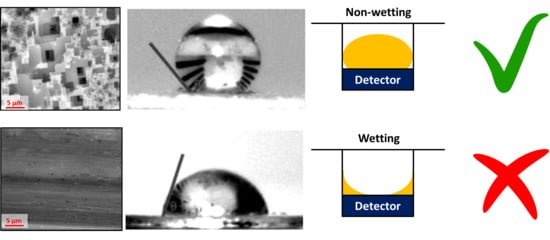
Wettability Control for Correct Thermophysical Properties Determination of Molten Salts and Their Nanofluids †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
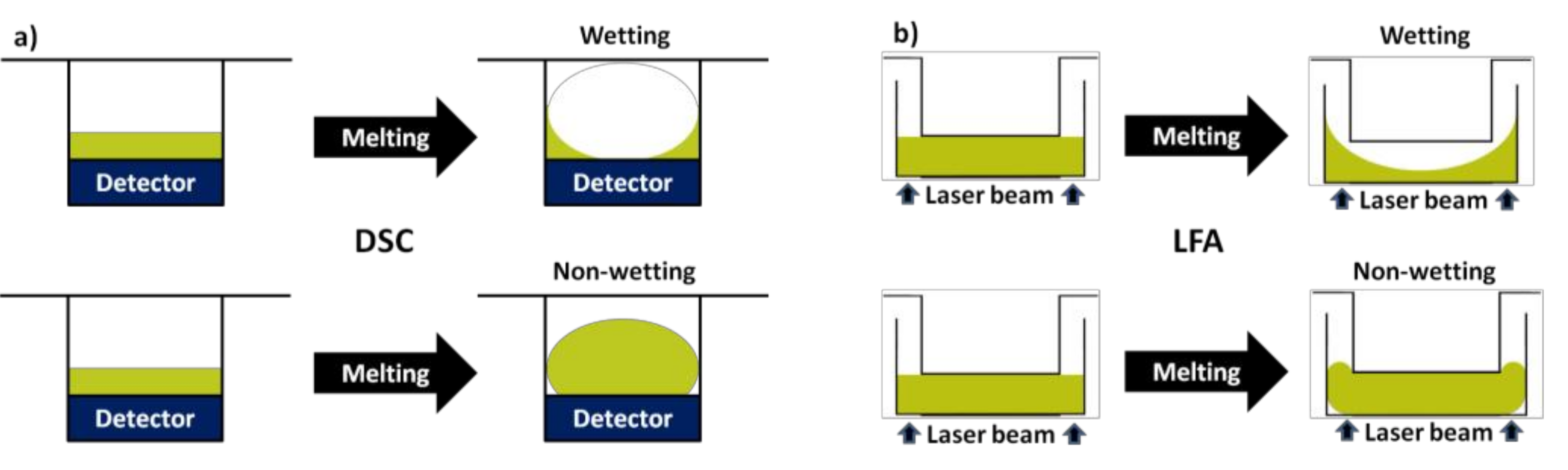
2.2. Differential Scanning Calorimetry (DSC)
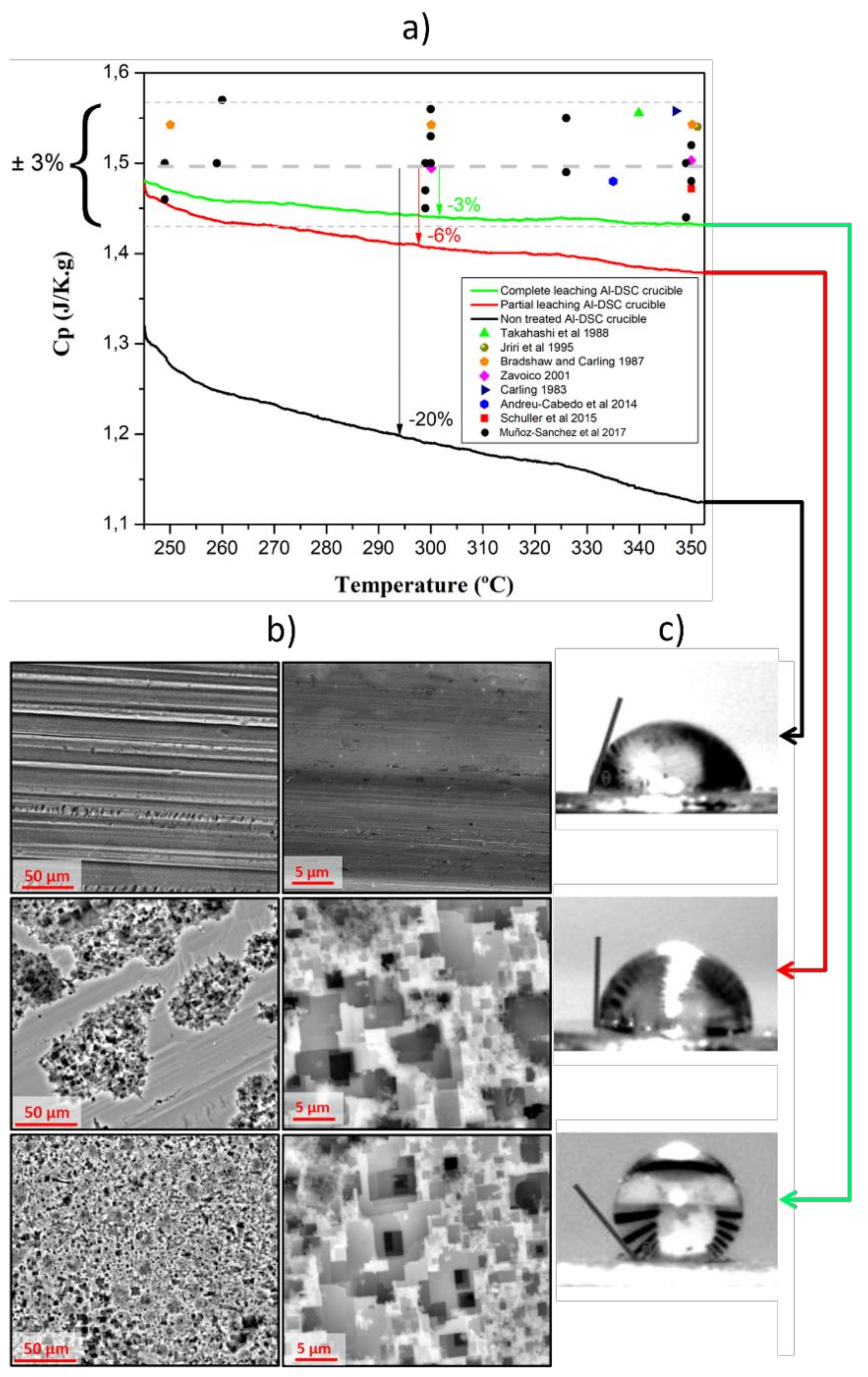
- As received Al-DSC crucible from the supplier.
- Partially leached Al-DSC crucible. These crucibles were prepared as by introducing 10 mL of 37% HCl acid into the pristine non-treated Al-DSC crucible for 15 min, followed by distilled water washing and drying at 60 °C.
- Completely leached Al-DSC crucible was prepared the same way as the partially leached Al-DSC crucible, but by repeating the leaching procedure 5 times.
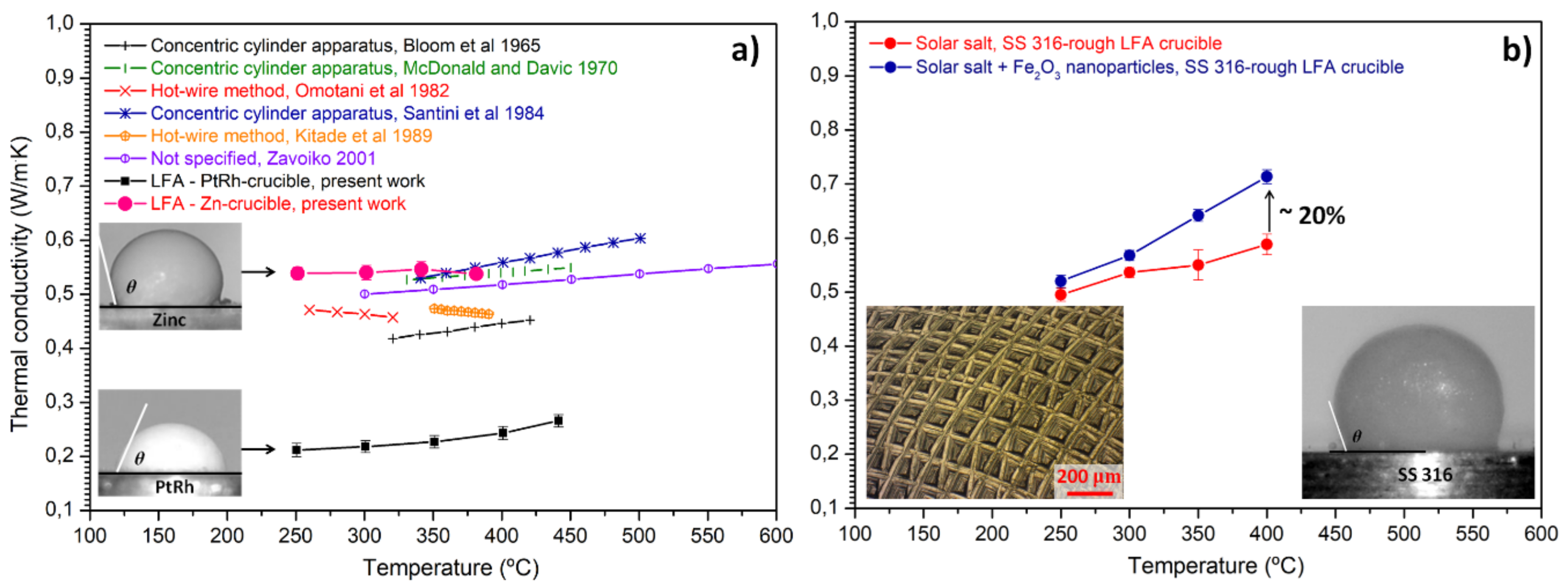
2.3. Laser Flash Apparatus (LFA)
- As received PtRh-crucible from Netzsch.
- Similar crucible, but custom-made from zinc (Zn-crucible).
- Similar crucible, but custom-made from Stainless Steel (SS) 316-crucible. The interior of this crucible was also decorated with roughness by means of mechanical treatment.
2.4. Scanning Electron Microscopy (SEM)
2.5. Optical Microscope
2.6. Contact Angle
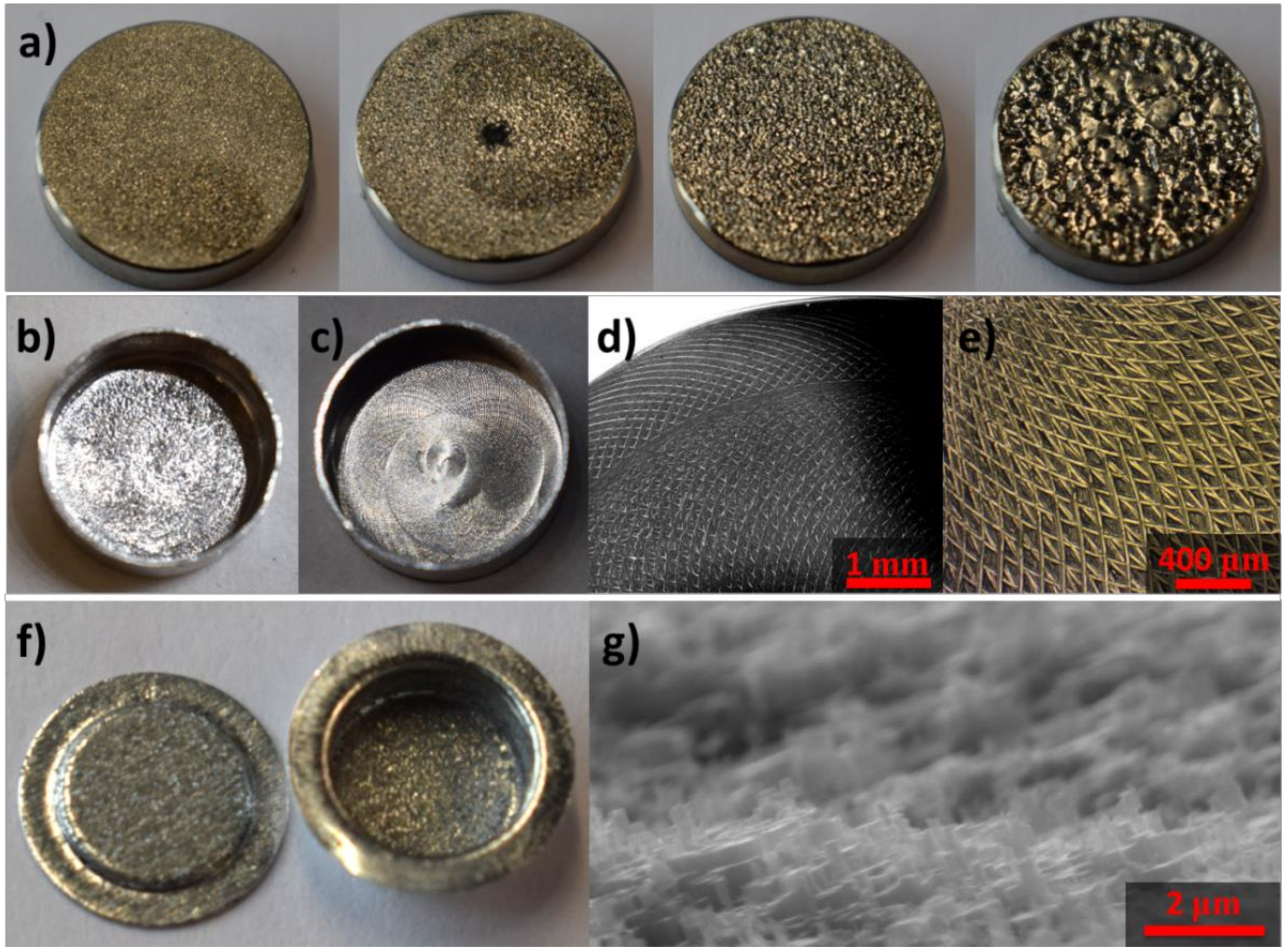
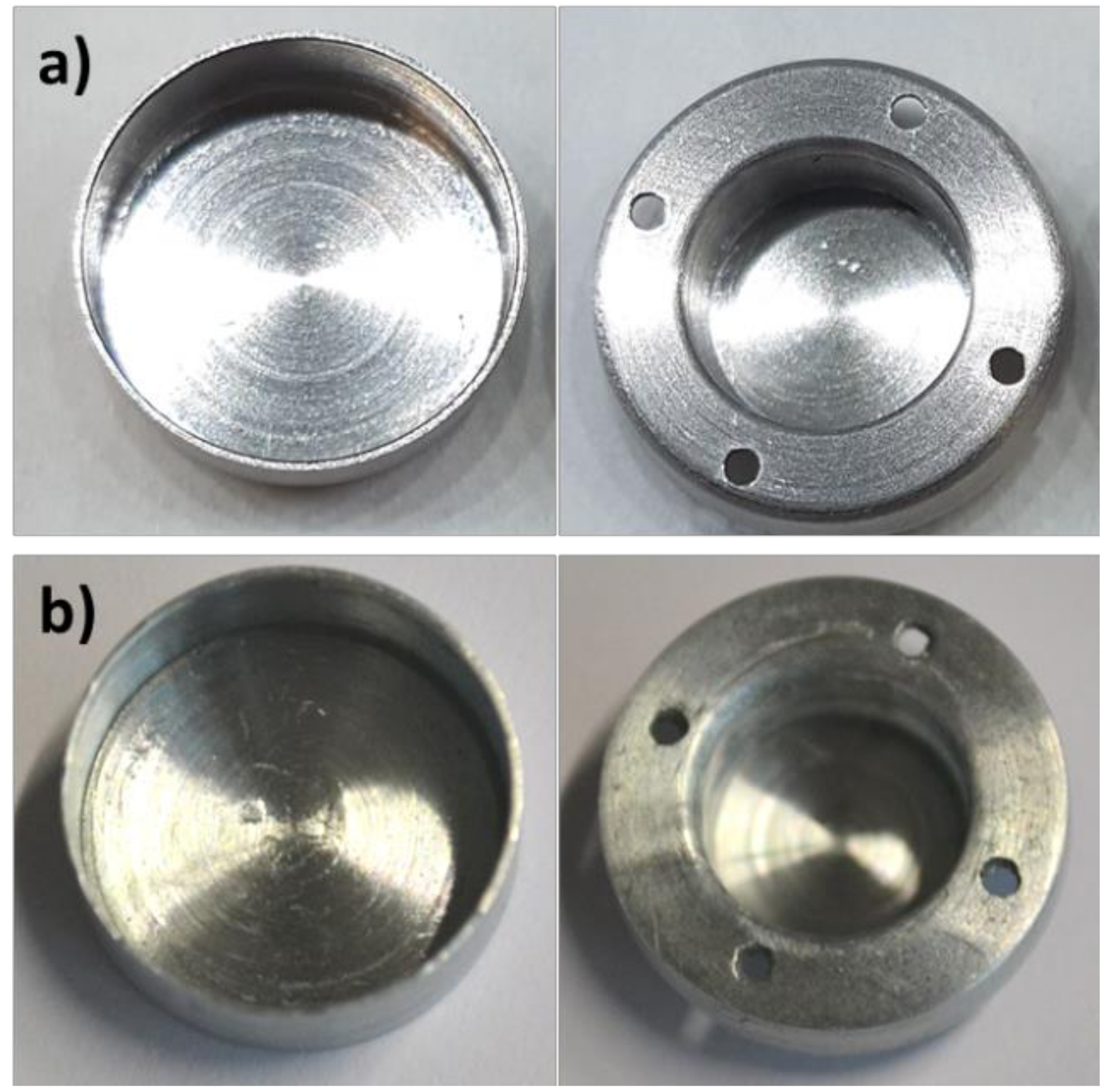
