Dynamic Viscosity, Surface Tension and Wetting Behavior Studies of Paraffin–in–Water Nano–Emulsions †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nano–Emulsion Formulation
2.3. Rheological Behavior
2.4. Surface Properties
3. Results and Discussion
3.1. Rheological Studies
3.1.1. Shear Viscosity–Shear Rate Flow Curves
3.1.2. Shear Viscosity–Temperature Sweeps
3.2. Surface Properties
3.2.1. Interfacial Tension
3.2.2. Surface Tension
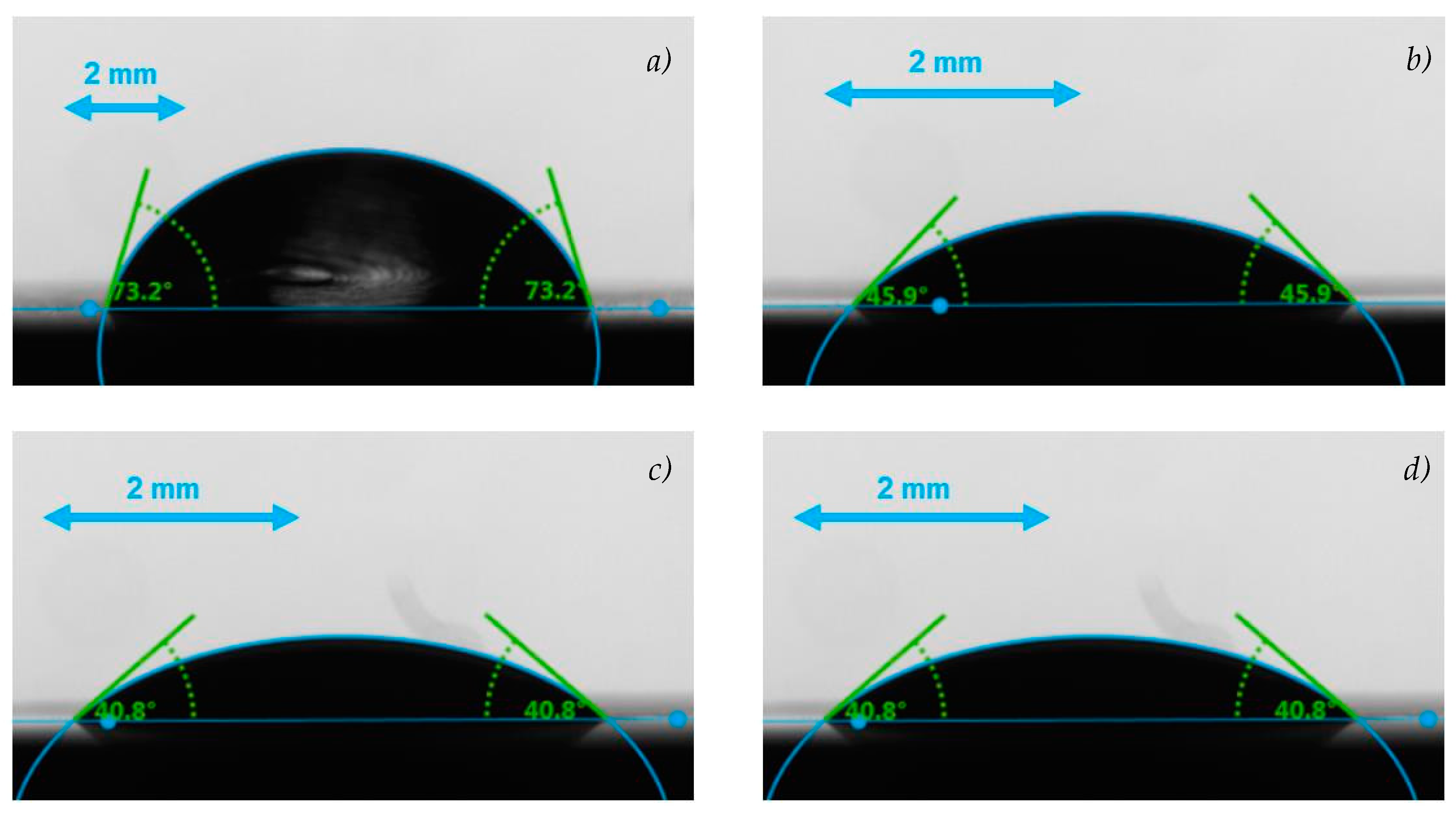
3.2.3. Wetting Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| AAD% | percentage absolute average deviations |
| CA | contact angle [°] |
| CMC | critical micelle concentration |
| ∆N1 | first normal force difference [Pa] |
| intrinsic viscosity | |
| ϕ | volume fraction |
| ϕeff | effective volume fraction |
| ϕm | maximum packing volume fraction |
| ϕs | solvated/swollen droplet volume fraction |
| ITF, Γ | interfacial tension [mN/m] |
| ka | aggregation coefficient |
| K–D | Krieger and Dougherty viscosity model |
| kH | Huggins coefficient |
| pc | geometrical percolation threshold |
| PCM | phase change material |
| PCME | phase change material emulsion |
| pdl | polydispersity index |
| r | radius of PCM droplet [m] |
| RT21HC | Rubitherm RT21HC paraffin |
| RT55 | Rubitherm RT55 paraffin wax |
| SDS | sodium dodecyl sulfate |
| SFT | surface tension [mN/m] |
| T | temperature [K] |
| W | water |
| z | dispersed phase to continuous phase viscosity ratio (z = μPCM/μwater) |
| δ | thickness of solvated layer |
| λd | drop relaxation time |
| μ | shear dynamic viscosity [mPa·s] |
| μr | relative viscosity of sample to corresponding water+SDS mixture (μr = μPCM/μwater+SDS) |
| Subscripts | |
| melt. | melting |
| PCM | phase change materials |
| PCME | phase change material emulsion |
| sol. | solidification |
References
- Singh, S.; Gaikwad, K.K.; Suk, Y. Phase change materials for advanced cooling packaging. Environ. Chem. Lett. 2018, 16, 845–859. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.W.; Wang, R.Z. An overview of phase change material slurries: MPCS and CHS. Renew. Sustain. Energy Rev. 2010, 14, 598–614. [Google Scholar] [CrossRef]
- Huang, L.; Petermann, M.; Doetsch, C. Evaluation of paraffin/water emulsion as a phase change slurry for cooling applications. Energy 2009, 34, 1145–1155. [Google Scholar] [CrossRef]
- Huang, L.; Petermann, M. An experimental study on rheological behaviors of paraffin/water phase change emulsion. Int. J. Heat Mass Transf. 2015, 83, 479–486. [Google Scholar] [CrossRef]
- Youssef, Z.; Delahaye, A.; Huang, L.; Trinquet, F.; Fournaison, L.; Pollerberg, C.; Doetsch, C. State of the art on phase change material slurries. Energy Convers. Manag. 2013, 65, 120–132. [Google Scholar] [CrossRef]
- Tesfai, W.; Singh, P.; Shatilla, Y.; Iqbal, M.Z.; Abdala, A.A. Rheology and microstructure of dilute graphene oxide suspension. J. Nanoparticle Res. 2013, 15, 1989. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Zalba, B. Review on phase change material emulsions and microencapsulated phase change material slurries: Materials, heat transfer studies and applications. Renew. Sustain. Energy Rev. 2012, 16, 253–273. [Google Scholar] [CrossRef]
