Abstract
Methane production was carried out in two different types of reactors using a thermophilic and hydrogenotrophic methanogen, Methanothermobacter sp. KEPCO-1, which converts hydrogen and carbon dioxide into methane at 60 °C. The two reactors used for methane production were stirred-tank reactor (ST) and a bubble column reactor (BC), which were selected because they can provide a good comparison between the medium agitation type and gas–liquid mass transfer. The specific growth rate of KEPCO-1 in the ST and BC was 0.03 h−1 and 0.07 h−1, respectively. The methane conversion rate increased to 77.8 L/L/d in the ST and 19.8 L/L/d in the BC. To prevent the dilution of nutrients in the medium by the water generated during the hydrogenotrophic methanation reaction, a membrane distillation (MD) process was applied to selectively remove water from the culture medium. The MD process selectively removed only water from the medium. Fouling by KEPCO-1 had a negligible effect on flux and showed a high removal performance flux of 16.3 ± 3.1 L/m2/h. By operating the MD process in conjunction with the hydrogenotrophic methanation process, it is possible to prevent the dilution of the nutrients in the medium by the water generated during the methanation process, thereby maintaining stable microbial growth and methanation activity.
1. Introduction
Global warming due to greenhouse gas emissions has sparked interest in reducing the world’s carbon footprint. As a carbon utilization method, CO2 reduction into methane (CH4) with hydrogen produced from electrolysis has recently emerged as a promising option to store surplus renewable electricity [1,2]. In contrast to H2, CH4 is good for long-term storage without additional special storage materials and is already widely used as an energy carrier in compressed natural gas, transport fuel, etc. throughout the natural gas grid [3,4]. In particular, biomethanation for microbial reduction of CO2 into CH4 with renewably produced hydrogen (H2) is catalyzed by methanogenic archaea and operates at a lower temperature (40–70 °C) and lower pressure (≤10 bar) than chemical methanation [2].
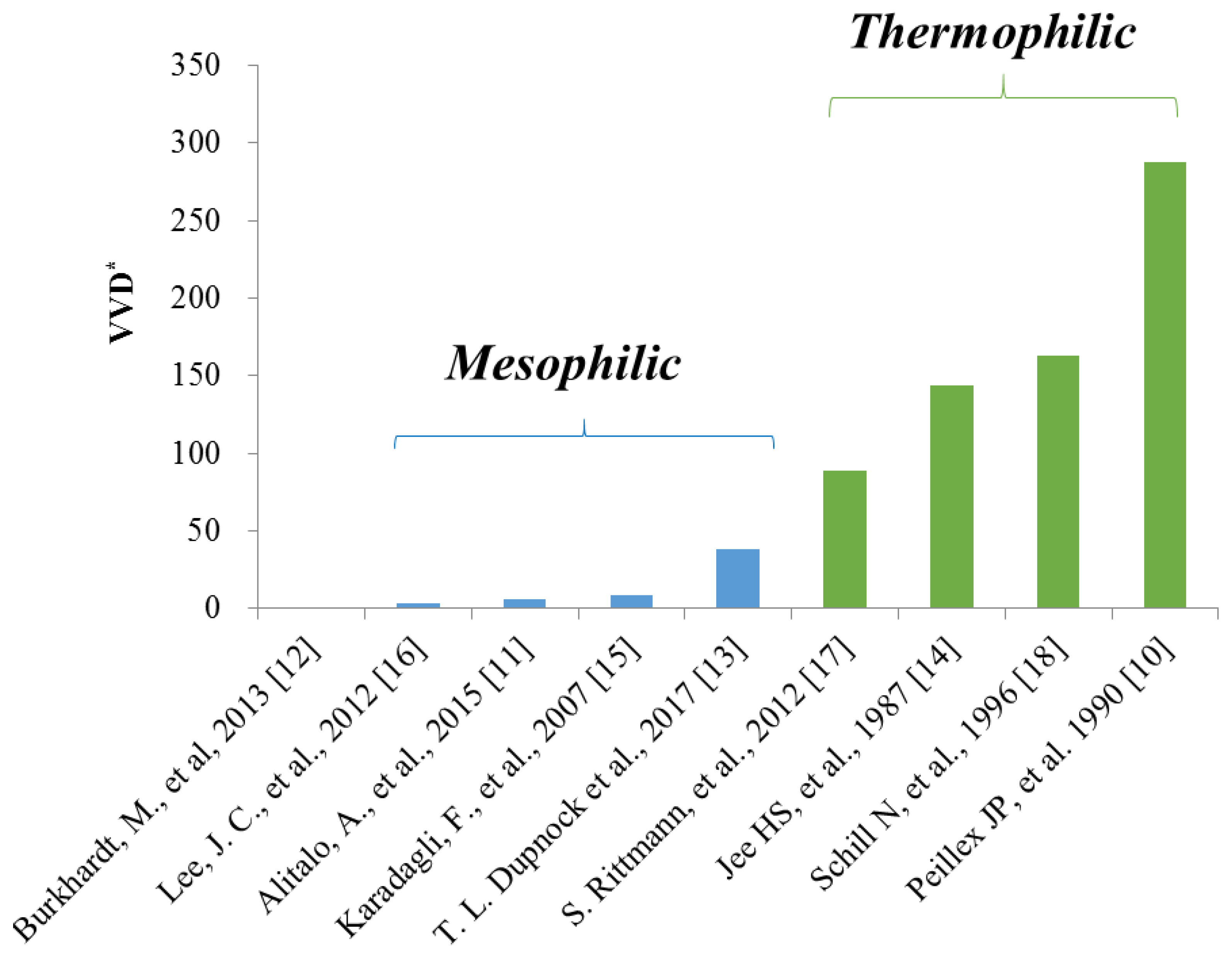
Methanogenic archaea that produce methane using acetate (acetoclastic) or CO2 (hydrogenotrophic) are capable of thriving under extreme conditions, such as high temperatures or high salt environments [5]. The methanation rate of hydrogenotrophic methanogens was greater than that of acetoclastic methanogens [6], and among hydrogenotrophic methanogens, thermophilic hydrogenotrophic methanogens demonstrated a shorter doubling time [7] and a higher methane production rate than those of mesophilic hydrogenotrophic methanogens (Figure 1). The gas-liquid mass transfer of H2, known as the main obstacle to the successful application of biomethanation with hydrogenotrophic methanogens [8], has been improved by the use of pressurized fermenters [9] and a high stirring speed [10].
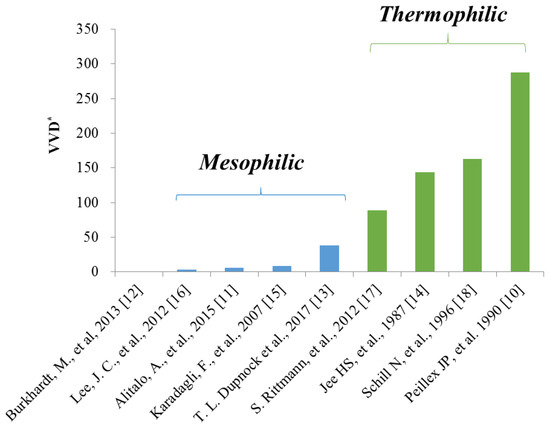
Figure 1.
Comparison of the methane production rate between mesophilic and thermophilic hydrogenotrophic methanogens. VVD: Volumetric production of methane per working volume per day [10,11,12,13,14,15,16,17,18].
Another main obstacle is water generation during biomethanation, as shown in Equation (1) [19].