2.7. Details on the Development of Crucibles with Roughness
2.7.1. Electroetching
2.7.2. Mechanical Treatment
2.7.3. Chemical Etching
3. Results
3.1. Microroughness for Wettability Control of Molten Salts
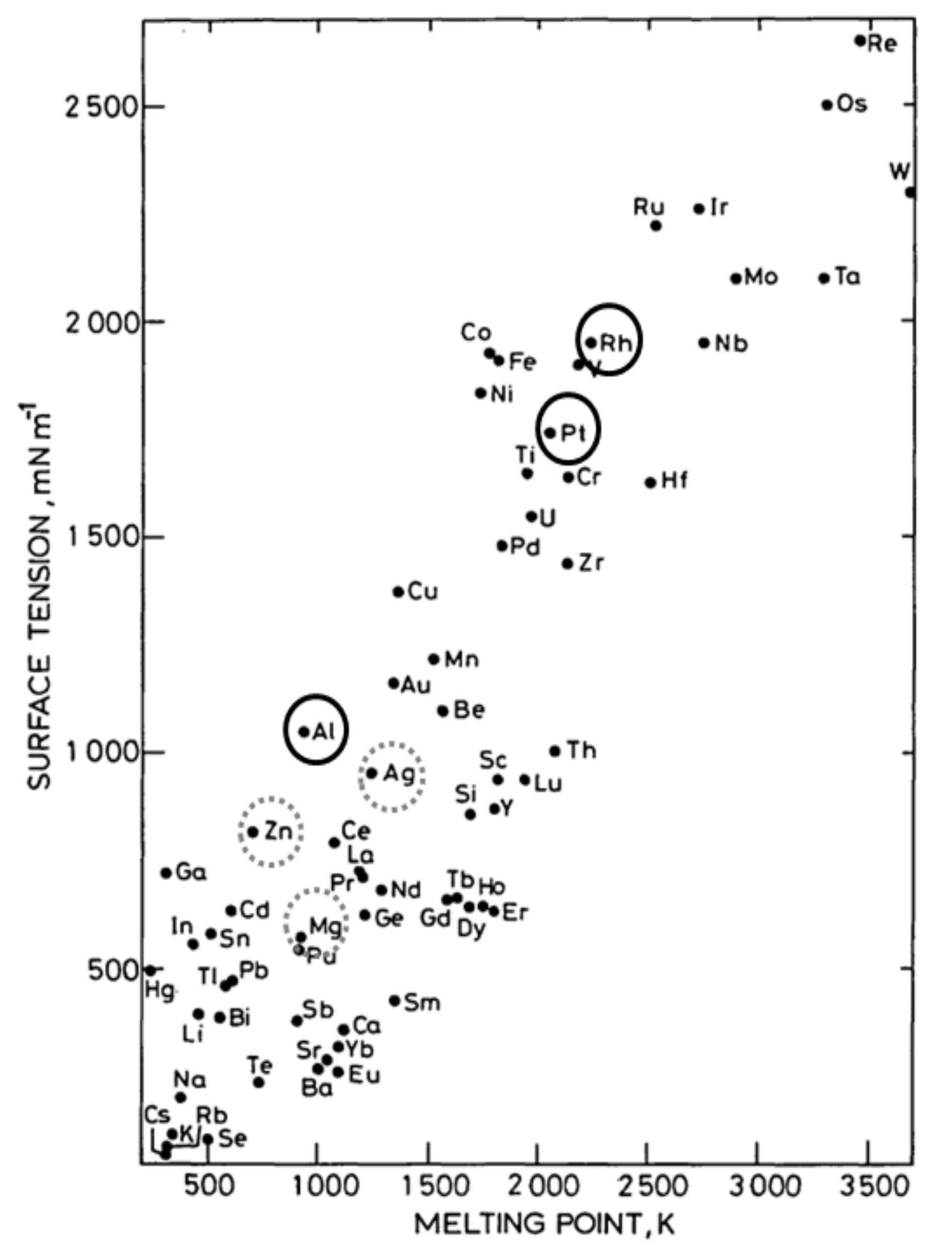
3.2. Surface Energy for Wettability Control of Molten Salts
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat transfer fluids for concentrating solar power systems—A review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Burgaleta, J.I.; Arias, S.; Ramirez, D. Gemasolar, the first tower thermosolar commercial plant with molten salt storage. Solarpaces 2011, 69, 1–8. [Google Scholar]
- Relloso, S.; Delgado, E.B. Experience with molten salt thermal storage in a commercial parabolic trough plant. In Andasol-1 commissioning and operation. In Proceedings of the 15th SolarPACES Symposium, Berlin, Germany, 14–18 September 2009. [Google Scholar]
- Bonk, A.; Sau, S.; Uranga, N.; Hernaiz, M.; Bauer, T. Advanced heat transfer fluids for direct molten salt line-focusing CSP plants. Prog. Energy Combust. Sci. 2018, 67, 69–87. [Google Scholar] [CrossRef]
