Thermal and Catalytic Cracking of Toluene Using Char from Commercial Gasification Systems
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Char Characterization
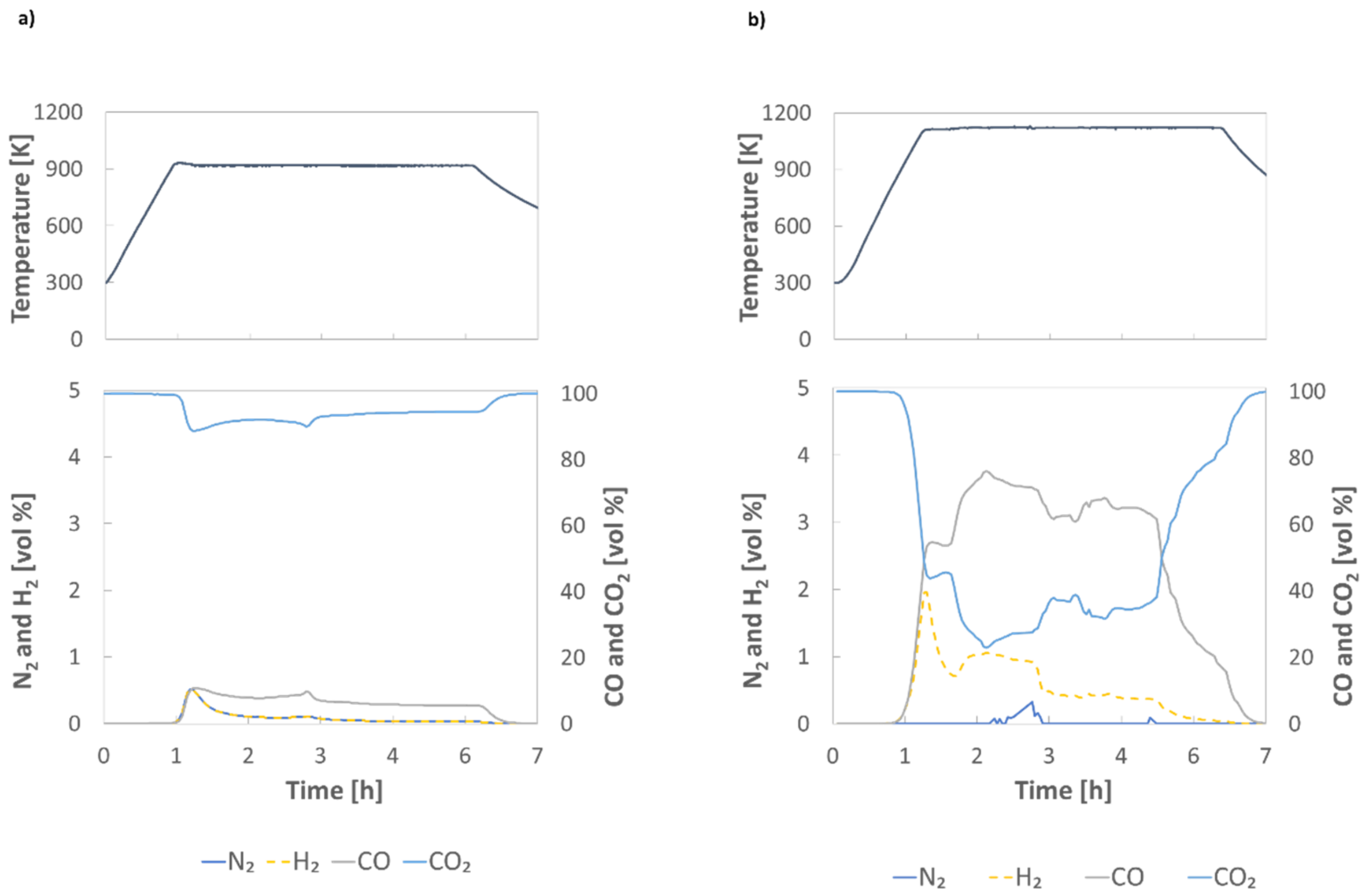
3.2. Thermal Degradation Tests in N2 and in CO2
3.3. Tar Cracking Reactor Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asadullah, M. Biomass gasification gas cleaning for downstream applications: A comparative critical review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Milne, T.A.; Evans, R.J.; Abatzaglou, N. Biomass Gasifier “Tars”: Their Nature, Formation, and Conversion; National Renewable Energy Laboratory: Golden, CO, USA, 1998. [Google Scholar]
- Hasler, P.; Nussbaumer, T. Gas cleaning for IC engine applications from fixed bed biomass gasification. Biomass Bioenergy 1999, 16, 385–395. [Google Scholar] [CrossRef]
- Bridgwater, A.V. The technical and economic feasibility of biomass gasification for power generation. Fuel 1995, 74, 631–653. [Google Scholar] [CrossRef]
- Baratieri, M.; Baggio, P.; Bosio, B.; Grigiante, M.; Longo, G.A. The use of biomass syngas in IC engines and CCGT plants: A comparative analysis. Appl. Therm. Eng. 2009, 29, 3309–3318. [Google Scholar] [CrossRef]
- Hofmann, P.; Panopoulos, K.D.; Aravind, P.V.; Siedlecki, M.; Schweiger, A.; Karl, J.; Ouweltjes, J.P.; Kakaras, E. Operation of solid oxide fuel cell on biomass product gas with tar levels >10 g Nm−3. Int. J. Hydrog. Energy 2009, 34, 9203–9212. [Google Scholar] [CrossRef]
- Rakesh, N.; Dasappa, S. Biosyngas for Electricity Generation Using Fuel Cells—A Gas Quality Assessment; ETA: Florence, Italy, 2018. [Google Scholar] [CrossRef]
- Tijmensen, M.J.A.; Faaija, A.P.C.; Hamelinck, C.N.; van Hardeveldb, M.R.M. Exploration of the possibilities for production of Fischer Tropsch liquids and power via biomass gasification. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Aravind, P.V.; de Jong, W. Evaluation of high temperature gas cleaning options for biomass gasification product gas for Solid Oxide Fuel Cells. Progress Energy Combust. Sci. 2012, 38, 737–764. [Google Scholar] [CrossRef]
