Chemical and Mineralogical Composition of Soot and Ash from the Combustion of Peat Briquettes in Household Boilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Mineralogical Methods
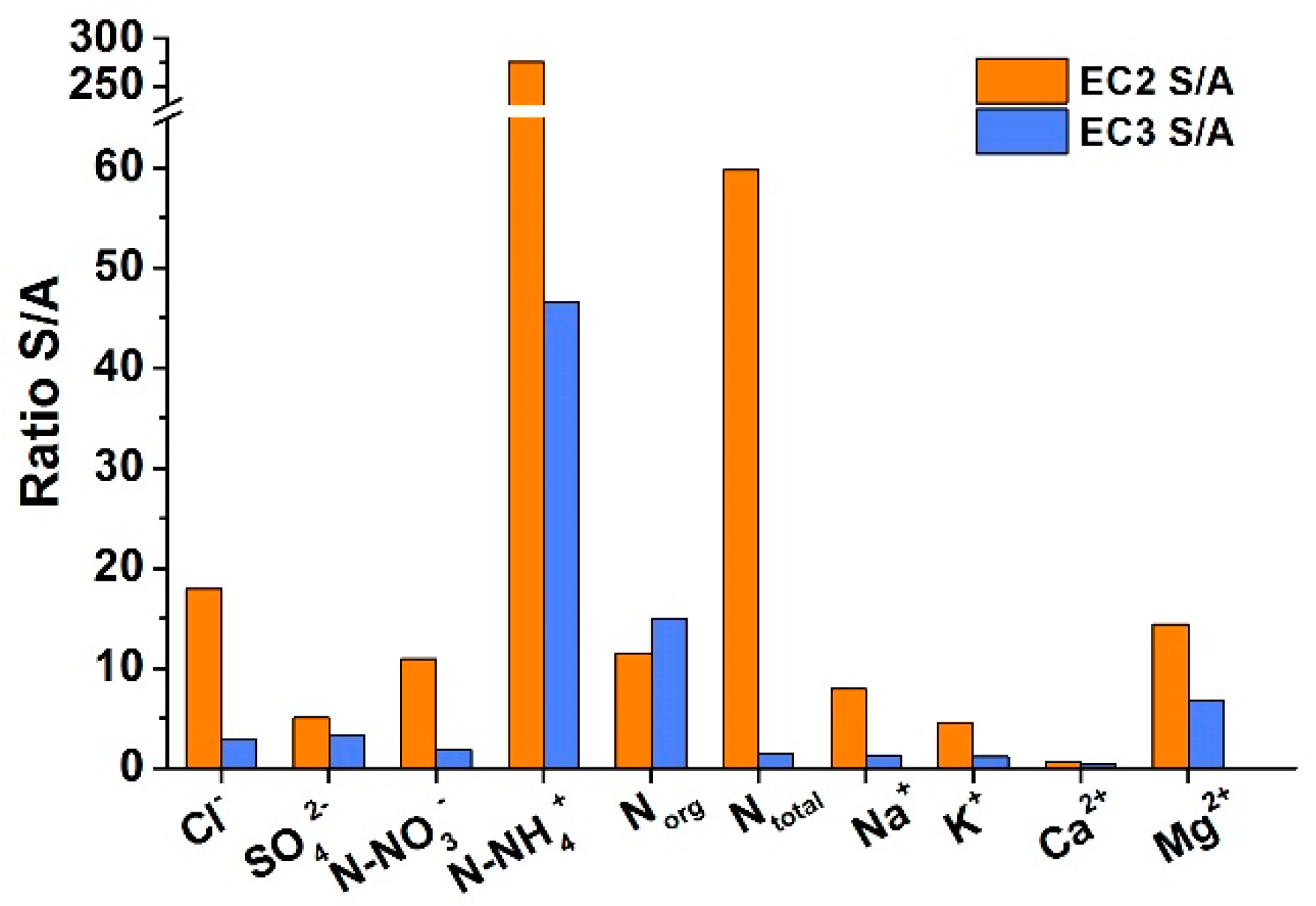
2.2. Leachability
3. Results and Discussion
3.1. The Conditions of the Combustion of Peat Briquettes
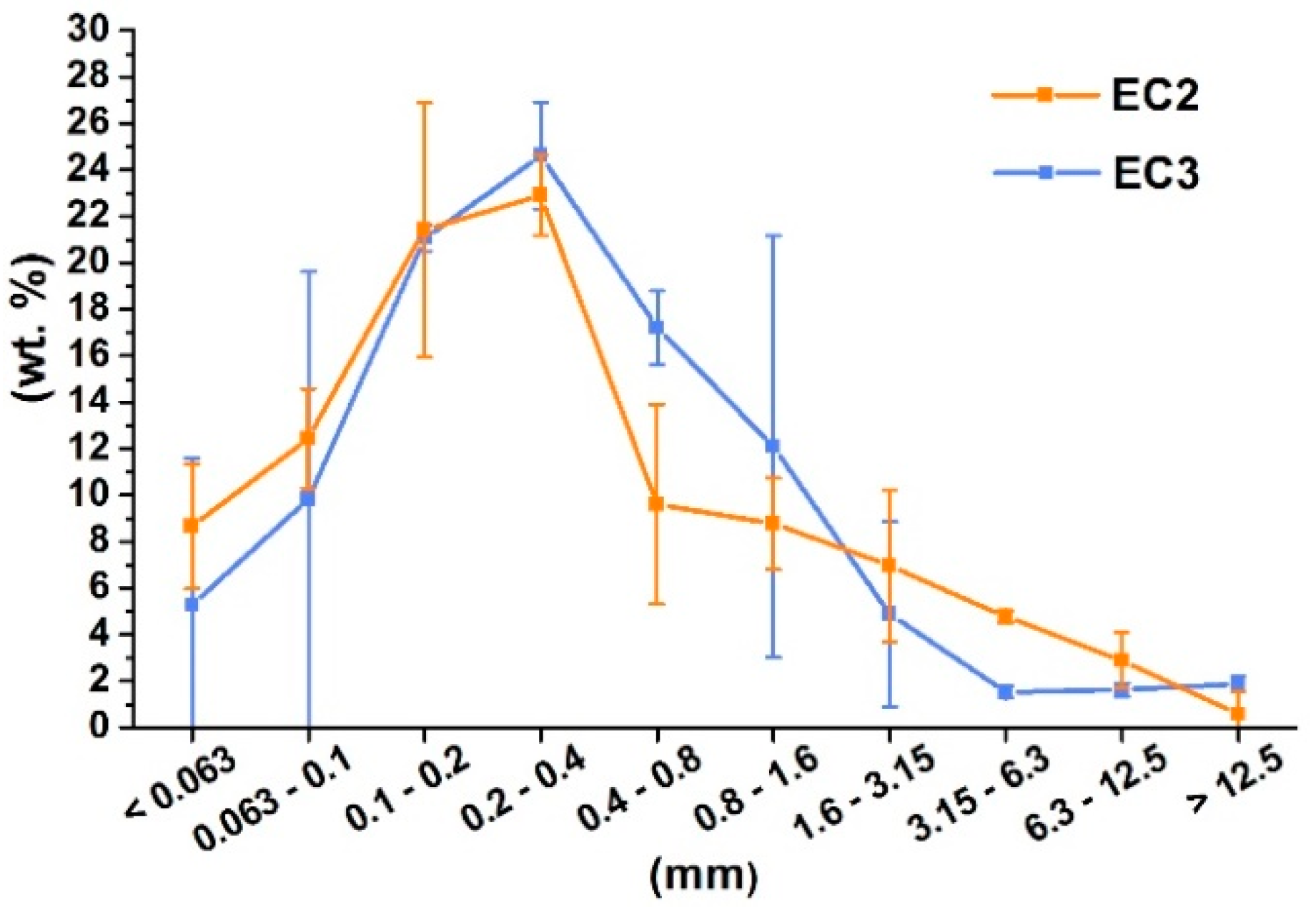
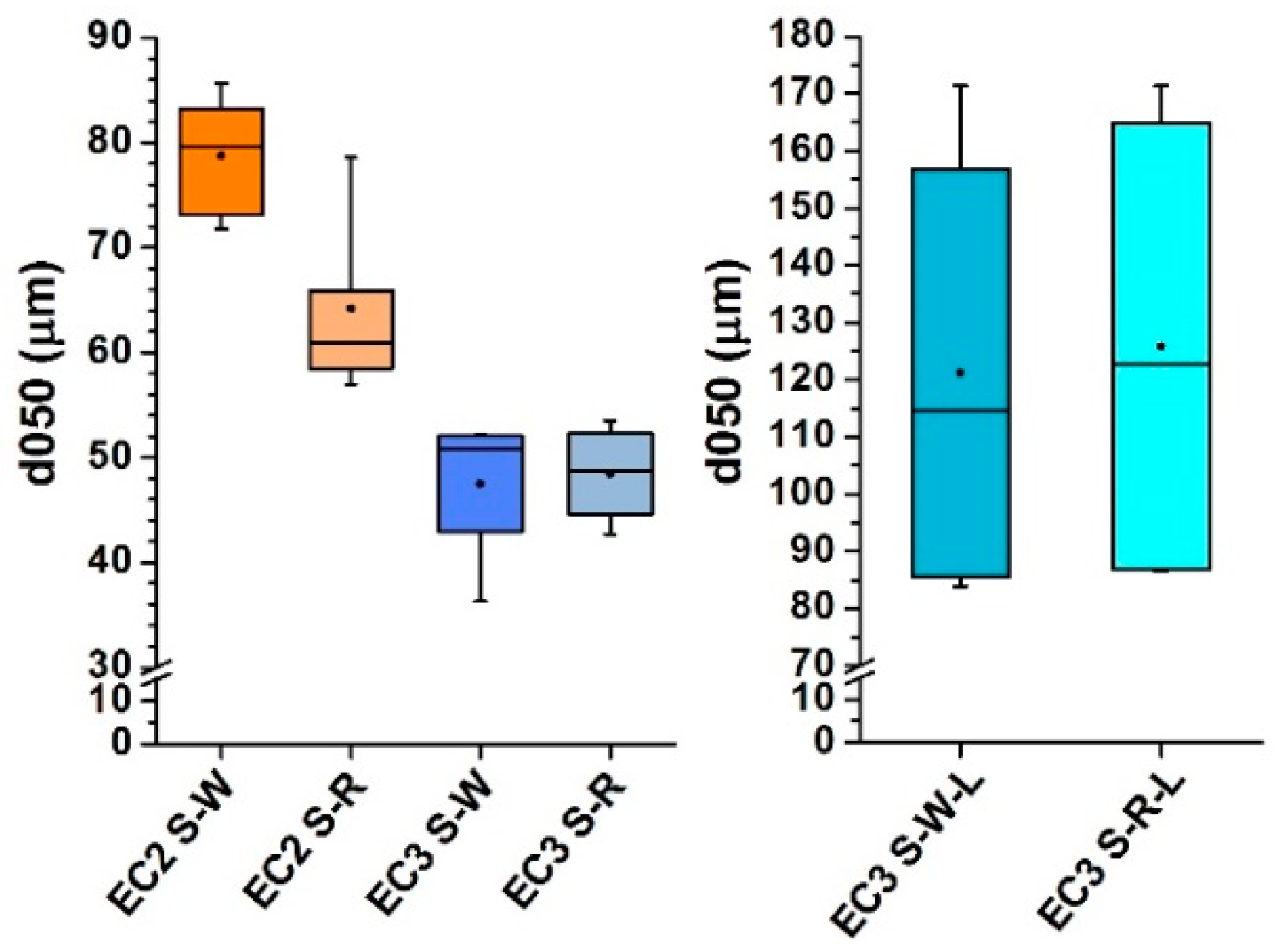
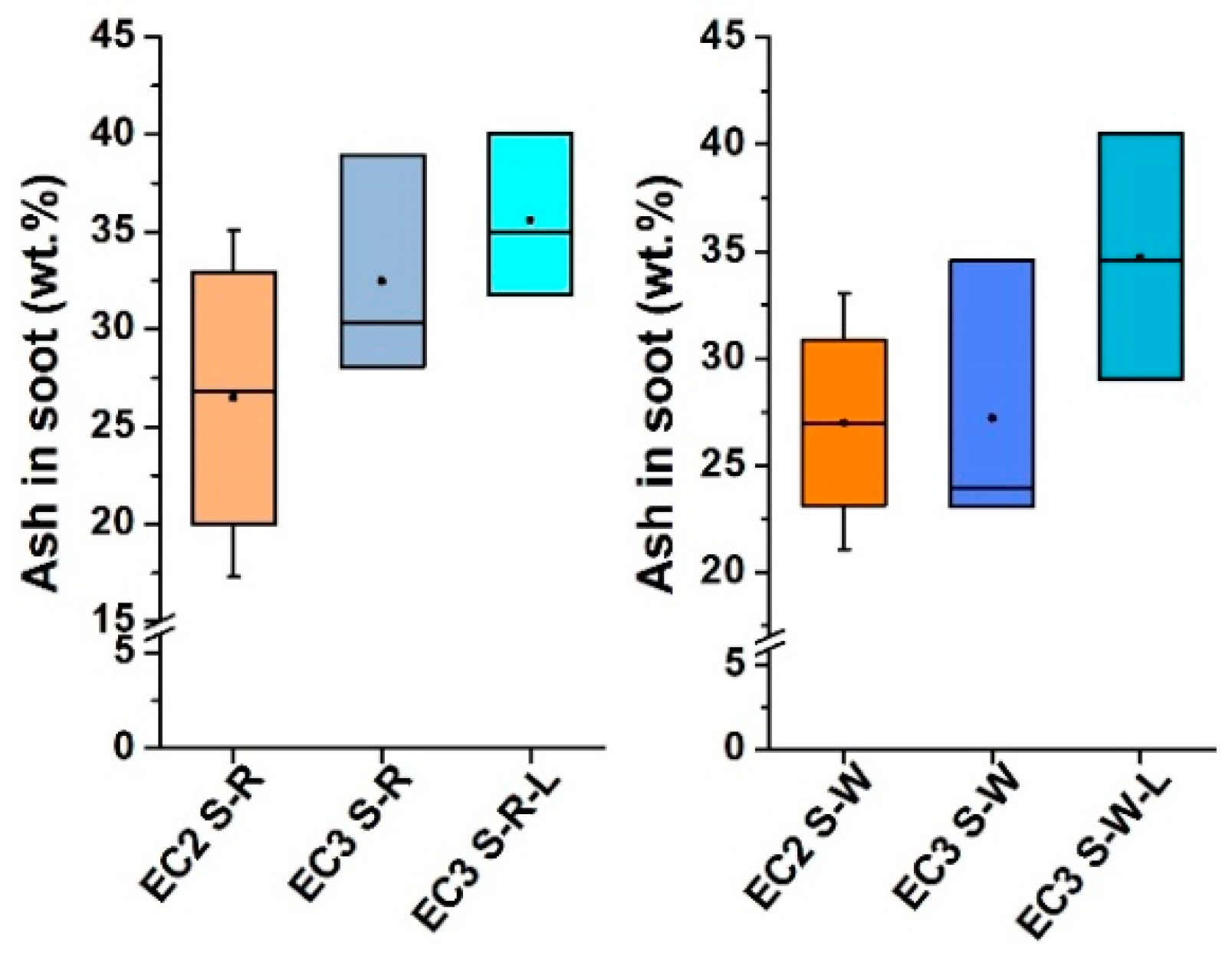
3.2. Product of the Combustion
3.3. Identification of Organic Compounds in Soot
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kowalczyk-Juśko, A.; Onuch, J.; Kościk, B.; Skowron, P.; Chołody, M.; Kosidło, A.; Rawski, J. Environmental and practical aspects of the use of peat for agriculture and energy aims. J. Ecol. Eng. 2016, 17, 138–142. [Google Scholar] [CrossRef]
- Kasiuliene, A.; Carabante, I.; Bhattacharya, P.; Caporale, A.G.; Adamo, P.; Kumpiene, J. Removal of metal(oid)s from contaminated water using iron-coated peat sorbent. Chemosphere 2018, 198, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Naafs, B.D.A.; Inglis, G.N.; Blewett, J.; McClymont, E.L.; Lauretano, V.; Xie, S.; Evershed, R.P.; Pancost, R.D. The potential of biomarker proxies to trace climate, vegetation, and biogeochemical processes in peat: A review. Glob. Planetary Chang. 2019, 179, 57–79. [Google Scholar] [CrossRef]
- Joseph, I.V.; Roncaglia, G.; Tosheva, L.; Doyle, A.M. Waste peat ash mineralogy and transformation to microporous zeolites. Fuel Process. Technol. 2019, 194, 106124. [Google Scholar] [CrossRef]
- Montanarella, L.; Jones, R.J.A.; Hiederer, R. The distribution of peatland in Europe. Mires Peat 2006, 1, 1–11. [Google Scholar]
- Köchy, M.; Hiederer, R.; Freibauer, A. Global distribution of soil organic carbon–Part 1: Masses and frequency distributions of SOC stocks for the tropics, permafrost regions, wetlands, and the world. Soil 2015, 1, 351–365. [Google Scholar] [CrossRef]
- Tanneberger, F.; Moen, A.; Joosten, H.; Nilsen, N. The peatland map of Europe. Mires Peat 2017, 19, 1–17. [Google Scholar]
- Xu, J.; Morris, P.J.; Liu, J.; Holden, J. PEATMAP: Refining estimates of global peatland distribution based on a meta-analysis. Catena 2018, 160, 134–140. [Google Scholar] [CrossRef]
- Minayeva, T.; Sirin, A.; Bragg, O. A Quick Scan of Peatlands in Central and Eastern Europe; Wetlands International: Wageningen, The Netherlands, 2009. [Google Scholar]
- Volkova, I.I.; Kolesnichenko, L.G.; Kirpotin, S.N.; Pokrovsky, O.S.; Vorobyev, S.N. Peat deposits and peat-forming plants in the mires of the West Siberian northern taiga (based on studies of the Khanymei site). IOP Conf. Ser. Earth Environ. Sci. 2019, 232, 012018. [Google Scholar] [CrossRef]
- Gerasimov, Y. Energy Sector in Belarus: Focus on Wood and Peat Fuels; Finnish Forest Research Institute: Vantaa, Finland, 2010. [Google Scholar]