- Arthur, O.; Karim, M.A. An investigation into the thermophysical and rheological properties of nanofluids for solar thermal applications. Renew. Sustain. Energy Rev. 2016, 55, 739–755. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, B.; Nieto-Maestre, J.; González-Aguilar, J.; Julia, J.E.; Navarrete, N.; Faik, A.; Bauer, T.; Bonk, A.; Navarro, M.E.; Ding, Y.; et al. Round robin test on the measurement of the specific heat of solar salt. AIP Conf. Proc. 2017, 1850, 080017. [Google Scholar]
- Muñoz-Sánchez, B.; Nieto-Maestre, J.; Imbuluzqueta, G.; Marañón, I.; Iparraguirre-Torres, I.; García-Romero, A. A precise method to measure the specific heat of solar salt-based nanofluids. J. Therm. Anal. Calorim. 2017, 129, 905–914. [Google Scholar] [CrossRef]
- Thoms, M.W. Adsorption at the Nanoparticle Interface for Increased Thermal Capacity in Solar Thermal Systems. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 28 June 2012. [Google Scholar]
- Hohne, G.; Hemminger, W.F.; Flammersheim, H.-J. Differential Scanning Calorimetry, 2nd ed.; Springer-Verlag: Heidelberg, Germany, 2003; 298p, Available online: http://www.spring er.com/la/book/9783540004677 (accessed on 3 September 2019).
- Hernaiz, M.; Alonso, V.; Estellé, P.; Wu, Z.; Sundén, B.; Doretti, L.; Mancin, S.; Çobanoğlu, N.; Karadeniz, Z.H.; Garmendia, N.; et al. The contact angle of nanofluids as thermophysical property. J. Colloid Interface Sci. 2019, 547, 393–406. [Google Scholar] [CrossRef]
