progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Yi, S.; Babadagli, T.; Li, H.A. Use of nickel nanoparticles for promoting aquathermolysis reaction during cyclic steam stimulation. SPE J. 2018, 23, 145–156. [Google Scholar] [CrossRef]
- Jorshari, K.; O’Hara, B. A new SAGD-well-pair placement: A field case review. J. Can. Pet. Technol. 2013, 52, 12–19. [Google Scholar] [CrossRef]
- Kar, T.; Ovalles, C.; Rogel, E.; Vien, J.; Hascakir, B. The residual oil saturation determination for Steam Assisted Gravity Drainage (SAGD) and Solvent-SAGD. Fuel 2016, 172, 187–195. [Google Scholar] [CrossRef]
- Burger, J.G. Chemical aspects of in-situ combustion-heat of combustion and kinetics. Soc. Pet. Eng. J. 1972, 12, 410–422. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Rodriguez, E.; Franco, C.A.; Cortés, F.B. Effect of Pressure on the Oxidation Kinetics of Asphaltenes. Energy Fuels 2019, 33, 10734–10744. [Google Scholar] [CrossRef]
- Franco, C.A.; Zabala, R.; Cortés, F.B. Nanotechnology applied to the enhancement of oil and gas productivity and recovery of Colombian fields. J. Pet. Sci. Eng. 2017, 157, 39–55. [Google Scholar] [CrossRef]
- Franco, C.; Cardona, L.; Lopera, S.; Mejía, J.; Cortés, F. Heavy oil upgrading and enhanced recovery in a continuous steam injection process assisted by nanoparticulated catalysts. In Proceedings of the SPE improved oil recovery conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Iskandar, F.; Dwinanto, E.; Abdullah, M.; Muraza, O. Viscosity reduction of heavy oil using nanocatalyst in aquathermolysis reaction. KONA Powder Part. J. 2016, 33, 3–16. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. Enhanced heavy oil recovery by in situ prepared ultradispersed multimetallic nanoparticles: A study of hot fluid flooding for Athabasca bitumen recovery. Energy Fuels 2013, 27, 2194–2201. [Google Scholar] [CrossRef]
- Montoya, T.; Argel, B.L.; Nassar, N.N.; Franco, C.A.; Cortés, F.B. Kinetics and mechanisms of the catalytic thermal cracking of asphaltenes adsorbed on supported nanoparticles. Pet. Sci. 2016, 13, 561–571. [Google Scholar] [CrossRef]
- Husein, M.M.; Alkhaldi, S.J. In Situ Preparation of Alumina Nanoparticles in Heavy Oil and Their Thermal Cracking Performance. Energy Fuels 2014, 28, 6563–6569. [Google Scholar] [CrossRef]
- Hou, J.; Li, C.; Gao, H.; Chen, M.; Huang, W.; Chen, Y.; Zhou, C. Recyclable oleic acid modified magnetic NiFe2O4 nanoparticles for catalytic aquathermolysis of Liaohe heavy oil. Fuel 2017, 200, 193–198. [Google Scholar] [CrossRef]
- Kaminski, T.; Anis, S.F.; Husein, M.M.; Hashaikeh, R. Hydrocracking of Athabasca VR Using NiO-WO3 Zeolite-Based Catalysts. Energy Fuels 2018, 32, 2224–2233. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Montoya, T.; Ruíz, M.A.; Cortés, F.B. Influence of Asphaltene Aggregation on the Adsorption and Catalytic Behavior of Nanoparticles. Energy Fuels 2015, 29, 1610–1621. [Google Scholar] [CrossRef]
- Amrollahi Biyouki, A.; Hosseinpour, N.; Nassar, N.N. Pyrolysis and Oxidation of Asphaltene-Born Coke-like Residue Formed onto in Situ Prepared NiO Nanoparticles toward Advanced in Situ Combustion Enhanced Oil Recovery Processes. Energy Fuels 2018, 32, 5033–5044. [Google Scholar] [CrossRef]
- Tang, X.-D.; Liang, G.-J.; Li, J.-J.; Wei, Y.-T.; Dang, T. Catalytic effect of in-situ preparation of copper oxide nanoparticles on the heavy oil low-temperature oxidation process in air injection recovery. Pet. Sci. Technol. 2017, 35, 1321–1326. [Google Scholar] [CrossRef]
- Franco-Ariza, C.A.; Guzmán-Calle, J.D.; Cortés-Correa, F.B. Adsorption and catalytic oxidation of asphaltenes in fumed silica nanoparticles: Effect of the surface acidity. Dyna 2016, 83, 171–179. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Comparative oxidation of adsorbed asphaltenes onto transition metal oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 145–149. [Google Scholar] [CrossRef]
- Hosseinpour, N.; Mortazavi, Y.; Bahramian, A.; Khodatars, L.; Khodadadi, A.A. Enhanced pyrolysis and oxidation of asphaltenes adsorbed onto transition metal oxides nanoparticles towards advanced in-situ combustion EOR processes by nanotechnology. Appl. Catal. A Gen. 2014, 477, 159–171. [Google Scholar] [CrossRef]
- Amrollahi Biyouki, A.; Hosseinpour, N.; Bahramian, A.; Vatani, A. In-situ upgrading of reservoir oils by in-situ preparation of NiO nanoparticles in thermal enhanced oil recovery processes. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 289–300. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Thermogravimetric studies on catalytic effect of metal oxide nanoparticles on asphaltene pyrolysis under inert conditions. J. Therm. Anal. Calorim. 2012, 110, 1327–1332. [Google Scholar] [CrossRef]
- Nassar, N.N.; Franco, C.A.; Montoya, T.; Cortés, F.B.; Hassan, A. Effect of oxide support on Ni–Pd bimetallic nanocatalysts for steam gasification of n-C7 asphaltenes. Fuel 2015, 156, 110–120. [Google Scholar] [CrossRef]