- Sutejo, Y.; Saggaff, A.; Rahayu, W.; Hanafiah, I. Physical and chemical characteristics of fibrous peat. In Proceedings of the 3rd International Conference on Construction and Building Engineering, Palembang, Indonesia, 14–17 August 2017; AIP Publishing: Melville, NY, USA, 2017; p. 090006. [Google Scholar]
- Chow, J.C.; Cao, J.; Chen, L.-W.A.; Wang, X.; Wang, Q.; Tian, J.; Ho, S.S.H.; Carlson, T.B.; Kohl, S.D.; Watson, J.G. Changes in PM2.5 Peat Combustion Source Profiles with Atmospheric Aging in an Oxidation Flow Reactor. Atmospheric Measurement Techniques Discussions, 2019; 1–44, (under review). [Google Scholar] [CrossRef]
- Hu, Y.; Christensen, E.; Restuccia, F.; Rein, G. Transient gas and particle emissions from smouldering combustion of peat. Proceed. Combust. Inst. 2019, 37, 4035–4042. [Google Scholar] [CrossRef]
- Palamba, P.; Ramadhan, M.L.; Imran, F.A.; Kosasih, E.A.; Nugroho, Y.S. Investigation of smoldering combustion propagation of dried peat. In Proceedings of the International Tropical Renewable Energy Conference, Bogor, Indonesia, 26–28 October 2016; AIP Publishing: Melville, NY, USA, 2017; p. 020017. [Google Scholar]
- Huang, X.; Rein, G. Upward-and-downward spread of smoldering peat fire. Proceed. Combust. Inst. 2019, 37, 4025–4033. [Google Scholar] [CrossRef]
- Kohlenberg, A.J.; Turetsky, M.R.; Thompson, D.K.; Branfireun, B.A.; Mitchell, C.P.J. Controls on boreal peat combustion and resulting emissions of carbon and mercury. Environ. Res. Lett. 2018, 13, 035005. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Benscoter, B.; Page, S.; Rein, G.; van der Werf, G.R.; Watts, A. Global vulnerability of peatlands to fire and carbon loss. Nature Geosci. 2015, 8, 11–14. [Google Scholar] [CrossRef]
- Black, R.R.; Aurell, J.; Holder, A.; George, I.J.; Gullett, B.K.; Hays, M.D.; Geron, C.D.; Tabor, D. Characterization of gas and particle emissions from laboratory burns of peat. Atmos. Environ. 2016, 132, 49–57. [Google Scholar] [CrossRef]
- Geron, C.; Hays, M. Air emissions from organic soil burning on the coastal plain of North Carolina. Atmos. Environ. 2013, 64, 192–199. [Google Scholar] [CrossRef]
- Othman, M.; Latif, M.T. Dust and Gas Emissions from Small-Scale Peat Combustion. Aerosol Air Qual. Res. 2013, 13, 1045–1059. [Google Scholar] [CrossRef]
- Telford, P.G. Peat fuel—A sustainable bioenergy resource. In Proceedings of the International Conference on Environmental Management and Engineering, Banff, AB, Canada, 6–8 July 2009; Hamza, M.H., Ed.; ACTA Press: Calgary, AB, Canada, 2009; pp. 650–815. [Google Scholar]
- Lempinen, H. “Barely surviving on a pile of gold”: Arguing for the case of peat energy in 2010s Finland. Energy Policy 2019, 128, 1–7. [Google Scholar] [CrossRef]
- Tcvetkov, P.S. The history, present status and future prospects of the Russian fuel peat industry. Mires Peat 2017, 19, 1–12. [Google Scholar]