- Simell, P.; Kurkela, E.; Ståhlberg, P.; Hepola, J. Catalytic hot gas cleaning of gasification gas. Catal. Today 1996, 27, 55–62. [Google Scholar] [CrossRef]
- Myrén, C.; Hörnell, C.; Björnbom, E.; Sjöström, K. Catalytic tar decomposition of biomass pyrolysis gas with a combination of dolomite and silica. Biomass Bioenergy 2002, 23, 217–227. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B.; Sharrock, P. A review of catalysts for the gasification of biomass char, with some reference to coal. Energy 2013, 58, 305–317. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Review of Catalysts for Tar Elimination in Biomass Gasification Processes. Ind. Eng. Chem. Res. 2004, 43, 6911–6919. [Google Scholar] [CrossRef]
- Dayton, D. Review of the Literature on Catalytic Biomass Tar Destruction; Milestone Completion Report; National Renewable Energy Lab.: Golden, CO, USA, 2002. [Google Scholar]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G.; van Paasen, S.V.B.; Bergman, P.C.A.; Kiel, J.H.A. Catalytic decomposition of biomass tars: Use of dolomite and untreated olivine. Renew. Energy 2005, 30, 565–587. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, S.; Wang, F.; Wang, J. Decomposition and gasification of pyrolysis volatiles from pine wood through a bed of hot char. Fuel 2011, 90, 1041–1048. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.; Al-Gharabli, S.; Brem, G. Impact of Char Properties and Reaction Parameters on Naphthalene Conversion in a Macro-TGA Fixed Char Bed Reactor. Catalysts 2019, 9, 307. [Google Scholar] [CrossRef]
- Benedetti, V.; Patuzzi, F.; Baratieri, M. Characterization of char from biomass gasification and its similarities with activated carbon in adsorption applications. Appl. Energy 2018, 227, 92–99. [Google Scholar] [CrossRef]
- Liu, Y.; Paskevicius, M.; Wang, H.; Parkinson, G.; Veder, J.-P.; Hu, X.; Li, C.-Z. Role of O-containing functional groups in biochar during the catalytic steam reforming of tar using the biochar as a catalyst. Fuel 2019, 253, 441–448. [Google Scholar] [CrossRef]