- Huang, L.; Doetsch, C.; Pollerberg, C. Low temperature paraffin phase change emulsions. Int. J. Refrig. 2010, 33, 1583–1589. [Google Scholar] [CrossRef]
- Inaba, H.; Morita, S.; Nozu, S. Fundamental study of cold heat-storage system of phase-change-type emulsion having cold latent heat dispersion material. Trans. JSME 1993, 59, 2882–2889. [Google Scholar] [CrossRef]
- Inaba, H.; Morita, S. Flow and cold heat-storage characteristics of phase-change emulsion in a coiled double- tube heat exchanger. J. Heat Transf. 2016, 117, 440–446. [Google Scholar] [CrossRef]
- Royon, L. Physical properties and thermorheological behaviour of a dispersio having cold latent heat-storage material. Energy Convers. Manag. 1998, 39, 1529–1535. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Fang, X.; Zhang, Z. Graphite nanoparticles-dispersed paraffin/water emulsion with enhanced thermal-physical property and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2016, 147, 101–107. [Google Scholar] [CrossRef]
- Agresti, F.; Fedele, L.; Rossi, S.; Cabaleiro, D.; Bobbo, S.; Ischia, G.; Barison, S. Nano-encapsulated PCM emulsions prepared by a solvent-assisted method for solar applications. Sol. Energy Mater Sol. Cells 2019, 194, 268–275. [Google Scholar] [CrossRef]
- Lu, W.; Tassou, S.A. Experimental study of the thermal characteristics of phase change slurries for active cooling. Appl. Energy 2012, 91, 366–374. [Google Scholar] [CrossRef]
- Wang, F.; Fang, X.; Zhang, Z. Preparation of phase change material emulsions with good stability and little supercooling by using a mixed polymeric emulsifier for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 381–390. [Google Scholar] [CrossRef]
- Wang, F.; Ling, Z.; Fang, X.; Zhang, Z. Optimization on the photo-thermal conversion performance of graphite nanoplatelets decorated phase change material emulsions. Sol. Energy Mater. Sol. Cells 2018, 186, 340–348. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Ling, X. A molecular dynamics study of nano-encapsulated phase change material slurry. Appl. Therm. Eng. 2016, 98, 835–840. [Google Scholar] [CrossRef]
- Kong, M.; Alvarado, J.L.; Terrell, W.; Thies, C. Performance characteristics of microencapsulated phase change material slurry in a helically coiled tube. Int. J. Heat Mass Transf. 2016, 101, 901–914. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental research of viscosity of microencapsulated PCM slurry at the phase change temperature. Int. J. Heat Mass Transf. 2019, 134, 1209–1217. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental investigation of the effects of mass fraction and temperature on the viscosity of microencapsulated PCM slurry. Int. J. Heat Mass Transf. 2018, 126, 390–399. [Google Scholar] [CrossRef]
- Frank, A.J. TA Instruments Normal Stresses in Shear Flow. Available online: www.tainstruments.com/pdf/literature/AAN007e_Normal_forces_in_Shear_flow.pdf (accessed on 24 August 2019).
