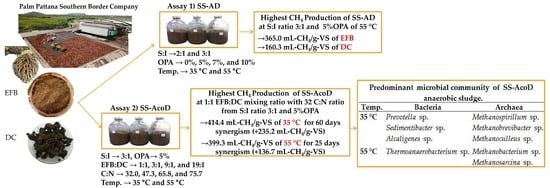
Biogas Production from Oil Palm Empty Fruit Bunches and Palm Oil Decanter Cake using Solid-State Anaerobic co-Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum Preparation
2.2. Solid-state Mono-anaerobic Digestion
2.3. Solid-state Anaerobic co-Digestion
2.4. Analytical Methods
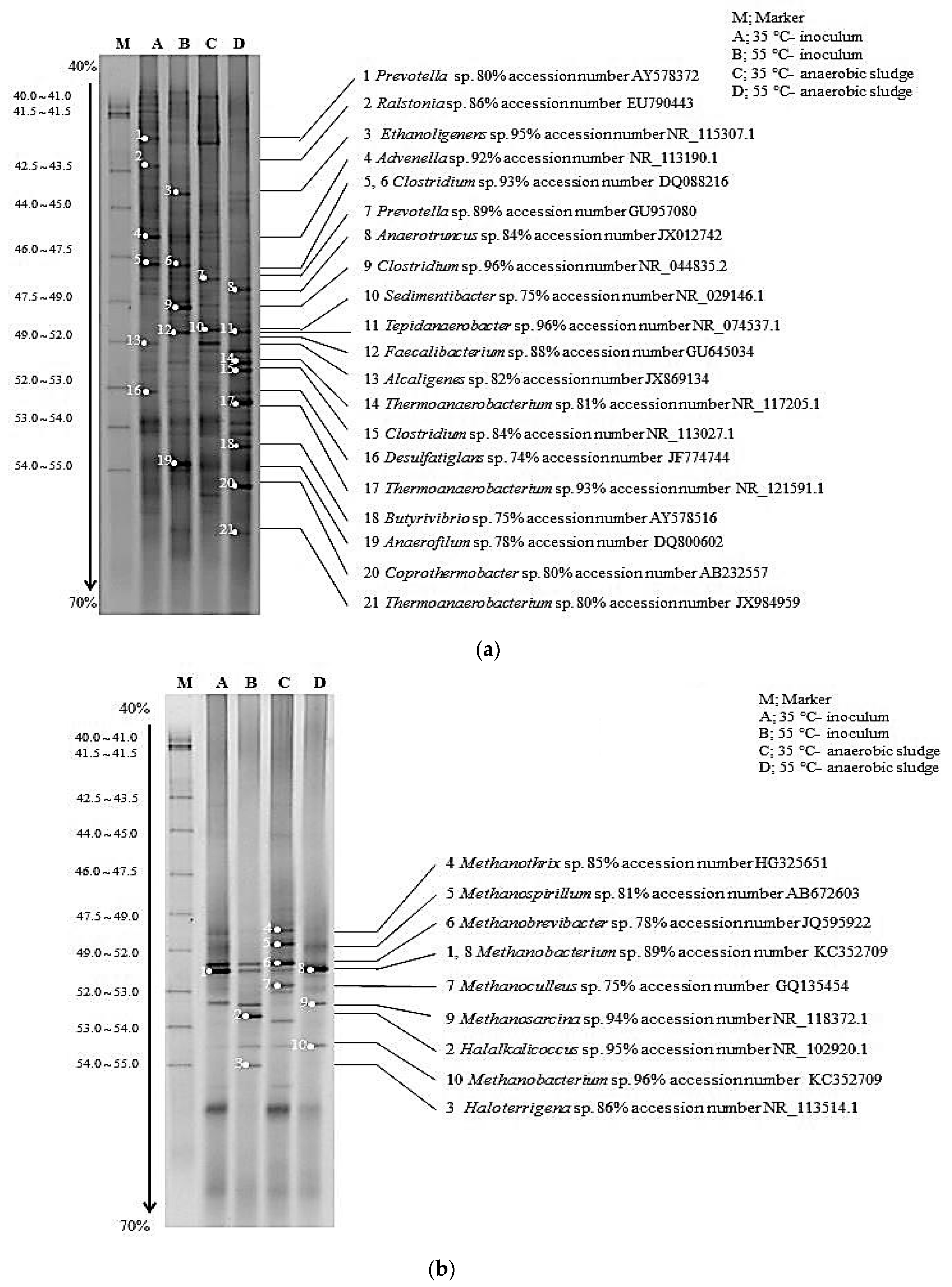
2.5. Microbial Community Analysis
3. Results and Discussion
3.1. Characteristics of EFB, DC, Anaerobic Sludge, Oxide Component of Oil Palm Ash
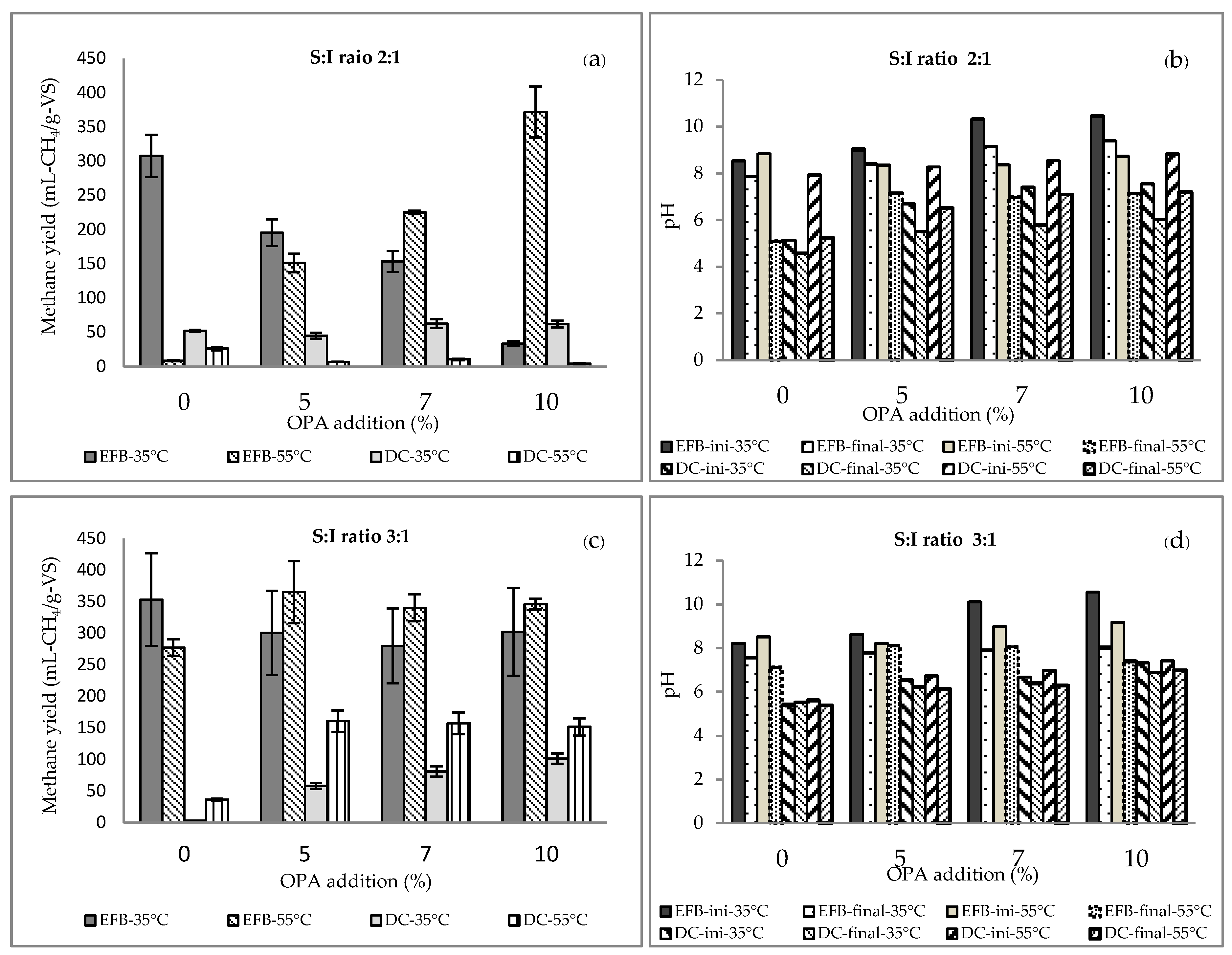
3.2. Solid-state Mono-anaerobic Digestion of EFB and DC
3.3. Solid-state anaerobic co-digestion of EFB and DC
3.4. Microbial Community
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AcoD | Anaerobic co-digestion |
| BMP | Bio-methane potential |
| C:N | Carbon to nitrogen ratio |
| CPO | Crude palm oil |
| DC | Decanter cake |
| EFB | Empty fruit bunches |
| FFB | Fresh fruit bunches |
| L-AD | Liquid state anaerobic digestion |
| OPA | Oil palm ash |
| PCR-DGGE | Polymerase chain reaction-denaturing gradient gel electrophoresis analysis |
| POME | Palm oil mill effluent |
| S:I | Substrate to inoculum |
| SS-AcoD | Solid-state anaerobic co-digestion |
| SS-AD | Solid state anaerobic digestion |
| TS | Total solid content |
| VFAs | Volatile fatty acids |
References
- Chavalparit, O.; Rulkens, W.; Mol, A.; Khaodhair, S. Options for environmental sustainability of the crude palm oil industry in Thailand through enhancement of industrial ecosystems. Environ. Dev. Sustain. 2006, 8, 271–287. [Google Scholar] [CrossRef]
- Sompong, O.; Boe, K.; Angelidaki, I. Thermophilic anaerobic co-digestion of oil palm empty fruit bunches with palm oil mill effluent for efficient biogas production. Appl. Energy 2012, 93, 648–654. [Google Scholar]
- Tan, C.; Saritpongteeraka, K.; Kungsanant, S.; Charnnok, B.; Chaiprapat, S. Low temperature hydrothermal treatment of palm fiber fuel for simultaneous potassium removal, enhanced oil recovery and biogas production. Fuel 2018, 234, 1055–1063. [Google Scholar] [CrossRef]