- Fuentes-Cano, D.; Parrillo, F.; Ruoppolo, G.; Gómez-Barea, A.; Arena, U. The influence of the char internal structure and composition on heterogeneous conversion of naphthalene. Fuel Process. Technol. 2018, 172, 125–132. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Hu, M.; Laghari, M.; Cui, B.; Xiao, B.; Zhang, B.; Guo, D. Catalytic cracking of biomass tar over char supported nickel catalyst. Energy 2018, 145, 228–237. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Experimental comparison of biomass chars with other catalysts for tar reduction. Fuel 2008, 87, 2243–2252. [Google Scholar] [CrossRef]
- Klinghoffer, N.B.; Castaldi, M.J.; Nzihou, A. Influence of char composition and inorganics on catalytic activity of char from biomass gasification. Fuel 2015, 157, 37–47. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A. Reforming of lignin-derived tars over char-based catalyst using Py-GC/MS. Fuel 2015, 162, 47–54. [Google Scholar] [CrossRef]
- Buentello-Montoya, D.A.; Zhang, X.; Li, J. The use of gasification solid products as catalysts for tar reforming. Renew. Sustain. Energy Rev. 2019, 107, 399–412. [Google Scholar] [CrossRef]
- Dias, D.; Lapa, N.; Bernardo, M.; Godinho, D.; Fonseca, I.; Miranda, M.; Pinto, F.; Lemos, F. Properties of chars from the gasification and pyrolysis of rice waste streams towards their valorisation as adsorbent materials. Waste Manag. 2017, 65, 186–194. [Google Scholar] [CrossRef]
- Ravenni, G.; Elhami, O.H.; Ahrenfeldt, J.; Henriksen, U.B.; Neubauer, Y. Adsorption and decomposition of tar model compounds over the surface of gasification char and active carbon within the temperature range 250–800 °C. Appl. Energy 2019, 241, 139–151. [Google Scholar] [CrossRef]
- Choi, C.; Shima, K.; Kudo, S.; Norinaga, K.; Gao, X.; Hayashi, J.-I. Continuous monitoring of char surface activity toward benzene. Carbon Resour. Convers. 2019, 2, 43–50. [Google Scholar] [CrossRef]