- Macosko, C.W. Rheology. Principles, Measurements and Applications; Wiley-VCH: Weinheim, Germany, 1994; ISBN 1560815795. [Google Scholar]
- Chatterjee, T.; Van Dyk, A.K.; Ginzburg, V.V.; Nakatani, A.I. Formulation-controlled positive and negative first normal stress differences in waterborne hydrophobically modified ethylene oxide urethane (HEUR)-latex suspensions. ACS Macro Lett. 2017, 6, 716–720. [Google Scholar] [CrossRef]
- Aral, B.K.; Kalyon, D.M. Viscoelastic material functions of noncolloidal suspensions with spherical particles. J. Rheol. 1997, 41, 599–620. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Q.; Lü, Y.; Wu, G. Determination of critical micelle concentration of sodium dodecyl sulfate in butyl acrylate emulsions. Polym. Bull. 2015, 72, 2215–2225. [Google Scholar] [CrossRef]
- Helgeson, M.E. Colloidal behavior of nanoemulsions: Interactions, structure and rheology. Curr. Opin. Colloid Interface Sci. 2016, 25, 39–50. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Solè, I.; Maestro, A.; González, C.; Solans, C. Optimization of nano-emulsion preparation by low-energy methods in an ionic surfactant system. Langmuir 2006, 22, 8326–8332. [Google Scholar]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Estellé, P.; Cabaleiro, D.; Żyła, G.; Lugo, L.; Murshed, S.M.S. Current trends in surface tension and wetting behavior of nanofluids. Renew. Sustain. Energy Rev. 2018, 94, 931–944. [Google Scholar] [CrossRef]
- Adzima, B.J.; Velankar, S.S. Pressure drops for droplet flows in microfluidic channels. J. Micromech. Microeng 2006, 16, 1504–1510. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Agresti, F.; Barison, S.; Marcos, M.A.; Prado, J.I.; Rossi, S.; Bobbo, S.; Fedele, L. Development of paraffinic phase change material nanoemulsions for thermal energy storage and transport in low-temperature applications. Appl. Therm. Eng. 2019, 159, 113868. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S.; Barison, S.; Agresti, F. Experimental stability analysis of different water- based nanofluids. Nanoscale Res. Lett. 2011, 6, 300. [Google Scholar] [CrossRef] [PubMed]
- Halelfadl, S.; Estellé, P.; Aladag, B.; Doner, N.; Maré, T. Viscosity of carbon nanotubes water-based nanofluids: Influence of concentration and temperature. Int. J. Therm. Sci. 2013, 71, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Villarejo, R.; Aguilar, T.; Hamze, S.; Estellé, P.; Navas, J. Experimental analysis of water-based nanofluids using boron nitride nanotubes with improved thermal properties. J. Mol. Liq. 2019, 277, 93–103. [Google Scholar] [CrossRef]
- Hernaiz, M.; Alonso, V.; Estellé, P.; Wu, Z.; Sundén, B.; Doretti, L.; Mancin, S.; Çobanoğlu, N.; Karadeniz, Z.H.; Garmendia, N.; et al. The contact angle of nanofluids as thermophysical property. J. Colloid Interface Sci. 2019, 547, 393–406. [Google Scholar] [CrossRef] [PubMed]
- COST Action NanoUptake Website. Available online: http://nanouptake.uji.es/ (accessed on 24 August 2019).
- Vargaftik, N.B.; Volkov, B.N.; Voljak, L.D. International tables of surface tension of water with air. J. Phy. Chem. Ref. Data 1983, 12, 817–820. [Google Scholar] [CrossRef]
- Żyła, G.; Fal, J.; Estellé, P. Thermophysical and dielectric profiles of ethylene glycol based titanium nitride (TiN–EG) nanofluids with various size of particles. Int. J. Heat Mass Transf. 2017, 113, 1189–1199. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties (REFPROP), Version 9.0. Physical and Chemical Properties; 2010. Available online: https://www.nist.gov/programs-projects/reference-fluid-thermodynamic-and-transport-properties-database-refprop (accessed on 24 August 2019).