As one mole of methane is produced, the medium volume of the fermenter is inevitably increased, and the medium is diluted by two moles of generated water. For the growth of hydrogenotrophic methanogens, H2 and CO2 are used as energy and carbon sources, and ammonium ion (NH4+), hydrogen sulfide (H2S), and other trace elements are required. In particular, the optimum concentration of NH4+ and H2S should be maintained to obtain the maximum methanation rate, but during biomethanation, their optimum concentrations can be diluted by the generated water. This phenomenon indicates that the development of a process to remove only the generated water from biomethanation fermenters is important for stable CH4 production with hydrogenotrophic methanogens. By discharging water containing very low concentrations of NH4+ and H2S using the water removing technology, the operation cost of wastewater treatment following the biomethanation process can be reduced.
Membrane distillation (MD) is a useful separation process for the removal of only water from the culture medium during the thermophilic methanation process. During MD, low-grade heat can be used to remove water by a vapor pressure gradient from the feed through a phase change. Direct contact membrane distillation (DCMD) condenses steam by bringing cold permeate or distillate streams into direct contact with the membrane. MD has been widely applied in desalination [20,21,22] and wastewater treatment [23,24,25]. This widespread use is due to the low probability of contamination of the MD compared to the pressure-driven process, the ability to achieve very high concentration factors, and the excellent permeation quality [26]. In particular, hydrophobic membranes only allow the passage of vapor [27].
In this study, the MD process was applied to remove the water produced during the methanation process using two different types of mixer-operated fermenters: By a stirrer (stirred tank reactor) and by a diffuser (bubble column). This is a feasibility study and our results suggested that the integrated system of biomethanation and MD could be applied to remove only water without losing nutrients in the methanation process. Thermophilic autotrophic methanation has the advantage of saving thermal energy and reducing the fouling of the MD system.
2. Methods and Materials
2.1. Methanogen
A thermophilic hydrogenotrophic methanogen, Methanothermobacter sp. KEPCO-1 (KEPCO-1), was isolated from anaerobic sludge from a local wastewater treatment plant in Seoul, Korea. The strain was stored in a 50% glycerol stock at −80 °C and was serially transferred from a 125 mL serum bottle to a 1 L Duran® bottle (Daehan Scientific Co. Ltd., Seoul, Korea) for inoculation. The bottles were filled with mixed gas (CO2:H2 = 1:4) and capped with a rubber stopper and an aluminum cap. ATCC medium 2133 (OSU967) was used, containing (per liter): 1 g NH4Cl, 0.3 g KH2PO4, 0.6 g NaCl, 0.1 g MgCl·6H2O, 0.06 g CaCl2·2H2O, 4.0 g NaHCO3, 0.5 g L-cysteine·HCl, 0.5 g Na2S·9H2O, and 10 mL × 100 trace elements. Trace elements contained (per l L): 1.35 g FeCl3·6H2O, 0.1 g MnCl3·4H2O, 0.1 g CaCl2·6H2O, 0.1 g ZnCl2, 0.01 g H3BO3, 1.0 g NaCl, 0.15 g NiCl2·6H2O, 0.05 g AlCl3·6H2O, 24.0 mg CoCl2·6H2O, 25.0 mg CuCl2·2H2O, 24.0 mg Na2MoO4·2H2O, and 26.0 mg Na2SeO4·6H2O. The pH was adjusted to 7.5 using 6N NaOH. Methanogens were incubated at 60 °C with shaking at 200 rpm. Two different types of thermophilic hydrogenotrophic methanation reactors were operated in the laboratory at 60 °C for about three months while purging the mixed gas of H2 and CO2 with a volumetric ratio of 4:1.
2.2. Methanation Reactors
2.2.1. Stirred-Tank Reactor (ST)
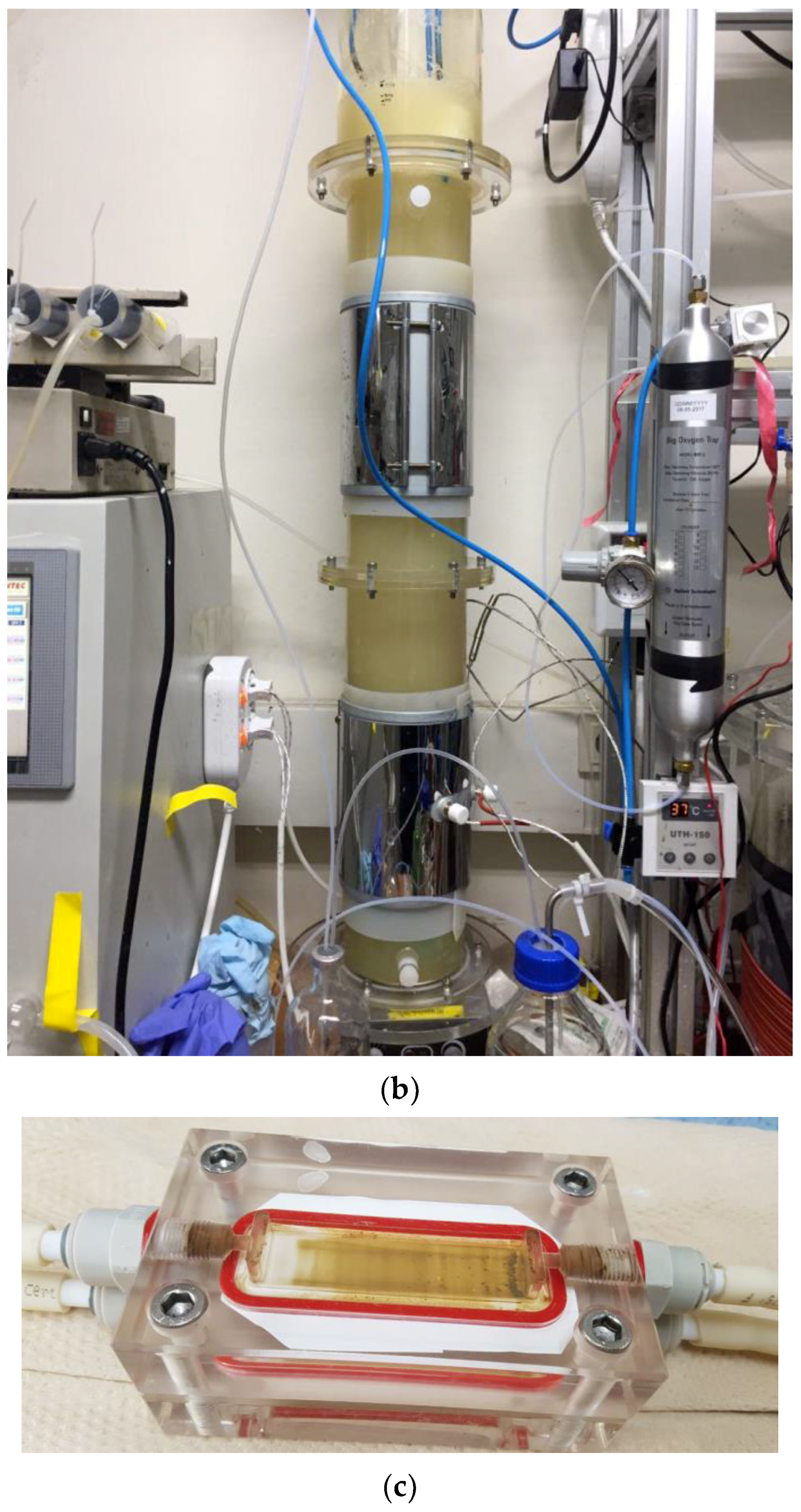
A bioreactor (3 L, Fermentec Co. Ltd., Cheongju-si, Korea) was used as the stirred-tank reactor (Figure 2a). The overhead mixer was used with an impeller speed from 150 rpm to 600 rpm. The working volume was 1.5 L, and the temperature was maintained at 60 ± 2 °C using an external heating jacket. The influent rate of mixed gases was controlled at 600 mL/min (CO2:H2 = 1:4, 24 h−1 inflow gas per working volume) using digital mass flow controllers (HFC-D-302B, infoRAD corp., Gyeonggi-do, Korea). The pH was adjusted to 7.3 ± 0.2 using 6 N NaOH. A mixed solution containing ammonium chloride (200 g/L) and Na2S·9H2O (100 g/L) was manually injected to maintain NH4+ and sulfide concentration at ~1 g/L of NH4Cl and 0.5 g/L of Na2S∙9H2O (the concentration of the medium), respectively, when the daily measurement was lower than 0.5 g/L of ammonium chloride.