- Toner, E. Power from peat—more polluting than coal—is on its way out in Ireland. Science. 2018. Available online: https://www.sciencemag.org/news/2018/12/power-peat-more-polluting-coal-its-way-out-ireland (accessed on 4 October 2019).
- Werner, S. District heating and cooling in Sweden. Energy 2017, 126, 419–429. [Google Scholar] [CrossRef]
- Lukoševičius, V. Regarding wider usage of local fuel—Energy peat—in Lithuanian heat and electricity sector; Lithuanian Energy Consultants Association: Vilnius, Lithuania, 2014. [Google Scholar]
- Mitchell, E.J.S.; Lea-Langton, A.R.; Jones, J.M.; Williams, A.; Layden, P.; Johnson, R. The impact of fuel properties on the emissions from the combustion of biomass and other solid fuels in a fixed bed domestic stove. Fuel Process. Technol. 2016, 142, 115–123. [Google Scholar] [CrossRef]
- Dehmer, J. Petrological and organic geochemical investigation of recent peats with known environments of deposition. Int. J. Coal Geol. 1995, 28, 111–138. [Google Scholar] [CrossRef]
- Orru, M.; Übner, M.; Orru, H. Chemical properties of peat in three peatlands with balneological potential in Estonia. Est. J. Earth Sci. 2011, 60, 43. [Google Scholar] [CrossRef]
- Jayarathne, T.; Stockwell, C.E.; Gilbert, A.A.; Daugherty, K.; Cochrane, M.A.; Ryan, K.C.; Putra, E.I.; Saharjo, B.H.; Nurhayati, A.D.; Albar, I.; et al. Chemical characterization of fine particulate matter emitted by peat fires in Central Kalimantan, Indonesia, during the 2015 El Niño. Atmos. Chem. Phys. 2018, 18, 2585–2600. [Google Scholar] [CrossRef]
- Fabiańska, M.J.; Szymczyk, A.; Chłapik, M. Fossil fuel compounds from fly dust in recent organic matter of southern Poland peats. Geochemistry 2014, 74, 237–250. [Google Scholar] [CrossRef]
- Schellekens, J.; Bradley, J.A.; Kuyper, T.W.; Fraga, I.; Pontevedra-Pombal, X.; Vidal-Torrado, P.; Abbott, G.D.; Buurman, P. The use of plant-specific pyrolysis products as biomarkers in peat deposits. Quat. Sci. Rev. 2015, 123, 254–264. [Google Scholar] [CrossRef]
- Mikhailov, A.V. Coal-peat compositions for co-combustion in local boilers. J. Min. Inst. 2016, 220, 538–544. [Google Scholar]
- Zulkifley, M.T.M.; Ng, T.F.; Abdullah, W.H.; Raj, J.K.; Shuib, M.K.; Ghani, A.A.; Ashraf, M.A. Geochemical characteristics of a tropical lowland peat dome in the Kota Samarahan-Asajaya area, West Sarawak, Malaysia. Environ. Earth Sci. 2015, 73, 1443–1458. [Google Scholar] [CrossRef]
- Roulston, C.; Paton-Walsh, C.; Smith, T.E.L.; Guérette, É.-A.; Evers, S.; Yule, C.M.; Rein, G.; Van der Werf, G.R. Fine particle emissions from tropical peat fires decrease rapidly with time dince ignition. J. Geophys. Res. Atmos. 2018, 123, 5607–5617. [Google Scholar] [CrossRef]