- López, D.; Giraldo, L.J.; Salazar, J.P.; Zapata, D.M.; Ortega, D.C.; Franco, C.A.; Cortés, F.B. Metal Oxide Nanoparticles Supported on Macro-Mesoporous Aluminosilicates for Catalytic Steam Gasification of Heavy Oil Fractions for On-Site Upgrading. Catalysts 2017, 7, 319. [Google Scholar] [CrossRef]
- Vélez, J.F.; Chejne, F.; Valdés, C.F.; Emery, E.J.; Londoño, C.A. Co-gasification of Colombian coal and biomass in fluidized bed: An experimental study. Fuel 2009, 88, 424–430. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Luna, G.; Pereira-Almao, P. Kinetics of the catalytic thermo-oxidation of asphaltenes at isothermal conditions on different metal oxide nanoparticle surfaces. Catal. Today 2013, 207, 127–132. [Google Scholar] [CrossRef]
- Cardona, L.; Arias-Madrid, D.; Cortés, F.B.; Lopera, S.H.; Franco, C.A. Heavy Oil Upgrading and Enhanced Recovery in a Steam Injection Process Assisted by NiO-and PdO-Functionalized SiO2 Nanoparticulated Catalysts. Catalysts 2018, 8, 132. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Almao, P.P. Nanoparticle technology for heavy oil in-situ upgrading and recovery enhancement: Opportunities and challenges. Appl. Energy 2014, 133, 374–387. [Google Scholar] [CrossRef]
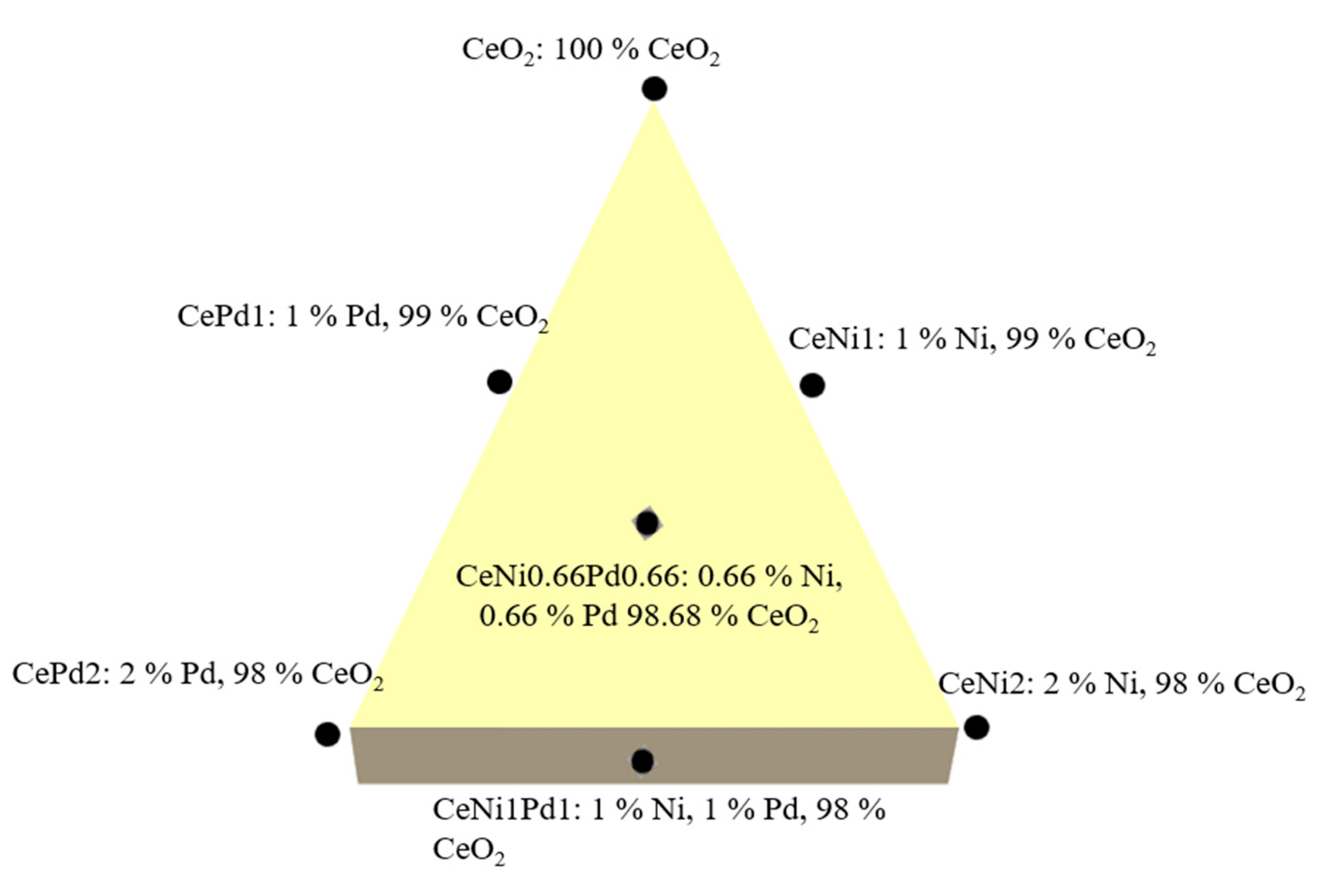
- Medina, O.E.; Gallego, J.; Restrepo, L.G.; Cortés, F.B.; Franco, C.A. Influence of the Ce4+/Ce3+ Redox-Couple on the Cyclic Regeneration for Adsorptive and Catalytic Performance of NiO-PdO/CeO2±δ Nanoparticles for n-C7 Asphaltene Steam Gasification. Nanomaterials 2019, 9, 734. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V. Enhanced Heavy Oil Recovery Using TiO2 Nanoparticles: Investigation of Deposition during Transport in Core Plug. Energy Fuels 2015, 29, 1–8. [Google Scholar] [CrossRef]
- Amanam, U.U.; Kovscek, A.R. Analysis of the effects of copper nanoparticles on in-situ combustion of extra heavy-crude oil. J. Pet. Sci. Eng. 2017, 152, 406–415. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. Metallic Nanoparticles for Enhanced Heavy Oil Recovery: Promises and Challenges. Energy Procedia 2015, 75, 2068–2073. [Google Scholar] [CrossRef]
- Heim, W.; Wolf, F.J.; Savery, W.T. Heavy Oil Recovering. Google Patents No. 4,456,065, 26 June 1984. [Google Scholar]
- Yaws, C.L. Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Dianatnasab, F.; Nikookar, M.; Hosseini, S.; Sabeti, M. Study of reservoir properties and operational parameters influencing in the steam assisted gravity drainage process in heavy oil reservoirs by numerical simulation. Petroleum 2016, 2, 236–251. [Google Scholar] [CrossRef]
- McCain, W.D., Jr. Properties of Petroleum Fluids; PennWell Corporation: Tulsa, OK, USA, 2017. [Google Scholar]
- Rudyk, S. Relationships between SARA fractions of conventional oil, heavy oil, natural bitumen and residues. Fuel 2018, 216, 330–340. [Google Scholar] [CrossRef]
- Liu, D.; Song, Q.; Tang, J.; Zheng, R.; Yao, Q. Interaction between saturates, aromatics and resins during pyrolysis and oxidation of heavy oil. J. Pet. Sci. Eng. 2017, 154, 543–550. [Google Scholar] [CrossRef]
- Aske, N.; Kallevik, H.; Sjöblom, J. Determination of saturate, aromatic, resin, and asphaltenic (SARA) components in crude oils by means of infrared and near-infrared spectroscopy. Energy Fuels 2001, 15, 1304–1312. [Google Scholar] [CrossRef]
- Vitolo, S.; Seggiani, M.; Filippi, S.; Brocchini, C. Recovery of vanadium from heavy oil and Orimulsion fly ashes. Hydrometallurgy 2000, 57, 141–149. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sarica, C.; Zhang, H.-Q.; Rhyne, L.; Greenhill, K. Assessment and development of heavy oil viscosity correlations. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium, Calgary, AB, Canada, 1–3 November 2005. [Google Scholar]
- Spiecker, P.M.; Gawrys, K.L.; Kilpatrick, P.K. Aggregation and solubility behavior of asphaltenes and their subfractions. J. Colloid Interface Sci. 2003, 267, 178–193. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sheu, E.Y.; Hammami, A.; Marshall, A.G. Asphaltenes, Heavy Oils, and Petroleomics; Springer Science & Business Media: New York, NY, USA, 2007. [Google Scholar]
- Murgich, J. Intermolecular forces in aggregates of asphaltenes and resins. Pet. Sci. Technol. 2002, 20, 983–997. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L. Advances in asphaltene science and the Yen–Mullins model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Mullins, O.C.; Pomerantz, A.E.; Andrews, A.B.; Majumdar, R.D.; Hazendonk, P.; Ruiz-Morales, Y.; Goual, L.; Zare, R.N. Asphaltenes. In Springer Handbook of Petroleum Technology; Springer: Cham, Switzerland, 2017; pp. 221–250. [Google Scholar]
- Acevedo, S.; Castro, A.; Negrin, J.G.; Fernández, A.; Escobar, G.; Piscitelli, V.; Delolme, F.; Dessalces, G. Relations between asphaltene structures and their physical and chemical properties: The rosary-type structure. Energy Fuels 2007, 21, 2165–2175. [Google Scholar] [CrossRef]
- Acevedo, S.; Castillo, J.; Vargas, V.; Castro, A.; Delgado, O.Z.; Ariza, C.A.F.; Cotés, F.B.; Bouyssiere, B. Suppression of Phase Separation as a Hypothesis to Account for Nuclei or Nanoaggregate Formation by Asphaltenes in Toluene. Energy Fuels 2018, 32, 6669–6677. [Google Scholar] [CrossRef]