- Patuzzi, F.; Prando, D.; Vakalis, S.; Rizzo, A.M.; Chiaramonti, D.; Tirler, W.; Mimmo, T.; Gasparella, A.; Baratieri, M. Small-scale biomass gasification CHP systems: Comparative performance assessment and monitoring experiences in South Tyrol (Italy). Energy 2016, 112, 285–293. [Google Scholar] [CrossRef]
- Ahmad, J.; Rashid, U.; Patuzzi, F.; Baratieri, M.; Taufiq-Yap, Y.H. Synthesis of char-based acidic catalyst for methanolysis of waste cooking oil: An insight into a possible valorization pathway for the solid by-product of gasification. Energy Convers. Manag. 2018, 158, 186–192. [Google Scholar] [CrossRef]
- Basso, D.; Patuzzi, F.; Gasparella, A.; Tirler, W.; Dal Savio, S.; Rizzo, A.M.; Chiaramonti, D.; Baratieri, M. Valorization Pathways for Char from Small Scale Gasification Systems in South-Tyrol: The Next Generation Project; ETA: Florence, Italy, 2017. [Google Scholar]
- Cordioli, E.; Patuzzi, F.; Baratieri, M. Analysis of Biomass Chars Thermal Decomposition: Experimental Tests and Modelling in N2 and CO2 Atmosphere; ETA: Florence, Italy, 2017. [Google Scholar]
- Zhang, Y.; Wu, W.; Zhao, S.; Long, Y.; Luo, Y. Experimental study on pyrolysis tar removal over rice straw char and inner pore structure evolution of char. Fuel Process. Technol. 2015, 134, 333–344. [Google Scholar] [CrossRef]
- El-Rub, Z.Y. Biomass Char as an in-situ Catalyst for tar Removal in Gasification Systems; University of Twente: Enschede, The Netherlands, 2008. [Google Scholar]
- Wang, H.; Dlugogorski, B.Z.; Kennedy, E.M. Coal oxidation at low temperatures: Oxygen consumption, oxidation products, reaction mechanism and kinetic modelling. Prog. Energy Combust. Sci. 2003, 29, 487–513. [Google Scholar] [CrossRef]
- Raman, C.V.; Bhagavantam, S. The relation between colour and molecular structure in organic compounds. Indian J. Phys. 1929, 4, 57–78. [Google Scholar]
- Mani, S.; Kastner, J.R.; Juneja, A. Catalytic decomposition of toluene using a biomass derived catalyst. Fuel Process. Technol. 2013, 114, 118–125. [Google Scholar] [CrossRef]