- Fujii, Y.; Iriana, W.; Oda, M.; Puriwigati, A.; Tohno, S.; Lestari, P.; Mizohata, A.; Huboyo, H.S. Characteristics of carbonaceous aerosols emitted from peatland fire in Riau, Sumatra, Indonesia. Atmos. Environ. 2014, 87, 164–169. [Google Scholar] [CrossRef]
- Růžičková, J.; Kucbel, M.; Raclavská, H.; Švédová, B.; Raclavský, K.; Juchelková, D. Comparison of organic compounds in char and soot from the combustion of biomass in boilers of various emission classes. J. Environ. Manag. 2019, 236, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Turpin, B.J.; LIM, H.-J. Species contributions to PM2.5 mass concentrations: Revisiting common assumptions for estimating organic mass. Aerosol Sci. Technol. 2001, 35, 602–610. [Google Scholar] [CrossRef]
- Fachinger, F.; Drewnick, F.; Gieré, R.; Borrmann, S. How the user can influence particulate emissions from residential wood and pellet stoves: Emission factors for different fuels and burning conditions. Atmos. Environ. 2017, 158, 216–226. [Google Scholar] [CrossRef]
- Nkosi, N.C.; Piketh, S.J.; Burger, R.P. Fine PM emission factors from residential burning of solid fuels using traditional cast-iron coal stoves. Clean Air J. 2018, 28, 35–41. [Google Scholar] [CrossRef]
- Raclavská, H.; Corsaro, A.; Hartmann-Koval, S.; Juchelková, D. Enrichment and distribution of 24 elements within the sub-sieve particle size distribution ranges of fly ash from wastes incinerator plants. J. Environ. Manag. 2017, 203, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Esenlik, S.; Karayigit, A.I.; Bulut, Y.; Querol, X.; Alastuey, A.; Font, O. Element behaviour during combustion in coal-fired Orhaneli power plant, Bursa-Turkey. Geologica Acta 2006, 4, 439–449. [Google Scholar]
- Meij, R. Trace element behavior in coal-fired power plants. Fuel Process. Technol. 1994, 39, 199–217. [Google Scholar] [CrossRef]
- Dolníčková, D.; Drozdová, J.; Raclavský, K.; Juchelková, D. Geochemistry of trace elements in fly ashes from lignite fired power stations. Inżynieria Miner. J. Pol. Miner. Eng. Soc. 2012, 28, 59–68. [Google Scholar]
- Smieja-Król, B.; Fiałkiewicz-Kozieł, B.; Sikorski, J.; Palowski, B. Heavy metal behaviour in peat–A mineralogical perspective. Sci. Total Environ. 2010, 408, 5924–5931. [Google Scholar] [CrossRef]
- Biester, H.; Hermanns, Y.-M.; Martinez Cortizas, A. The influence of organic matter decay on the distribution of major and trace elements in ombrotrophic mires–a case study from the Harz Mountains. Geochimica et Cosmochimica Acta 2012, 84, 126–136. [Google Scholar] [CrossRef]
- Sippula, O.; Lamberg, H.; Leskinen, J.; Tissari, J.; Jokiniemi, J. Emissions and ash behavior in a 500 kW pellet boiler operated with various blends of woody biomass and peat. Fuel 2017, 202, 144–153. [Google Scholar] [CrossRef]