- Acevedo, S.; Castro, A.; Vásquez, E.; Marcano, F.; Ranaudo, M.A. Investigation of physical chemistry properties of asphaltenes using solubility parameters of asphaltenes and their fractions A1 and A2. Energy Fuels 2010, 24, 5921–5933. [Google Scholar] [CrossRef]
- León, O.; Contreras, E.; Rogel, E.; Dambakli, G.; Acevedo, S.; Carbognani, L.; Espidel, J. Adsorption of native resins on asphaltene particles: A correlation between adsorption and activity. Langmuir 2002, 18, 5106–5112. [Google Scholar] [CrossRef]
- Lozano, M.M.; Franco, C.A.; Acevedo, S.A.; Nassar, N.N.; Cortés, F.B. Effects of resin I on the catalytic oxidation of n-C 7 asphaltenes in the presence of silica-based nanoparticles. RSC Adv. 2016, 6, 74630–74642. [Google Scholar] [CrossRef]
- Pérez, N.A.; Rincón, G.; Velásquez, J. Effect of resin and asphaltene content present on the vacuum residue on the yield of delayed coking products (Efecto del contenido de resinas y asfaltenos presente en el residuo de vacio sobre el rendimiento de los productos de la coquización retardada). Rev. Latinoam. de Metal. y Mater. 2019, 39, 1–10. [Google Scholar]
- Pereira, J.; López, I. Interacciones resinas-asfaltenos: Correlación con la precipitación de asfaltenos. Ciencia 2006, 14, 132–142. [Google Scholar]
- Navarro, L.; Álvarez, M.; Grosso, J.-L.; Navarro, U. Separación y caracterización de resinas y asfaltenos provenientes del crudo Castilla. Evaluación de su interacción molecular. CT&F-Ciencia, Tecnología y Futuro 2004, 2, 53–67. [Google Scholar]
- Andersen, S.I.; Speight, J.G. Petroleum resins: Separation, character, and role in petroleum. Pet. Sci. Technol. 2001, 19, 1–34. [Google Scholar] [CrossRef]
- Behura, J.; Batzle, M.; Hofmann, R.; Dorgan, J. Heavy oils: Their shear story. Geophysics 2007, 72, E175–E183. [Google Scholar] [CrossRef]
- Wang, J.; Anthony, E.J. A study of thermal-cracking behavior of asphaltenes. Chem. Eng. Sci. 2003, 58, 157–162. [Google Scholar] [CrossRef]
- Inoue, S.-I.; Takatsuka, T.; Wada, Y.; Nakata, S.-I.; Ono, T. A new concept for catalysts of asphaltene conversion. Catal. Today 1998, 43, 225–232. [Google Scholar] [CrossRef]
- Liao, Z.; Geng, A. Characterization of nC7-soluble fractions of the products from mild oxidation of asphaltenes. Org. Geochem. 2002, 33, 1477–1486. [Google Scholar] [CrossRef]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Pereira-Almao, P.; Cortés, F.B. Adsorption and subsequent oxidation of colombian asphaltenes onto nickel and/or palladium oxide supported on fumed silica nanoparticles. Energy Fuels 2013, 27, 7336–7347. [Google Scholar] [CrossRef]
- Stell, R.C.; Dinicolantonio, A.R.; Frye, J.M.; Spicer, D.B.; Mccoy, J.N.; Strack, R.D. Process for Steam Cracking Heavy Hydrocarbon Feedstocks. Google Patents Application No. 7,138,047, 21 November 2006. [Google Scholar]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Cortés, F.B. NiO and PdO Supported on Fumed Silica Nanoparticles for Adsorption and Catalytic Steam Gasification of Colombian n-C7 Asphaltenes. In Handbook on Oil Production Research; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 101–145. [Google Scholar]
- Gray, M.R. Consistency of asphaltene chemical structures with pyrolysis and coking behavior. Energy Fuels 2003, 17, 1566–1569. [Google Scholar] [CrossRef]
- Ambalae, A.; Mahinpey, N.; Freitag, N. Thermogravimetric studies on pyrolysis and combustion behavior of a heavy oil and its asphaltenes. Energy Fuels 2006, 20, 560–565. [Google Scholar] [CrossRef]
- Cardona Rojas, L. Efecto de nanopartículas en procesos con inyección de vapor a diferentes calidades. Master’s Thesis, Universidad Nacional de Colombia-Sede Medellín, Medellín, Colombia, 2018. [Google Scholar]
- Medina, O.E.; Gallego, J.; Arias-Madrid, D.; Cortés, F.B.; Franco, C.A. Optimization of the Load of Transition Metal Oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 Nanoparticles in Catalytic Steam Decomposition of n-C7 Asphaltenes at Low Temperatures. Nanomaterials 2019, 9, 401. [Google Scholar] [CrossRef]
- Niu, B.; Ren, S.; Liu, Y.; Wang, D.; Tang, L.; Chen, B. Low-temperature oxidation of oil components in an air injection process for improved oil recovery. Energy Fuels 2011, 25, 4299–4304. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene adsorption, a literature review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Cortés, F.B.; Montoya, T.; Acevedo, S.; Nassar, N.N.; Franco, C.A. Adsorption-desorption of n-c7 asphaltenes over micro-and nanoparticles of silica and its impact on wettability alteration. CT F-Ciencia, Tecnología y Futuro 2016, 6, 89–106. [Google Scholar] [CrossRef]
- Taborda, E.A.; Alvarado, V.; Cortés, F.B. Effect of SiO2-based nanofluids in the reduction of naphtha consumption for heavy and extra-heavy oils transport: Economic impacts on the Colombian market. Energy Convers. Manag. 2017, 148, 30–42. [Google Scholar] [CrossRef]
- Taborda, E.A.; Franco, C.A.; Lopera, S.H.; Alvarado, V.; Cortés, F.B. Effect of nanoparticles/nanofluids on the rheology of heavy crude oil and its mobility on porous media at reservoir conditions. Fuel 2016, 184, 222–232. [Google Scholar] [CrossRef]