- Ferrer, G.; Gschwander, S.; Solé, A.; Barreneche, C.; Fernández, A.I.; Schossig, P.; Cabeza, L.F. Empirical equation to estimate viscosity of paraffin. J. Energy Storage 2017, 11, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Mikkola, V.; Puupponen, S.; Saari, K.; Ala-Nissila, T.; Seppälä, A. Thermal properties and convective heat transfer of phase changing paraffin nanofluids. Int. J. Therm. Sci. 2017, 117, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Murshed, S.M.S.; Estellé, P. A state of the art review on viscosity of nanofluids. Renew. Sustain. Energy Rev. 2017, 76, 1134–1152. [Google Scholar] [CrossRef]
- Bullard, J.W.; Pauli, A.T.; Garboczi, E.J.; Martys, N.S. A comparison of viscosity–concentration relationships for emulsions. J. Colloid Interface Sci. 2009, 330, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Rhodes, E. Viscosity/concentration relationships for emulsions viscosity. J. Rheol. 1989, 33, 1021–1045. [Google Scholar] [CrossRef]
- Taylor, G.I. The viscosity of a fluid containing small drops of another fluid. Philos. Trans. R. Soc. London Ser. A 1932, 138, 41–48. [Google Scholar] [CrossRef]
- Einstein, A. A new determination of molecular dimension. Ann. Phys. 1906, 19, 289–305. [Google Scholar] [CrossRef]