- Bhandari, P.N.; Kumar, A.; Bellmer, D.D.; Huhnke, R.L. Synthesis and evaluation of biochar-derived catalysts for removal of toluene (model tar) from biomass-generated producer gas. Renew. Energy 2014, 66, 346–353. [Google Scholar] [CrossRef]
- Korus, A.; Samson, A.; Szlęk, A.; Katelbach-Woźniak, A.; Sładek, S. Pyrolytic toluene conversion to benzene and coke over activated carbon in a fixed-bed reactor. Fuel 2017, 207, 283–292. [Google Scholar] [CrossRef]
- Tamhankar, S.S.; Tsuchiya, K.; Riggs, J.B. Catalytic cracking of benzene on iron oxide-silica: Catalyst activity and reaction mechanism. Appl. Catal. 1985, 16, 103–121. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Y.; Hu, X.; Zhang, L.; Sun, S.; Li, C.Z. Destruction of tar during volatile-char interactions at low temperature. Fuel Process. Technol. 2018, 171, 215–222. [Google Scholar] [CrossRef]
- Burhenne, L.; Aicher, T. Benzene removal over a fixed bed of wood char: The effect of pyrolysis temperature and activation with CO2 on the char reactivity. Fuel Process. Technol. 2014, 127, 140–148. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Ravenni, G.; Sárossy, Z.; Ahrenfeldt, J.; Henriksen, U.B. Activity of chars and activated carbons for removal and decomposition of tar model compounds—A review. Renew. Sustain. Energy Rev. 2018, 94, 1044–1056. [Google Scholar] [CrossRef]
- Jess, A. Mechanisms and kinetics of thermal reactions of aromatic hydrocarbons from pyrolysis of solid fuels. Fuel 1996, 75, 1441–1448. [Google Scholar] [CrossRef]
- Song, E.; Song, J. Modeling of kerosene combustion under fuel-rich conditions. Adv. Mech. Eng. 2017, 9, 168781401771138. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, J.; Zhang, T.; Qi, F. Kinetic modeling study of toluene pyrolysis at low pressure. Combust. Flame 2010, 157, 1686–1697. [Google Scholar] [CrossRef]
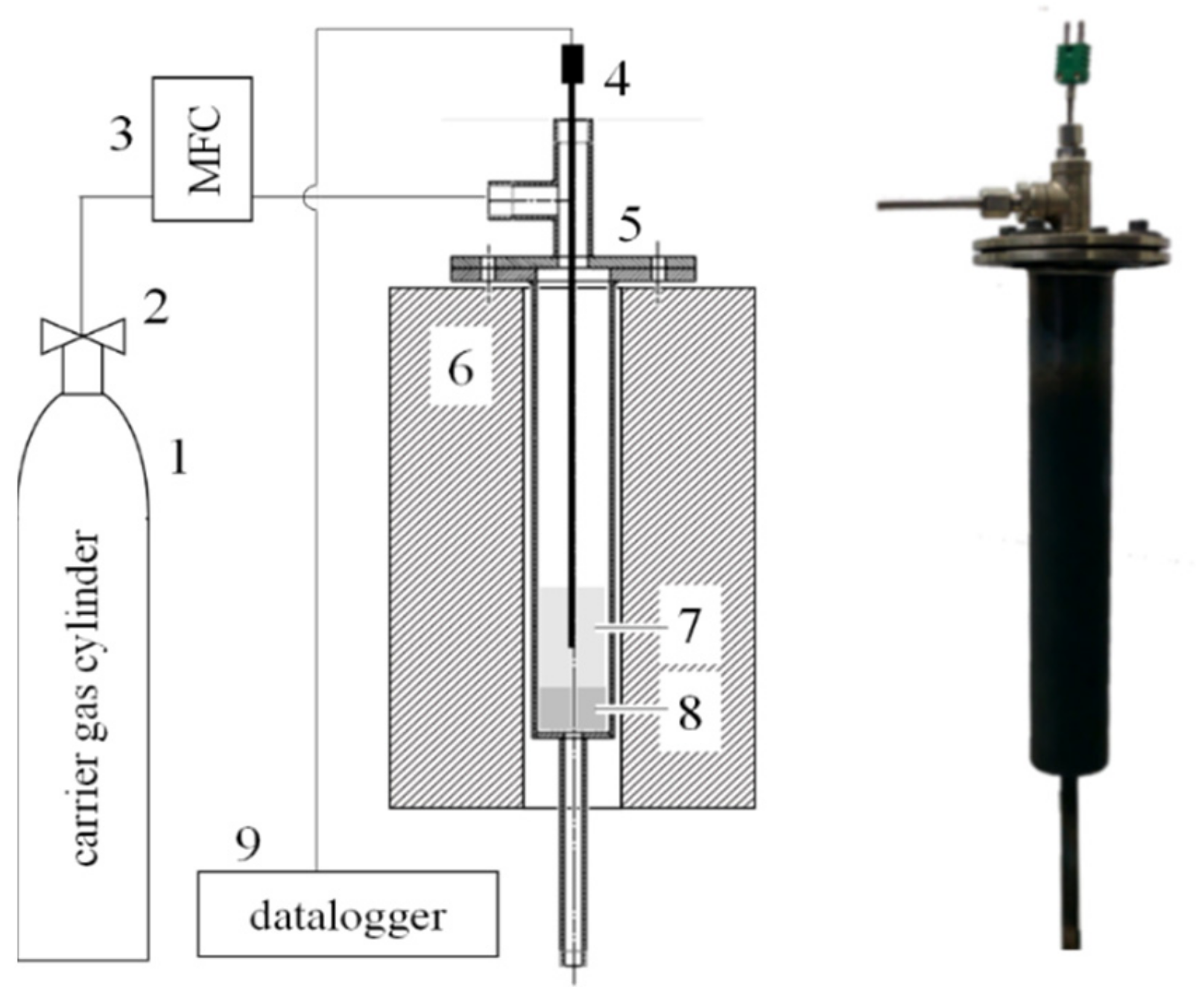
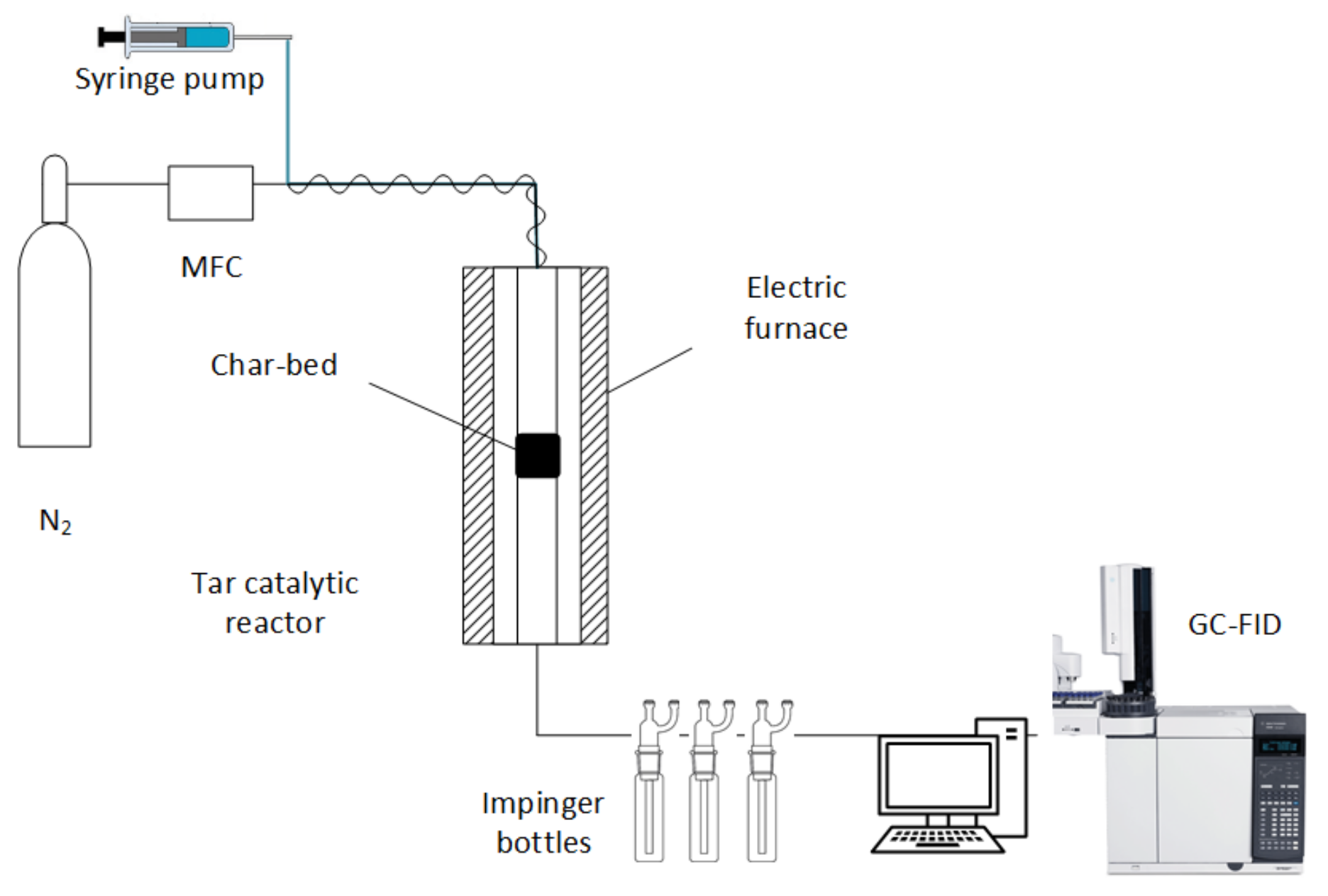


| Tar Cracking Tests | Blank with Char Bed | Empty Reactor | With Char Bed |