- Taborda, E.A.; Alvarado, V.; Franco, C.A.; Cortés, F.B. Rheological demonstration of alteration in the heavy crude oil fluid structure upon addition of nanoparticles. Fuel 2017, 189, 322–333. [Google Scholar] [CrossRef]
- Acevedo, S.C.; García, L.A.; Rodríguez, P. Changes of diameter distribution with temperature measured for asphaltenes and their fractions A1 and A2. Impact of these measurements in colloidal and solubility issues of asphaltenes. Energy Fuels 2012, 26, 1814–1819. [Google Scholar] [CrossRef]
- Nassar, N.N. Asphaltene adsorption onto alumina nanoparticles: Kinetics and thermodynamic studies. Energy Fuels 2010, 24, 4116–4122. [Google Scholar] [CrossRef]
- Márquez, S.B.; Cortés, F.B.; Marín, F.C. Desarrollo de Nanopartículas Basadas en Sílice para la Inhibición de la Precipitación/Depositación de Asfaltenos. In Química y Petróleos; Universidad Nacional de Colombia: Medellín, Colombia, 2015; p. 96. [Google Scholar]
- Nassar, N.N.; Montoya, T.; Franco, C.A.; Cortés, F.B.; Pereira-Almao, P. A new model for describing the adsorption of asphaltenes on porous media at a high pressure and temperature under flow conditions. Energy Fuels 2015, 29, 4210–4221. [Google Scholar] [CrossRef]
- Cortés, F.B.; Mejía, J.M.; Ruiz, M.A.; Benjumea, P.; Riffel, D.B. Sorption of asphaltenes onto nanoparticles of nickel oxide supported on nanoparticulated silica gel. Energy Fuels 2012, 26, 1725–1730. [Google Scholar] [CrossRef]
- Franco, C.A.; Lozano, M.M.; Acevedo, S.; Nassar, N.N.; Cortés, F.B. Effects of resin I on asphaltene adsorption onto nanoparticles: A novel method for obtaining asphaltenes/resin isotherms. Energy Fuels 2015, 30, 264–272. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Effect of surface acidity and basicity of aluminas on asphaltene adsorption and oxidation. J. Colloid Interface Sci. 2011, 360, 233–238. [Google Scholar] [CrossRef]
- Shayan, N.N.; Mirzayi, B. Adsorption and removal of asphaltene using synthesized maghemite and hematite nanoparticles. Energy Fuels 2015, 29, 1397–1406. [Google Scholar] [CrossRef]
- Marei, N.N.; Nassar, N.N.; Vitale, G. The effect of the nanosize on surface properties of NiO nanoparticles for the adsorption of Quinolin-65. Phys. Chem. Chem. Phys. 2016, 18, 6839–6849. [Google Scholar] [CrossRef] [PubMed]
- Diez, R.; Giraldo, L.J.; Arias Madrid, D.; Gallego Marin, J.; Cortés, F.B.; Franco Ariza, C. Síntesis y caracterización de nanopartículas Janus de sílice/níquel para procesos EOR por inyección de vapor. In Proceedings of the Congreso Mexicano del Petróleo, Mexico city, Mexico, 26–29 September 2018. [Google Scholar]
- Giraldo, L.J.; Gallego, J.; Villegas, J.P.; Franco, C.A.; Cortés, F.B. Enhanced waterflooding with NiO/SiO2 0-D Janus nanoparticles at low concentration. J. Pet. Sci. Eng. 2019, 174, 40–48. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Ruiz, M.A.; Pereira-Almao, P.; Cortés, F.B. Nanoparticles for inhibition of asphaltenes damage: Adsorption study and displacement test on porous media. Energy Fuels 2013, 27, 2899–2907. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Metal oxide nanoparticles for asphaltene adsorption and oxidation. Energy Fuels 2011, 25, 1017–1023. [Google Scholar] [CrossRef]
- Polanyi, M. The potential theory of adsorption. Science 1963, 141, 1010–1013. [Google Scholar] [CrossRef]
- Montoya, T.; Coral, D.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. A novel solid–liquid equilibrium model for describing the adsorption of associating asphaltene molecules onto solid surfaces based on the “chemical theory”. Energy Fuels 2014, 28, 4963–4975. [Google Scholar] [CrossRef]
- Aristizábal-Fontal, J.E.; Cortés, F.B.; Franco, C.A. Viscosity reduction of extra heavy crude oil by magnetite nanoparticle-based ferrofluids. Adsorpt. Sci. Technol. 2017, 36, 23–45. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. Effects of nano-sized metals on viscosity reduction of heavy oil/bitumen during thermal applications. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar]
- Taborda, E.A.; Franco, C.A.; Ruiz, M.A.; Alvarado, V.; Cortés, F.B. Experimental and Theoretical Study of Viscosity Reduction in Heavy Crude Oils by Addition of Nanoparticles. Energy Fuels 2017, 31, 1329–1338. [Google Scholar] [CrossRef]
- Montes, D.; Orozco, W.; Taborda, E.A.; Franco, C.A.; Cortés, F.B. Development of Nanofluids for Perdurability in Viscosity Reduction of Extra-Heavy Oils. Energies 2019, 12, 1068. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Y.; Huan, R.; Castanier, L.M.; Kovscek, A.R. An experimental investigation of the in-situ combustion behavior of Karamay crude oil. J. Pet. Sci. Eng. 2015, 127, 82–92. [Google Scholar] [CrossRef]
- Ado, M.R.; Greaves, M.; Rigby, S.P. Dynamic Simulation of the THAI Heavy Oil Recovery Process. Energy Fuels 2017, 31, 1276–1284. [Google Scholar]
- Xia, T.; Greaves, M.; Turta, A. Main Mechanism for Stability of THAI-" Toe-to-Heel Air Injection". In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 10–12 June 2003. [Google Scholar]