- Bicerano, J.; Douglas, J.F.; Brune, D.A. Polymer model for the viscosity of particle dispersions model for the viscosity of particle dispersions. J. Macromol. Sci. Part C 1999, 39, 561–642. [Google Scholar] [CrossRef]
- Pal, R. Modeling the viscosity of concentrated nanoemulsions and nanosuspensions. Fluids 2016, 1, 11. [Google Scholar] [CrossRef]
- Pal, R. A new model for the viscosity of asphaltene solutions. Can. J. Chem. Eng. 2015, 93, 747–755. [Google Scholar] [CrossRef]
- Pal, R. A new linear viscoelastic model for emulsions and suspensions. Polym. Eng. Sci. 2008, 48, 1250–1253. [Google Scholar] [CrossRef]
- Lewis, T.B.; Nielsen, L.E.; Company, M. Dynamic mechanical properties of particulate-filled composites. J. Appl. Polym. Sci. 1970, 14, 1449–1471. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A mechanism for non-Newtonian flow in suspensions of rigid spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Brinkman, H.C. The viscosity of concentrated suspensions and solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Roscoe, R. The viscosity of suspensions of rigid spheres. Br. J. Appl. Phys. 1952, 3, 267–269. [Google Scholar] [CrossRef]
- Chiesa, M.; Garg, J.; Kang, Y.T.; Chen, G. Thermal conductivity and viscosity of water-in-oil nanoemulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 326, 67–72. [Google Scholar] [CrossRef]