- Kazi, T.G.; Lashari, A.A.; Ali, J.; Baig, J.A.; Afridi, H.I. Volatilization of toxic elements from coal samples of Thar coal field, after burning at different temperature and their mobility from ash: Risk assessment. Chemosphere 2019, 217, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ting, G. An overview of peat related chemistry. Bachelor’s Thesis, Central University of Applied Sciences, Kokkola, Finland, April 2015. [Google Scholar]
- Teirumnieka, Ē.; Kļaviņš, M.; Teirumnieks, E. Major and Trace Elements in Peat from Bogs of East Latvia. In Mires Peat; Klavins, M., Ed.; University of Latvia Press: Riga, Latvia, 2010; pp. 115–123. [Google Scholar]
- Klavins, M.; Silamikele, I.; Nikodemus, O.; Kalnina, L.; Kuske, E.; Rodinov, V.; Purmalis, O. Peat properties, major and trace element accumulation in bog peat in Latvia. Baltica 2009, 22, 37–49. [Google Scholar]
- Cao, W.; Martí-Rosselló, T.; Li, J.; Lue, L. Prediction of potassium compounds released from biomass during combustion. Appl. Energy 2019, 250, 1696–1705. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.; Peng, N.; Lang, Q.; Xia, Y.; Gai, C.; Liu, Z. Nitrogen transformation among char, tar and gas during pyrolysis of sewage sludge and corresponding hydrochar. J. Anal. Appl. Pyrolysis 2017, 126, 298–306. [Google Scholar] [CrossRef]
- Riaza, J.; Mason, P.; Jones, J.M.; Gibbins, J.; Chalmers, H. High temperature volatile yield and nitrogen partitioning during pyrolysis of coal and biomass fuels. Fuel 2019, 248, 215–220. [Google Scholar] [CrossRef]
- Broer, K.M.; Brown, R.C. The role of char and tar in determining the gas-phase partitioning of nitrogen during biomass gasification. Appl. Energy 2015, 158, 474–483. [Google Scholar] [CrossRef]
- Jansen, B.; Nierop, K.G.J. Methyl ketones in high altitude Ecuadorian Andosols confirm excellent conservation of plant-specific n-alkane patterns. Organic Geochem. 2009, 40, 61–69. [Google Scholar] [CrossRef]
- Ralph, J.; Quideau, S.; Grabber, J.H.; Hatfield, R.D. Identification and synthesis of new ferulic acid dehydrodimers present in grass cell walls. J. Chem. Society Perkin Trans. 1994, 1, 3485–3498. [Google Scholar] [CrossRef]
- Bocchini, P.; Galletti, G.C.; Camarero, S.; Martinez, A.T. Absolute quantitation of lignin pyrolysis products using an internal standard. J. Chromatogr. A 1997, 773, 227–232. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Fabbri, D.; Torri, C.; Spokas, K.A. Analytical pyrolysis of synthetic chars derived from biomass with potential agronomic application (biochar). Relationships with impacts on microbial carbon dioxide production. J. Anal. Appl. Pyrolysis 2012, 93, 77–84. [Google Scholar] [CrossRef]
- Simoneit, B.R.T. Organic matter of the troposphere-V: Application of molecular marker analysis to biogenic emissions into the troposphere for source reconciliations. J. Atmos. Chem. 1989, 8, 251–275. [Google Scholar] [CrossRef]