- Thomas, S. Enhanced oil recovery-an overview. Oil Gas Sci. Technol. -Revue de l’IFP 2008, 63, 9–19. [Google Scholar] [CrossRef]
- Greaves, M.; Xia, T.X.; Ayasse, C. Underground upgrading of heavy oil using THAI-‘toe-to-heel air injection’. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium, Calgary, AB, Canada, 1–3 November 2005. [Google Scholar]
- Hart, A. Advanced Studies of Catalytic Upgrading of Heavy Oils. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2014. [Google Scholar]
- Greaves, M.; Xia, T. CAPRI-Downhole catalytic process for upgrading heavy oil: Produced oil properties and composition. In Proceedings of the Canadian international petroleum conference, Calgary, AB, Canada, 12–14 June 2001. [Google Scholar]
- Hart, A.; Wood, J. In Situ Catalytic Upgrading of Heavy Crude with CAPRI: Influence of Hydrogen on Catalyst Pore Plugging and Deactivation due to Coke. Energies 2018, 11, 636. [Google Scholar] [CrossRef]
- Marei, N.N.; Nassar, N.N.; Vitale, G.; Hassan, A.; Zurita, M.J.P. Effects of the size of NiO nanoparticles on the catalytic oxidation of Quinolin-65 as an asphaltene model compound. Fuel 2017, 207, 423–437. [Google Scholar] [CrossRef]
- Marei, N.N.; Nassar, N.N.; Hmoudah, M.; El-Qanni, A.; Vitale, G.; Hassan, A. Nanosize effects of NiO nanosorbcats on adsorption and catalytic thermo-oxidative decomposition of vacuum residue asphaltenes. Can. J. Chem. Eng. 2017, 95, 1864–1874. [Google Scholar] [CrossRef]
- Mirzayi, B.; Shayan, N.N. Adsorption kinetics and catalytic oxidation of asphaltene on synthesized maghemite nanoparticles. J. Pet. Sci. Eng. 2014, 121, 134–141. [Google Scholar] [CrossRef]
- Yuan, C.; Varfolomeev, M.A.; Emelianov, D.A.; Suwaid, M.A.; Khachatrian, A.A.; Starshinova, V.L.; Vakhitov, I.R.; Al-Muntaser, A.A. Copper stearate as a catalyst for improving the oxidation performance of heavy oil in in-situ combustion process. Appl. Catal. A Gen. 2018, 564, 79–89. [Google Scholar] [CrossRef]
- Druetta, P.; Raffa, P.; Picchioni, F. Plenty of Room at the Bottom: Nanotechnology as Solution to an Old Issue in Enhanced Oil Recovery. Appl. Sci. 2018, 8, 2596. [Google Scholar] [CrossRef]
- Luft, H.; Pelensky, P.; George, G. Development and operation of a new insulated concentric coiled tubing string for continuous steam injection in heavy oil production. In Proceedings of the SPE International Heavy Oil Symposium, Calgary, AB, Canada, 19–21 June 1995. [Google Scholar]
- Boberg, T.C. Thermal Methods of Oil Recovery; Wiley: Hoboken, NJ, USA, 1988. [Google Scholar]
- Hassan, A.; Carbognani-Arambarri, L.; Nassar, N.N.; Vitale, G.; Bartolini, M.; Pereira-Almao, P. Catalytic Steam Gasification of Athabasca Visbroken Residue by NiO–Kaolin-Based Catalysts in a Fixed-Bed Reactor. Energy Fuels 2017, 31, 7396–7404. [Google Scholar] [CrossRef]
- Hassan, A.; Lopez-Linares, F.; Nassar, N.N.; Carbognani-Arambarri, L.; Pereira-Almao, P. Development of a support for a NiO catalyst for selective adsorption and post-adsorption catalytic steam gasification of thermally converted asphaltenes. Catal. Today 2013, 207, 112–118. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Application of nanotechnology for heavy oil upgrading: Catalytic steam gasification/cracking of asphaltenes. Energy Fuels 2011, 25, 1566–1570. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. In-situ upgrading of heavy oil/bitumen during steam injection by use of metal nanoparticles: A study on in-situ catalysis and catalyst transportation. SPE Reser. Eval. Eng. 2013, 16, 333–344. [Google Scholar] [CrossRef]
- Jeong, G.; Kim, C.H.; Hur, Y.G.; Han, G.-H.; Lee, S.H.; Lee, K.-Y. Ni-Doped MoS2 Nanoparticles Prepared via Core–Shell Nanoclusters and Catalytic Activity for Upgrading Heavy Oil. Energy Fuels 2018, 32, 9263–9270. [Google Scholar] [CrossRef]
- Betancur, S.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Franco, C.A.; Jiménez, J.; Manrique, E.J.; Quintero, H.; Cortés, F.B. Effect of Magnetic Iron Core–Carbon Shell Nanoparticles in Chemical Enhanced Oil Recovery for Ultralow Interfacial Tension Region. Energy Fuels 2019, 33, 4158–4168. [Google Scholar] [CrossRef]
- Maity, S.; Ancheyta, J.; Soberanis, L.; Alonso, F.; Llanos, M. Alumina–titania binary mixed oxide used as support of catalysts for hydrotreating of Maya heavy crude. Appl. Catal. A Gen. 2003, 244, 141–153. [Google Scholar] [CrossRef]
- Gao, Y.; Ghorbanian, B.; Gargari, H.N.; Gao, W. Steam reforming of gaseous by-products from bitumen oil using various supported Ni-based catalysts. Pet. Sci. Technol. 2018, 36, 34–39. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira-Almao, P. Transport behavior of multimetallic ultradispersed nanoparticles in an oil-sands-packed bed column at a high temperature and pressure. Energy Fuels 2012, 26, 1645–1655. [Google Scholar] [CrossRef]
- Farooqui, J.; Babadagli, T.; Li, H.A. Improvement of the recovery factor using nano-metal particles at the late stages of cyclic steam stimulation. In Proceedings of the SPE Canada Heavy Oil Technical Conference, Calgary, AB, Canada, 9–11 June 2015. [Google Scholar]