- Suksong, W.; Kongjan, P.; Prasertsan, P.; Imai, T.; Sompong, O. Optimization and microbial community analysis for production of biogas from solid waste residues of palm oil mill industry by solid-state anaerobic digestion. Bioresour. Technol. 2016, 214, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Corsi, G.; Pino-Cortés, E.; Díaz-Robles, L.A.; Campos, V.; Cubillos, F.; Pelz, S.K.; Paczkowski, S.; Carrasco, S.; Silva, J. Modeling and simulation of a continuous biomass hydrothermal carbonization process. Chem. Eng. Commun. 2019. [Google Scholar] [CrossRef]
- Paepatung, N.; Nopharatana, A.; Songkasiri, W. Bio-methane potential of biological solid materials and agricultural wastes. Asian J. Energy Environ. 2009, 10, 19–27. [Google Scholar]
- Suksong, W.; Jehlee, A.; Singkhala, A.; Kongjan, P.; Prasertsan, P.; Imai, T.; Sompong, O. Thermophilic solid-state anaerobic digestion of solid waste residues from palm oil mill industry for biogas production. Ind. Crop. Prod. 2017, 95, 502–511. [Google Scholar] [CrossRef]
- Meng, L.; Xie, L.; Kinh, C.T.; Suenaga, T.; Hori, T.; Riya, S.; Terada, A.; Hosomi, M. Influence of feedstock-to-inoculum ratio on performance and microbial community succession during solid-state thermophilic anaerobic co-digestion of pig urine and rice straw. Bioresour. Technol. 2018, 252, 127–133. [Google Scholar] [CrossRef]
- Rouches, E.; Escudié, R.; Latrille, E.; Carrère, H. Solid-state anaerobic digestion of wheat straw: Impact of S/I ratio and pilot-scale fungal pretreatment. Waste Manag. 2019, 85, 464–476. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Yin, C.Y.; Kadir, S.A.S.A.; Lim, Y.P.; Syed-Ariffin, S.N.; Zamzuri, Z. An investigation into physicochemical characteristics of ash produced from combustion of oil palm biomass wastein a boiler. Fuel Process. Technol. 2008, 89, 693–696. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, L.; Li, Y. Comparison of premixing methods for solid-state anaerobic digestion of corn stover. Bioresour. Technol. 2015, 175, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, F.; Ge, X.; Li, Y. Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2015, 44, 824–834. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2019, 12, 26. [Google Scholar] [CrossRef]
- APHA, A.W.; Greenberg, W.I.A.; Clesceri, L.; Eaton, A. Standard Methods for the Examination of Water and Waste Water; American Public Health Association: Wasghington, DC, USA, 2012. [Google Scholar]