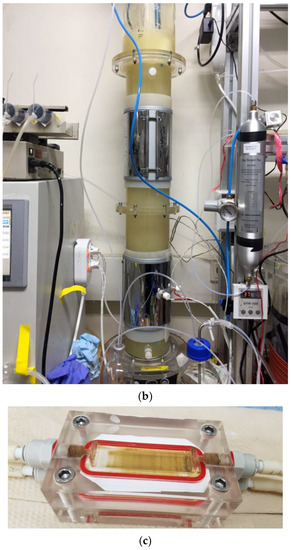
Figure 2.
Images of the stirred-tank reactor (ST) (a), the bubble column reactor (BC) (b), and the membrane distillation (MD) module (c).
2.2.2. Bubble Column (BC)
A cylindrical reactor (15 L) was customized and prepared with polycarbonate (Figure 2b). The diameter and height were 120 mm and 1410 mm, respectively. A ceramic membrane diffuser (25 cm × 40 mm, 0.01–0.5 μm pore size, Korea Ceramic International, Gyeonggi-do, Korea) was used as a sparger to make 100–200 μm bubbles. To support the mixing of biomass, a peristaltic pump (GT-150D, Green Tech, Gyeonggi-do, Korea) was used for circulation with 1 L/min between the upper and lower points. The supply for ammonium and sulfide supplementation was the same as above in the ST. The working volume was 5 L, and the influent rate of mixed gases was 140 mL/min (CO2:H2 = 1:4, 8.4 h−1 inflow gas per working volume), which was controlled using digital mass flow controllers. Ammonium was added intermittently via syringe as an NH4Cl solution when ammonia deficiency was indicated by the cell concentration.
2.3. Membrane Distillation
As a hydrophobic membrane, a PVDF (polyvinylidene fluoride) membrane with a pore size of 0.2 μm (GVS Korea Ltd., Namyangju-si, Korea) with an effective membrane area of 0.3 cm2 was used for the MD process. The acrylic module was used and consisted of a feed compartment (10 × 6 × 2 cm3), PVDF membrane (7 × 2.5 cm2) and permeate compartment (10 × 6 × 2 cm3) (Figure 2c). The performance of MD was compared with feed without cells (medium) and with cells (culture solution, after growth in medium). Two types of feeds at 60 °C, i.e., fresh medium and culture solution (optical density (O.D.) at 600 nm = ~4, equal to 1.6 g MLVSS/L), were used to compare the water removal rates. To prevent cell fracture, the pump for the cell culture was a magnetic gear pump (EMS Tech, Gyeonggi-do, Korea) operated at 800 mL/min at 60 °C, which was controlled by a heat exchanger. The permeate was anaerobically treated with distilled water and pumped with a peristaltic pump (Cole-Parmer, Vernon Hills, IL, USA) at 600 mL/min at 20 °C, which was controlled by a low-temperature circulator (CCA-1112A, Sunileyela, Gyeonggi-do, Korea). The Reynolds numbers of feed and permeate are 3302.36 and 1171.78, respectively. The mass of the water collected by the condenser was measured by an electronic balance [28]. The conductivity was measured using an edge® multiparameter EC/TDS/salinity meter (HI2030-01, Hanna instruments, Smithfield, RI, USA) to ensure that only water, and not ions, was removed from the feed. In addition, ammonium was analyzed in the permeate to ensure that only water was removed by using a Merck RQflex reflectometer (10007723, Merck, Darmstadt, Germany).
2.4. Monitoring and Experimental Analysis
The methanogen concentration was measured by optical density using UV-VIS spectrophotometry (Libra S22, Biochrom, UK) at 600 nm. Methane production was measured by GC-TCD (gas chromatography thermal-conductivity detector 7890A, Agilent, Santa Clara, CA, USA) with a Porapak Q Column (Supelco, Inc, 6 ft × 1/8 in, SS, 80/100 mesh) and oven, injector, and detector temperatures of 80 °C, 0 °C, and 230 °C, respectively. Gas analysis using GC-TCD was expressed as gas composition (Pgas, %) using standard calibration prepared with a known gas composition sets containing H2, CO2, and CH4. The effluent gas amount (volume) decreased because of the decompression reaction (4H2 + CO2 → CH4 + 2H2O) and can be deduced from the methane production, as shown in Equation (2). The methane production rate was calculated as VVD (volume of methane production per working volume per day), as shown in Equation (3).
The nitrogen concentration of ammonium was determined reflectometrically, using test strips (RQflex10, Merck, Darmstadt, Germany). The increase in conductivity due to the supplementation with nitrogen, sulfide, and base solution for pH control was monitored using a multiparameter EC/TDS/salinity meter (HI2030-01, Hanna Instruments, Woonsocket, RI, USA).