- Shen, Y.; Yoshikawa, K. Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 21, 371–392. [Google Scholar] [CrossRef]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. A review of pyrolysis, aquathermolysis, and oxidation of Athabasca bitumen. Fuel Proc. Technol. 2015, 131, 270–289. [Google Scholar] [CrossRef]
- Douda, J.; Llanos, M.E.; Alvarez, R.; Franco, C.L.; de la Fuente, J.A.M. Pyrolysis applied to the study of a Maya asphaltene. J. Anal. Appl. Pyrolysis 2004, 71, 601–612. [Google Scholar] [CrossRef]
- Magaril, R.; Aksenova, É. Investigation of the mechanism of coke formation during thermal decomposition of asphaltenes. Chem. Technol. Fuels Oils 1970, 6, 509–512. [Google Scholar] [CrossRef]
- Rezaei, M.; Schaffie, M.; Ranjbar, M. Thermocatalytic in situ combustion: Influence of nanoparticles on crude oil pyrolysis and oxidation. Fuel 2013, 113, 516–521. [Google Scholar] [CrossRef]
- Chhetri, A.; Islam, M. A critical review of electromagnetic heating for enhanced oil recovery. Pet. Sci. Technol. 2008, 26, 1619–1631. [Google Scholar] [CrossRef]
- Mohd Zaid, H.; Latiff, A.; Rasyada, N.; Yahya, N.; Soleimani, H.; Shafie, A. Application of Electromagnetic Waves and Dielectric Nanoparticles in Enhanced Oil Recovery. J. Nano Res. 2014, 26, 135–142. [Google Scholar] [CrossRef]
- Al-Shehri, A.A.; Ellis, E.S.; Servin, J.M.F.; Kosynkin, D.V.; Kanj, M.Y.; Schmidt, H.K. Illuminating the reservoir: Magnetic nanomappers. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 10–13 March 2013. [Google Scholar]
- Greff, J.; Babadagli, T. Catalytic effects of nano-size metal ions in breaking asphaltene molecules during thermal recovery of heavy-oil. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- Yahya, N.; Kashif, M.; Nasir, N.; Niaz Akhtar, M.; Yusof, N.M. Cobalt ferrite nanoparticles: An innovative approach for enhanced oil recovery application. J. Nano Res. 2012, 17, 115–126. [Google Scholar] [CrossRef]
- Hascakir, B.; Akin, S. Effect of metallic additives on upgrading heavy oil with microwave heating. In Proceedings of the First World Heavy Oil Conference, Beijing, China, 15 January 2006. [Google Scholar]
- Shokrlu, Y.H.; Babadagli, T. Transportation and interaction of nano and micro size metal particles injected to improve thermal recovery of heavy-oil. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- Bennetzen, M.V.; Mogensen, K. Novel applications of nanoparticles for future enhanced oil recovery. In Proceedings of the International petroleum technology conference, Kuala Lumpur, Malaysia, 10–12 December 2014. [Google Scholar]
- Nassar, N.N.; Betancur, S.; Acevedo, S.; Franco, C.A.; Cortés, F.B. Development of a Population Balance Model to Describe the Influence of Shear and Nanoparticles on the Aggregation and Fragmentation of Asphaltene Aggregates. Ind. Eng. Chem. Res. 2015, 54, 8201–8211. [Google Scholar] [CrossRef]
- Guzmán, J.D.; Betancur, S.; Carrasco-Marín, F.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. Importance of the Adsorption Method Used for Obtaining the Nanoparticle Dosage for Asphaltene-Related Treatments. Energy Fuels 2016, 30, 2052–2059. [Google Scholar] [CrossRef]
- Li, L.; Yuan, X.; Sun, J. Vital role of nanotechnology and nanomaterials in the field of oilfield chemistry Soc. Pet. Eng. 2013, 1, 85–91. [Google Scholar]
- Loria, H.; Trujillo-Ferrer, G.; Sosa-Stull, C.; Pereira-Almao, P. Kinetic Modeling of Bitumen Hydroprocessing at In-Reservoir Conditions Employing Ultradispersed Catalysts. Energy Fuels 2011, 25, 1364–1372. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. In Situ Upgrading of Athabasca Bitumen Using Multimetallic Ultradispersed Nanocatalysts in an Oil Sands Packed-Bed Column: Part 2. Solid Analysis and Gaseous Product Distribution. Energy Fuels 2014, 28, 1351–1361. [Google Scholar] [CrossRef]
- Galarraga, C.E.; Pereira-Almao, P. Hydrocracking of Athabasca Bitumen Using Submicronic Multimetallic Catalysts at Near In-Reservoir Conditions. Energy Fuels 2010, 24, 2383–2389. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. In Situ Upgrading of Athabasca Bitumen Using Multimetallic Ultradispersed Nanocatalysts in an Oil Sands Packed-Bed Column: Part 1. Produced Liquid Quality Enhancement. Energy Fuels 2013, 28, 1338–1350. [Google Scholar] [CrossRef]
- Tajmiri, M.; Ehsani, M.R. The Potential of ZnO Nanoparticles to Reduce Water Consuming in Iranian Heavy Oil Reservoir. J. Water Environ. Nanotechnol. 2016, 1, 84–90. [Google Scholar] [CrossRef]
- Al-Maamari, R.S.H.; Buckley, J.S. Asphaltene Precipitation and Alteration of Wetting: The Potential for Wettability Changes During Oil Production. SPE Reserv. Eval. Eng. 2003, 6, 210–214. [Google Scholar] [CrossRef]
- Gharfeh, S.; Yen, A.; Asomaning, S.; Blumer, D. Asphaltene Flocculation Onset Determinations for Heavy Crude Oil and Its Implications. Pet. Sci. Technol. 2004, 22, 1055–1072. [Google Scholar] [CrossRef]