- Lundblad, A.; Bergman, B. Determination of contact angle in porous molten-carbonate fuel-cell electrodes. J. Electrochem. Soc. 1997, 144, 984–987. [Google Scholar] [CrossRef]
- Baumli, P.; Kaptay, G. Wettability of carbon surfaces by pure molten alkali chlorides and their penetration into a porous graphite substrate. Mater. Sci. Eng. A 2008, 495, 192–196. [Google Scholar] [CrossRef]
- Faik, A.; Grosu, Y.; Udayashankar, N.; González-Fernández, L. Thermal Analysis Sample Container. European Patent application No 18382688.2-1003, 16 April 2019. [Google Scholar]
- Grosu, Y.; Udayashankar, N.; Bondarchuk, O.; González-Fernández, L.; Faik, A. Unexpected effect of nanoparticles doping on the corrosivity of molten nitrate salt for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 178, 91–97. [Google Scholar] [CrossRef]
- Coquard, R.; Panel, B. Adaptation of the FLASH method to the measurement of the thermal conductivity of liquids or pasty materials. Int. J. Therm. Sci. 2009, 48, 747–760. [Google Scholar] [CrossRef]
- Herminghaus, S. Roughness-induced non-wetting. Europhys. Lett. 2000, 52, 165–170. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Liu, T.; Kim, C.J. Turning a surface superrepellent even to completely wetting liquids. Science 2014, 346, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sakamoto, R.; Kamimoto, M. Heat capacities and latent heats of LiNO3, NaNO3, and KNO3. Int. J. Thermophys. 