- Xiang, N.; Yuan, Y.; Sun, L.; Cao, X.; Zhao, J. Simultaneous decrease in supercooling and enhancement of thermal conductivity of paraffin emulsion in medium temperature range with graphene as additive. Thermochim. Acta 2018, 664, 16–25. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.A.; Xu, Y.; Dong, J. A review on supercooling of phase change materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Zeppieri, S.; Rodríguez, J.; López De Ramos, A.L. Interfacial tension of alkane + water systems. J. Chem. Eng. Data 2001, 46, 1086–1088. [Google Scholar] [CrossRef]
- Goebel, A.; Lunkenheimer, K. Interfacial tension of the water/ n-alkane interface. Langmuir 2002, 13, 369–372. [Google Scholar] [CrossRef]
- Oh, S.G.; Shah, D.O. Effect of counterions on the interfacial tension and emulsion droplet size in the oil/water/dodecyl sulfate system. J. Phys. Chem. 1993, 97, 284–286. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Lylyk, S.V.; Aksenenko, E.V.; Petkov, J.T.; Yorke, J.; Miller, R. Surface tension isotherms, adsorption dynamics and dilational visco-elasticity of sodium dodecyl sulphate solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 354, 8–15. [Google Scholar] [CrossRef]


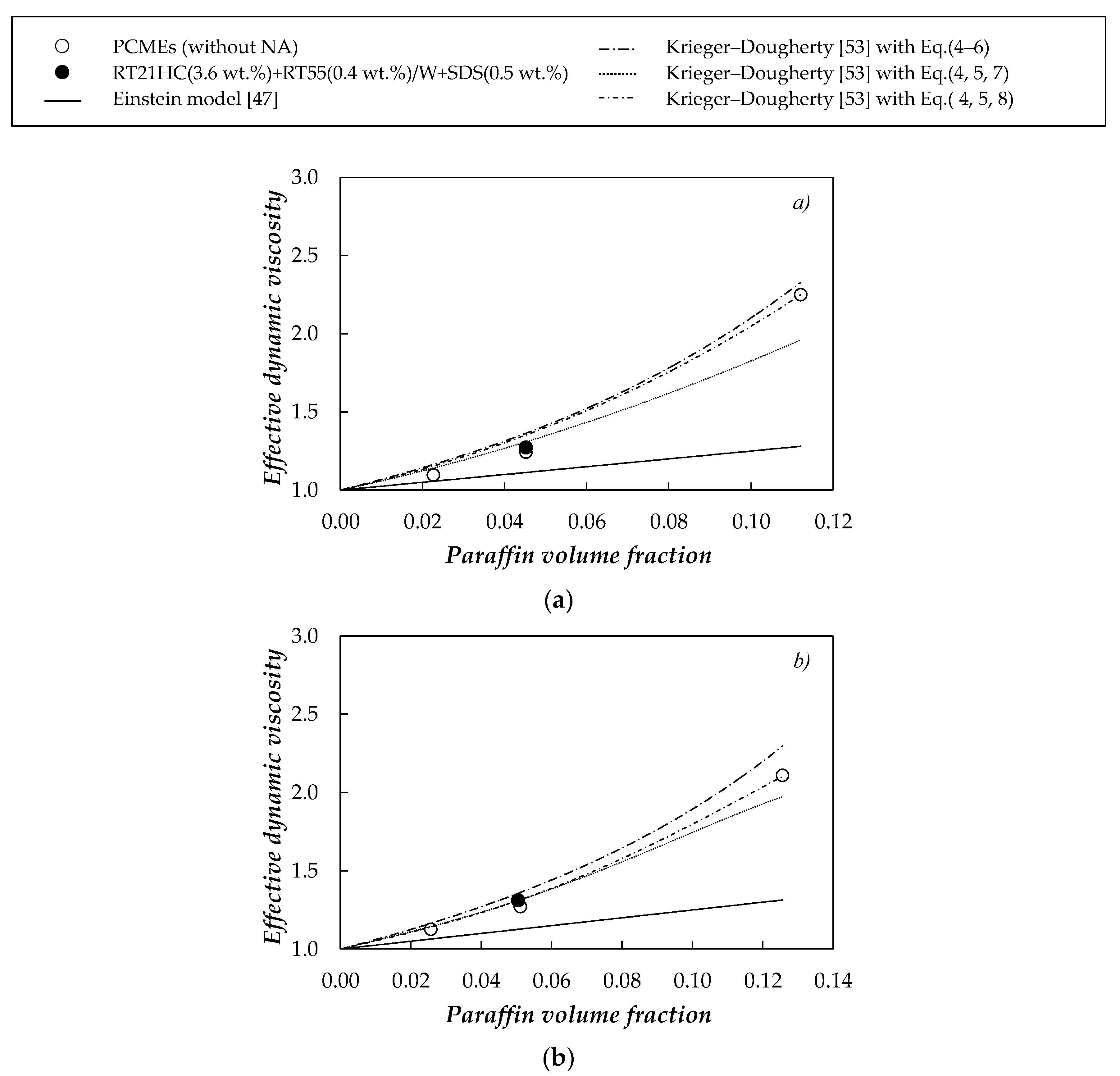
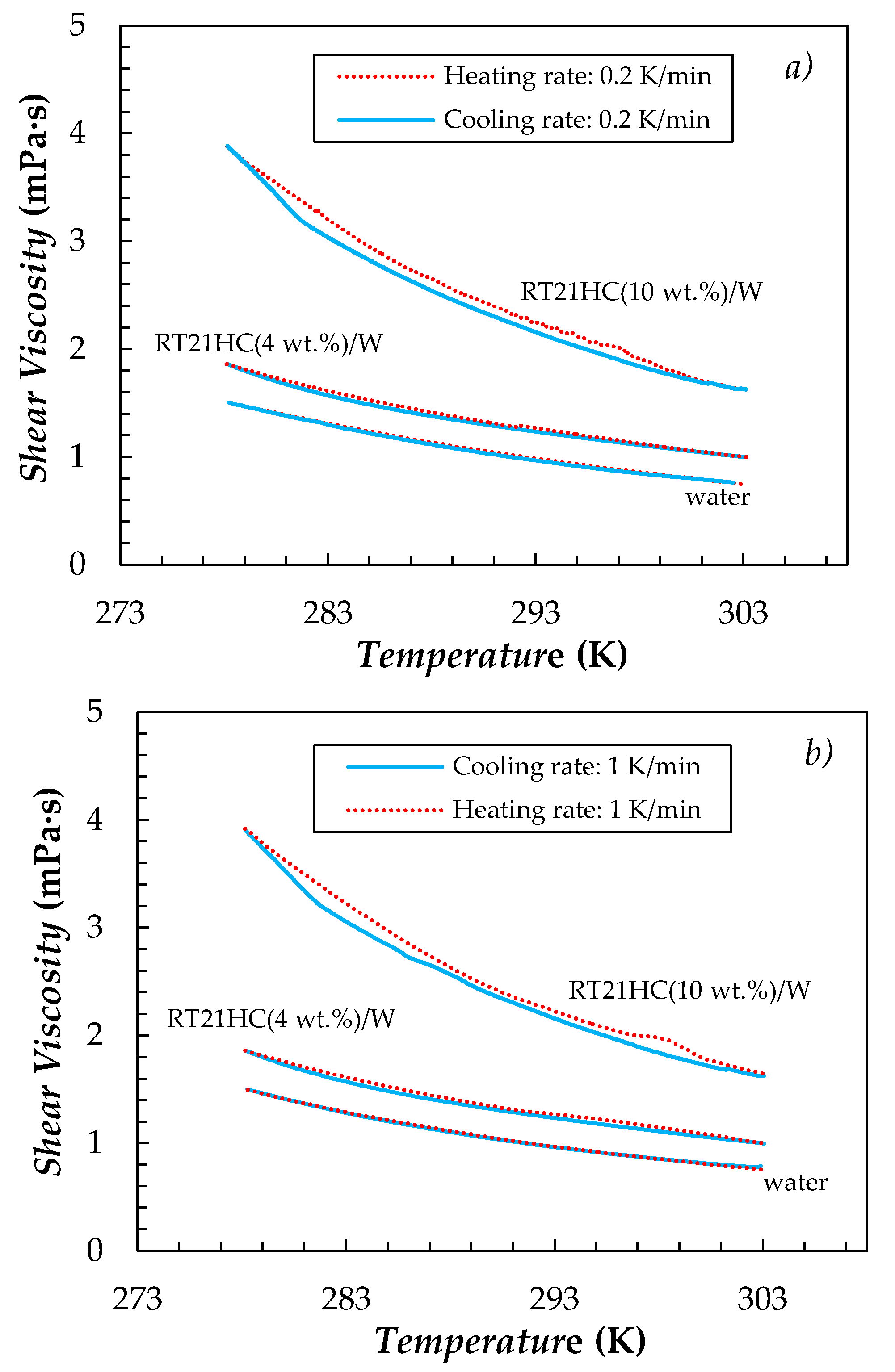
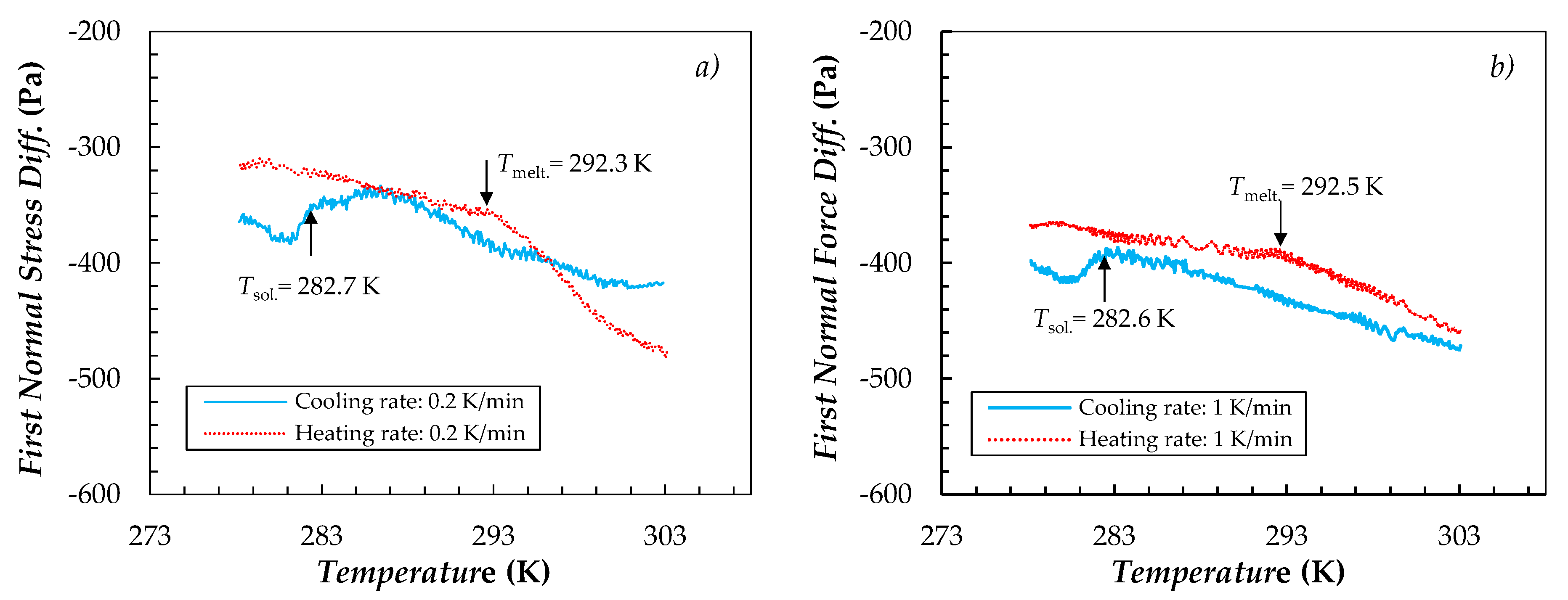
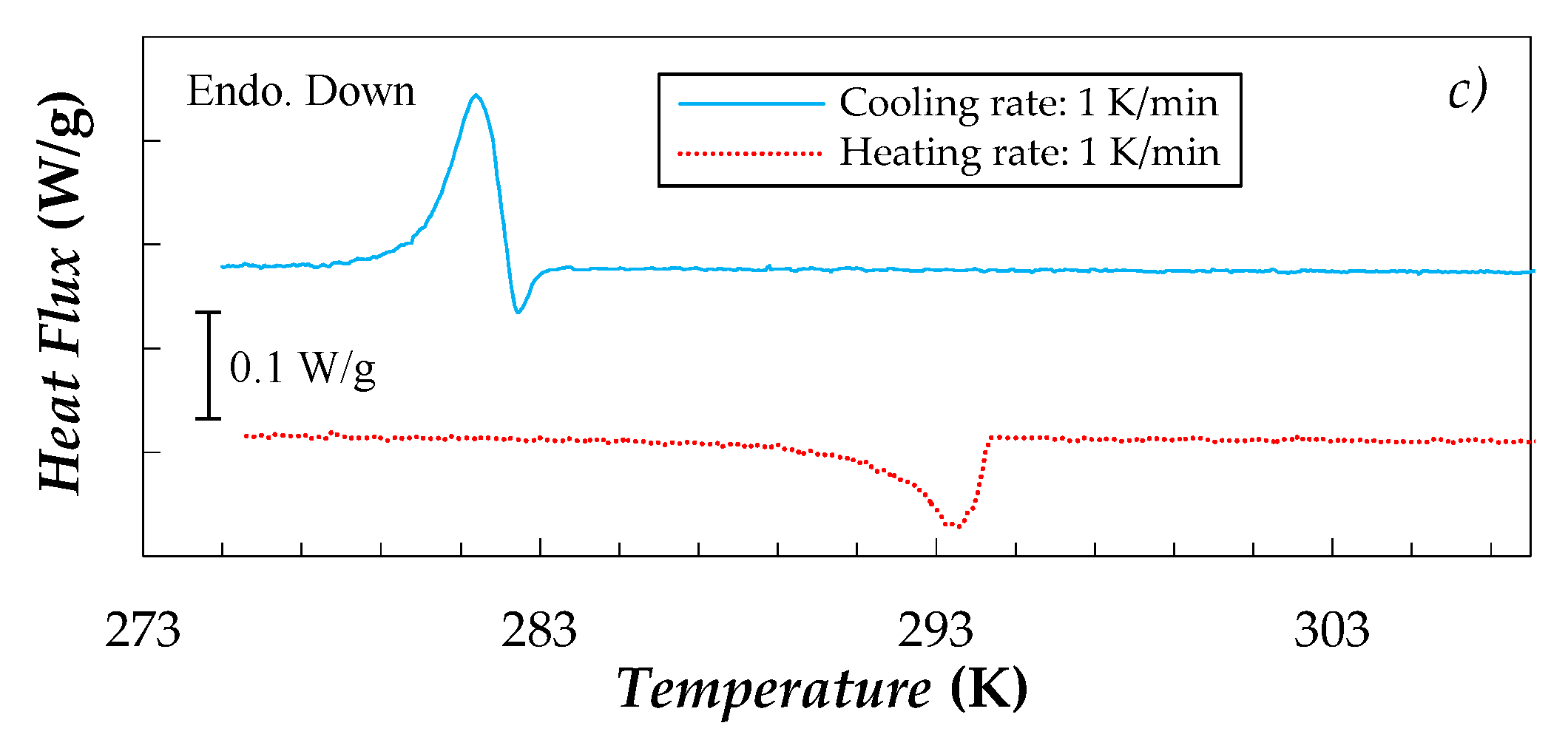
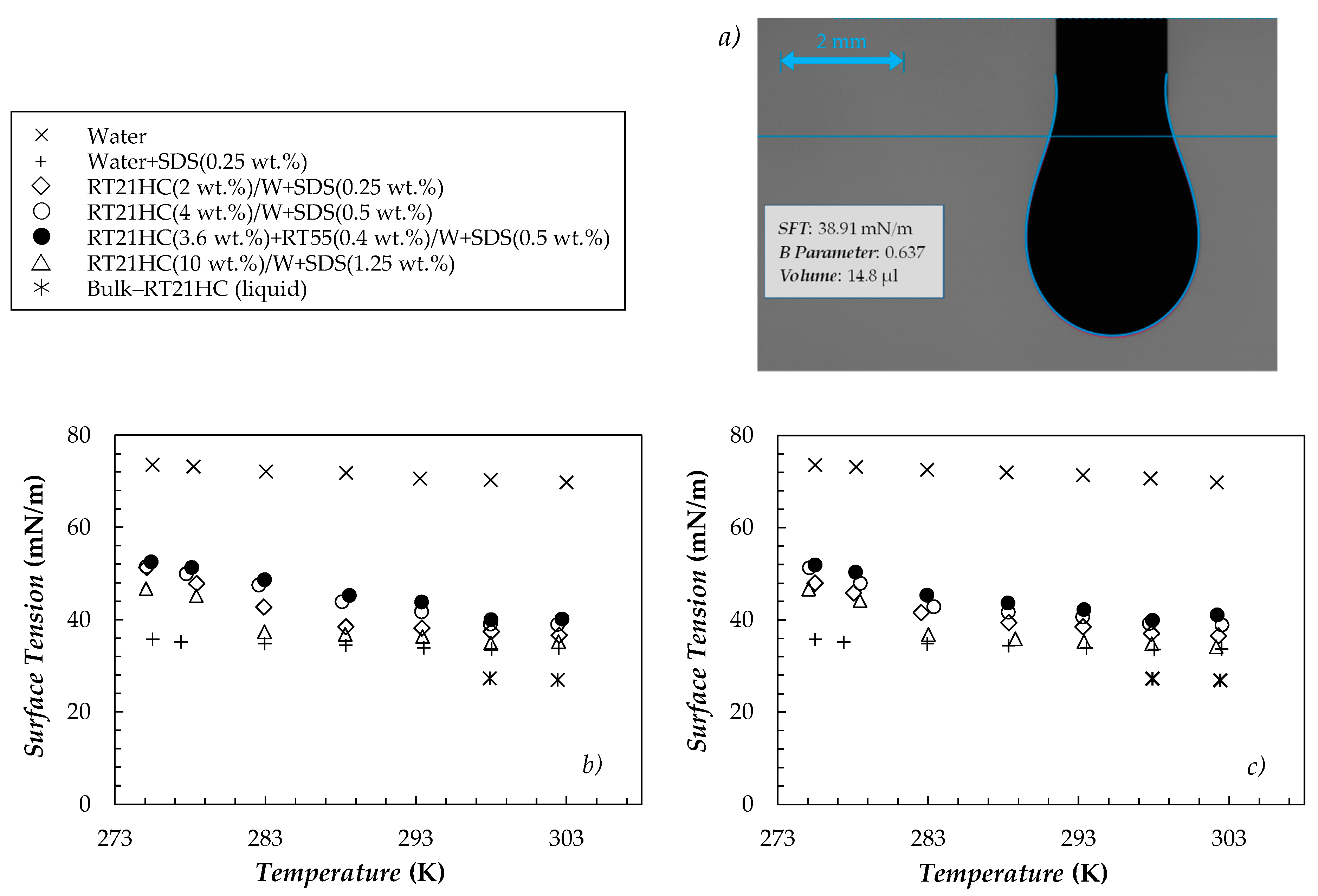
| Sample | T = 278.15 K | T = 303.15 K | ||
|---|---|---|---|---|
| µ (mPa·s) | µ/µwater | µ (mPa·s) | µ/µwater | |
| Water | 1.50 | 1.00 | 0.791 | 1.000 |
| Water+SDS(0.25 wt.%) | 1.55 | 1.03 | 0.802 | 1.014 |
| Water+SDS(0.5 wt.%) | 1.61 | 1.07 | 0.813 | 1.028 |
| Water+SDS(1.25 wt.%) | 1.67 | 1.11 | 0.865 | 1.094 |
| RT21HC(2 wt.%)/water+SDS(0.25 wt.%) | 1.70 | 1.13 | 0.89 | 1.13 |
| RT21HC(4 wt.%)/water+SDS(0.5 wt.%) | 2.01 | 1.34 | 1.01 | 1.28 |
| RT21HC(3.6 wt.%)+RT55(0.4 wt.%)/water+SDS(0.5 wt.%) | 2.05 | 1.37 | 1.04 | 1.31 |
| RT21HC(10 wt.%)/water+SDS(1.25 wt.%) | 3.77 | 2.51 | 1.67 | 2.11 |
| Bulk–RT21HC | - | - | 3.32 | - |
| System | T (K) | ITF (mN/m) |
|---|---|---|
| RT21HC–Water | 297.06 ± 0.04 | 41.95 ± 0.34 |
| RT21HC–Water+SDS(0.25 wt.%) | 296.57 ± 0.07 | 8.32 ± 0.09 |
| RT21HC–Water+SDS(0.5 wt.%) | 297.21 ± 0.03 | 8.20 ± 0.08 |
| RT21HC–Water+SDS(1.25 wt.%) | 297.44 ± 0.02 | 8.19 ± 0.14 |
| Sample | CA (°) | |
|---|---|---|
| T = 278 K | T = 298 K | |
| Water | 72 ± 2 | 69 ± 2 |
| RT21HC(2 wt.%)/W+SDS(0.25 wt.%) | 46 ± 2 | 37 ± 2 |