3. Results and Discussion
3.1. Methane Production in the ST and BC
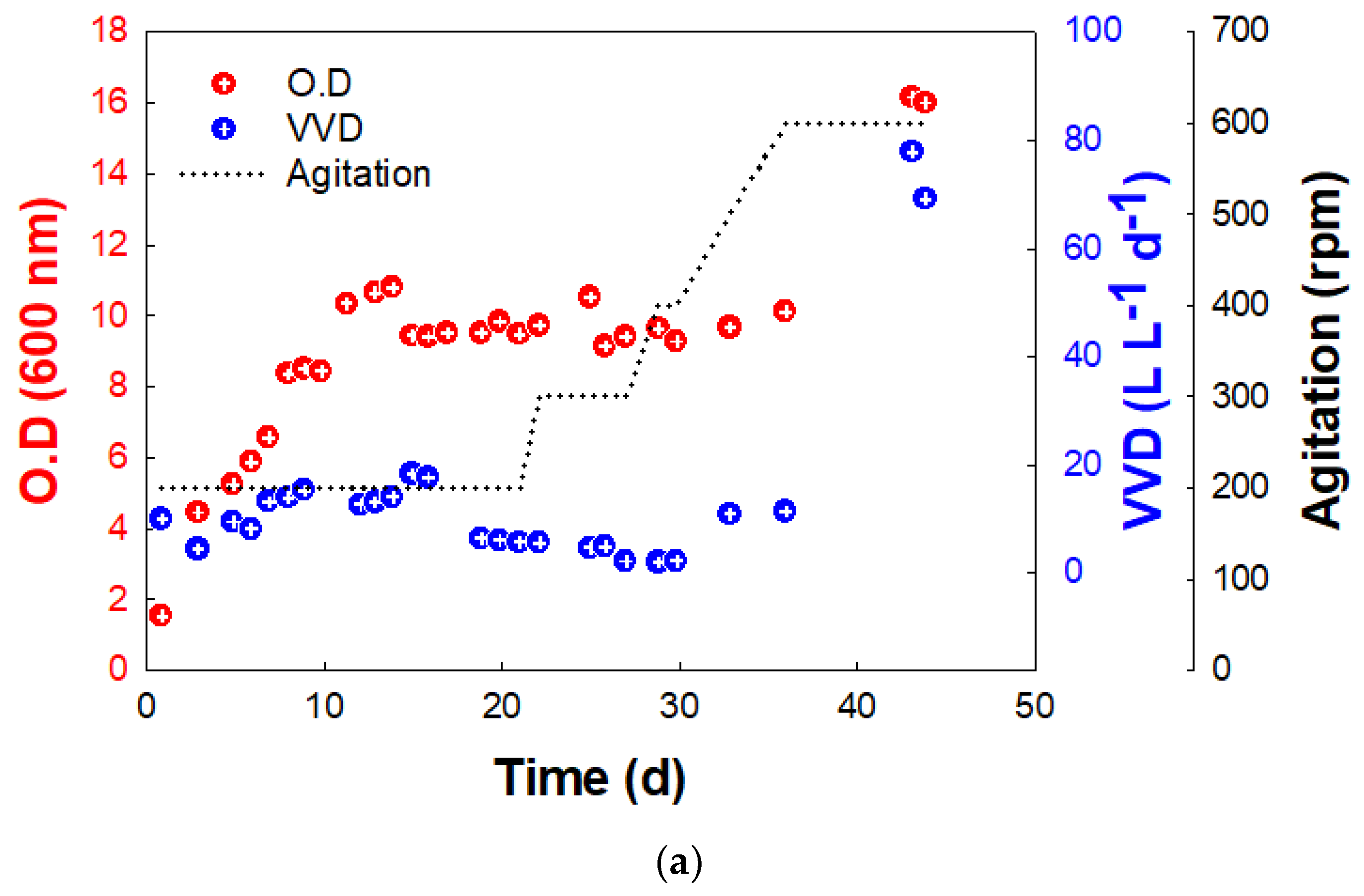
In the ST, KEPCO-1 showed a specific growth rate of 0.035 h−1 without a lag period (Figure 3a). After the 13th day, the concentration of KEPCO-1 was not significantly changed until 37 days, and the mean VVD was 9.5 ± 5.6 (Figure 3a). On the 32nd day, the agitation rate was changed to 500 rpm, and both O.D. and VVD began to increase to 16.2 and 77.8, respectively (Figure 3a). Previous studies have shown that the rate of methane production was significantly enhanced when the agitation rate was increased in the reactor [10]. The intense mixing of the substrate in the gas and the biocatalyst in the liquid (gas-liquid mass transfer) increased the CO2 conversion to CH4.
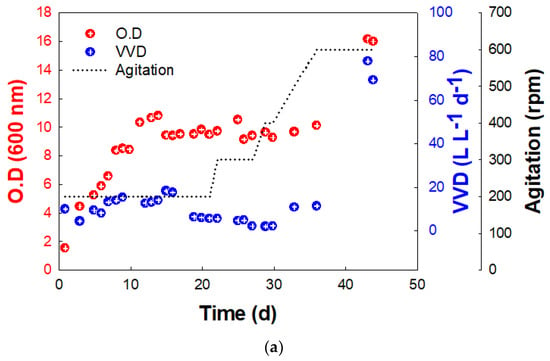
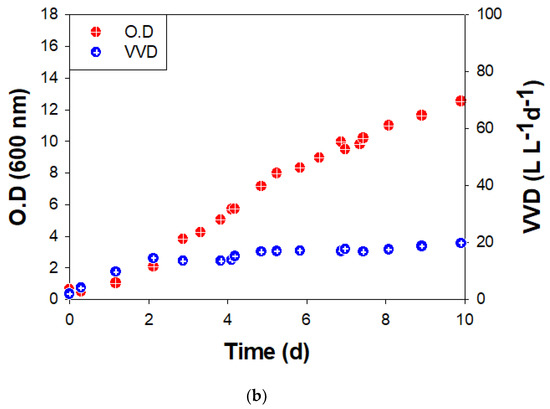
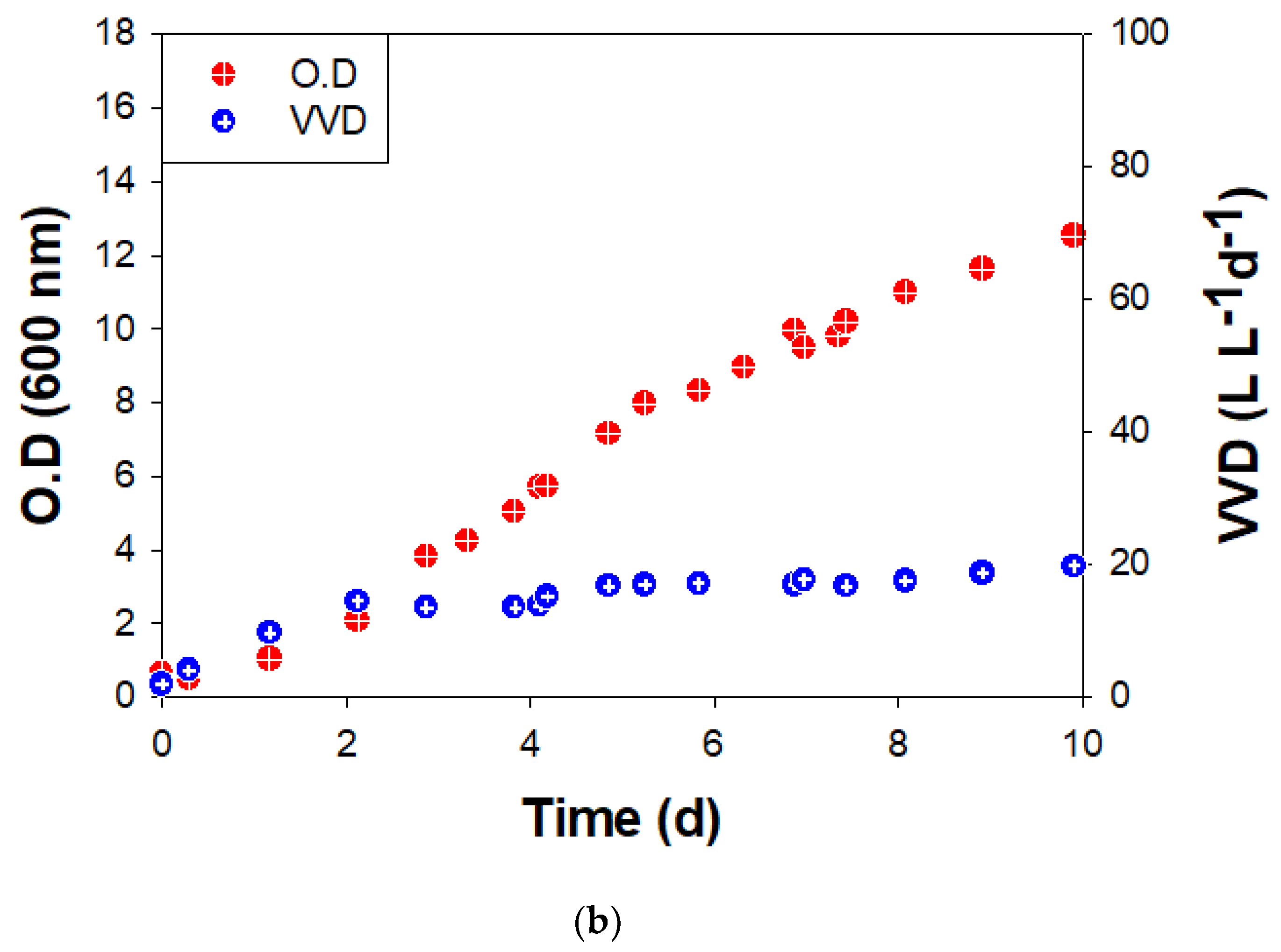
Figure 3.