- Luna, G.M.; Stumm, K.; Pusceddu, A.; Danovaro, R. Archaeal diversity in deep-sea sediments estimated by means of different terminal-restriction fragment length polymorphisms (T-RFLP) protocols. Curr. Microbiol. 2009, 59, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Kongjan, P.; O-Thong, S.; Kotay, M.; Min, B.; Angelidaki, I. Biohydrogen production from wheat straw hydrolysate by dark fermentation using extreme thermophilic mixed culture. Biotechnol. Bioeng. 2010, 105, 899–908. [Google Scholar] [CrossRef]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2008, 37 (Suppl. 1), D26–D31. [Google Scholar] [CrossRef]
- Mshandete, A.; Björnsson, L.; Kivaisi, A.K.; Rubindamayugi, M.S.; Mattiasson, B. Effect of particle size on biogas yield from sisal fibre waste. Renew. Energy 2006, 31, 2385–2392. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Brown, D.; Shi, J.; Li, Y. Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour. Technol. 2012, 124, 379–386. [Google Scholar] [CrossRef]
- Sangyoka, S.; Reungsang, A.; Lin, C.-Y. Optimization of biohydrogen production from sugarcane bagasse by mixed cultures using a statistical method. Sustain. Environ. Res. 2016, 26, 235–242. [Google Scholar] [CrossRef]
- Taherdanak, M.; Zilouei, H. Improving biogas production from wheat plant using alkaline pretreatment. Fuel 2014, 115, 714–719. [Google Scholar] [CrossRef]
- Zhou, H.; Wen, Z. Solid-State Anaerobic Digestion for Waste Management and Biogas Production. In Solid State Fermentation; Advances in Biochemical Engineering/Biotechnology; Steudler, S., Werner, A., Cheng, J., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Kengen, S.W.; Goorissen, H.P.; Verhaart, M.; van Niel, E.; Claassen, P.; Stams, A. Biological Hydrogen Production by Anaerobic Microorganisms. In Biofuels; John Wiley Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- De Bok, F.; Plugge, C.; Stams, A. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 2004, 38, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.N.; Shi, J.; Li, Y. Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 2012, 46, 125–132. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ge, X.; Li, Y. Solid-state anaerobic co-digestion of spent mushroom substrate with yard trimmings and wheat straw for biogas production. Bioresour. Technol. 2014, 169, 468–474. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L. Codigestion of manure and organic wastes in centralized biogas plants. Appl. Biochem. Biotechnol. 2003, 109, 95–105. [Google Scholar] [CrossRef]