- Oskui, G.; Reza, P.; Jumaa, M.A.; Folad, E.G.; Rashed, A.; Patil, S. Systematic approach for prevention and remediation of asphaltene problems during CO2/hydrocarbon injection project. In Proceedings of the Twenty-first International Offshore and Polar Engineering Conference, Maui, HI, USA, 19–24 June 2011. [Google Scholar]
- Kojima, T.; Tahara, K. Refinement and transportation of petroleum with hydrogen from renewable energy. Energy Convers. Manag. 2001, 42, 1839–1851. [Google Scholar] [CrossRef]
- Aminzadeh, B.; Chung, D.; Bryant, S.L.; Huh, C.; DiCarlo, D.A. CO2 leakage prevention by introducing engineered nanoparticles to the in-situ brine. Energy Procedia 2013, 37, 5290–5297. [Google Scholar] [CrossRef]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef]
- Yunus, I.S.; Harwin; Kurniawan, A.; Adityawarman, D.; Indarto, A. Nanotechnologies in water and air pollution treatment. Environ. Technol. Rev. 2012, 1, 136–148. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Youssif, M.I.; El-Maghraby, R.M.; Saleh, S.M.; Elgibaly, A. Silica nanofluid flooding for enhanced oil recovery in sandstone rocks. Egypt. J. Pet. 2018, 27, 105–110. [Google Scholar] [CrossRef]
- Weaire, D.; Phelan, R. The physics of foam. J. Phys. Condens. Matter 1996, 8, 9519. [Google Scholar] [CrossRef]
- Al-Hashim, H.S.; Celik, M.S.; Oskay, M.M.; Al-Yousef, H.Y. Adsorption and precipitation behaviour of petroleum sulfonates from Saudi Arabian limestone. J. Pet. Sci. Eng. 1988, 1, 335–344. [Google Scholar] [CrossRef]
- Figdore, P.E. Adsorption of surfactants on kaolinite: NaCl versus CaCl2 salt effects. J. Colloid Interface Sci. 1982, 87, 500–517. [Google Scholar] [CrossRef]
- Khatib, Z.I.; Hirasaki, G.J.; Falls, A.H. Effects of Capillary Pressure on Coalescence and Phase Mobilities in Foams Flowing Through Porous Media. SPE Reserv. Eng. 1988, 3, 919–926. [Google Scholar] [CrossRef]
- Farzaneh, S.A.; Sohrabi, M. A Review of the Status of Foam Application in Enhanced Oil Recovery. In Proceedings of the EAGE Annual Conference & Exhibition incorporating SPE Europec, London, UK, 10 June 2013; p. 15. [Google Scholar]
- Friedmann, F.; Chen, W.H.; Gauglitz, P.A. Experimental and Simulation Study of High-Temperature Foam Displacement in Porous Media. SPE Reserv. Eng. 1991, 6, 37–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Chen, B.; Li, H.K.; Wang, Y.T.; Ren, S.R. Evaluation and experimental study on CO2 foams at high pressure and temperature. J. Chem. Eng. Chin. Univ. 2014, 28, 535–541. [Google Scholar]
- Hurtado, Y.; Beltrán, C.; Zabala, R.D.; Lopera, S.H.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. Effects of Surface Acidity and Polarity of SiO2 Nanoparticles on the Foam Stabilization Applied to Natural Gas Flooding in Tight Gas-Condensate Reservoirs. Energy Fuels 2018, 32, 5824–5833. [Google Scholar] [CrossRef]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef]
- Binks, B.P.; Horozov, T.S. Aqueous Foams Stabilized Solely by Silica Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 3722–3725. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xue, G.; Tian, B.; Li, S.; Chen, K.; Wang, D.; Sun, Y.; Xu, H.; Petkov, J.T.; Li, Z. Interaction between Surfactants and SiO2 Nanoparticles in Multiphase Foam and Its Plugging Ability. Energy Fuels 2017, 31, 408–417. [Google Scholar] [CrossRef]
- Yusuf, S.; Manan, M.; Jaafar, M. Aqueous foams stabilized by hydrophilic silica nanoparticles via in-situ physisorption of nonionic TX100 Surfactant. Iran. J. Energy Environ. 2013, 4, 8–16. [Google Scholar]
- Zhu, Y.; Pei, X.; Jiang, J.; Cui, Z.; Binks, B.P. Responsive aqueous foams stabilized by silica nanoparticles hydrophobized in situ with a conventional surfactant. Langmuir 2015, 31, 12937–12943. [Google Scholar] [CrossRef]
- Manan, M.; Farad, S.; Piroozian, A.; Esmail, M. Effects of nanoparticle types on carbon dioxide foam flooding in enhanced oil recovery. Pet. Sci. Technol. 2015, 33, 1286–1294. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Samin, A.M.; Risal, A.R. Experimental investigation of minimization in surfactant adsorption and improvement in surfactant-foam stability in presence of silicon dioxide and aluminum oxide nanoparticles. J. Pet. Sci. Eng. 2017, 159, 115–134. [Google Scholar] [CrossRef]
- Yekeen, N.; Idris, A.K.; Manan, M.A.; Samin, A.M.; Risal, A.R.; Kun, T.X. Bulk and bubble-scale experimental studies of influence of nanoparticles on foam stability. Chin. J. Chem. Eng. 2017, 25, 347–357. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Mohamed, A.; Samin, A.R.R. Influence of silicon oxide and aluminum oxide nanoparticles on air and CO2 foams stability in presence and absence of oil. Chem. Eng. 2017, 56, 1243–1248. [Google Scholar]
- Cui, Z.-G.; Cui, Y.-Z.; Cui, C.-F.; Chen, Z.; Binks, B. Aqueous foams stabilized by in situ surface activation of CaCO3 nanoparticles via adsorption of anionic surfactant. Langmuir 2010, 26, 12567–12574. [Google Scholar] [CrossRef]
- Lee, D.; Cho, H.; Lee, J.; Huh, C.; Mohanty, K. Fly ash nanoparticles as a CO2 foam stabilizer. Powder Technol. 2015, 283, 77–84. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, A.; Mohanty, K.K.; Huh, C.; Lee, D.; Cho, H. Fly Ash Nanoparticle-Stabilized CO2-in-Water Foams for Gas Mobility Control Applications. In Proceedings of the SPE annual technical conference and exhibition, Houston, TX, USA, 28–30 September 2015. [Google Scholar]