Growth (O.D.at 600 nm) and methane production rate (VVD) of BS-9 (a) in ST and (b) BC reactors.
In the BC, O.D. also increased without a lag period after 10% inoculation at an initial O.D. = 0.63. The maximum specific growth rate (μmax) was 0.034 h−1, and the final O.D. was 12.5 on the 10th day (Figure 3b). The methane conversion rate was relatively stable as ~20 VVD (Figure 3b). The final O.D. was not changed after 10 days. The limit of the methane production rate (VVD) seems to be due to the low solubility of hydrogen and limitations of mass transfer, such as low mixing efficiency between cells in liquid (biocatalyst) and gas (substrate) in the BC [29,30].
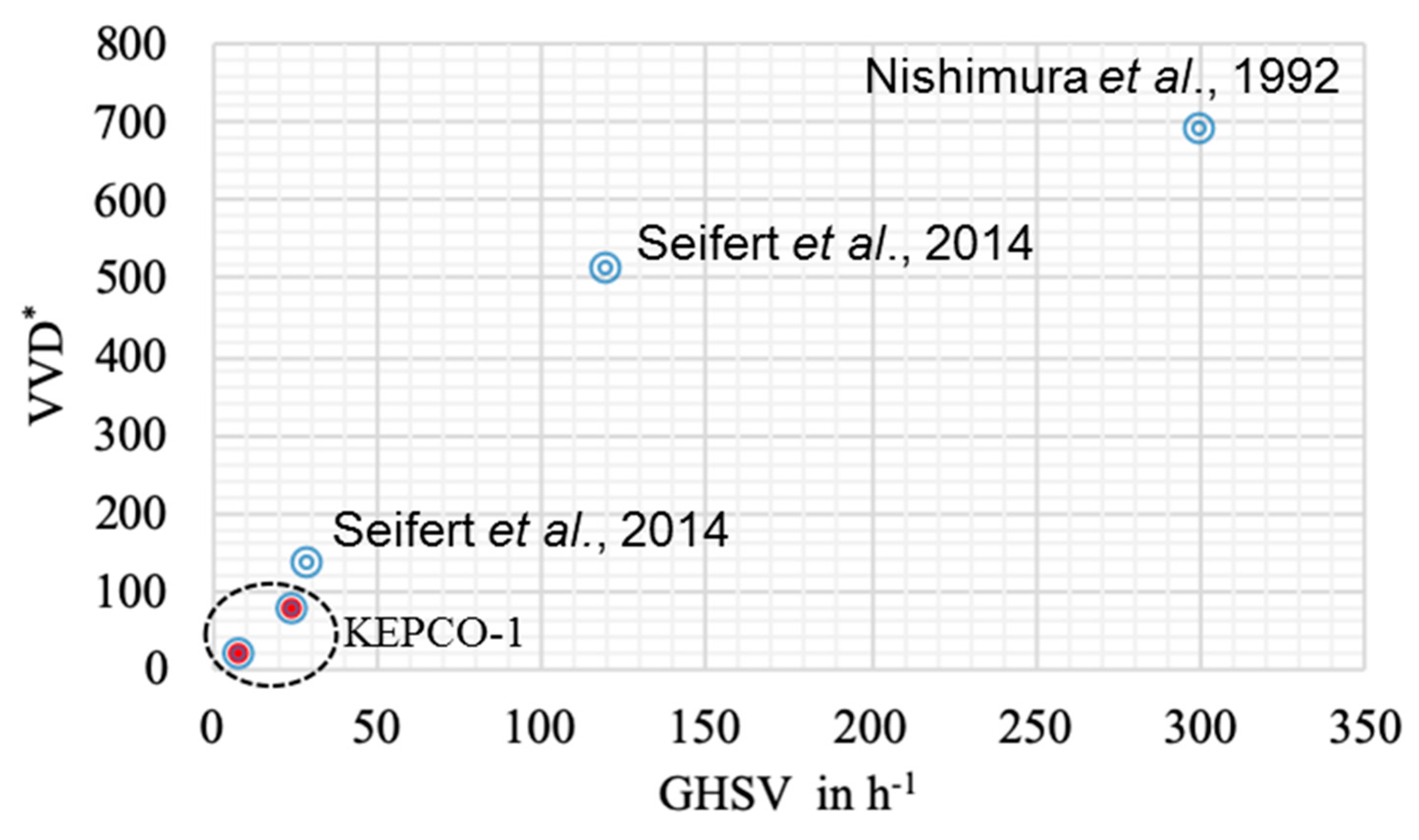
VVD is dependent on the gas inflow rate, as shown in Equation (1). Previous studies have also reported that a higher gas hourly space velocity (GHSV) led to higher methane productivity (Figure 4). Compared with previous reports [9,31] that show a high methane production rate at high GHSV, the methane production rate in this study showed a convincing conversion rate at low GHSV (Figure 4). The ratio of VVD versus O.D. can indicate the activity of methanogen activity as biocatalysts. Comparing the ST and BC data, it can be seen that the three-fold higher value of VVD/O.D. in the ST (4.8 vs. 1.6) was due to the GHSV value of the ST being three times larger (24 vs. 8.4, Table 1). In addition, the agitation speed in the ST increased the ratio of VVD/O.D.
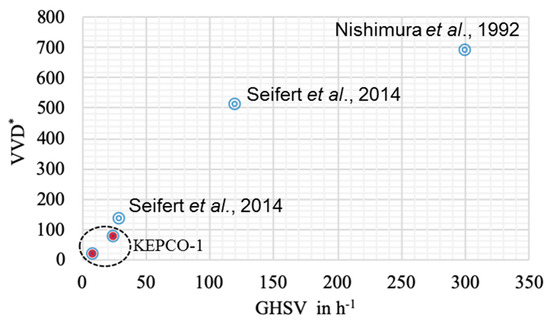
Figure 4.
Comparison of the methane production rate with previous reports. The methane production rate (* VVD, volume of methane per volume of reactor per day) as a function of gas hourly space velocity (GHSV).

Table 1.
Comparison of operation parameters and biomethanation process between the -ST and BC reactors.
The characterization of the gas mass transfer is described by the volumetric mass transfer coefficient, which is dependent on various parameters, such as bioreactor design and operating conditions [32]. In detail, volumetric mass transfer was affected by changes in the stirrer speed and gas superficial velocity in the ST [33] and by the superficial gas velocity, the properties of the fluid and the geometric factors (height of the column, diameter of the column, and the sparger characteristics) in the BC [34]. Mass transfer between gas and liquid could be one of the main considerations in the design of biomethanation reactors. Microbubble generation (100–200 μm bubbles size advertised by the manufacturer) in the BC showed a comparable methane conversion rate with that in the ST operated with high agitation speed. This result suggested that microbubble generation could be used from an economic point perspective to increase the mass transfer depending on the reaction scale.