1988, 9, 1081–1090. [Google Scholar] [CrossRef]
- Jriri, T.; Rogez, J.; Bergman, C.; Mathieu, J.C. Thermodynamic study of the condensed phases of NaNO3, KNO3 and CsNO3 and their transitions. Thermochim. Acta 1995, 266, 147–161. [Google Scholar] [CrossRef]
- Bradshaw, R.W.; Carling, R.W. A Review of the Chemical and Physical Properties of Molten Alkali Nitrate Salts and Their Effect on Materials Used for Solar Central Receivers; Report SAND87-8005; Sandia National Laboratories: Albuquerque, NM, USA, 1987. [Google Scholar]
- Zavoico, A.B. Design Basis Document; SAND2001-2100; Sandia National Laboratories: Albuquerque, NM, USA, 2001. [Google Scholar]
- Carling, R.W. Heat capacities of NaNO3 and KNO3 from 350 to 800 K. Thermochim. Acta 1983, 60, 265–275. [Google Scholar] [CrossRef]
- Andreu-Cabelo, P.; Mondragon, R.; Hernandez, L.; Martines-Cuenca, R.; Cabedo, L.; Julia, J.E. Increment of specific heat of Solar Salt with SiO2 nanoparticles. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar]
- Schuller, M.; Shao, Q.; Lalk, T. Experimental investigation of the specific heat of a nitrate-alumina nanofluid for solar thermal energy storage systems. Int. J. Therm. Sci. 2015, 91, 142–145. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Abdelsalam, M.E.; Bartlett, P.N.; Kelf, T.; Baumberg, J. Wetting of regularly structured gold surfaces. Langmuir 2005, 21, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Martines, E.; Seunarine, K.; Morgan, H.; Gadegaard, N.; Wilkinson, C.D.; Riehle, M.O. Air-trapping on biocompatible nanopatterns. Langmuir 2006, 22, 11230–11233. [Google Scholar] [CrossRef] [PubMed]
- Nithiyanantham, U.; Grosu, Y.; González-Fernández, L.; Zaki, A.; Igartua, J.M.; Faik, A. Corrosion aspects of molten nitrate salt-based nanofluids for thermal energy storage applications. Sol. Energy 2019, 189, 219–227. [Google Scholar] [CrossRef]
- Keene, B.J. Review of data for the surface tension of pure metals. Int. Mater. Rev. 1993, 38, 157–192. [Google Scholar] [CrossRef]
- Tricklebank, S.B. Molten Salt Mixtures. IX. The Thermal Conductivities of Molten Nitrate Systems. Aust. J. Chem. 1965, 18, 1171–1176. [Google Scholar]
- McDonald, J.; Davis, H.T. Thermal conductivity of binary mixtures of alkali nitrates. J. Phys. Chem. 1970, 74, 725–730. [Google Scholar] [CrossRef]