| RT21HC(4 wt.%)/W+SDS(0.5 wt.%) | 41 ± 2 | 35 ± 2 |
| RT21HC(3.6 wt.%)+RT55(0.4 wt.%)/W+SDS(0.5 wt.%) | 41 ± 1 | 36 ± 2 |
| RT21HC(10 wt.%)/W+SDS(1.25 wt.%) | 40 ± 2 | 33 ± 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabaleiro, D.; Hamze, S.; Agresti, F.; Estellé, P.; Barison, S.; Fedele, L.; Bobbo, S. Dynamic Viscosity, Surface Tension and Wetting Behavior Studies of Paraffin–in–Water Nano–Emulsions. Energies 2019, 12, 3334. https://doi.org/10.3390/en12173334
Cabaleiro D, Hamze S, Agresti F, Estellé P, Barison S, Fedele L, Bobbo S. Dynamic Viscosity, Surface Tension and Wetting Behavior Studies of Paraffin–in–Water Nano–Emulsions. Energies. 2019; 12(17):3334. https://doi.org/10.3390/en12173334
Chicago/Turabian StyleCabaleiro, David, Samah Hamze, Filippo Agresti, Patrice Estellé, Simona Barison, Laura Fedele, and Sergio Bobbo. 2019. "Dynamic Viscosity, Surface Tension and Wetting Behavior Studies of Paraffin–in–Water Nano–Emulsions" Energies 12, no. 17: 3334. https://doi.org/10.3390/en12173334
APA StyleCabaleiro, D., Hamze, S., Agresti, F., Estellé, P., Barison, S., Fedele, L., & Bobbo, S. (2019). Dynamic Viscosity, Surface Tension and Wetting Behavior Studies of Paraffin–in–Water Nano–Emulsions. Energies, 12(17), 3334. https://doi.org/10.3390/en12173334