|---|---|---|---|
| Furnace temperature | 1173 K, 1273 K | ||
| N2 feed flow | 0.03 m3/h | ||
| Char bed mass | 0.6 g | n.a. | 0.6 g |
| GHSV | 24,000 h−1 | n.a. | 24,000 h−1 |
| Toluene injected | n.a. | 8.67 g | 8.67 g |
| Test duration | 40 min | ||
| Plant Type | Dual-Stage Gasifier | |
|---|---|---|
| Feedstock | Woodchips | |
| Char Analysis (%wtdry) | C | 78.97 |
| H | 0.68 | |
| N | 0.20 | |
| S | 0.31 | |
| Ash | 22.20 | |
| Moisture (%wtas received) | 2.58 | |
| SBET (m2/g) | 587 | |
| Pore volume (cm3/g) | 0.30 | |
| Element | Mass Fraction (%) |
|---|---|
| Ca | 17.47 |
| Mg | 2.18 |
| Fe | 1.12 |
| P | 0.84 |
| Mn | 0.56 |
| Na | 0.40 |
| Al | 0.38 |
| S | 0.37 |
| Cr | 0.30 |
| Ba | 0.22 |
| Temperature (K) | Feed Gas: 100% N2 | Feed Gas: 100% CO2 |
|---|---|---|
| 973 | 92.3% | 96.6% |
| 1173 | 91.7% | 33.7% |
| Tar Cracking Tests | Empty Reactor | With Char Bed | |||
|---|---|---|---|---|---|
| Temperature | (K) | 1173 | 1273 | 1173 | 1273 |
| mo | (g) | 5.21 | 0.23 | 3.44 | 0.08 |
| X | (%) | 39.9 | 97.3 | 60.3 | 99.0 |
| Xb | (%) | 7.8 | 22.5 | 9.5 | 17.3 |
| Xc | (%) | 1.0 | 21.4 | 5.1 | 25.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordioli, E.; Patuzzi, F.; Baratieri, M. Thermal and Catalytic Cracking of Toluene Using Char from Commercial Gasification Systems. Energies 2019, 12, 3764. https://doi.org/10.3390/en12193764
Cordioli E, Patuzzi F, Baratieri M. Thermal and Catalytic Cracking of Toluene Using Char from Commercial Gasification Systems. Energies. 2019; 12(19):3764. https://doi.org/10.3390/en12193764
Chicago/Turabian StyleCordioli, Eleonora, Francesco Patuzzi, and Marco Baratieri. 2019. "Thermal and Catalytic Cracking of Toluene Using Char from Commercial Gasification Systems" Energies 12, no. 19: 3764. https://doi.org/10.3390/en12193764
APA StyleCordioli, E., Patuzzi, F., & Baratieri, M. (2019). Thermal and Catalytic Cracking of Toluene Using Char from Commercial Gasification Systems. Energies, 12(19), 3764. https://doi.org/10.3390/en12193764