- David, A.; Govil, T.; Tripathi, A.; McGeary, J.; Farrar, K.; Sani, R. Thermophilic Anaerobic Digestion: Enhanced and Sustainable Methane Production from Co-Digestion of Food and Lignocellulosic Wastes. Energies 2018, 11, 2058. [Google Scholar] [CrossRef]
- Gavala, H.N.; Skiadas, I.V.; Ahring, B.K. Biological hydrogen production in suspended and attached growth anaerobic reactor systems. Int. J. Hydrogen Energy 2006, 31, 1164–1175. [Google Scholar] [CrossRef]
- Han, R.; Yuan, Y.; Cao, Q.; Li, Q.; Chen, L.; Zhu, D.; Liu, D. PCR–DGGE Analysis on Microbial Community Structure of Rural Household Biogas Digesters in Qinghai Plateau. Curr. Microbiol. 2018, 75, 541–549. [Google Scholar] [CrossRef]
- Wirth, R.; Kovács, E.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnol. Biofuels 2012, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.N.; Gharbia, S.E. Biochemical and chemical studies on strains designated Prevotella intermedia and proposal of a new pigmented species, Prevotella nigrescens sp. nov. Int. J. Syst. Evol. Microbiol. 1992, 42, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Zhou, A.; Zhang, J.; Liu, Z.; Wang, G.; Liu, W.; Wang, A.; Yue, X. Characterization of biocarbon-source recovery and microbial community shifts from waste activated sludge by conditioning with cornstover: Assessment of cellulosic compositions. Sci. Rep. 2017, 7, 42887. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Kumar Jha, A. Psychrophilic dry anaerobic digestion of cow dung for methane production: Effect of inoculum. Sci. Asia 2013, 39, 500. [Google Scholar] [CrossRef]
- Usman, M.; Hao, S.; Chen, H.; Ren, S.; Tsang, D.C.; Sompong, O.; Luo, G.; Zhang, S. Molecular and microbial insights towards understanding the anaerobic digestion of the wastewater from hydrothermal liquefaction of sewage sludge facilitated by granular activated carbon (GAC). Environ. Int. 2019, 133, 105257. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Alkasrawi, M. Process enhancement of hydrogen and methane production from palm oil mill effluent using two-stage thermophilic and mesophilic fermentation. Int. J. Hydrogen Energy 2016, 41, 12888–12898. [Google Scholar] [CrossRef]