- Zhang, T.; Davidson, D.; Bryant, S.L.; Huh, C. Nanoparticle-stabilized emulsions for applications in enhanced oil recovery. In Proceedings of the SPE improved oil recovery symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Zhang, T.; Roberts, M.; Bryant, S.L.; Huh, C. Foams and emulsions stabilized with nanoparticles for potential conformance control applications. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 20–22 April 2009. [Google Scholar]
- Son, H.; Kim, H.; Lee, G.; Kim, J.; Sung, W. Enhanced oil recovery using nanoparticle-stabilized oil/water emulsions. Korean J. Chem. Eng. 2014, 31, 338–342. [Google Scholar] [CrossRef]
- Zhang, T.; Espinosa, D.; Yoon, K.Y.; Rahmani, A.R.; Yu, H.; Caldelas, F.M.; Ryoo, S.; Roberts, M.; Prodanovic, M.; Johnston, K.P. Engineered nanoparticles as harsh-condition emulsion and foam stabilizers and as novel sensors. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2011. [Google Scholar]
- Khosravani, S.; Alaei, M.; Rashidi, A.; Ramazani, A.; Ershadi, M. O/W emulsions stabilized with γ-alumina nanostructures for chemical enhanced oil recovery. Mater. Res. Bull. 2013, 48, 2186–2190. [Google Scholar] [CrossRef]
- Nguyen, N.; Tu, T.; Bae, W.; Dang, C.; Chung, T.; Nguyen, H. Gelation time optimization for an HPAM/chromium acetate system: The successful key of conformance control technology. Energy Sour. Part A Recovery Util. Environ. Eff. 2012, 34, 1305–1317. [Google Scholar] [CrossRef]
- Cordova, M.; Cheng, M.; Trejo, J.; Johnson, S.J.; Willhite, G.P.; Liang, J.-T.; Berkland, C. Delayed HPAM gelation via transient sequestration of chromium in polyelectrolyte complex nanoparticles. Macromolecules 2008, 41, 4398–4404. [Google Scholar] [CrossRef]
- Jang, H.Y.; Zhang, K.; Chon, B.H.; Choi, H.J. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015, 21, 741–745. [Google Scholar] [CrossRef]
- Sveistrup, M.; van Mastrigt, F.; Norrman, J.; Picchioni, F.; Paso, K. Viability of biopolymers for enhanced oil recovery. J. Dispers. Sci. Technol. 2016, 37, 1160–1169. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Pérez-Robles, S.; Cortés, F.B.; Franco, C.A. Effect of the nanoparticles in the stability of hydrolyzed polyacrylamide/resorcinol/formaldehyde gel systems for water shut-off/conformance control applications. J. Appl. Polym. Sci. 2019, 136, 47568. [Google Scholar] [CrossRef]
- Michael, F.M.; Fathima, A.; Alyemni, E.; Huang, J.; Almohsin, A.; Alsharaeh, E.H. Enhanced PAM Polymer Gels Using Zirconium Hydroxide Nanoparticles for Water Shutoff at High Temperatures: Thermal and Rheological Investigations. Ind. Eng. Chem. Res. 2018, 57, 16347–16357. [Google Scholar] [CrossRef]
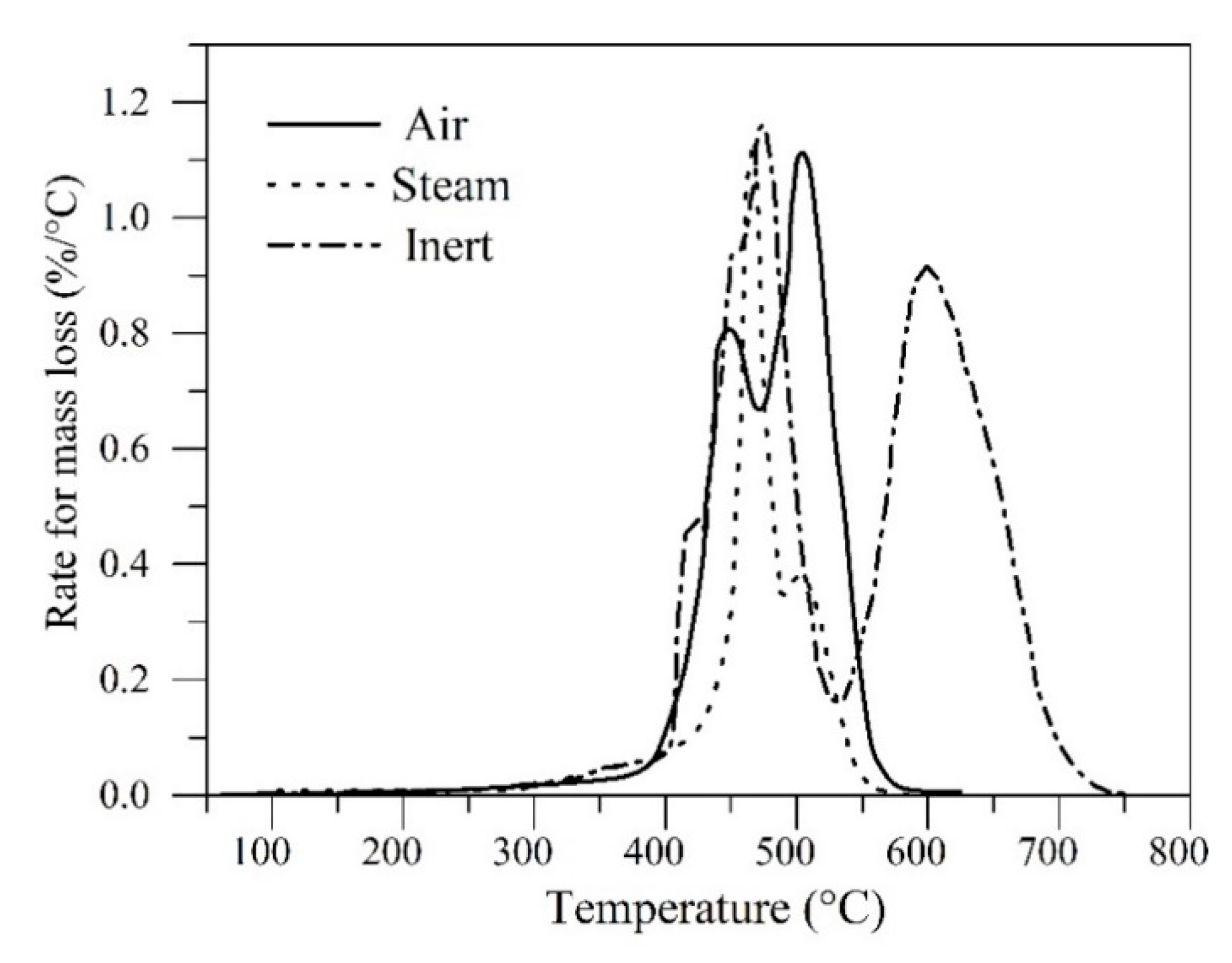
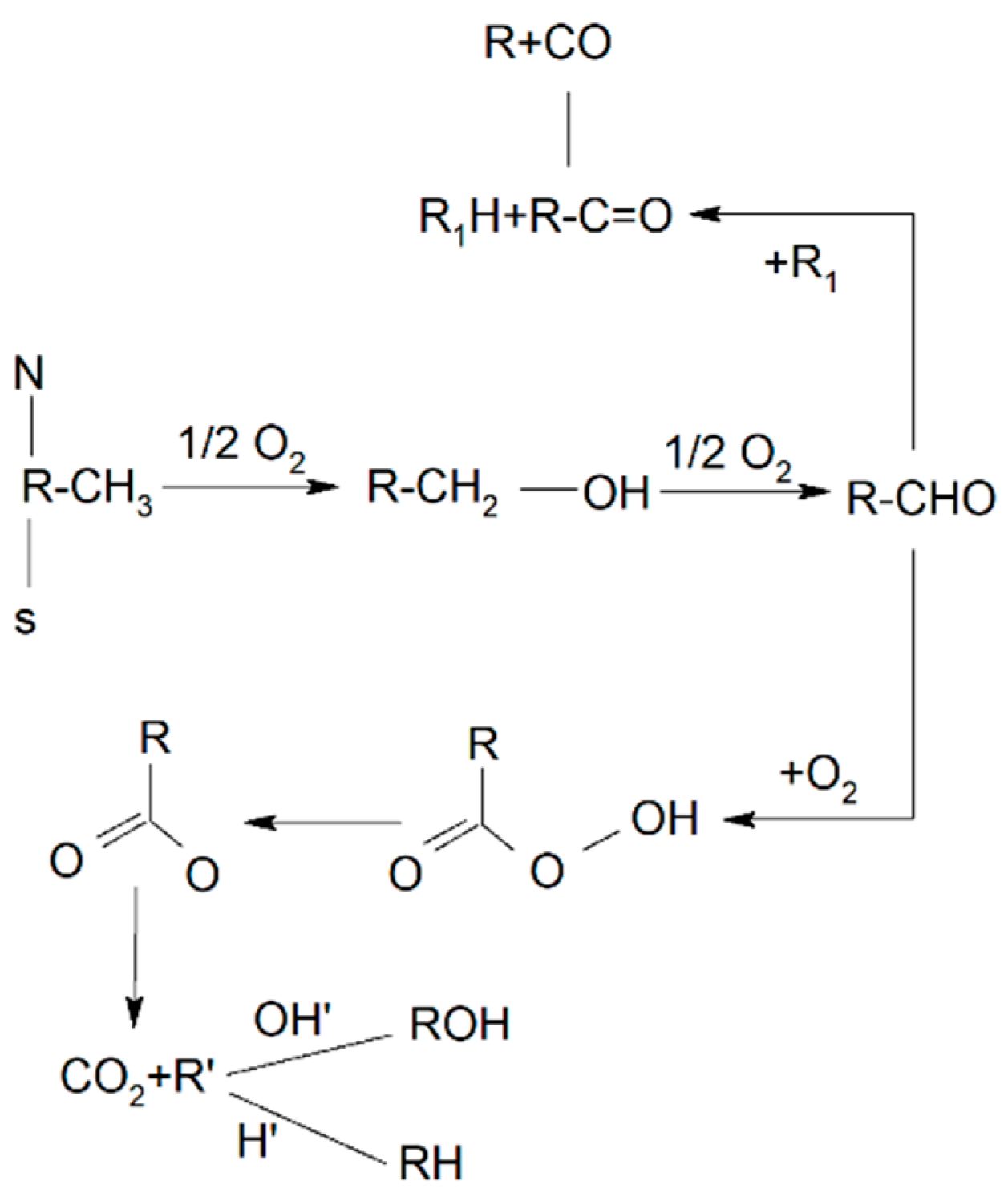
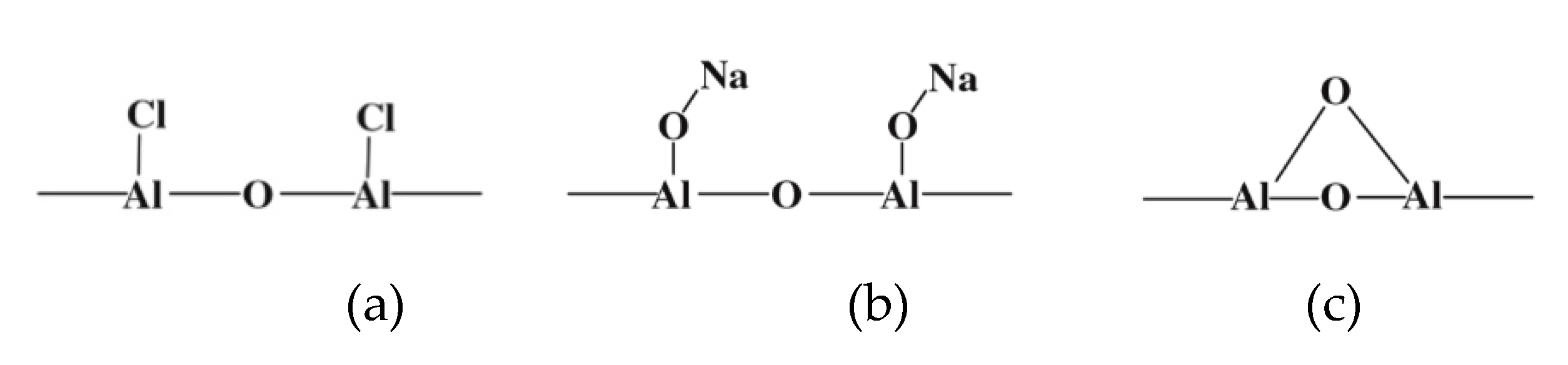
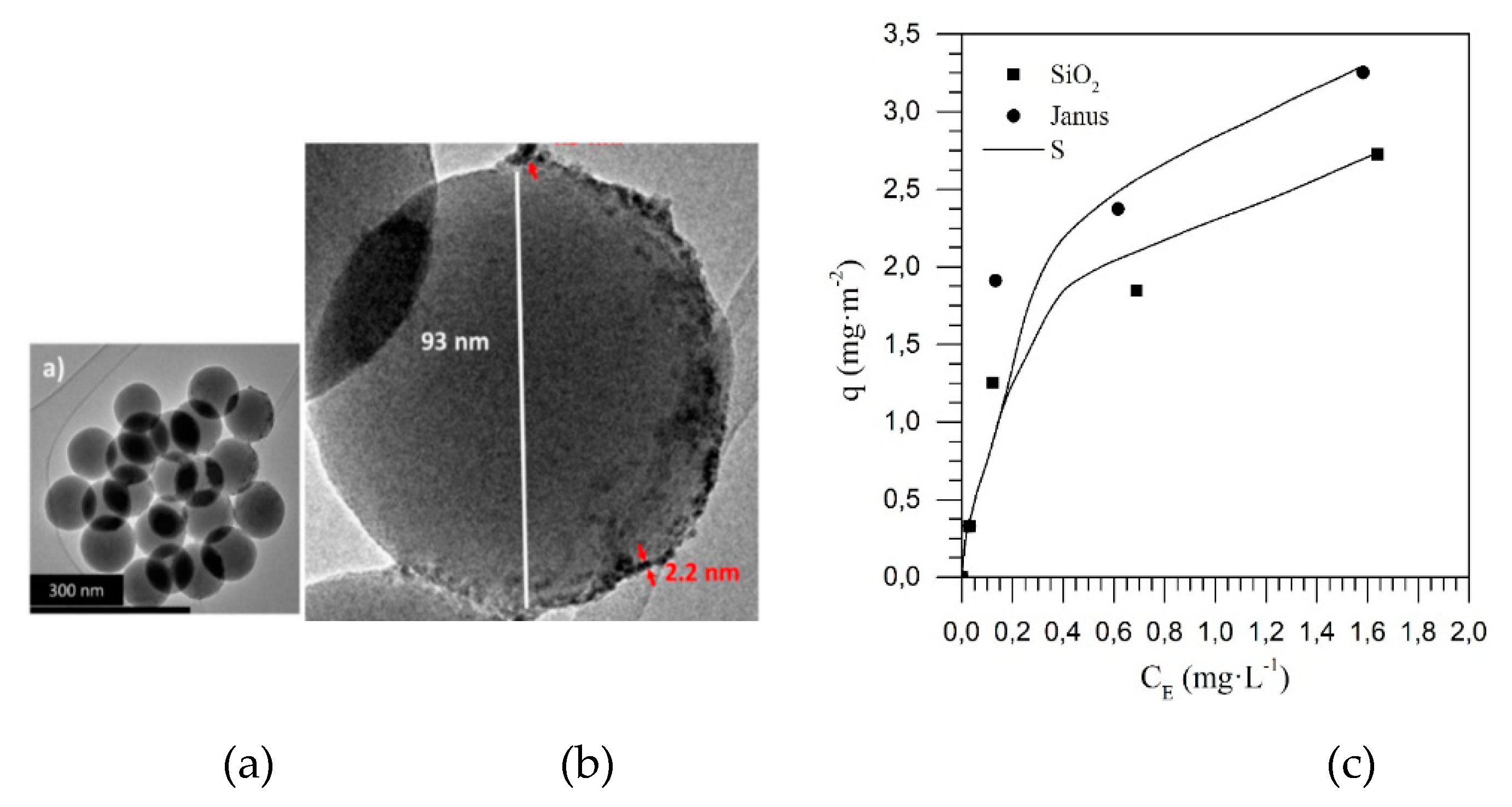
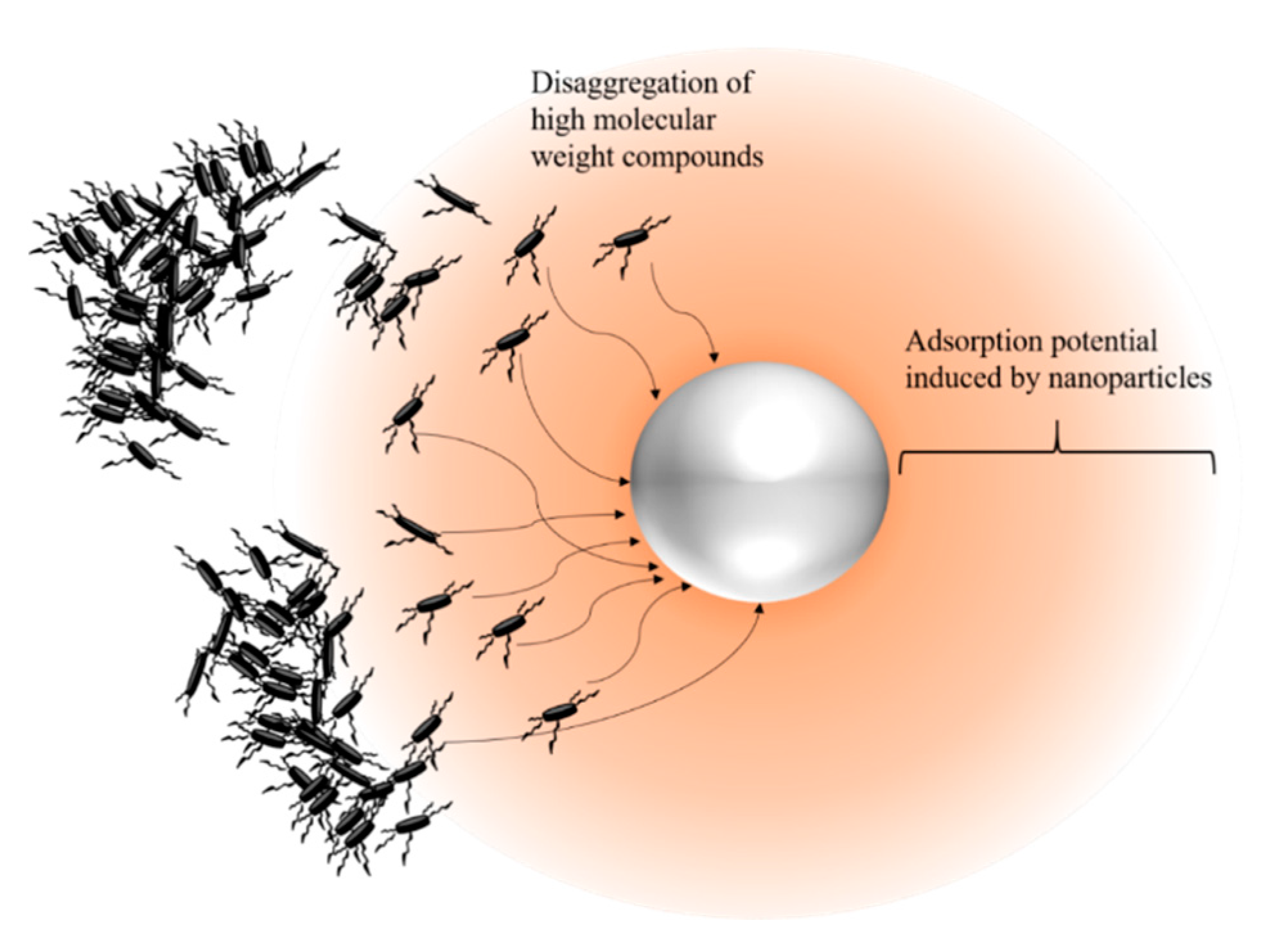
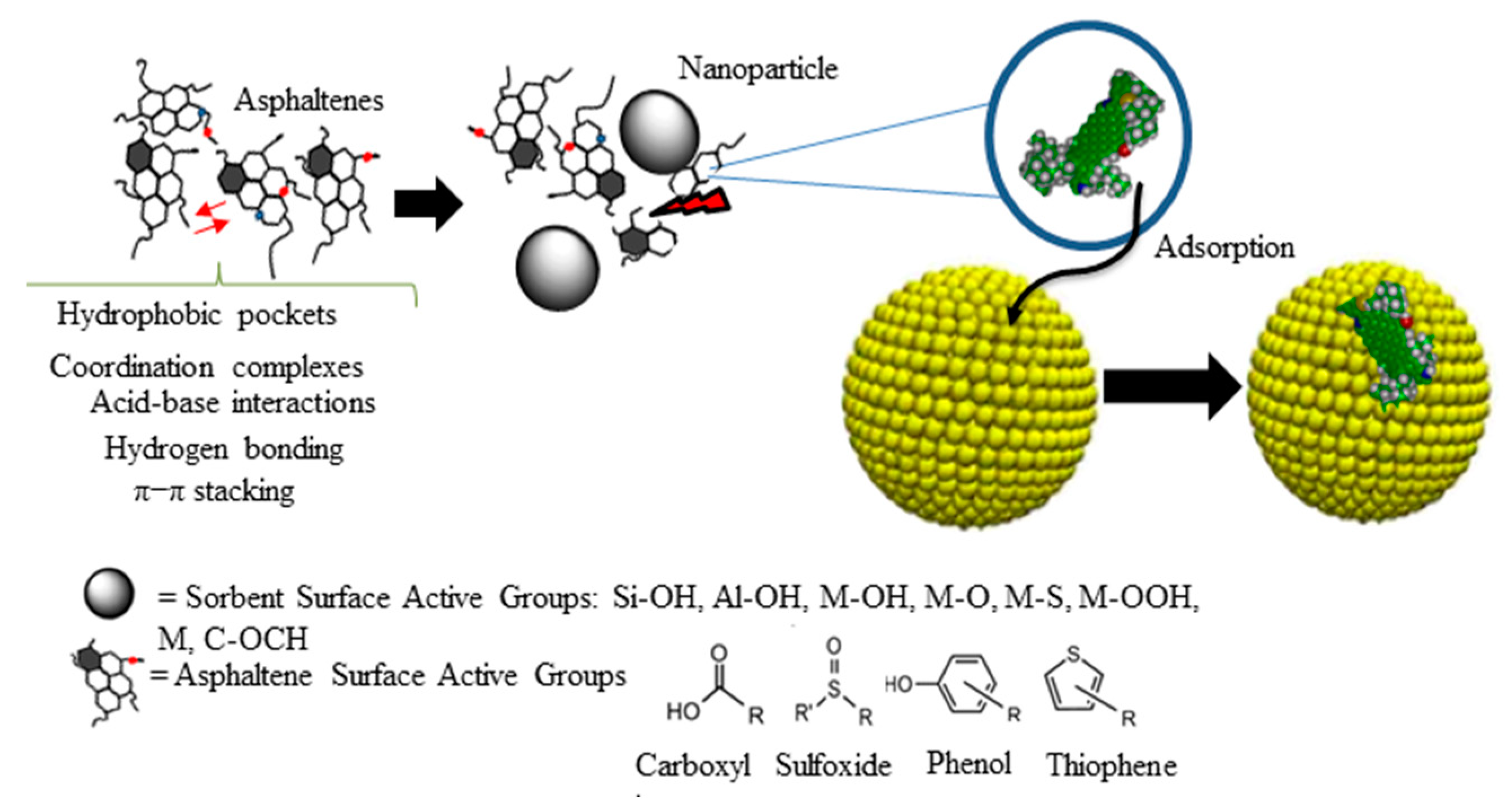
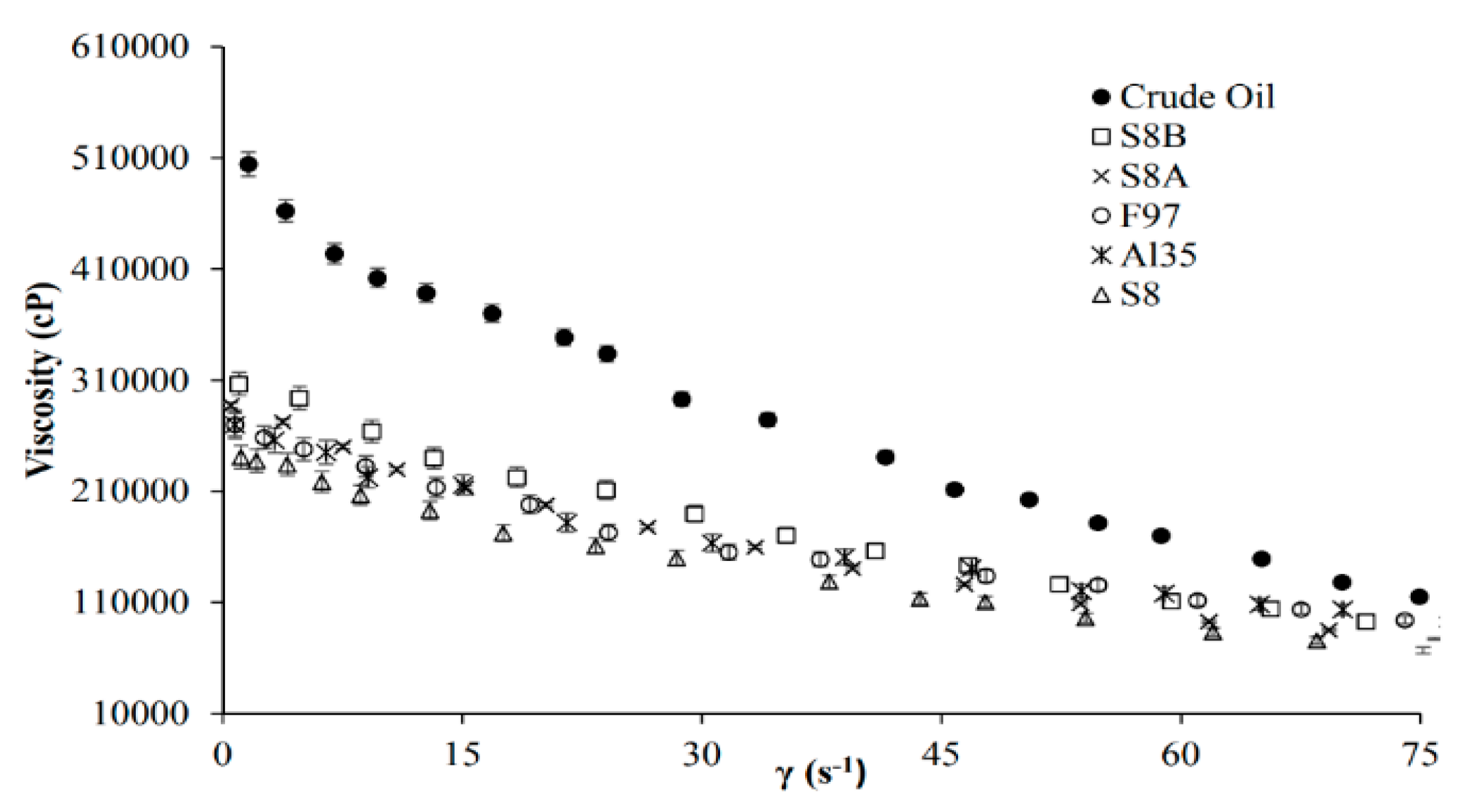
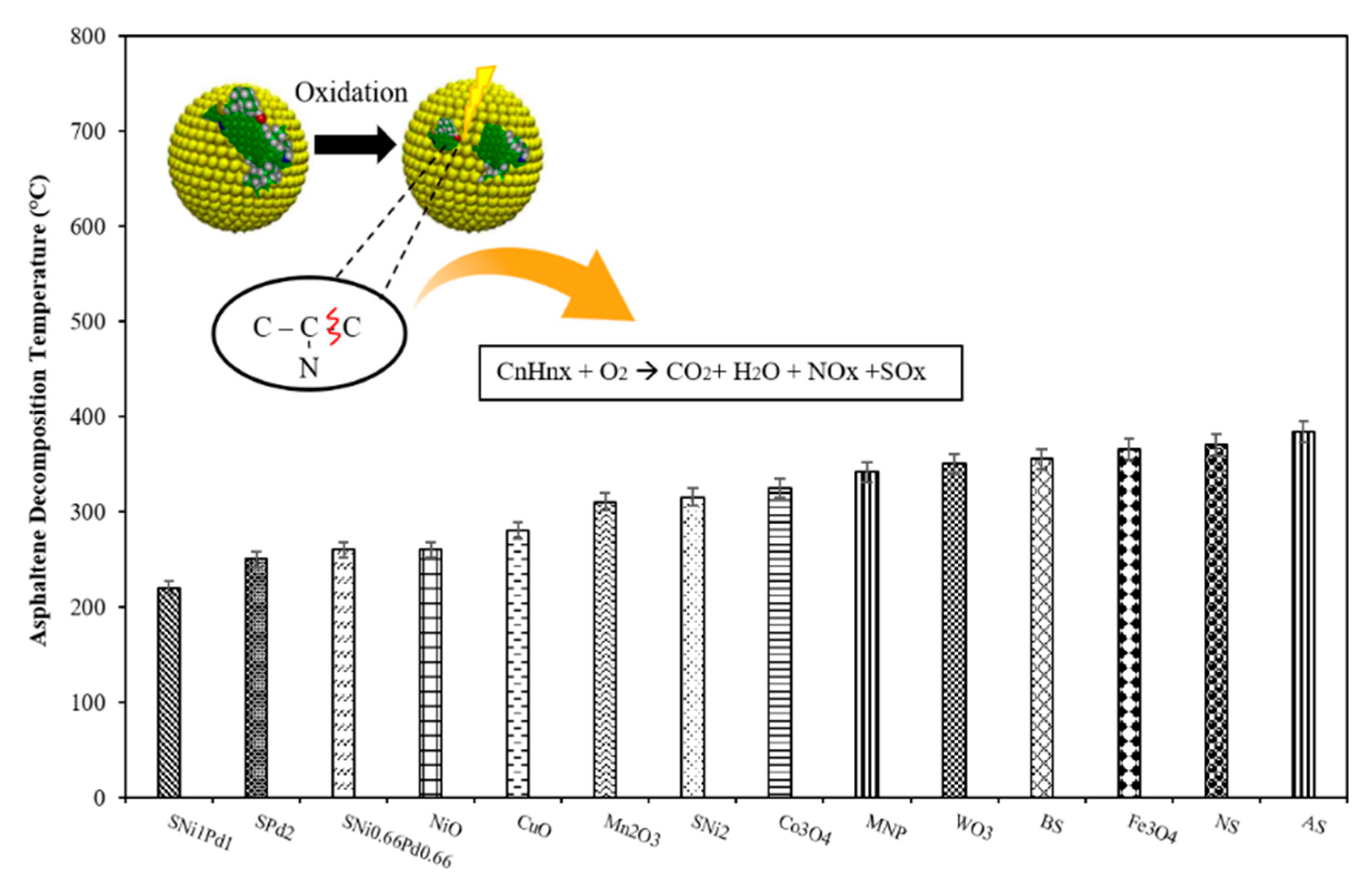
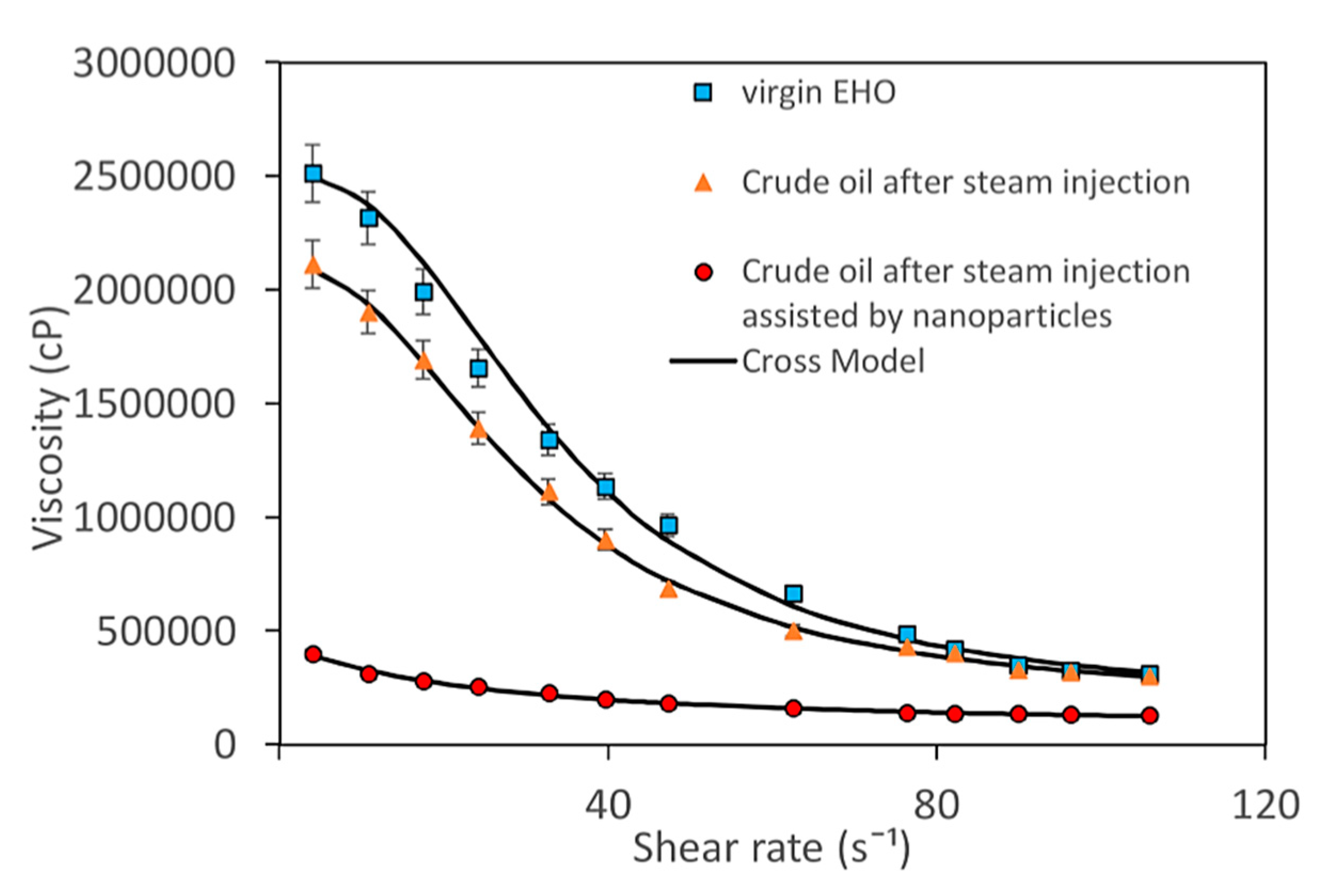
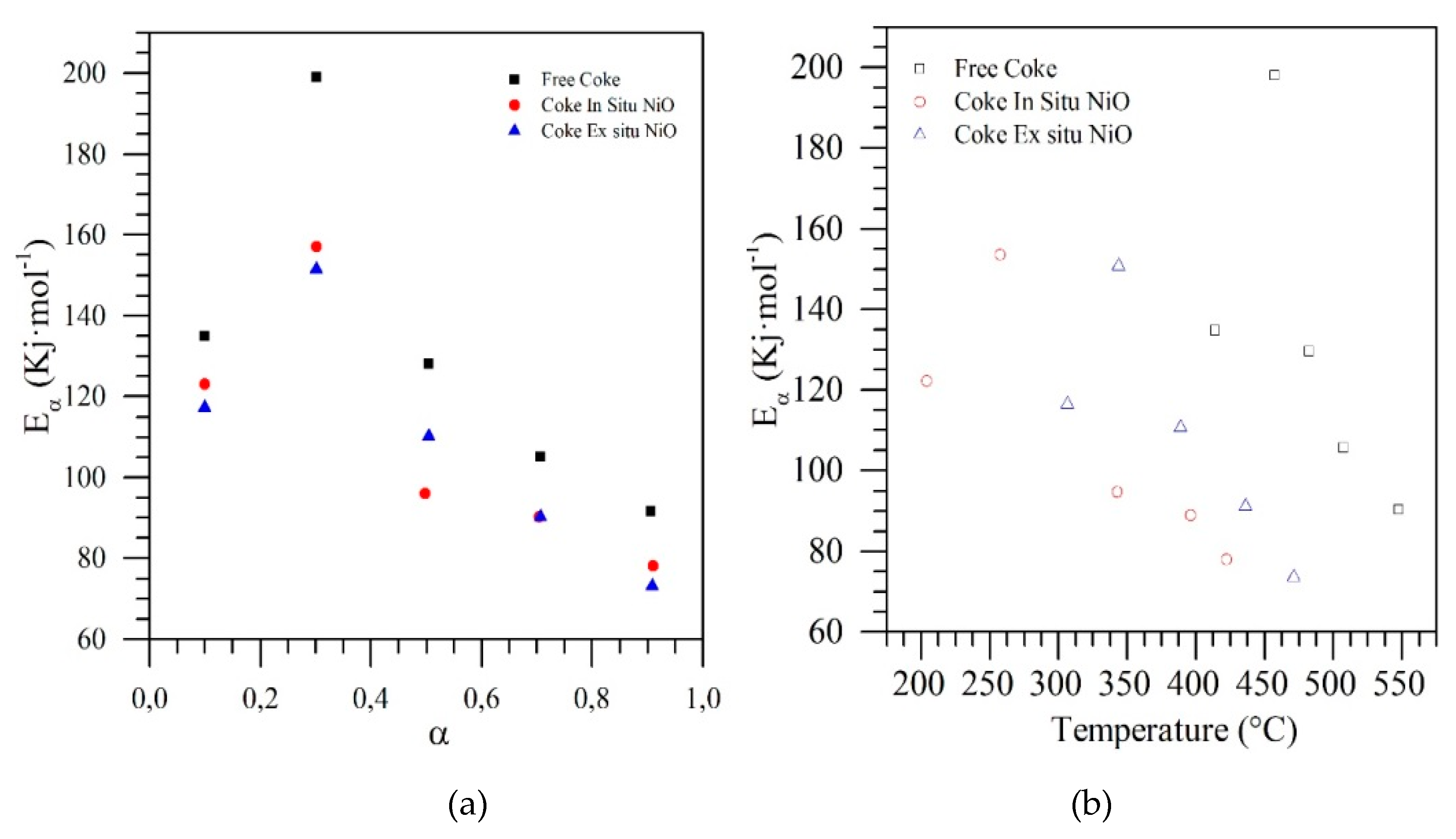
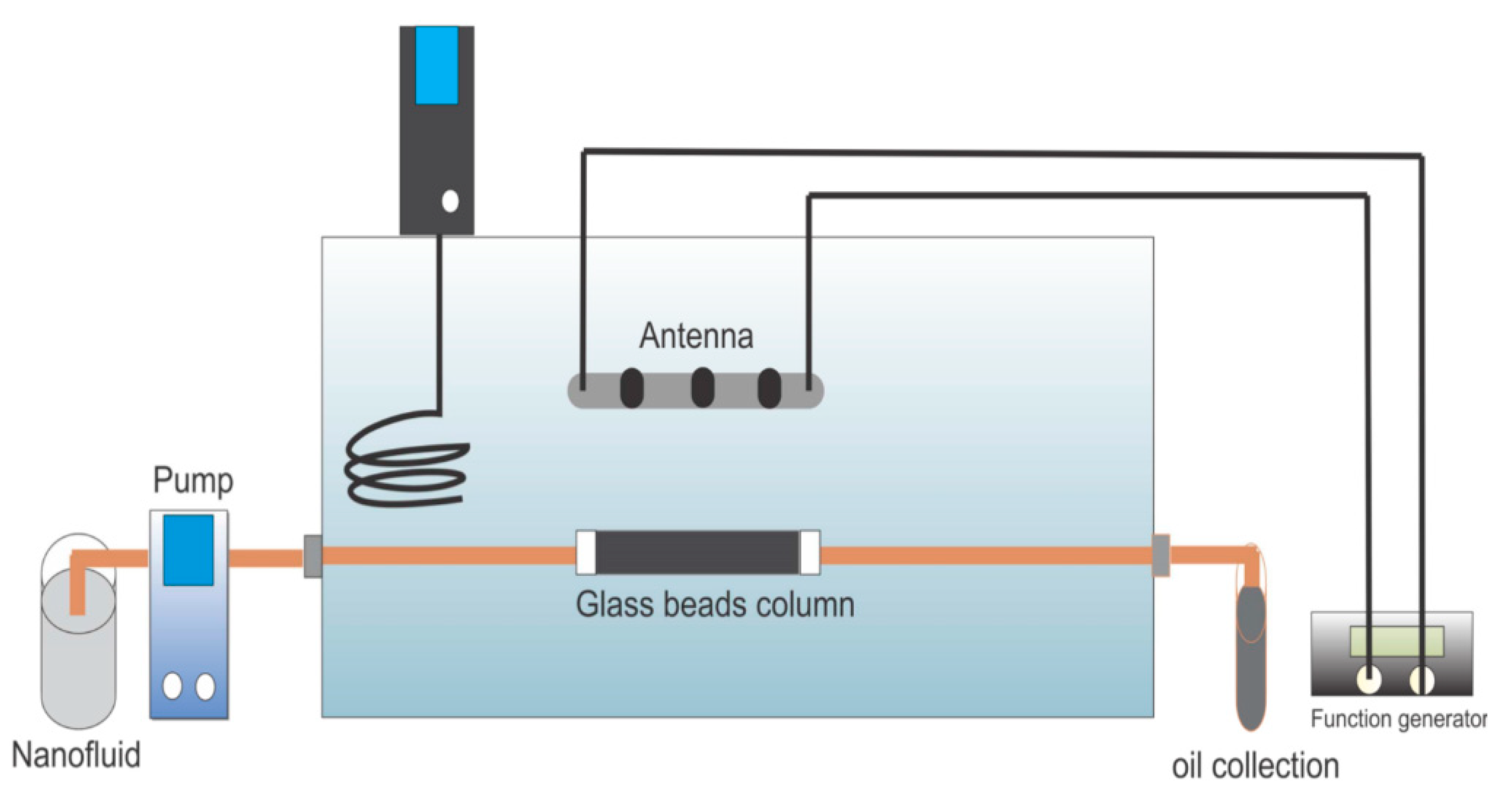
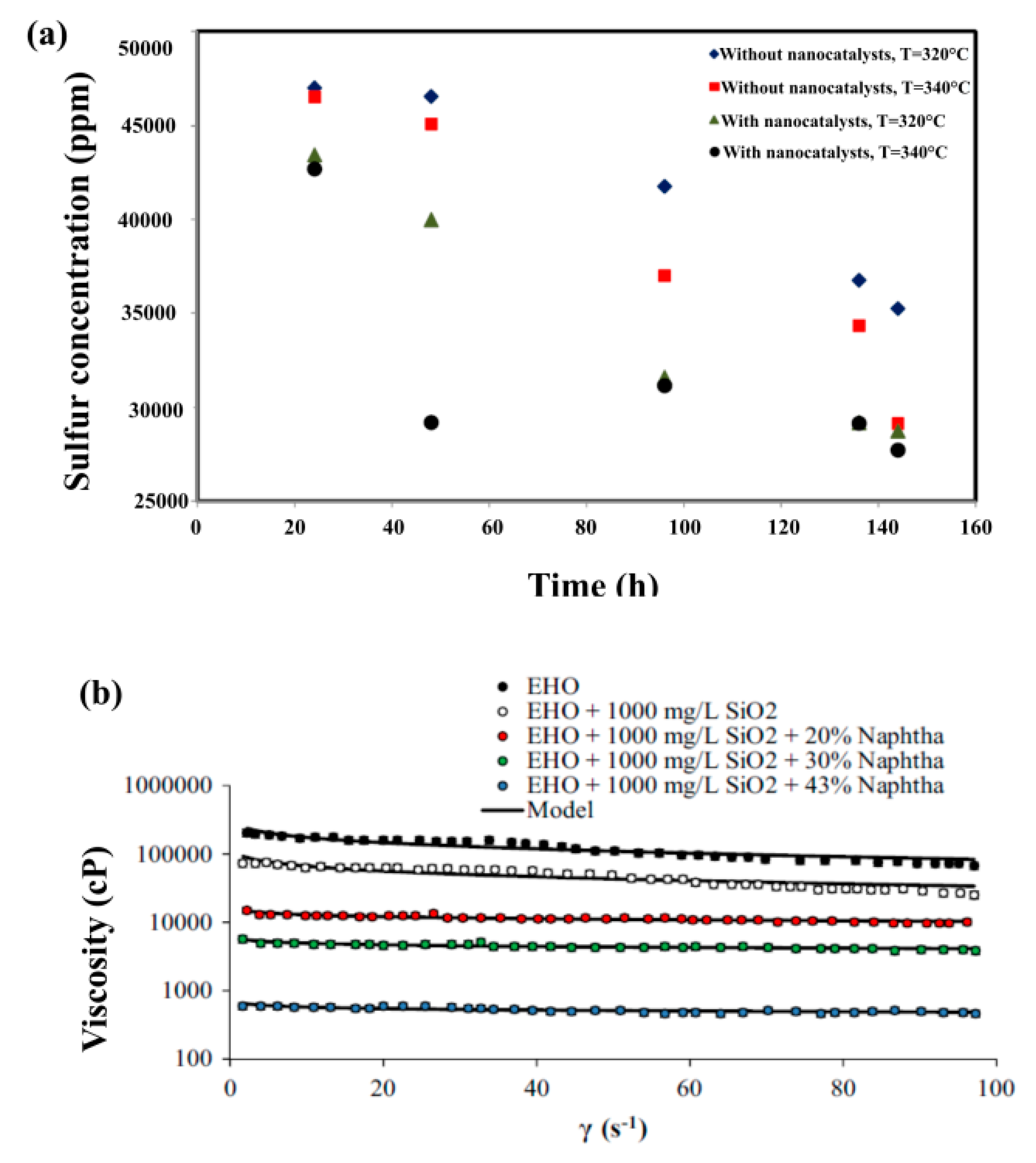
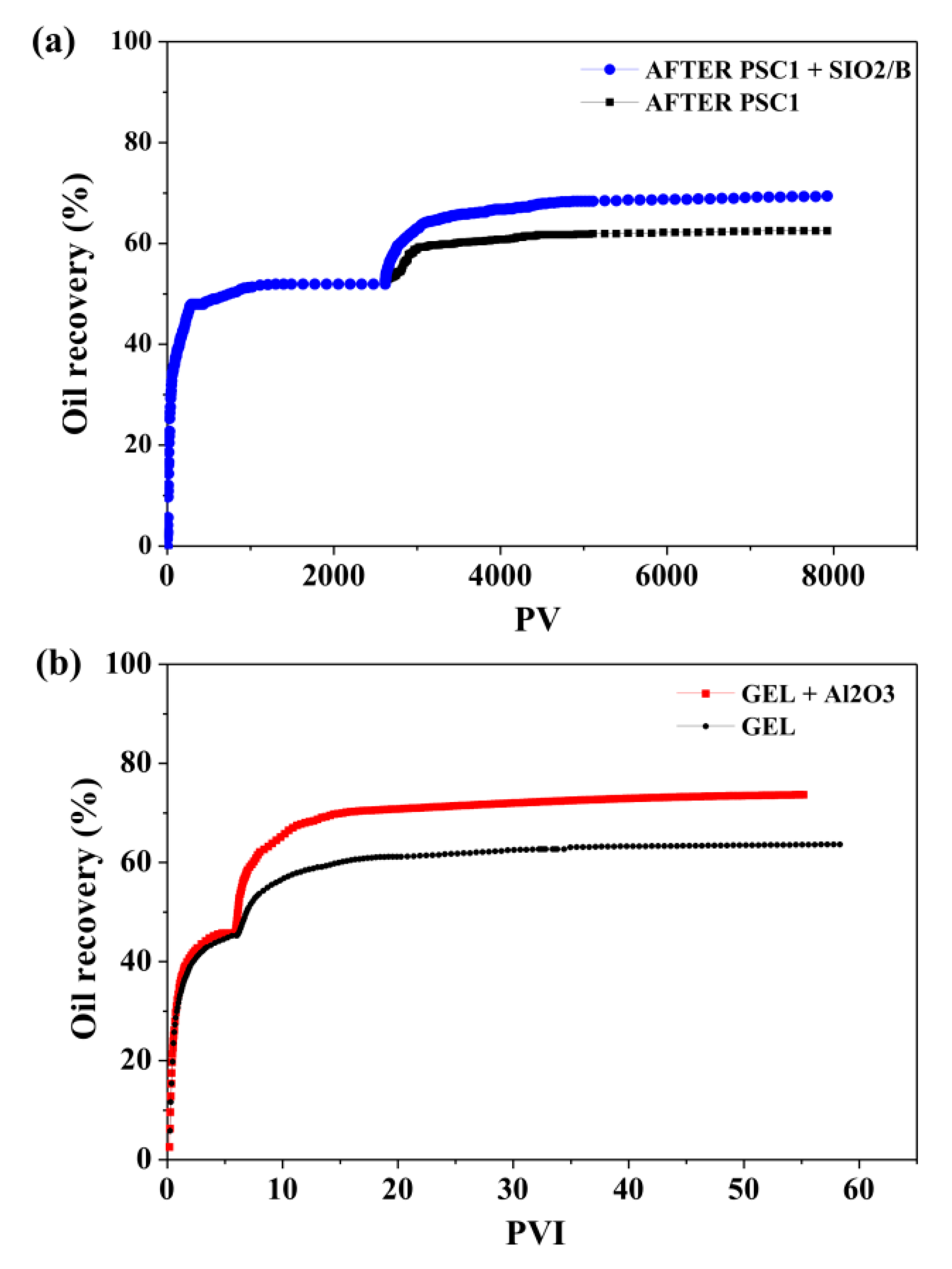
| Method | EOR Mechanism | Limitation |
|---|---|---|
| Cyclic steam stimulation (CSS) | Viscosity reduction | High energy cost |
| In-situ combustion (ISC) | Distillation and breaking of heavy crude oil fractions | Heat leakage to the undesired layers |
| SAGD | Oil expansion | Low effective thermal degradation |
| Electrical heating | Gravity drainage | Heat loss from heat generator to the reservoir |
| Stage | Original Oil in Place (mL) | Volume of Oil Recovered after Water Flooding (mL) | Residual Oil in Place after Water Flooding (mL) | Oil Recovered after Nanofluid Injection (mL) | Percentage Recovery Factor (%) |
|---|---|---|---|---|---|
| Nanofluid injection | 46.0 | 34.5 | 11.5 | 1.0 | 8.7 |
| Nanofluid injection with electromagnetic waver | 42.5 | 33.0 | 9.5 | 3.0 | 31.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, O.E.; Olmos, C.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Nanotechnology Applied to Thermal Enhanced Oil Recovery Processes: A Review. Energies 2019, 12, 4671. https://doi.org/10.3390/en12244671
Medina OE, Olmos C, Lopera SH, Cortés FB, Franco CA. Nanotechnology Applied to Thermal Enhanced Oil Recovery Processes: A Review. Energies. 2019; 12(24):4671. https://doi.org/10.3390/en12244671
Chicago/Turabian StyleMedina, Oscar E., Carol Olmos, Sergio H. Lopera, Farid B. Cortés, and Camilo A. Franco. 2019. "Nanotechnology Applied to Thermal Enhanced Oil Recovery Processes: A Review" Energies 12, no. 24: 4671. https://doi.org/10.3390/en12244671
APA StyleMedina, O. E., Olmos, C., Lopera, S. H., Cortés, F. B., & Franco, C. A. (2019). Nanotechnology Applied to Thermal Enhanced Oil Recovery Processes: A Review. Energies, 12(24), 4671. https://doi.org/10.3390/en12244671