3.2. Water Generation and Removal from Reactors
Hydrogenotrophic methanation generates two moles of water during one mole of methane production as shown in Equation (1). Thus, the amounts of water generated daily can be calculated from methane production (Equation (4), using ideal gas volume, 22.4 L/mol at STP, water density 1 g/mL, water molecular weight 18 g/mol).
Previous studies have reported water removal in chemical methanation processes using absorption on the catalyst [35] or adsorbent [36] (Sabatier process). On the other hand, during the microbial methanation process, the following two facts are inevitable: The active medium volume increases due to water generation from the methanation reaction and, consequently, the nutrient concentration utilized by methanogens for their growth and methane production decreases. Since severe nutrient dilution can occur due to water generation, except that utilized by methanogens, stable methanation performance requires the intermittent injections of concentrated nutrients and removal of generated water by pumps [30]. In particular, ammonium as the nitrogen source and sulfide to maintain redox potential should be sufficiently supplied for methanogen growth and methanation [37]. To remove only the generated water without discharging nutrients from the reactors, the MD process was integrated with the methanation process.
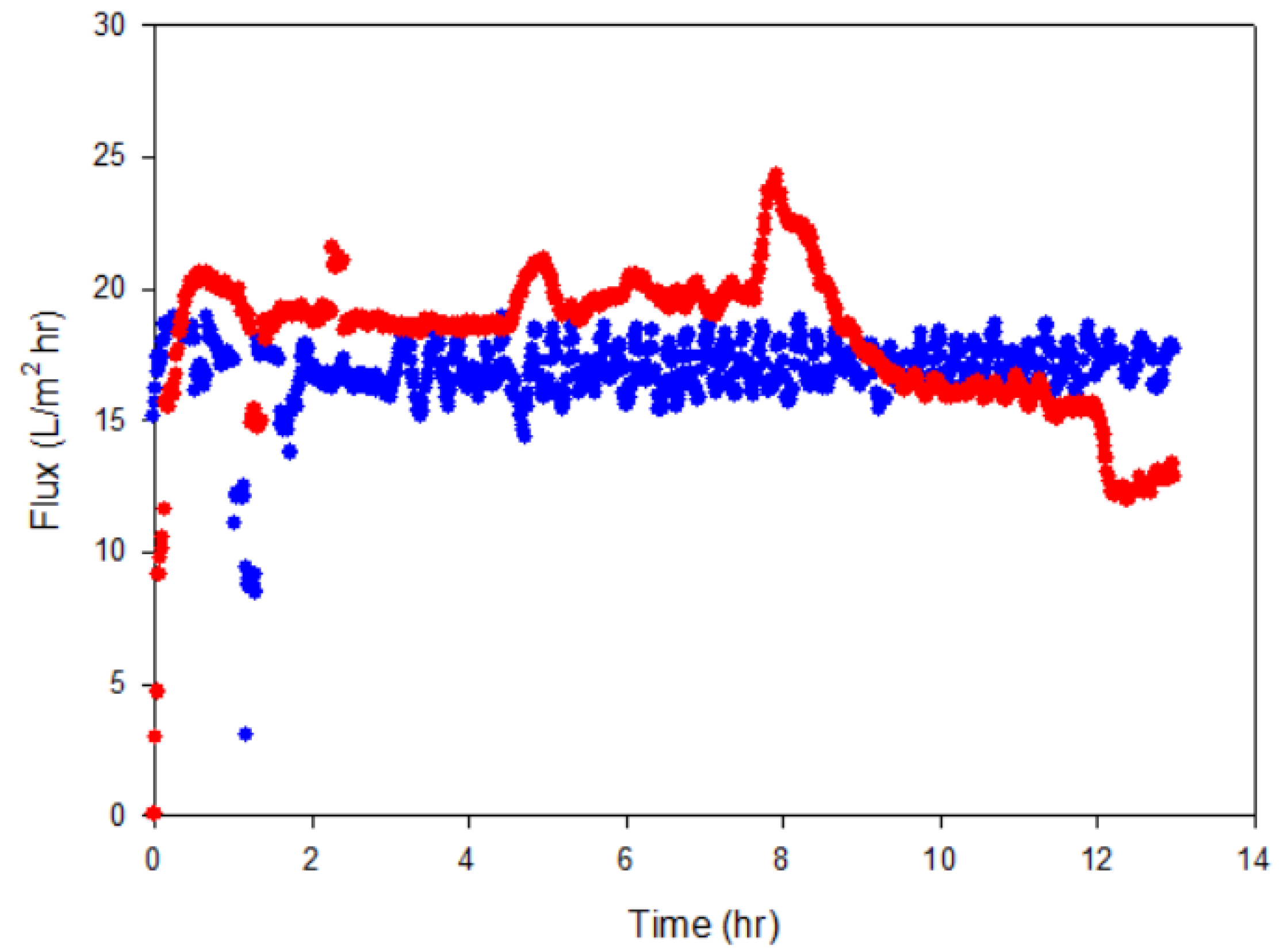
The water removal performance of MD in cell cultures was compared with that in the medium only. The membrane flux was 16.3 ± 3.1 L/m2/h and 19.2 ± 3.3 L/m2/h, respectively (Figure 5). This value was higher than that in previous reports of 4.5~6 L/m2/h using PVDF with the same temperature difference, 20 °C vs. 60 °C [38]. After operation, the membrane surface was visually checked and cell fouling was not significant, which seems to be due to low biomass (~1.6 g MLVSS/L).
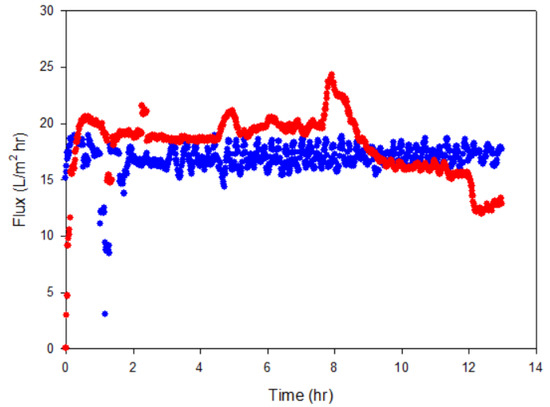
Figure 5.
The membrane flux during water removal from the biomethanation process using membrane distillation (MD) with medium (blue line) and with cell culture (red line).