- Wornat, M.J.; Ledesma, E.B.; Marsh, N.D. Polycyclic aromatic hydrocarbons from the pyrolysis of catechol (ortho-dihydroxybenzene), a model fuel representative of entities in tobacco, coal, and lignin. Fuel 2001, 80, 1711–1726. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Polycyclic Aromatic Hydrocarbon Formation from the Pyrolysis/Gasification of Lignin at Different Reaction Conditions. Energy Fuels 2014, 28, 6371–6379. [Google Scholar] [CrossRef]
- Fitzpatrick, E.M.; Ross, A.B.; Bates, J.; Andrews, G.; Jones, J.M.; Phylaktou, H.; Pourkashanian, M.; Williams, A. Emission of Oxygenated Species from the Combustion of Pine Wood and its Relation to Soot Formation. Process. Saf. Environ. Prot. 2007, 85, 430–440. [Google Scholar] [CrossRef]
- Song, J.; Peng, P. Characterisation of black carbon materials by pyrolysis–gas chromatography–mass spectrometry. J. Anal. Appl. Pyrolysis 2010, 87, 129–137. [Google Scholar] [CrossRef]
- Knicker, H.; Almendros, G.; González-Vila, F.J.; Martin, F.; Lüdemann, H.-D. 13C- and 15N-NMR spectroscopic examination of the transformation of organic nitrogen in plant biomass during thermal treatment. Soil Biol. Biochem. 1996, 28, 1053–1060. [Google Scholar] [CrossRef]
- Kuo, L.J.; Herbert, B.E.; Louchouarn, P. Can levoglucosan be used to characterize and quantify char/charcoal black carbon in environmental media? Org. Geochem. 2008, 39, 1466–1478. [Google Scholar] [CrossRef]
- Růžičková, J.; Kucbel, M.; Raclavská, H.; Švédová, B.; Raclavský, K.; Šafář, M. Organic compounds in char from the combustion of peat briquettes in household boilers. In Proceedings of the 2019 IEEE International Conference on Environment and Electrical Engineering and 2019 IEEE Industrial and Commercial Power Systems Europe, Genova, Italy, 11–14 June 2019; pp. 1439–1444. [Google Scholar]
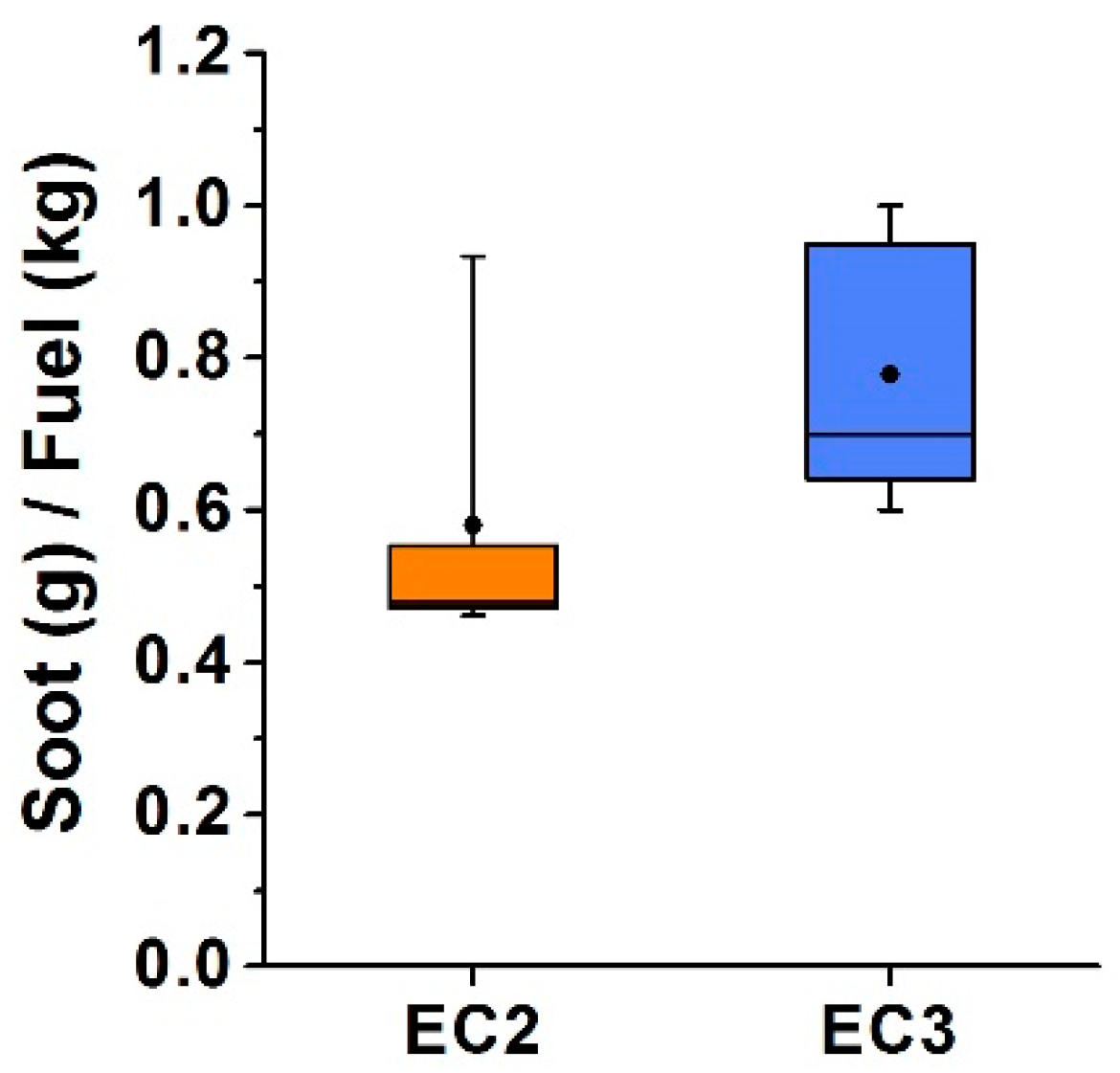



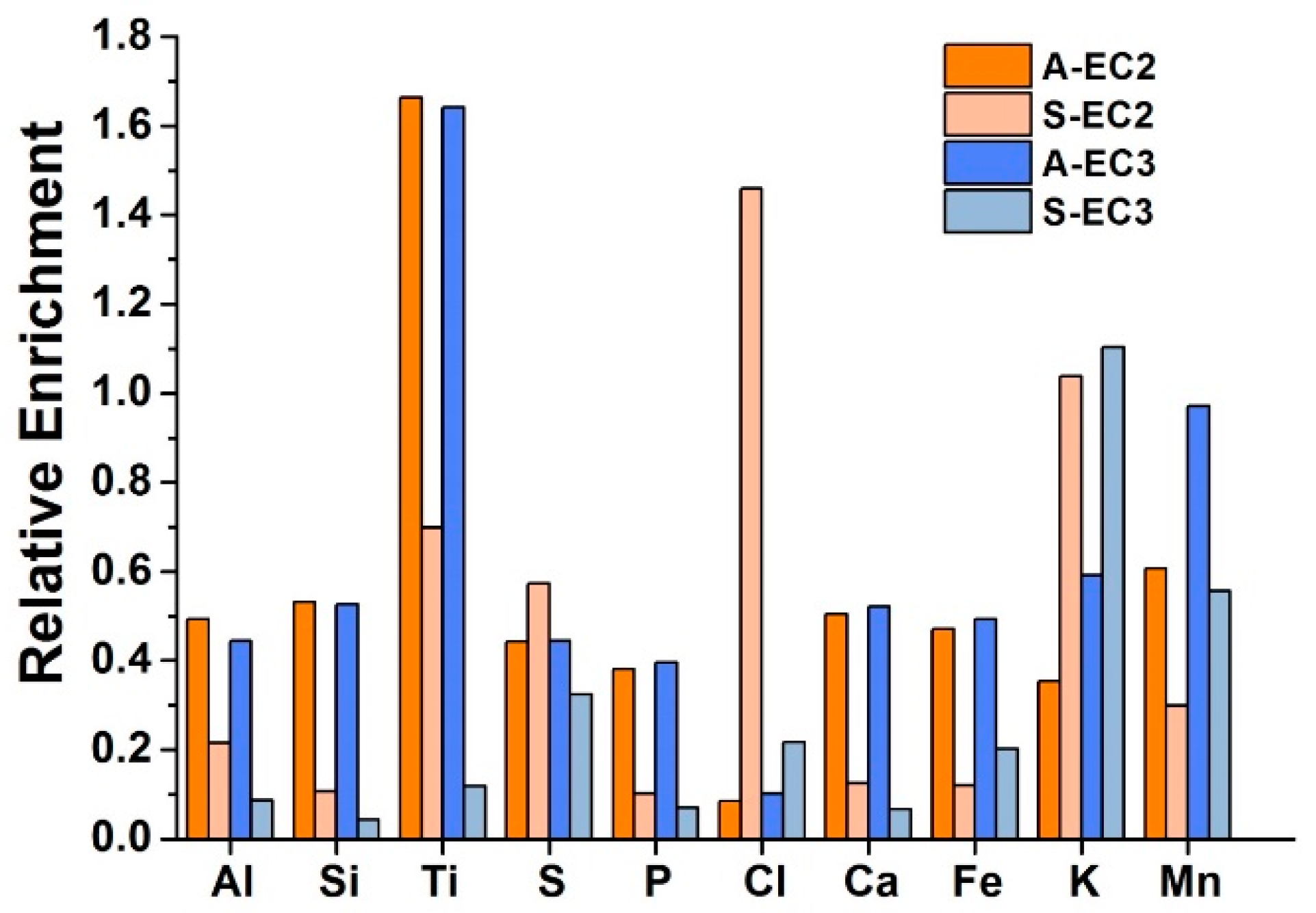
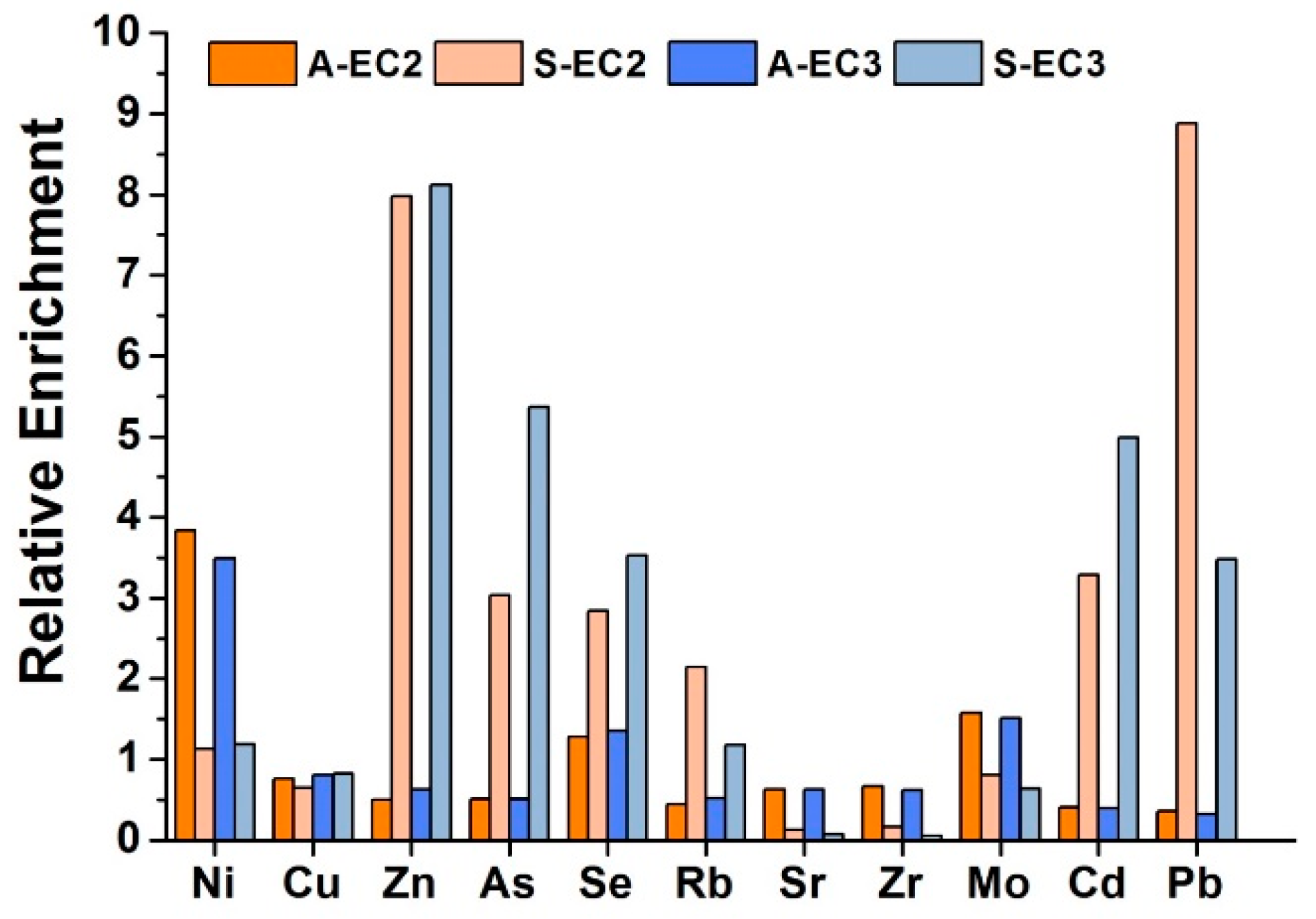
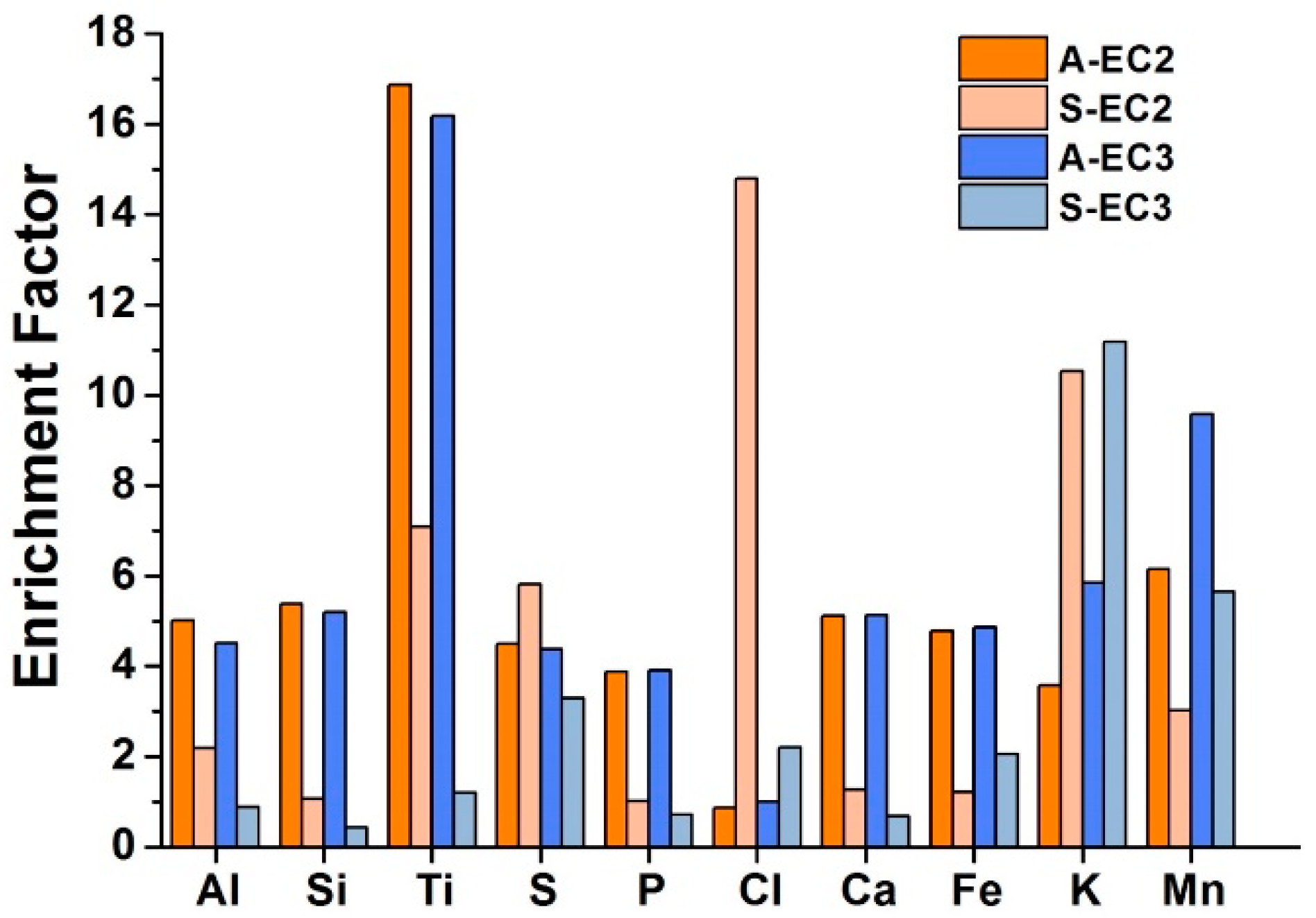



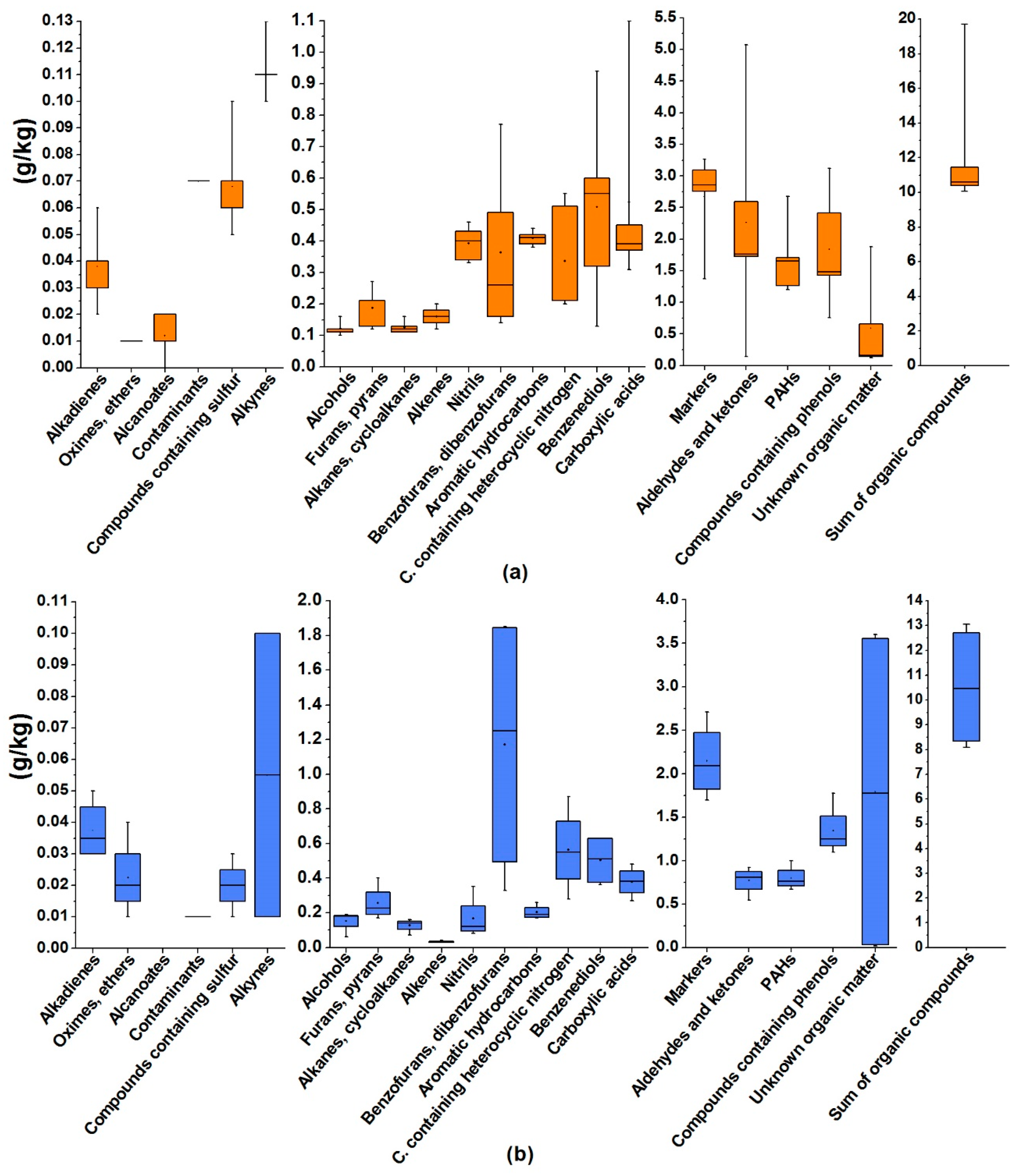
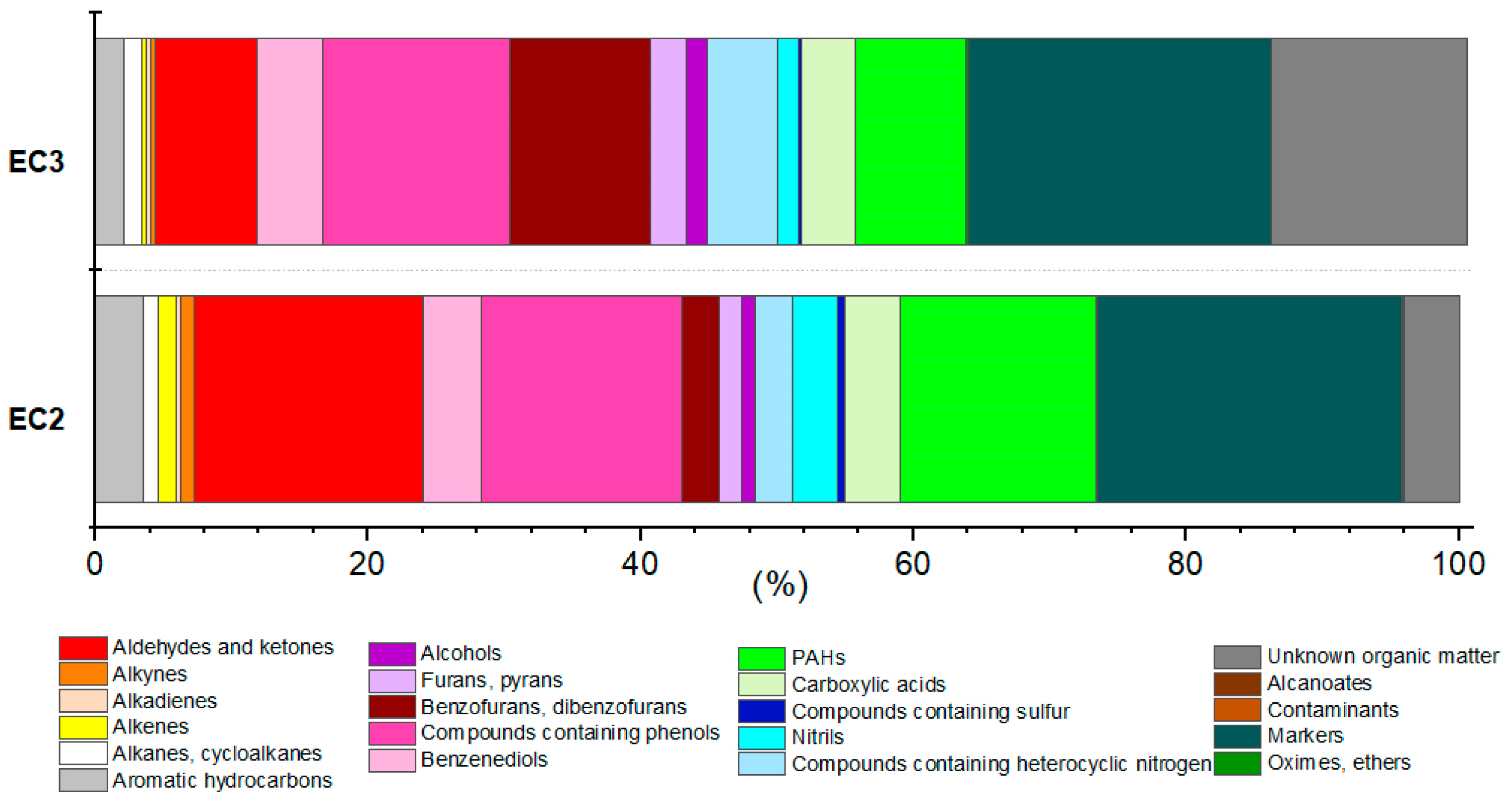
| EC2 | EC3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | 112 | 116 | 117 | 121 | 123 | 122 | 131 | 137 | 138 | 163 | |
| Weight of soot | g | 28.0 | 22.0 | 20.0 | 20.0 | 12.0 | 28.0 | 30.0 | 24.0 | 32.0 | 38.0 |
| Fuel consumption | kg | 30.0 | 39.8 | 42.4 | 41.6 | 26.0 | 40.0 | 30.0 | 40.0 | 50.0 | 40.0 |
| Burning time | hours | 8.00 | 8.00 | 11.0 | 8.00 | 7.50 | 14.0 | 11.0 | 11.0 | 28.0 | 24.0 |
| Soot production | g/kg fuel | 0.93 | 0.55 | 0.47 | 0.48 | 0.46 | 0.70 | 1.00 | 0.60 | 0.64 | 0.95 |
| Fuel/hours | kg/h | 3.75 | 4.98 | 3.85 | 5.20 | 3.47 | 2.86 | 2.73 | 3.64 | 1.79 | 1.67 |
| OC-soot | g/kg | 270 | 277 | 265 | 266 | 259 | 299 | 309 | 220 | 263 | 283 |
| EC-soot | g/kg | 382 | 382 | 370 | 287 | 365 | 315 | 321 | 280 | 307 | 320 |
| TC-soot | g/kg | 652 | 660 | 635 | 553 | 625 | 614 | 630 | 500 | 570 | 603 |