- Guermazi-Toumi, S.; Chouari, R.; Sghir, A. Molecular analysis of methanogen populations and their interactions within anaerobic sludge digesters. Environ. Technol. 2019, 40, 2864–2879. [Google Scholar] [CrossRef]
- Traore, S.I.; Khelaifia, S.; Armstrong, N.; Lagier, J.; Raoult, D. Isolation and culture of Methanobrevibacter smithii by co-culture with hydrogen-producing bacteria on agar plates. Clin. Microbiol. Infect. 2019, in press. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L.D. An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef]
- Patel, G. Characterization and nutritional properties of Methanothrix concilii sp. nov. a mesophilic, aceticlastic methanogen. Can. J. Microbiol. 1984, 30, 1383–1396. [Google Scholar] [CrossRef]
- Ziemiński, K.; Frąc, M. Methane fermentation process as anaerobic digestion of biomass: Transformations, stages and microorganisms. Afr. J. Biotechnol. 2012, 11, 4127–4139. [Google Scholar]
- Barret, M.; Gagnon, N.; Kalmokoff, M.L.; Topp, E.; Verastegui, Y.; Brooks, S.P.; Matias, F.; Neufeld, J.D.; Talbot, G. Identification of Methanoculleus spp. as active methanogens during anoxic incubations of swine manure storage tank samples. Appl. Environ. Microbiol. 2013, 79, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Boonpiyo, S.; Sittijunda, S.; Reungsang, A. Co-digestion of napier grass with food waste and napier silage with food waste for methane production. Energies 2018, 11, 3200. [Google Scholar] [CrossRef]

| Parameter (%w/w) | EFB | DC | Anaerobic Sludge | Oxide Component (%w/w) | OPA |
|---|---|---|---|---|---|
| Total solid (TS) | 96.38 ± 1.20 | 17.86 ± 0.25 | 5.14 ± 1.0 | Silicon oxide (SiO2) | 50.30 ± 1.2 |
| Volatile solid (VS) | 90.22 ± 1.52 | 15.51 ± 0.32 | 3.14 ± 0.88 | Calcium oxide (CaO) | 14.90 ± 0.07 |
| Lipid | 1.08 ± 0.052 | 2.56 ± 0.10 | 0.11 ± 0.01 | Potassium oxide (K2O) | 13.10 ± 0.013 |
| Ash | 6.16 ± 0.152 | 2.35 ± 0.11 | 2.0 ± 0.10 | Magnesium oxide (MgO) | 6.80 ± 0.18 |
| Nitrogen | 0.50 ± 0.002 | 2.22 ± 0.02 | 0.14 ± 0.001 | Phosphorus oxide (P2O5) | 4.80 ± 0.03 |
| Carbon | 44.29 ± 0.02 | 42.70 ± 0.14 | 1.12 ± 0.02 | Ferric oxide (Fe2O3) | 1.80 ± 0.01 |
| Hydrogen | 5.58 ± 0.02 | 5.77 ± 0.10 | 6.65 ± 0.02 | Aluminum oxide (Al2O3) | 0.87 ± 0.004 |
| Sulfur | 0.046 ± 0.002 | 0.260 ± 0.003 | N.D. | Sulfur oxide (SO3) | 0.81 ± 0.002 |
| Oxygen | 38.27 ± 0.23 | 32.89 ± 0.12 | 63.98 ± 0.02 | Manganese oxide (MnO2) | 0.31 ± 0.001 |