- Omotani, T.; Nagasaka, Y.; Nagashima, A. Measurement of the thermal conductivity of KNO3-NaNO3 mixtures using a transient hot-wire method with a liquid metal in a capillary probe. Int. J. Thermophys. 1982, 3, 17–26. [Google Scholar] [CrossRef]
- Santini, R.; Tadrist, L.; Pantaloni, J.; Cerisier, P. Measurement of thermal conductivity of molten salts in the range 100–500 °C. Int. J. Heat Mass Transf. 1984, 27, 623–626. [Google Scholar] [CrossRef]
- Kitade, S.; Kobayashi, Y.; Nagasaka, Y.; Nagashima, A. Measurement of the thermal conductivity of molten KNO3 and NaNO3 by the transient hot-wire method with ceramic-coated probes. High Temp. High Press. 1989, 21, 219–224. [Google Scholar]
- Grosu, Y.; González-Fernández, L.; Nithiyanantham, U.; Faik, A. Wettability control for correct thermophysical properties determination of molten salts and their nanofluids. In Proceedings of the 1st International Conference on Nanofluids (ICNf), Castello, Spain, 26–28 June 2019. [Google Scholar]
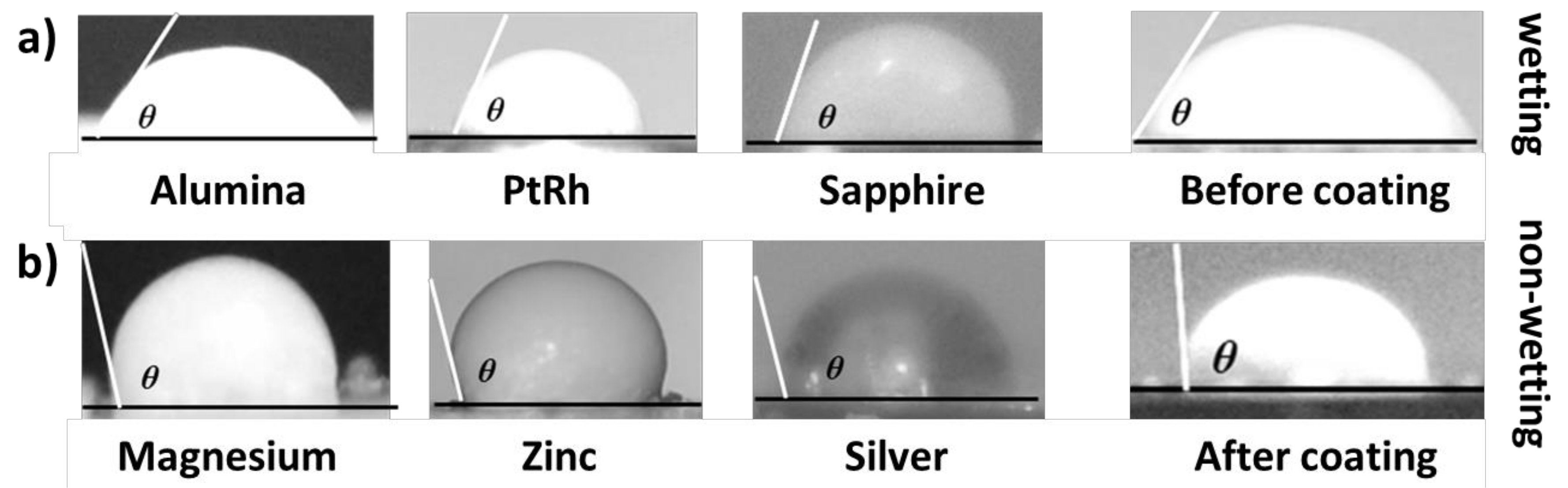
| Method | Scale of Roughness | Non-Wetting Condition |
|---|---|---|
| Electroetching 1 | 40 µm | No |
| Electroetching 2 | 110 µm | No |
| Electroetching 3 | 200 µm | No |
| Electroetching 4 | 800 µm | No |
| Mechanical treatment Dremel | 800 nm + 10–20 µm | Yes |
| Mechanical treatment 2 | 700–1200 nm + 100 µm | Yes |