| OC/EC | 0.71 | 0.72 | 0.72 | 0.92 | 0.71 | 0.95 | 0.96 | 0.79 | 0.86 | 0.88 | |
| OM-soot | g/kg | 378 | 388 | 371 | 372 | 363 | 418 | 432 | 308 | 368 | 396 |
| Ash in soot | wt.% | 24.0 | 23.0 | 25.9 | 34.1 | 27.2 | 26.7 | 24.7 | 41.2 | 32.5 | 28.4 |
| Char in ash | wt.% | 10.6 | 8.25 | 9.45 | 8.62 | 7.76 | 10.2 | 12.7 | 7.76 | 6.22 | 9.64 |
| Unit | Peat Briquettes | Peat Briquettes * | Hardwood | Softwood | |
|---|---|---|---|---|---|
| Moisture | wt. % | 13.2 | 38–60 | 8.90 | 9.22 |
| Calorific value | MJ/kg | 18.1 | 6.5–11.9 | 17.2 | 18.3 |
| Ash | wt. % | 13.5 | 3.0–7.0 | 1.31 | 0.55 |
| VM | 60.9 | 67–70 | 75–80 | ||
| FC | 25.6 | 20–30 | 20–25 | ||
| C | 48.1 | 48–65 | 47–50 | 50–53 | |
| N | 3.20 | 0.6–3.0 | 0.12 | 0.21 | |
| S | 0.50 | 0.1–1.5 | BDL | BDL | |
| O | 29.2 | 26–42 | 45.10 | 42.70 | |
| H | 5.47 | 5.60 | 6.22 | 6.38 | |
| Element | Unit | Finland | Finland | UK | Germany * | Norway | Norway-S | Norway-N | Latvia | Estonia | Estonia | Russia | Australia | Ukraine | Lignite |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | % | 0.21 | 0.26 | 0.31 | |||||||||||
| Ca | % | 0.41 | 0.31 | 0.55–0.18 | 0.38 | 0.47 | n.d. | 3.81 | |||||||
| Fe | % | 0.67 | 0.74 | 0.74–8.43 | 0.21 | 0.11 | 0.07 | 0.11 | n.d. | 0.11 | 0.17 | 0.16 | 1.95 | ||
| K | % | 0.079 | 0.14 | 0.0065 | n.d. | 0.098 | |||||||||
| Mg | % | 0.09 | 0.07 | 0.22 | 0.056 | n.d. | |||||||||
| Mn | % | 0.0081 | 0.003–0.034 | 0.0075 | 0.003 | 0.004 | 0.0014 | 0.0014 | 0.014 | 0.068 | 0.031 | ||||
| Na | % | 0.11 | 0.069 | 0.011 | n.d. | ||||||||||
| P | % | 0.048 | 0.063 | 0.25 | |||||||||||
| S | % | 0.17 | 0.163 | 0.54 | |||||||||||
| Si | % | 0.02 | 0.78 | 0.91–2.46 | 1.28 | ||||||||||
| N | % | 1.43 | |||||||||||||
| As | mg/kg | 2.9 | 5.5–104.4 | 0.5 | 1.19 | 0.89 | 2.4 | 6.9 | |||||||
| Ba | mg/kg | 36.6 | 208 | ||||||||||||
| Cd | mg/kg | 0.4 | 2.09 | n.d. | 1.2 | 0.24 | 0.14 | 9.62 | 17.4 | 2.7 | 2.2 | 4.46 | |||
| Cl | mg/kg | 520 | 175–466 | 486 | |||||||||||
| Co | mg/kg | 1.3 | 0.93 | 1.16 | 1.06 | 0.14 | 0.09 | n.d. | 10 | ||||||
| Cr | mg/kg | 5.6 | 15.9 | 8.3–36.4 | 12.5 | 0.8 | 0.9 | 1.17 | 0.39 | 63 | |||||
| Cu | mg/kg | 8.5 | 54.8 | 5 | 5.6 | 1.6 | 2.19 | 1.36 | 102.1 | 26.3 | 4.2 | 6.4 | 16 | ||
| Hg | µg/kg | 13.2–525 | |||||||||||||
| Mo | mg/kg | 1.2 | 0.85 | 0.32 | 0.21 | 0.8 | |||||||||
| Ni | mg/kg | 3.5 | 10.9 | 4.3 | 1.4 | 2.6 | 1.38 | 0.71 | 46.6 | n.d. | 1.2 | ||||
| Pb | mg/kg | 4.6 | 358 | 7–2256 | n.d. | 23.2 | 6.9 | 4.77 | 9.62 | 200 | 15 | 28.6 | 10 | 14 | |
| Se | mg/kg | 0.37 | 0.16 | n.d. | 1.18 | ||||||||||
| Sr | mg/kg | 26.9 | 183 | ||||||||||||
| Ti | mg/kg | 57.1 | 23.851–7 | 110 | |||||||||||
| U | mg/kg | 9.4 | |||||||||||||
| Th | mg/kg | 0.6 | 10 | ||||||||||||
| V | mg/kg | 11.3 | 3.75 | 1.33 | 0.51 | 48 | |||||||||