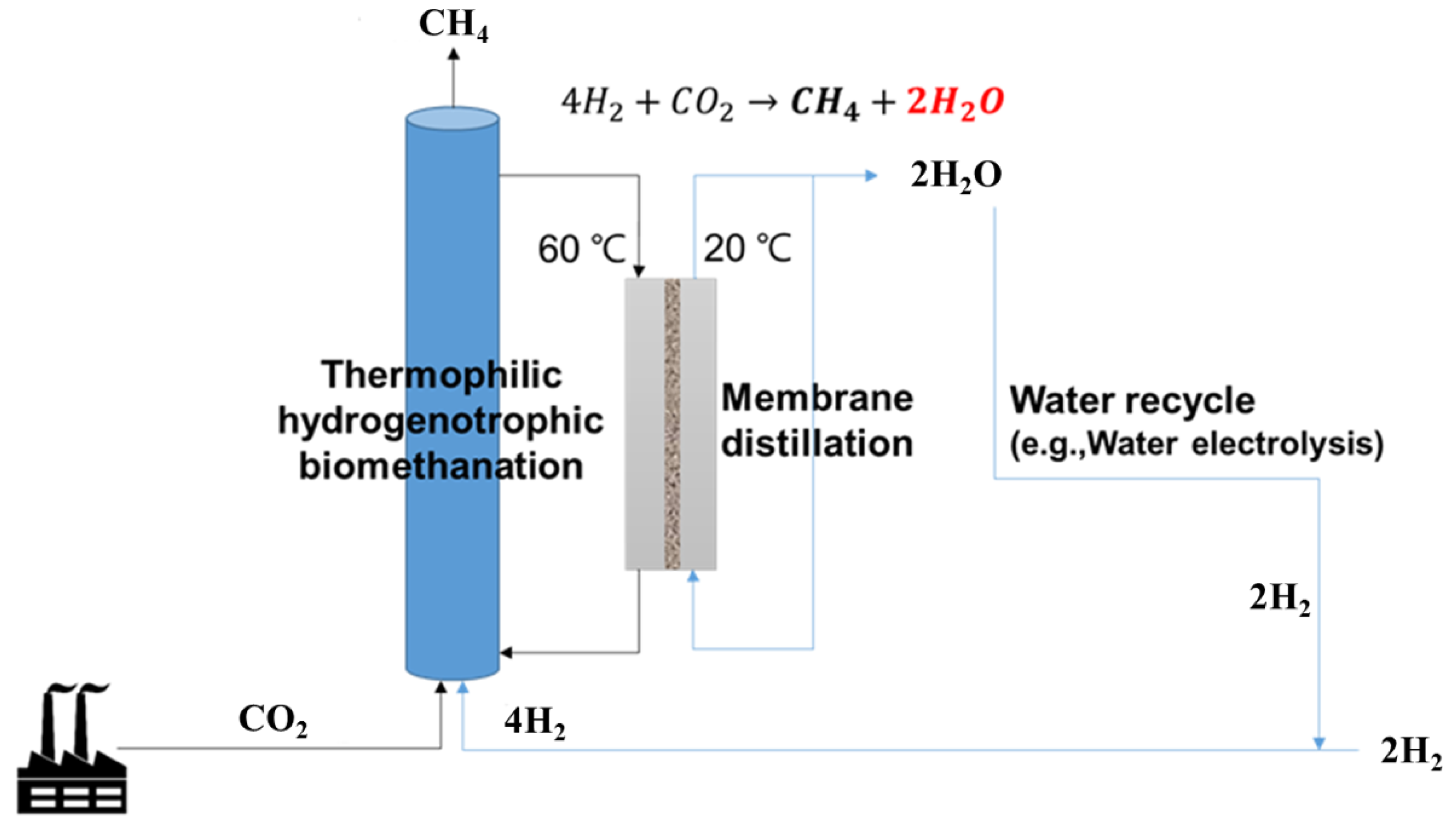
For hydrogenotrophic methanation, four moles of hydrogen are consumed to convert one mole of the CO2 into one mole of methane (Equation (1)). Four moles of hydrogen are equally divided into methane and water (Equation (1)). Thus, by using the water removed through the MD process for hydrogen production in an electrolyzer, water can be recycled, and the loss of hydrogen discarded as water can also be reduced. When small scale ex-situ biomethanation is built with a working volume of 120 L and a methane production rate of 400 VVD (Figure 6), the amount of generated water was 3.2 L/h (76.8 L/d), which was theoretically calculated as described in Equation (4). Based on our MD results, a membrane area of just 0.19 m2 is required, with an average water removal of 17 L/m2/h.
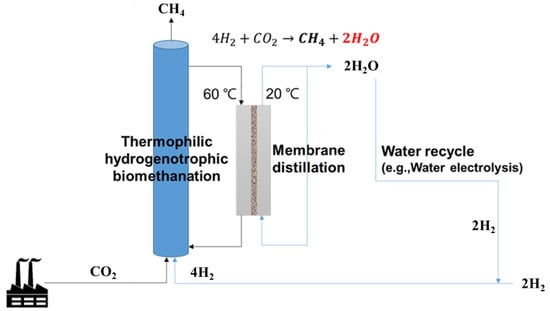
Figure 6.
The biomethanation–membrane distillation combination process.
To ensure that only water was removed from the culture medium in the MD process, the conductivity and ammonium concentration were measured. The conductivity and total N in the feed of the MD process were 13~16 mS/cm and 260 mg N/L at 1 g NH4Cl/L, respectively. The conductivity in the permeate was approximately 1/1000 of feed (up to ~30 μS/cm), and the total N was less than 5.5 mg N/L (see Figure S1 in Supplementary Materials). These results indicate that only water was removed from the medium through MD and that most of the nutrients in the medium were left in the reactor and could be used to continuously maintain the activity of the methanogens. However, the total nitrogen (TN) increased as the experiments continued (Figure S1), indicating membrane pore wetting [39,40]. Long-term operation requires the study of cell fouling and wetting phenomena during membrane distillation. If water is discharged from the reactor due to the amount of water produced from methane production and is not removed through the MD, a high concentration of nitrous wastewater of about 1 g/L is continuously generated and contained in the methanation reaction medium. By selectively removing only water from the methane reactor using MD, it is possible to reduce the process installation and operating costs associated with treating high concentrations of nitrous wastewater. This cost reduction is another important advantage that can be obtained by integrating the MD process with a methanation reactor.
4. Conclusions
Biomethanation used in two types of reactors showed that a high agitation speed increased the methane conversion rate. The use of a microbubble generator in a cylinder type reactor showed a relatively stable methane conversion rate. It is reasonable to consider the application of microbubbles for biomethanation to enhance gas–liquid mass transfer. The use of MD for water removal from biomethanation can be a useful method to recover hydrogen present in water, i.e., half of the hydrogen used for biomethanation, and the use of MD can reduce the cost of wastewater treatment from biomethanation reactor discharge.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1073/12/21/4130/s1, Figure S1: (a) Conductivity and (b) total nitrogen (TN) in the permeate.
Author Contributions
Data curation, M.K., B.K., Y.S., J.S.J., B.S.J. and B.-I.S.; Formal analysis, Y.G., Y.G.K. and B.-I.S.; Investigation, O.C.; Methodology, M.-G.H. and B.S.J.; Project administration, J.S.J.; Supervision, S.L. and B.-I.S.