| Chemical etching | 200–400 nm + 1–3 µm | Yes |
| Experiment Smooth | Experiment Rough | Calculated Rough | |||
|---|---|---|---|---|---|
| Al | 71 | 128 | 118 | 4.8 | 0.21 |
| PtRd | 77 | 117 | 108 | 2.2 | 0.46 |
| SS316 | 58 | 110 | 91 | 2.2 | 0.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu, Y.; González-Fernández, L.; Nithiyanantham, U.; Faik, A. Wettability Control for Correct Thermophysical Properties Determination of Molten Salts and Their Nanofluids. Energies 2019, 12, 3765. https://doi.org/10.3390/en12193765
Grosu Y, González-Fernández L, Nithiyanantham U, Faik A. Wettability Control for Correct Thermophysical Properties Determination of Molten Salts and Their Nanofluids. Energies. 2019; 12(19):3765. https://doi.org/10.3390/en12193765
Chicago/Turabian StyleGrosu, Yaroslav, Luis González-Fernández, Udayashankar Nithiyanantham, and Abdessamad Faik. 2019. "Wettability Control for Correct Thermophysical Properties Determination of Molten Salts and Their Nanofluids" Energies 12, no. 19: 3765. https://doi.org/10.3390/en12193765
APA StyleGrosu, Y., González-Fernández, L., Nithiyanantham, U., & Faik, A. (2019). Wettability Control for Correct Thermophysical Properties Determination of Molten Salts and Their Nanofluids. Energies, 12(19), 3765. https://doi.org/10.3390/en12193765