| Zn | mg/kg | 16.7 | 5.2 | 56.2 | 78–359 | 48 | 19.6 | 12.3 | 10.6 | 8.54 | 446 | 59.6 | 11.3 | 27 | 68 |
| Zr | mg/kg | 19 | |||||||||||||
| References | [50] | [48] | [51] | [47] | [52] | [51] | [51] | [52] | [52] | [51] | [51] | [51] | This study | [45] | |
| Unit | EC2 S-W | EC2 S-R | EC3 S-W | EC3 S-R | EC2 A | EC3 A | |
|---|---|---|---|---|---|---|---|
| pH | 7.91 ± 0.24 | 7.82 ± 0.12 | 7.46 ± 0.15 | 7.41 ± 0.15 | 11.40 ± 0.20 | 11.07 ± 0.45 | |
| Conductivity | µS/cm | 5920 ± 680 | 7820 ± 798 | 3440 ± 554 | 3300 ± 428 | 2096 ± 263 | 3102 ± 298 |
| Cl− | g/kg | 6.92 ± 1.14 | 9.93 ± 1.27 | 1.02 ± 0.41 | 1.08 ± 0.32 | 0.47 ± 0.37 | 0.37 ± 0.21 |
| SO42− | g/kg | 14.81 ± 2.32 | 18.03 ± 3.54 | 14.27 ± 1.98 | 13.61 ± 2.21 | 3.23 ± 0.37 | 4.21 ± 0.07 |
| PO43− | mg/kg | 5.87 ± 0.05 | 14.30 ± 1.14 | 20.82 ± 2.28 | 6.18 ± 0.08 | b.d. | b.d. |
| N-NO3− | mg/kg | 66.74 ± 11.2 | 86.41 ± 24.5 | 27.67 ± 6.4 | 26.59 ± 8.5 | 7.02 ± 1.2 | 14.66 ± 2.2 |
| N-NH4 | g/kg | 1.49 ± 0.27 | 3.69 ± 1.24 | 0.24 ± 0.06 | 0.19 ± 0.04 | 0.0094 ± 0.001 | 0.0045 ± 0.001 |
| Norg | g/kg | 0.56 ± 0.12 | 0.22 ± 0.05 | 0.44 ± 0.11 | 0.52 ± 0.15 | 0.034 ± 0.01 | 0.032 ± 0.01 |
| Ntotal | g/kg | 2.12 ± 0.4 | 3.99 ± 0.2 | 0.71 ± 0.2 | 0.73 ± 0.2 | 0.051 ± 0.017 | 0.052 ± 0.014 |
| Na+ | g/kg | 1.15 ± 0.21 | 1.08 ± 0.04 | 0.17 ± 0.05 | 0.17 ± 0.05 | 0.14 ± 0.03 | 0.13 ± 0.04 |
| K+ | g/kg | 2.29 ± 0.22 | 4.19 ± 0.54 | 3.14 ± 0.28 | 4.32 ± 0.41 | 0.96 ± 0.52 | 3.16 ± 2.81 |
| Ca2+ | g/kg | 1.68 ± 0.24 | 2.36 ± 0.58 | 2.51 ± 0.42 | 1.80 ± 0.21 | 3.03 ± 1.04 | 5.61 ± 2.81 |
| Mg2+ | g/kg | 2.11 ± 0.18 | 2.22 ± 0.22 | 1.87 ± 0.14 | 1.41 ± 0.17 | 0.15 ± 0.08 | 0.24 ± 0.021 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Růžičková, J.; Kucbel, M.; Raclavská, H.; Švédová, B.; Raclavský, K.; Šafář, M.; Kantor, P. Chemical and Mineralogical Composition of Soot and Ash from the Combustion of Peat Briquettes in Household Boilers. Energies 2019, 12, 3784. https://doi.org/10.3390/en12193784
Růžičková J, Kucbel M, Raclavská H, Švédová B, Raclavský K, Šafář M, Kantor P. Chemical and Mineralogical Composition of Soot and Ash from the Combustion of Peat Briquettes in Household Boilers. Energies. 2019; 12(19):3784. https://doi.org/10.3390/en12193784
Chicago/Turabian StyleRůžičková, Jana, Marek Kucbel, Helena Raclavská, Barbora Švédová, Konstantin Raclavský, Michal Šafář, and Pavel Kantor. 2019. "Chemical and Mineralogical Composition of Soot and Ash from the Combustion of Peat Briquettes in Household Boilers" Energies 12, no. 19: 3784. https://doi.org/10.3390/en12193784
APA StyleRůžičková, J., Kucbel, M., Raclavská, H., Švédová, B., Raclavský, K., Šafář, M., & Kantor, P. (2019). Chemical and Mineralogical Composition of Soot and Ash from the Combustion of Peat Briquettes in Household Boilers. Energies, 12(19), 3784. https://doi.org/10.3390/en12193784