| C:N ratio | 88.76 | 19.23 | 8.0 | Sodium oxide (Na2O) | - |
| EFB:DC | SubstrateC:N ratio | Temp | MY | WMY | MY-WMY | Kh | Modified Gompertz Model | ||
|---|---|---|---|---|---|---|---|---|---|
| B∞ | Rmax | l | |||||||
| VS basis | °C | mL-CH4/g-VS | d−1 | mL-CH4/g-VS | mL/d | d | |||
| 1:0 | 89 | 35 | 300.2 | 300.2 | 0 | N.D | N.D | N.D | N.D |
| 55 | 364.9 | 364.9 | 0 | N.D | N.D | N.D | N.D | ||
| 0:1 | 19.2 | 35 | 58.1 | 58.1 | 0 | N.D | N.D | N.D | N.D |
| 55 | 160.3 | 160.3 | 0 | N.D | N.D | N.D | N.D | ||
| 1:1 | 32.0 | 35 | 414.4 | 179.2 | +235.2 | 0.042 | 454.4 | 13.5 | 16.0 |
| 55 | 399.3 | 262.6 | +136.7 | 0.213 | 397.9 | 44.9 | 0.9 | ||
| 3:1 | 47.3 | 35 | 367.1 | 239.5 | +127.6 | 0.062 | 371.8 | 17.8 | 12.7 |
| 55 | 274.7 | 313.8 | −39.1 | 0.150 | 280.4 | 27.9 | 1.3 | ||
| 9:1 | 65.8 | 35 | 324.8 | 276.0 | +48.8 | 0.053 | 333.4 | 14.2 | 6.4 |
| 55 | 222.0 | 344.4 | −122.4 | 0.182 | 222.2 | 24.7 | 0.81 | ||
| 19:1 | 75.7 | 35 | 321.8 | 288.1 | +33.7 | 0.028 | 327.7 | 14.2 | 6.7 |
| 55 | 221.0 | 353.8 | −132.8 | 0.195 | 224.3 | 25.9 | 1.1 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepsour, M.; Usmanbaha, N.; Rattanaya, T.; Jariyaboon, R.; O-Thong, S.; Prasertsan, P.; Kongjan, P. Biogas Production from Oil Palm Empty Fruit Bunches and Palm Oil Decanter Cake using Solid-State Anaerobic co-Digestion. Energies 2019, 12, 4368. https://doi.org/10.3390/en12224368
Tepsour M, Usmanbaha N, Rattanaya T, Jariyaboon R, O-Thong S, Prasertsan P, Kongjan P. Biogas Production from Oil Palm Empty Fruit Bunches and Palm Oil Decanter Cake using Solid-State Anaerobic co-Digestion. Energies. 2019; 12(22):4368. https://doi.org/10.3390/en12224368
Chicago/Turabian StyleTepsour, Muthita, Nikannapas Usmanbaha, Thiwa Rattanaya, Rattana Jariyaboon, Sompong O-Thong, Poonsuk Prasertsan, and Prawit Kongjan. 2019. "Biogas Production from Oil Palm Empty Fruit Bunches and Palm Oil Decanter Cake using Solid-State Anaerobic co-Digestion" Energies 12, no. 22: 4368. https://doi.org/10.3390/en12224368
APA StyleTepsour, M., Usmanbaha, N., Rattanaya, T., Jariyaboon, R., O-Thong, S., Prasertsan, P., & Kongjan, P. (2019). Biogas Production from Oil Palm Empty Fruit Bunches and Palm Oil Decanter Cake using Solid-State Anaerobic co-Digestion. Energies, 12(22), 4368. https://doi.org/10.3390/en12224368