Funding
This work was supported by the Korea Electric Power Research Institute (KEPRI, Korea) grant funded by the Korea Electric Power Corporation (KEPCO, Korea) (No. R17VA01), by the Korea Institute of Energy Technology Evaluation and Planning(KETEP) and the Ministry of Trade, Industry & Energy(MOTIE) of the Republic of Korea (No. 2019281010007B), and by Hanyang University (HY-201100000000233-N).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| MD | Membrane distillation |
| ST | Stirred-tank reactor |
| BC | Bubble column |
| PVDF | Polyvinylidene fluoride |
| VSS | Volatile suspended solid |
| MLVSS | Mixed liquor volatile suspended solid |
| VVD | Methane volume per working volume per day, L/L/d |
| GHSV | Gas hourly space velocity |
References
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Götz, M.; McDaniel Koch, A.; Graf, F. State of the art and perspectives of CO2 methanation process concepts for power-to-gas applications. In Proceedings of the International Gas Union Research Conference (IGRC), Copenhagen, Denmark, 17–19 September 2014. [Google Scholar]
- Lindorfer, J.; Reiter, G.; Tichler, R.; Steinmüller, H. 14—Hydrogen fuel, fuel cells, and methane. In Managing Global Warming; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 419–453. [Google Scholar] [CrossRef]
- Ghaib, K.; Ben-Fares, F.-Z. Power-to-Methane: A state-of-the-art review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Taubner, R.S.; Schleper, C.; Firneis, M.G.; Rittmann, S.K. Assessing the ecophysiology of methanogens in the context of recent astrobiological and planetological studies. Life 2015, 5, 1652–1686. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Angelidaki, I.; Alvarado-Morales, M.; Liu, H.; Liu, Y.; Huang, X.; Zhu, G. Methane production from formate, acetate and H2/CO2; focusing on kinetics and microbial characterization. Bioresour. Technol. 2016, 218, 796–806. [Google Scholar] [CrossRef]
- Jabłoński, S.; Rodowicz, P.; Łukaszewicz, M. Methanogenic archaea database containing physiological and biochemical characteristics. Int. J. Syst. Evol. Microbiol. 2015, 65, 1360–1368. [Google Scholar] [CrossRef]
- Nishimura, N.; Kitaura, S.; Mimura, A.; Takahara, Y. Growth of thermophilic methanogen KN-15 on H2-CO2 under batch and continuous conditions. J. Ferment. Bioeng. 1991, 72, 280–284. [Google Scholar] [CrossRef]
- Nishimura, N.; Kitaura, S.; Mimura, A.; Takahara, Y. Cultivation of thermophilic methanogen KN-15 on H2-CO2 under pressurized conditions. J. Ferment. Bioeng. 1992, 73, 477–480. [Google Scholar] [CrossRef]
- Peillex, J.-P.; Fardeau, M.-L.; Belaich, J.-P. Growth of Methanobacterium thermoautotrophicum on on hydrogen-carbon dioxide: High methane productivities in continuous culture. Biomass 1990, 21, 315–321. [Google Scholar] [CrossRef]
- Alitalo, A.; Niskanen, M.; Aura, E. Biocatalytic methanation of hydrogen and carbon dioxide in a fixed bed bioreactor. Bioresour. Technol. 2015, 196, 600–605. [Google Scholar] [CrossRef]
- Burkhardt, M.; Busch, G. Methanation of hydrogen and carbon dioxide. Appl. Energy 2013, 111, 74–79. [Google Scholar] [CrossRef]
- Dupnock, T.L.; Deshusses, M.A. High-performance biogas upgrading using a biotrickling filter and hydrogenotrophic methanogens. Appl. Biochem. Biotechnol. 2017, 183, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.S.; Yano, T.; Nishio, N.; Nagai, S. Biomethanation of H2 and CO2 by Methanobacterium thermoautotrophicum in membrane and ceramic bioreactors. J. Ferment. Technol. 1987, 65, 413–418. [Google Scholar] [CrossRef]
- Karadagli, F.; Rittmann, B.E. Thermodynamic and kinetic analysis of the H2 threshold for Methanobacterium bryantii MoH. Biodegradation 2007, 18, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kim, J.H.; Chang, W.S.; Pak, D. Biological conversion of CO2 to CH4 using hydrogenotrophic methanogen in a fixed bed reactor. J. Chem. Technol. Biotechnol. 2012, 87, 844–847. [Google Scholar] [CrossRef]
- Rittmann, S.; Seifert, A.; Herwig, C. Quantitative analysis of media dilution rate effects on Methanothermobacter marburgensis grown in continuous culture on H2 and CO2. Biomass Bioenergy 2012, 36, 293–301. [Google Scholar] [CrossRef]
- Schill, N.; Van Gulik, W.; Voisard, D.; Von Stockar, U. Continuous cultures limited by a gaseous substrate: Development of a simple, unstructured mathematical model and experimental verification with Methanobacterium thermoautotrophicum. Biotechnol. Bioeng. 1996, 51, 645–658. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Hsu, S.T.; Cheng, K.T.; Chiou, J.S. Seawater desalination by direct contact membrane distillation. Desalination 2002, 143, 279–287. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard, V.J.H. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M.; Karakulski, K. Wastewater treatment by membrane distillation. Desalination 2006, 198, 67–73. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Hafidi, A.; Khayet, M.; García-Payo, M. Integrated direct contact membrane distillation for olive mill wastewater treatment. Desalination 2013, 323, 31–38. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M.; Grzechulska, J.; Morawski, A. Membrane distillation of NaCl solution containing natural organic matter. J. Membr. Sci. 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Park, S.-M.; Lee, S. Influence of Hydraulic Pressure on Performance Deterioration of Direct Contact Membrane Distillation (DCMD) Process. Membranes (Basel) 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Choi, Y.-J.; Lee, S.; Koo, J.; Huang, T. Comparison of hollow fiber membranes in direct contact and air gap membrane distillation (MD). Desalin. Water Treat. 2016, 57, 10012–10019. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wen, X.; Shimizu, K.; Lei, Z.; Kobayashi, M.; Zhang, Z.; Sumi, I.; Yao, Y.; Mogi, Y. Enhanced bioconversion of hydrogen and carbon dioxide to methane using a micro-nano sparger system: Mass balance and energy consumption. RSC Adv. 2018, 8, 26488–26496. [Google Scholar] [CrossRef]
- Martin, M.R.; Fornero, J.J.; Stark, R.; Mets, L.; Angenent, L.T. A single-culture bioprocess of Methanothermobacter thermautotrophicus to upgrade digester biogas by CO2-to-CH4 Conversion with H2. Archaea 2013, 2013, 11. [Google Scholar] [CrossRef]
- Seifert, A.H.; Rittmann, S.; Herwig, C. Analysis of process related factors to increase volumetric productivity and quality of biomethane with Methanothermobacter marburgensis. Appl. Energy 2014, 132, 155–162. [Google Scholar] [CrossRef]
- Braga, A.; Mesquita, D.P.; Amaral, A.L.; Ferreira, E.C.; Belo, I. Aroma production by Yarrowia lipolytica in airlift and stirred tank bioreactors: Differences in yeast metabolism and morphology. Biochem. Eng. J. 2015, 93, 55–62. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Prediction of gas-liquid mass transfer coefficient in sparged stirred tank bioreactors. Biotechnol. Bioeng. 2005, 92, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Borgschulte, A.; Gallandat, N.; Probst, B.; Suter, R.; Callini, E.; Ferri, D.; Arroyo, Y.; Erni, R.; Geerlings, H.; Züttel, A. Sorption enhanced CO2 methanation. Phys. Chem. Chem. Phys. 2013, 15, 9620–9625. [Google Scholar] [CrossRef] [PubMed]
- Walspurger, S.; Elzinga, G.D.; Dijkstra, J.W.; Sarić, M.; Haije, W.G. Sorption enhanced methanation for substitute natural gas production: Experimental results and thermodynamic considerations. Chem. Eng. J. 2014, 242, 379–386. [Google Scholar] [CrossRef]
- Gerhard, E.; Butsch, B.M.; Marison, I.W.; von Stockar, U. Improved growth and methane production conditions for Methanobacterium thermoautotrophicum. Appl. Microbiol. Biotechnol. 1993, 40, 432–437. [Google Scholar] [CrossRef]
- Macedonio, F.; Ali, A.; Poerio, T.; El-Sayed, E.; Drioli, E.; Abdel-Jawad, M. Direct contact membrane distillation for treatment of oilfield produced water. Sep. Purif. Technol. 2014, 126, 69–81. [Google Scholar] [CrossRef]
- Goh, S.; Zhang, J.; Liu, Y.; Fane, A.G. Fouling and wetting in membrane distillation (MD) and MD-bioreactor (MDBR) for wastewater reclamation. Desalination 2013, 323, 39–47. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).